Microwave-Assisted Reduction Technology for Recycling of Hematite Nanoparticles from Ferrous Sulfate Residue
Abstract
1. Introduction
2. Experimental Section
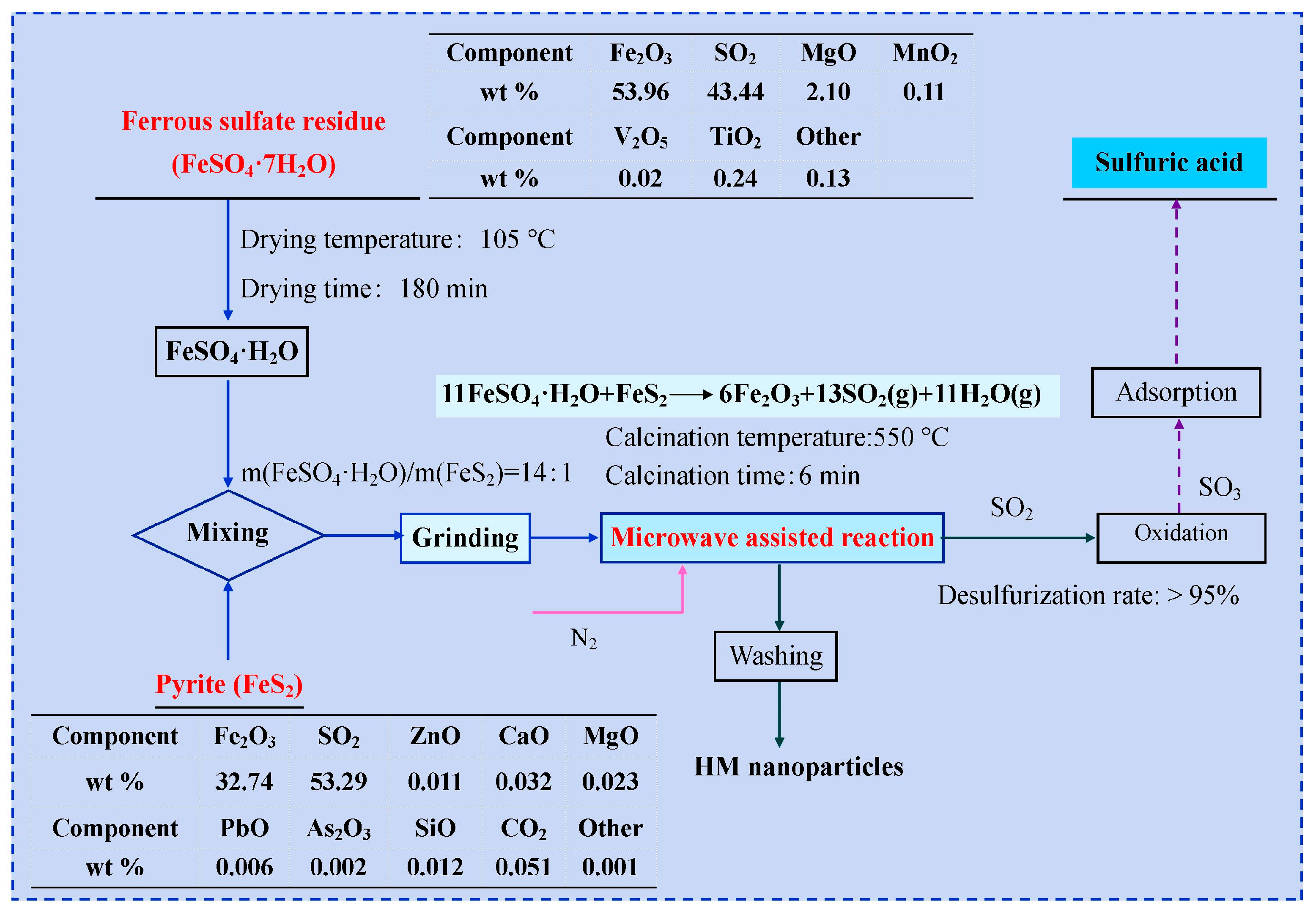
2.1. Materials
2.2. Fabrication of Hematite Nanoparticles
2.3. Characterization
3. Results and Discussion
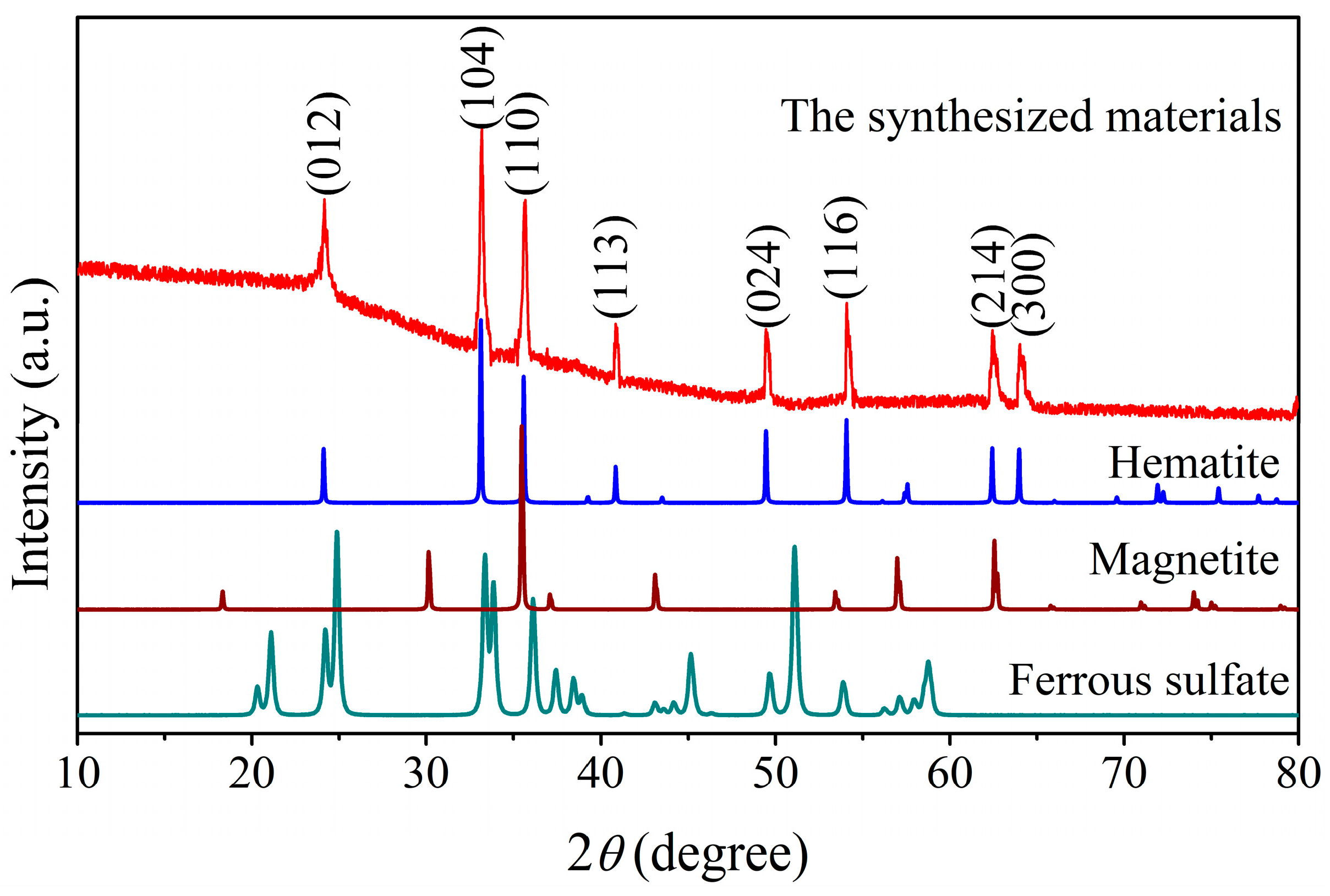
3.1. Crystal Structure
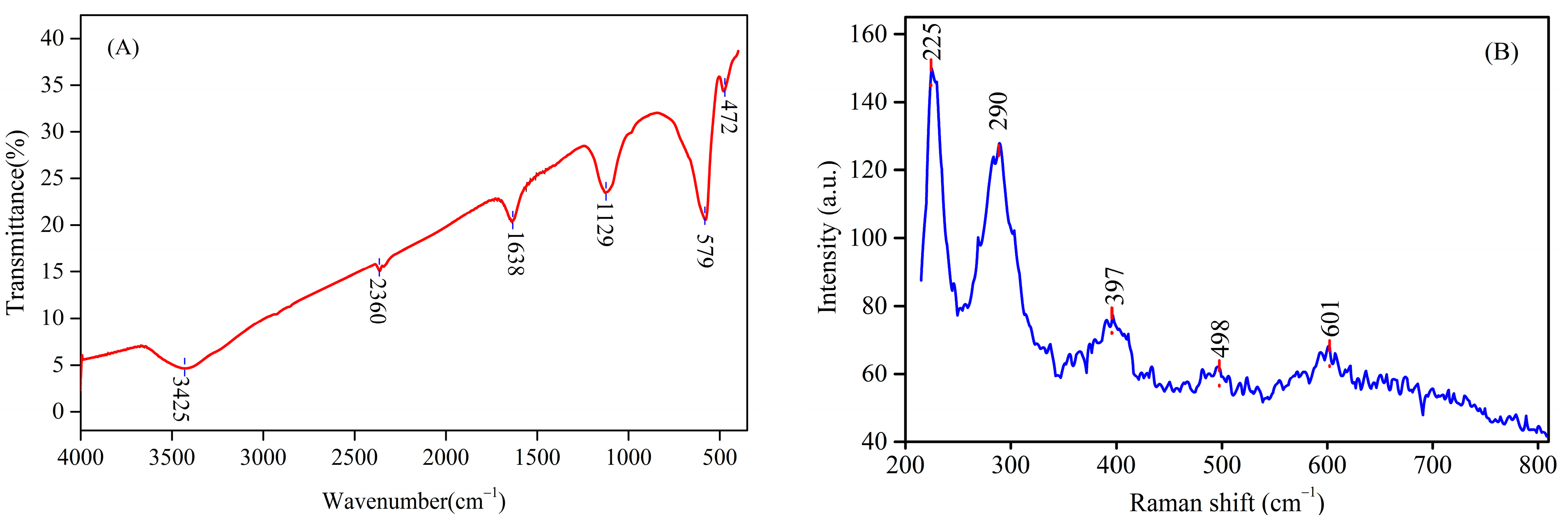
3.2. FTIR and RM Analysis
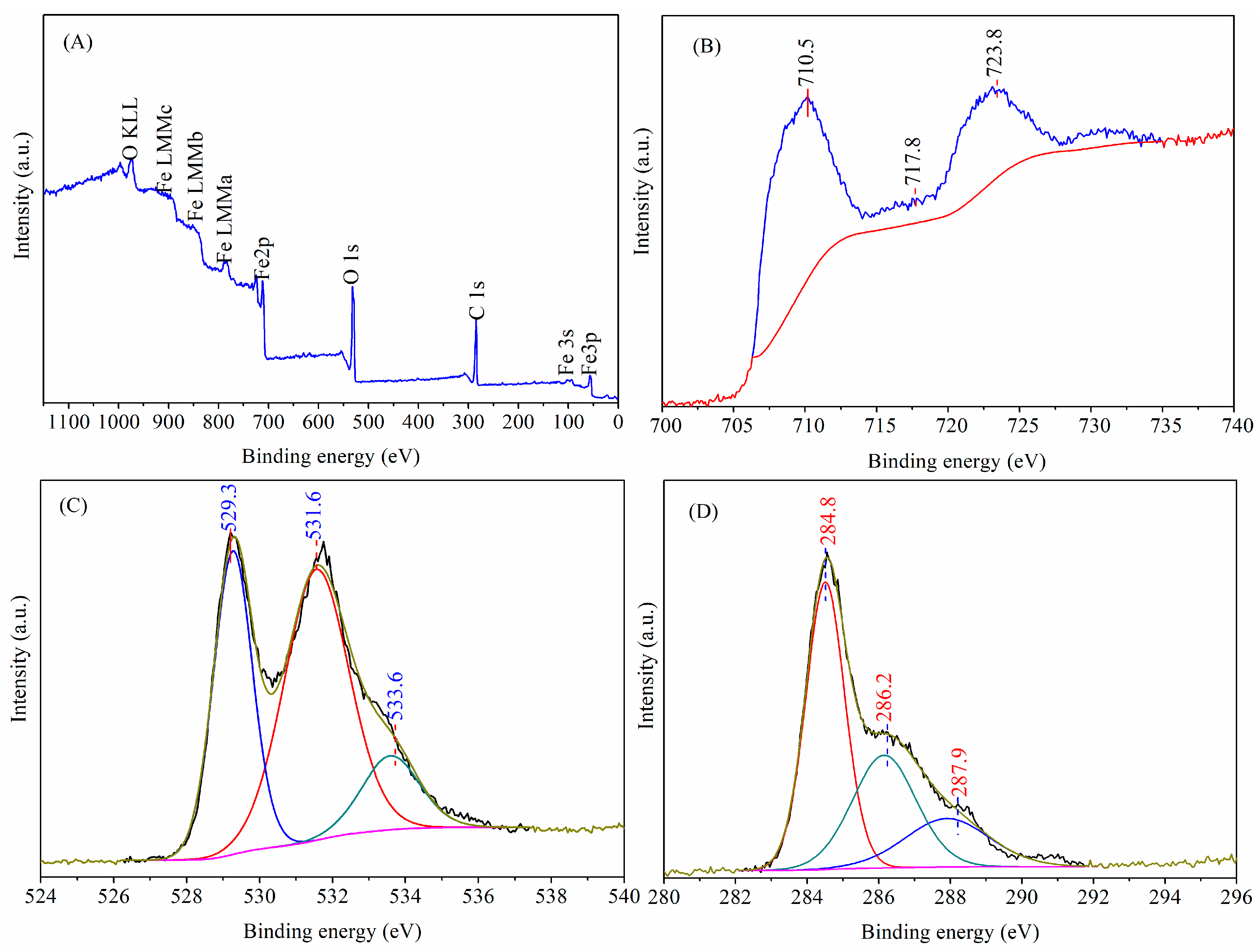
3.3. Chemical States
3.4. Microstructure and Element Composition
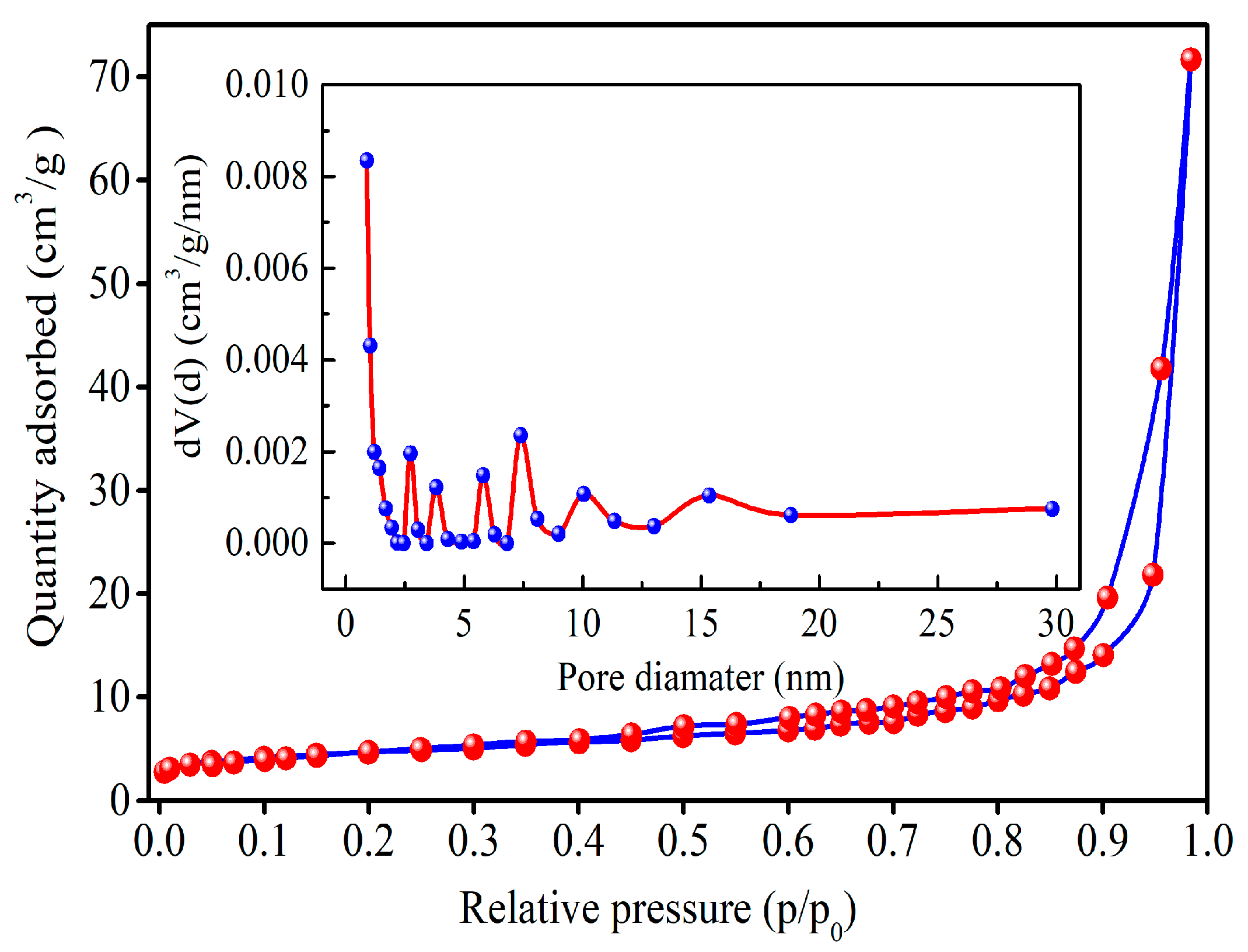
3.5. Specific Surface Area and Pore Size
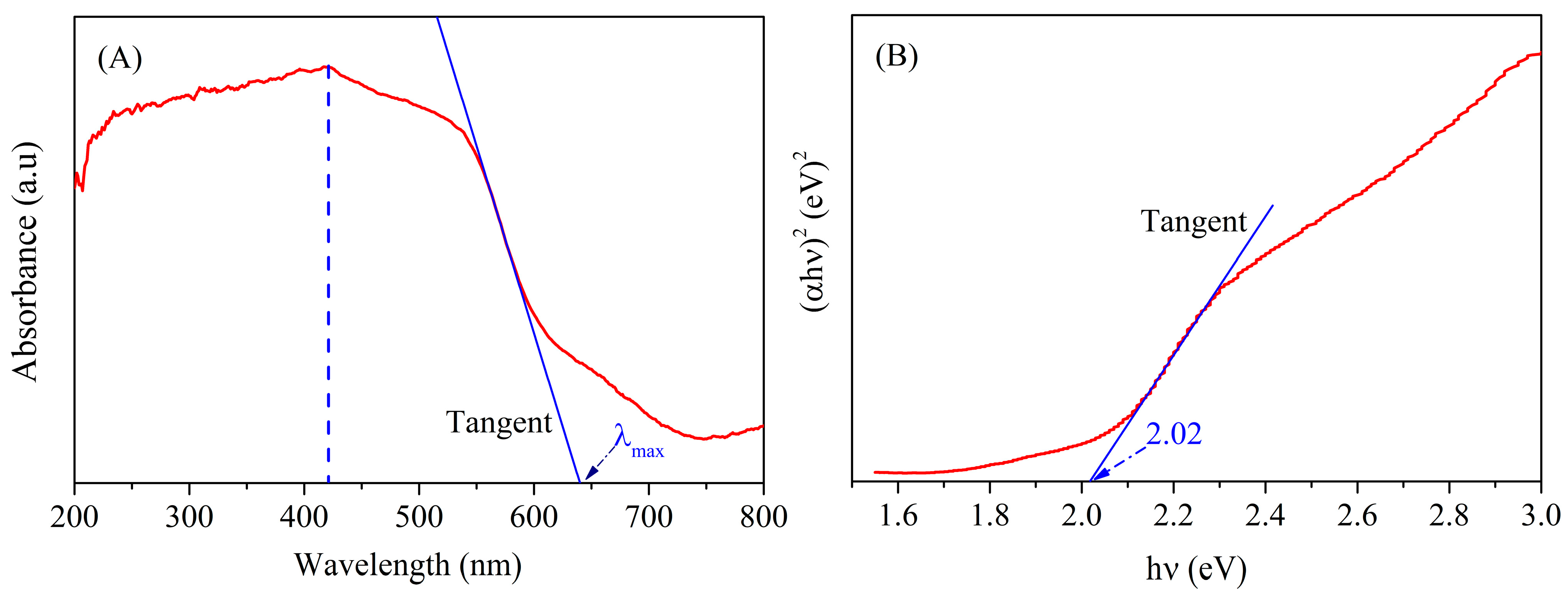
3.6. UV-Vis Spectrum
3.7. Magnetic Properties
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Li, M.; Deng, X.; Zhang, W.; Yao, M.; Zhang, X.; Huang, G.; Xu, S. Transforming waste to power: Selective control of associated impurity from ferrous sulfate waste for the preparation of self-doped LiFePO4. J. Energy Storage 2025, 117, 116168. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, X.; Han, Y.; Dong, Z. Preparation of battery-grade Fe2O3 and FePO4·2H2O materials from ferrous sulfate waste by chemical precipitation. J. Environ. Chem. Eng. 2025, 13, 116436. [Google Scholar] [CrossRef]
- Zhang, P.; Shu, Y.; Zhong, Y.; Yang, L.; Yang, X. CoZnFeO4 prepared by waste ferrous sulfate as iron source: Synthesis, characterization and photocatalytic degradation of methylene blue. J. Mater. Sci. Mater. Electron. 2021, 32, 20985–21011. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, C.; Zhou, K.; Hu, Z.; Zhang, G.; Wu, Y.; Zhang, J.; Chen, W. Recovery of iron from titanium white waste for the preparation of LiFePO4 battery. J. Clean. Prod. 2023, 415, 137817. [Google Scholar] [CrossRef]
