Investigation of Corrosion Resistance of 60Si2MnA Spring Steel Coated with Zn-Al in Atmospheric Environments
Abstract
1. Introduction
2. Experiment
2.1. Materials and Sample Preparation
2.2. Indoor Accelerated Corrosion Test
2.3. Weight Loss Analysis
2.4. Characterization
2.5. Electrochemical Tests
3. Results and Discussion
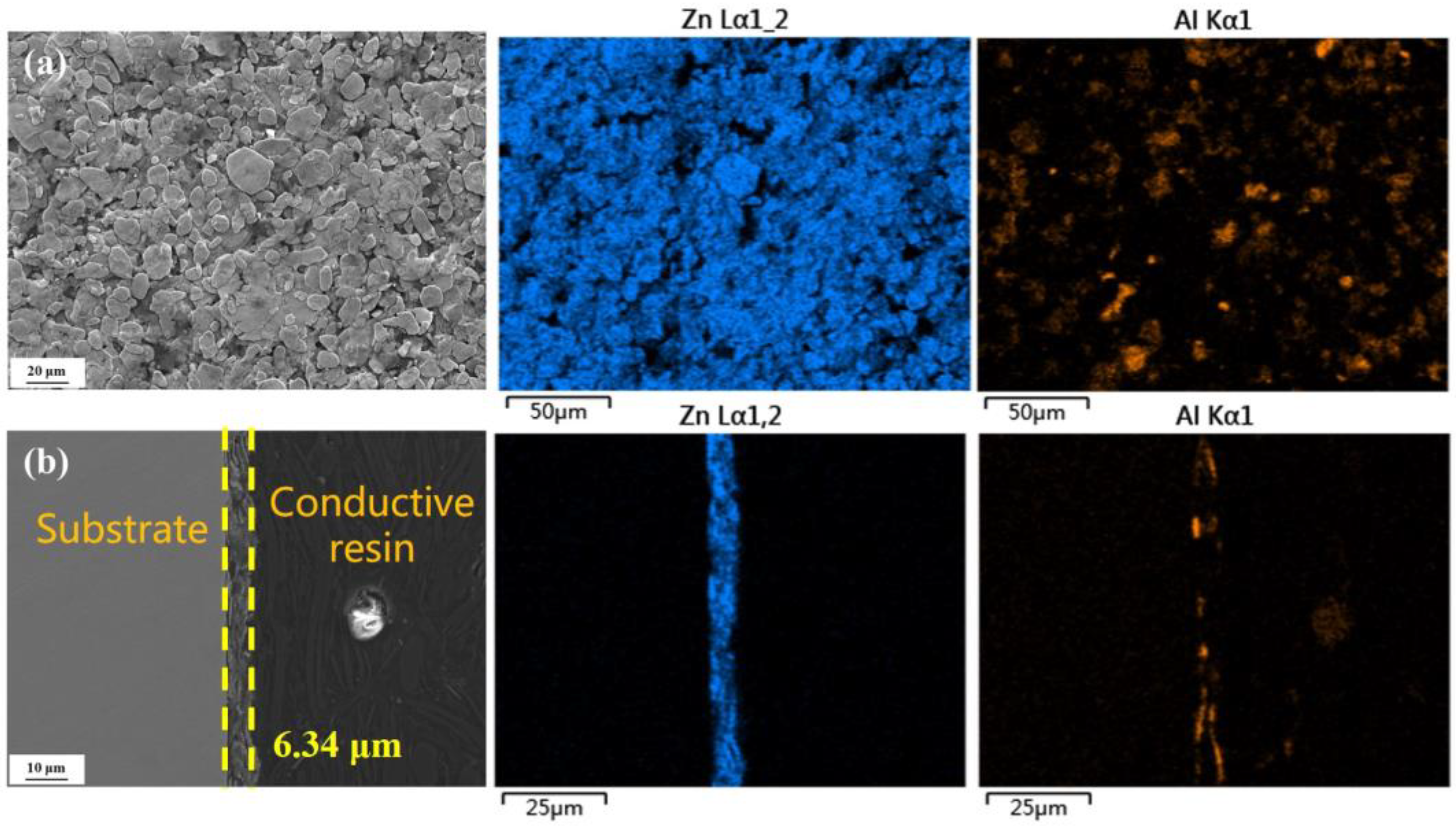
3.1. Characterization