- Meng, D.; Li, S.; Li, Z.; Li, Z.; Huang, P.; Guo, Y.; Li, H. Industrial by-products (ferrous sulfate minerals and stone powder) can serve as amendments to remediate Cd-As paddy soil, alleviating Cd-As accumulation in rice. Environ. Chem. Ecotoxicol. 2025, 7, 62–74. [Google Scholar] [CrossRef]
- Yu, W.; Peng, Y.; Zheng, Y. Recovery of iron from waste ferrous sulphate by co-precipitation and magnetic separation. Trans. Nonferrous Met. Soc. 2017, 27, 211–219. [Google Scholar] [CrossRef]
- Wang, W.; Shu, Y.; Xiang, H.; Xu, D.; Zhang, P.; Ren, G.; Zhong, Y.; Yang, X. Magnetic properties of Cu0.5Mg0.5Fe2O4 nanoparticles synthesized with waste ferrous sulfate. Mater. Today Commun. 2020, 25, 101516. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Zheng, B.; Zhang, Z.; Yang, L.; Yang, X. Fabrication of Mg doped magnetite nanoparticles by recycling of titanium slag and their application of arsenate adsorption. J. Clean. Prod. 2020, 252, 119599. [Google Scholar] [CrossRef]
- Peng, X.; Xu, D.; Yang, X.; Zhang, Z.; Ren, G.; Yang, L.; Zhong, Y.; Wang, X. A new green synthesis method of magnesium ferrite from ferrous sulfate waste. J. Alloys Compd. 2018, 756, 117–125. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zeng, Y.; Li, P.; Xie, T.; Zhang, Y. Bacteria-assisted preparation of nano α-Fe2O3 red pigment powders from waste ferrous sulfate. J. Hazard. Mater. 2016, 317, 563–569. [Google Scholar] [CrossRef]
- Quispe, L.T.; Mamani, L.G.L.; Baldárrago-Alcántara, A.A.; Félix, L.L.; Goya, G.F.; A Fuentes-García, J.; Pacheco-Salazar, D.G.; Coaquira, J.A.H. Synthesis and characterization of α-Fe2O3 nanoparticles showing potential applications for sensing quaternary ammonium vapor at room temperature. Nanotechnology 2022, 33, 335704. [Google Scholar] [CrossRef]
- Bouziani, A.; Yahya, M.; Naciri, Y.; Hsini, A.; Khan, M.A.; Sillanpää, M.; Celik, G. Development of polyaniline coated titania-hematite composite with enhanced photocatalytic activity under sun-like irradiation. Surf. Interfaces 2022, 34, 102328. [Google Scholar] [CrossRef]
- Tan, W.; Liang, Y.; Xu, Y.; Wang, M. Structural-controlled formation of nano-particle hematite and their removal performance for heavy metal ions: A review. Chemosphere 2022, 306, 135540. [Google Scholar] [CrossRef] [PubMed]
- Popov, N.; Ristić, M.; Bošković, M.; Perović, M.; Musić, S.; Stanković, D.; Krehula, S. Influence of Sn doping on the structural, magnetic, optical and photocatalytic properties of hematite (α-Fe2O3) nanoparticles. J. Phys. Chem. Solids 2022, 161, 110372. [Google Scholar] [CrossRef]
- Lu, P.A.; Manikandan, M.R.; Yang, P.F.; He, Y.L.; Yang, F.; Dang, S.T.; Shi, Y.C.; Lou, W.B.; Liu, R.; Wu, S.J.; et al. Synthesis, analysis and characterization of alpha-Fe2O3 nanoparticles and their applications in supercapacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 826. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Gadri, A.; Ammar, S. Synthesis, structural, optical and morphological characterization of hematite through the precipitation method: Effect of varying the nature of the base. J. Mol. Struct. 2017, 1141, 99–106. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R.; Shkir, M.; Ashraf, I.; Gedi, S. Hydrogen production and photocatalytic activity of HTAB assisted titanium doped α-Fe2O3 nanoparticles treated by microwave irradiation process. Inorg. Chem. Commun. 2022, 144, 109852. [Google Scholar] [CrossRef]
- Far, H.; Hamici, M.; Brihi, N.; Haddadi, K.; Boudissa, M.; Chihi, T.; Fatmi, M. High-performance photocatalytic degradation of NiO nanoparticles embedded on α-Fe2O3 nanoporous layers under visible light irradiation. J. Mater. Res. Technol. 2022, 19, 1944–1960. [Google Scholar] [CrossRef]
- Makalesi, A. Synthesis and Characterization of α-Fe2O3 Nanoparticles by Microemulsion Method. J. Sci. Technol. 2021, 13, 890–897. [Google Scholar]