of the Original Sample
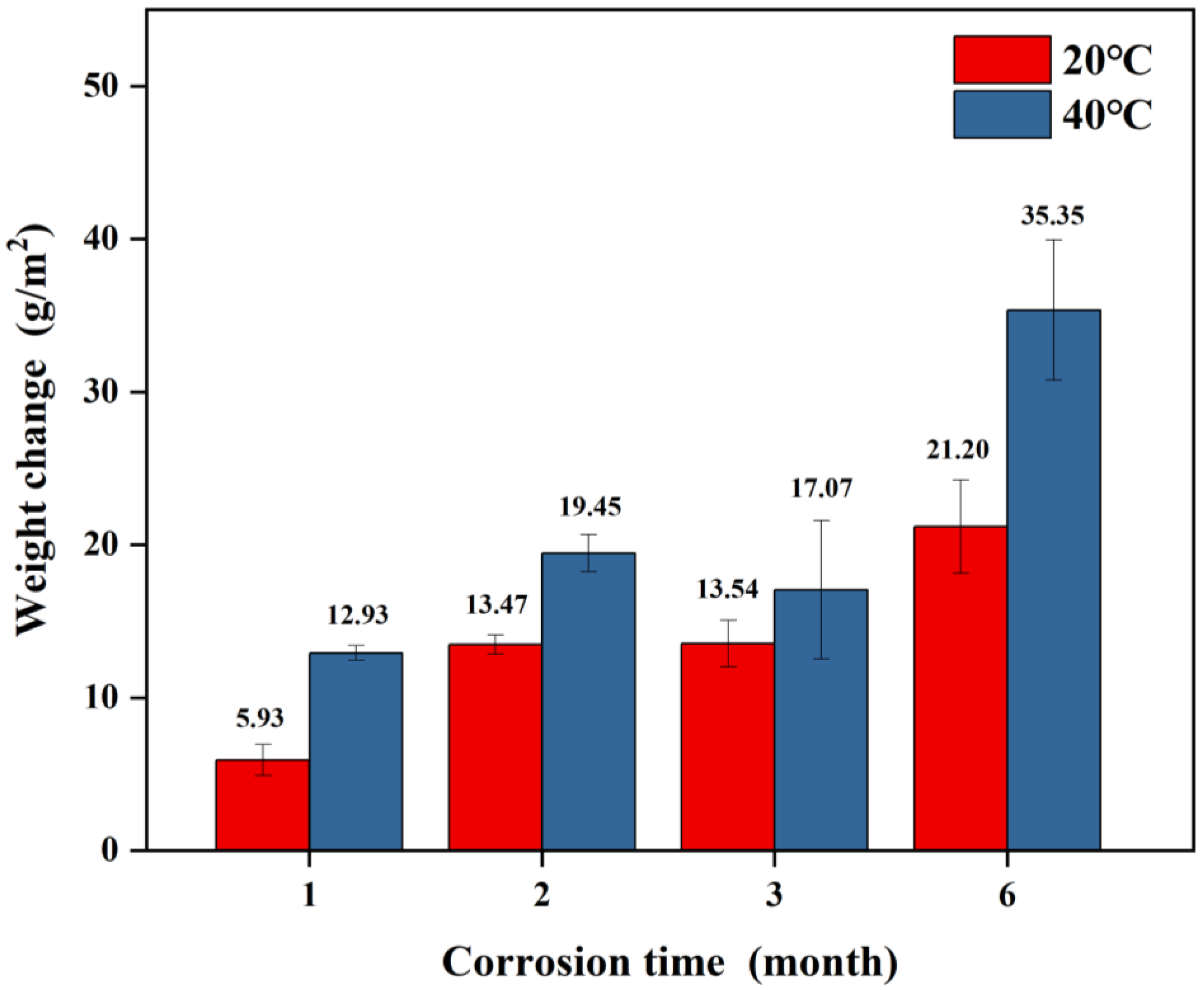
3.2. Corrosion Weight Change
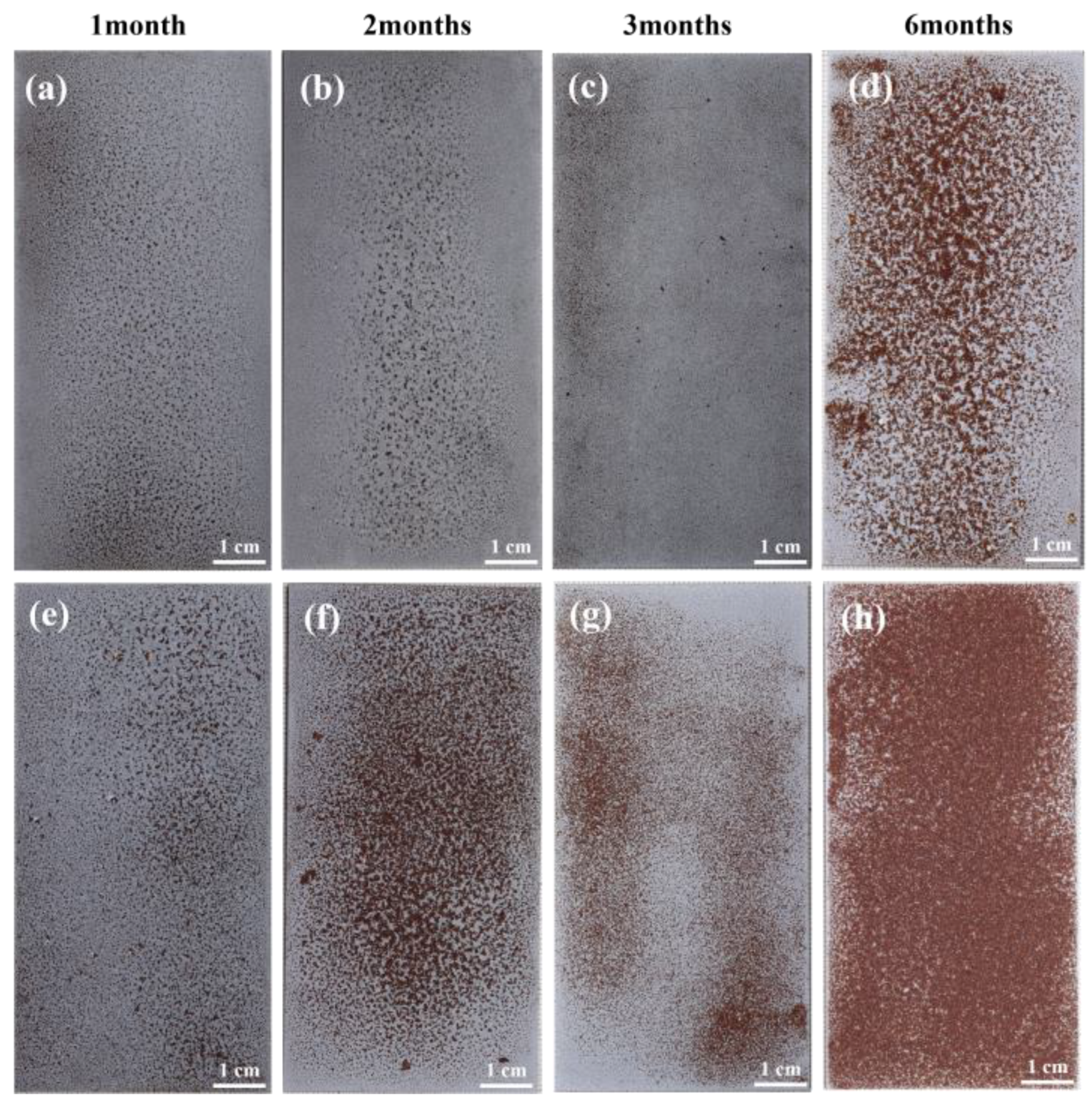