- Nagaraj, P.; Murugesan, N.; Balakrishnan, K. Biogenic synthesis of Hematite (α-Fe2O3) and Ni doped Hematite (α-Fe2O3) nanoparticles for enhanced In vitro cytotoxic studies on MCF-7 cell lines. J. Indian Chem. Soc. 2024, 101, 101273. [Google Scholar] [CrossRef]
- Tadic, M.; Lazovic, J.; Panjan, M.; Tadic, B.V.; Lalatonne, Y. Solvothermal synthesis of hematite (α-Fe2O3) nanoparticles: Influence of surfactants on morphology, magnetic anisotropy, MRI relaxivity and biocompatibility. Inorg. Chem. Commun. 2025, 176, 114290. [Google Scholar] [CrossRef]
- Catto, A.C.; Bernardini, S.; Aguir, K.; Longo, E.; da Silva, L.F. In-situ hydrothermal synthesis of oriented hematite nanorods for sub-ppm level detection of ozone gas. J. Alloys Compd. 2023, 947, 169444. [Google Scholar] [CrossRef]
- Tadic, M.; Lazovic, J.; Panjan, M.; Tadic, B.V.; Lalatonne, Y. Low-coercivity behavior and biomedical potential of cube-like and rounded hematite (α-Fe2O3) nanoparticles: Insights from hydrothermal synthesis. Mater. Sci. Eng. B 2025, 317, 118204. [Google Scholar] [CrossRef]
- Ren, G.; Deng, Y.; Yang, X. A Novel Fabrication of Hematite Nanoparticles via Recycling of Titanium Slag by Pyrite Reduction Technology. Nanomaterials 2024, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Comparison of microwave and conventional carbothermal reduction of red mud for recovery of iron values. Miner. Eng. 2019, 132, 202–210. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Anushkkaran, P.; Mahadik, M.A.; Chae, W.S.; Lee, H.H.; Choi, S.H.; Jang, J.S. Microwave-assisted sequential Pt/Al attachment on FeOOH for fabrication of highly efficient hematite photoanodes: Synergistic effect of Pt/Al co-doping and Al2O3 passivation layer. Appl. Surf. Sci. 2023, 623, 157035. [Google Scholar] [CrossRef]
- Potnuri, R.; Rao, C.S.; Lenka, M.; Sridevi, V.; Basak, T. Microwave-assisted torrefaction of lignocellulosic biomass: A critical review of its role in sustainable energy. Biomass Bioenergy 2025, 197, 107777. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Anushkkaran, P.; Hwang, J.B.; Chae, W.-S.; Kumar, M.; Lee, H.-H.; Choi, S.H.; Jang, J.S.; Lee, J.S. Microwave-assisted metal-ion attachment for ex-situ zirconium doping into hematite for enhanced photoelectrochemical water splitting. Renew. Energy 2022, 189, 694–703. [Google Scholar] [CrossRef]
- Hintsho-Mbita, N.C.; Maeko, M.; Mathipa, M.M.; Tetana, Z.N. Photodegradation of methylene blue dye and deactivation of E.coli and S.aureus using C.benghalensis zinc ferrite nanoparticles. Desalin. Water Treat. 2025, 321, 101003. [Google Scholar] [CrossRef]
- Dargahi, Z.; Ahmadi-Arpanah, A.; Moradpur-Tari, E.; Yarahmadi, M.; Kavanlouei, M.; Maleki-Ghaleh, H.; Arator, D.N.; Mehr, M.E.; Shakeri, M.S.; Paczesny, J.; et al. Synthesis and characterization of zinc-doped hematite nanoparticles for photocatalytic applications and their electronic structure studies by density functional theory. Opt. Mater. 2024, 157, 116234. [Google Scholar] [CrossRef]
- Vinayagam, R.; Patnaik, Y.; Brijesh, P.; Prabhu, D.; Quadras, M.; Pai, S.; Narasimhan, M.K.; Kaviyarasu, K.; Varadavenkatesan, T.; Selvaraj, R. Superparamagnetic hematite spheroids synthesis, characterization, and catalytic activity. Chemosphere 2022, 294, 133730. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Huang, P.; Zhong, B.; Zhang, Z.; Yang, L.; Yang, X. Chromium (VI) adsorption from wastewater using porous magnetite nanoparticles prepared from titanium residue by a novel solid-phase reduction method. Sci. Total Environ. 2017, 607–608, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, M.; Mani, R.J.; Parvathiraja, C.; Kader, S.M.A.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Photocatalytic Dye Degradation and Bio-Insights of Honey-Produced α-Fe2O3 Nanoparticles. Water 2022, 14, 2301. [Google Scholar] [CrossRef]
- Marshall, C.P.; Dufresne, W.J.B. Resonance Raman and polarized Raman scattering of single-crystal hematite. J. Raman Spectrosc. 2022, 53, 947–955. [Google Scholar] [CrossRef]
- Liang, X.; Wang, L.; Wen, T.; Liu, H.; Zhang, J.; Liu, Z.; Zhu, C.; Long, C. Mesoporous poorly crystalline alpha-Fe2O3 with abundant oxygen vacancies and acid sites for ozone decomposition. Sci. Total Environ. 2022, 804, 150161. [Google Scholar] [CrossRef] [PubMed]
- Noukelag, S.K.; Arendse, C.J.; Maaza, M. Biosynthesis of hematite phase α-Fe2O3 nanoparticles using an aqueous extract of Rosmarinus officinalis leaves. Mater. Today Proc. 2020, 43, 3679–3683. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, Z.; Chai, X.; Weng, Z.; Ding, R.; Dong, L. Fabrication of hematite (α-Fe2O3) nanoparticles using electrochemical deposition. Appl. Surf. Sci. 2016, 368, 303–308. [Google Scholar] [CrossRef]
- Demirci, S.; Yurddaskal, M.; Dikici, T.; Sarıoğlu, C. Fabrication and characterization of novel iodine doped hollow and mesoporous hematite (Fe2O3) particles derived from sol-gel method and their photocatalytic performances. J. Hazard. Mater. 2018, 345, 27–37. [Google Scholar] [CrossRef]
- Saiphaneendra, B.; Saxena, T.; Singh, S.A.; Madras, G.; Srivastava, C. Synergistic effect of co-existence of hematite (α-Fe2O3) and magnetite (Fe3O4 ) nanoparticles on graphene sheet for dye adsorption. J. Environ. Chem. Eng. 2017, 5, 26–37. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, R.; Chai, Z.; Pu, J.; Kang, R.; Wu, G.; Zeng, X. Synergies of Zn/P-co-doped α-Fe2O3 photoanode for improving photoelectrochemical water splitting performance. Int. J. Hydrogen Energ. 2024, 59, 22–29. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Han, D.; Liu, X.; Yu, X.; Xiong, J.; Li, Y.; Zhao, Z.; Liu, J.; Wei, Y. Activating well-defined α-Fe2O3 nanocatalysts by near-surface Mn atom functionality for auto-exhaust soot purification. Appl. Catal. B Environ. 2023, 321, 122077. [Google Scholar] [CrossRef]
- Cao, Z.; Qin, M.; Jia, B.; Gu, Y.; Chen, P.; Volinsky, A.A.; Qu, X. One pot solution combustion synthesis of highly mesoporous hematite for photocatalysis. Ceram. Int. 2015, 41, 2806–2812. [Google Scholar] [CrossRef]
- Sajjadi, S.H.; Goharshadi, E.K. Highly monodispersed hematite cubes for removal of ionic dyes. J. Environ. Chem. Eng. 2017, 5, 1096–1106. [Google Scholar] [CrossRef]
- Prabhu, P.; Rao, M.; Murugesan, G.; Narasimhan, M.K.; Varadavenkatesan, T.; Vinayagam, R.; Chi, N.T.L.; Pugazhendhi, A.; Selvaraj, R. Synthesis, characterization and anticancer activity of the green-synthesized hematite nanoparticles. Environ. Res. 2022, 214, 113864. [Google Scholar] [CrossRef]
- Mahmoudabadi, Z.S.; Rashidi, A.; Maklavany, D.M. Optimizing treatment of alcohol vinasse using a combination of advanced oxidation with porous α-Fe2O3 nanoparticles and coagulation- flocculation. Ecotoxicol. Environ. Saf. 2022, 234, 113354. [Google Scholar] [CrossRef]
- Alenad, A.M.; Waheed, M.S.; Aman, S.; Ahmad, N.; Khan, A.R.; Khosa, R.Y.; Ansari, M.Z.; Khan, S.A.; Farid, H.M.T.; Taha, T.A.M. Visible light driven Ni doped hematite for photocatalytic reduction of noxious methylene blue. Mater. Res. Bull. 2023, 165, 112306. [Google Scholar] [CrossRef]
- Algarni, S.A.; Aman, S.; Ahmad, N.; Khan, S.A.; Farid, H.M.T.; Taha, T.A.M. Processing of Nb doped hematite for visible light photocatalytic reduction of noxious methylene blue. Optik 2023, 287, 171097. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, P.; Duan, M.; He, B.; Li, X.; Xin, X. Preparation of direct Z-scheme heterojunction la-doped Bi2WO6/CdS with enhanced visible light absorption and its photocatalytic degradation of antibiotics. Sep. Purif. Technol. 2024, 338, 126524. [Google Scholar] [CrossRef]
- Guo, T.; Jiang, L.; Wang, K.; Li, Y.; Huang, H.; Wu, X.; Zhang, G. Efficient persulfate activation by hematite nanocrystals for degradation of organic pollutants under visible light irradiation: Facet-dependent catalytic performance and degradation mechanism. Appl. Catal. B Environ. 2021, 286, 119883. [Google Scholar] [CrossRef]
- Rivera, E.; Muñoz-Meneses, R.A.; Marín, L.; Mora, M.; Tabares, J.A.; Manotas-Albor, M.; Rodríguez, L.A.; Diosa, J.E.; Mosquera-Vargas, E. Structural, optical, and magnetic properties of submicron hematite (α-Fe2O3) particles synthesized from industrial steel waste. Mater. Sci. Eng. B 2023, 288, 116170. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Removal of Cu(II), Zn(II) and Pb(II) from water using microwave- assisted synthesized maghemite nanotubes. Chem. Eng. J. 2012, 211–212, 493–500. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G. Microwave-Assisted Reduction Technology for Recycling of Hematite Nanoparticles from Ferrous Sulfate Residue. Materials 2025, 18, 3214. https://doi.org/10.3390/ma18143214
Ren G. Microwave-Assisted Reduction Technology for Recycling of Hematite Nanoparticles from Ferrous Sulfate Residue. Materials. 2025; 18(14):3214. https://doi.org/10.3390/ma18143214
Chicago/Turabian StyleRen, Genkuan. 2025. "Microwave-Assisted Reduction Technology for Recycling of Hematite Nanoparticles from Ferrous Sulfate Residue" Materials 18, no. 14: 3214. https://doi.org/10.3390/ma18143214
APA StyleRen, G. (2025). Microwave-Assisted Reduction Technology for Recycling of Hematite Nanoparticles from Ferrous Sulfate Residue. Materials, 18(14), 3214. https://doi.org/10.3390/ma18143214