3.3. Macroscopic Morphology of Corrosion
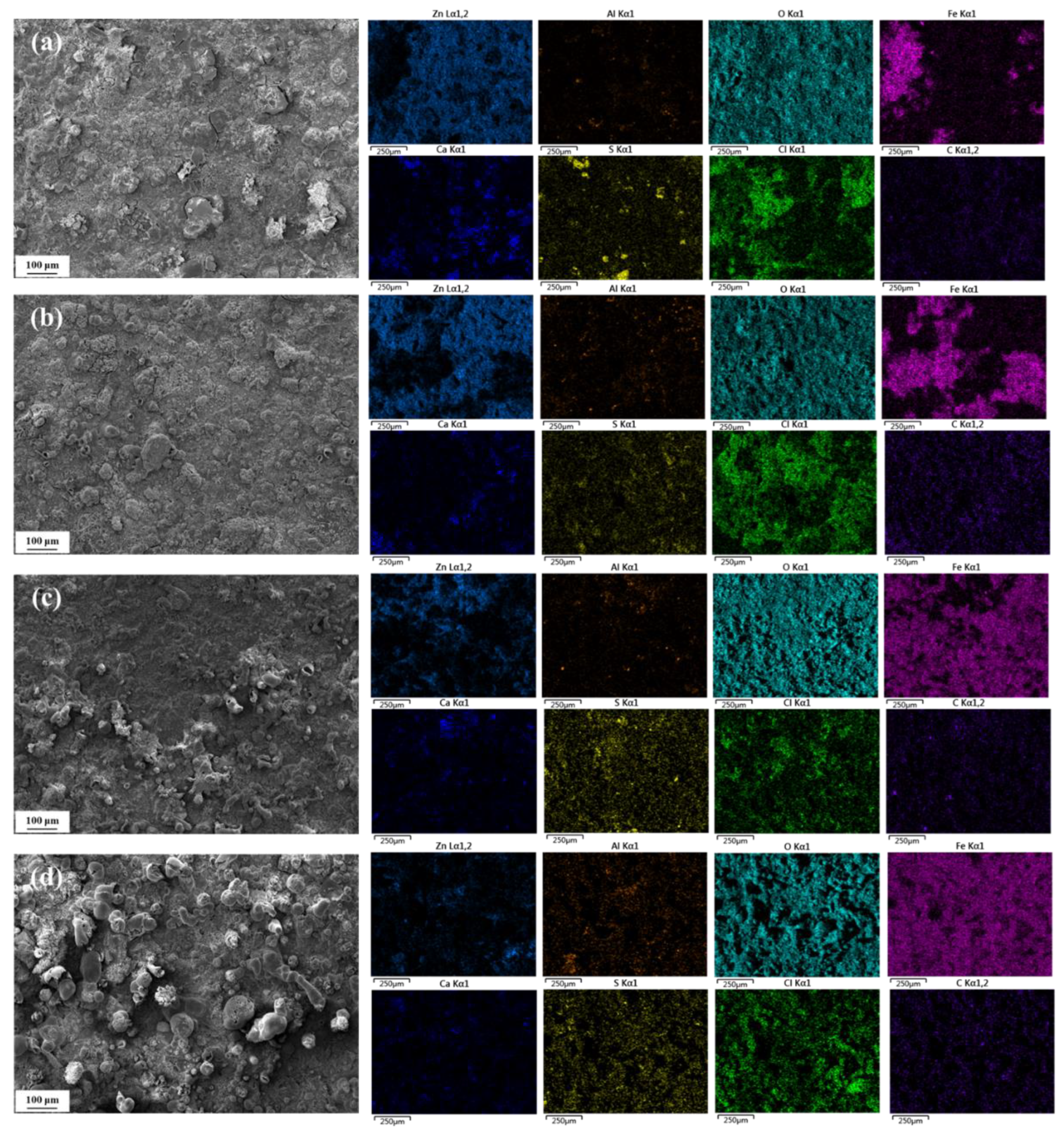
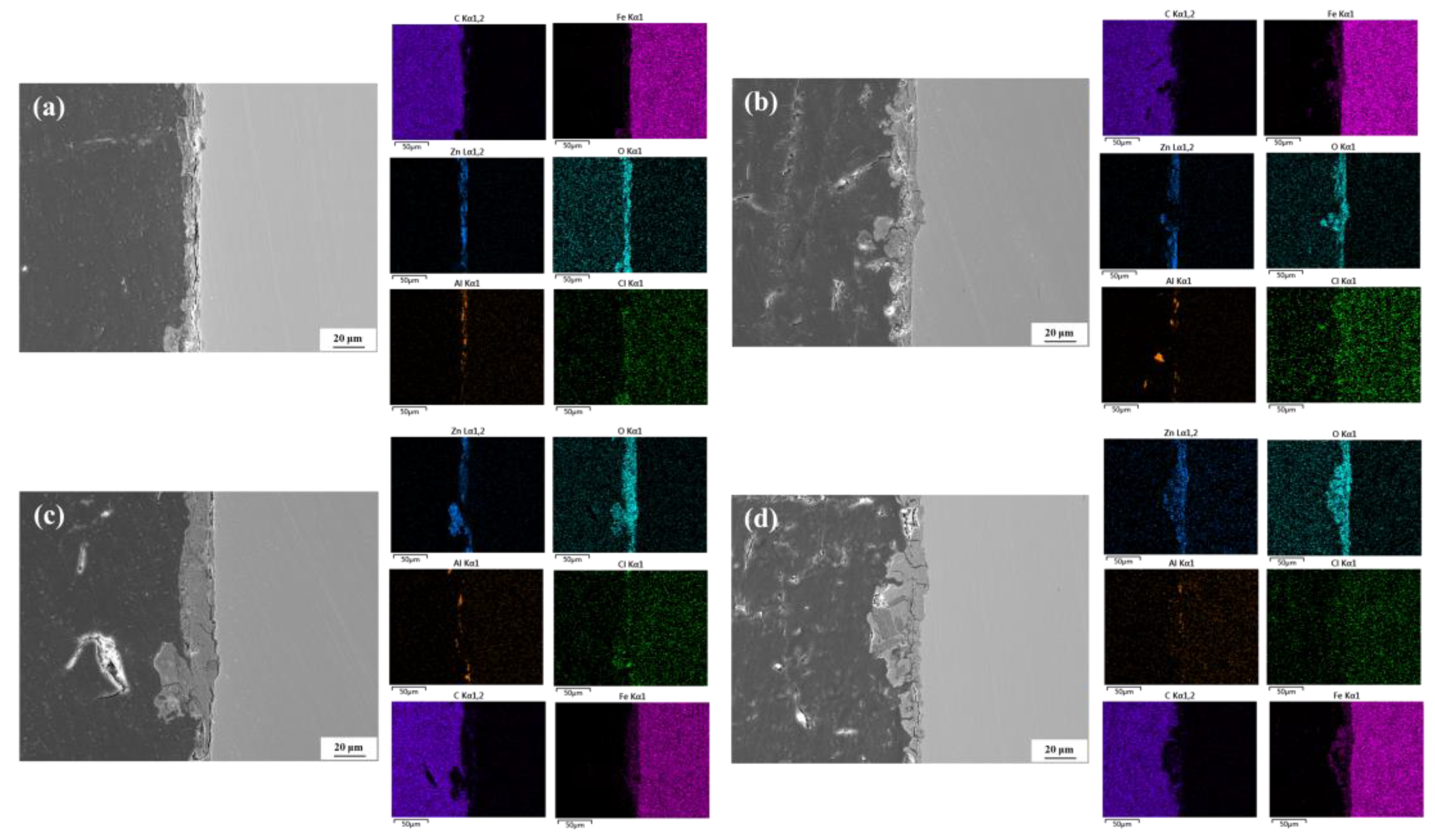
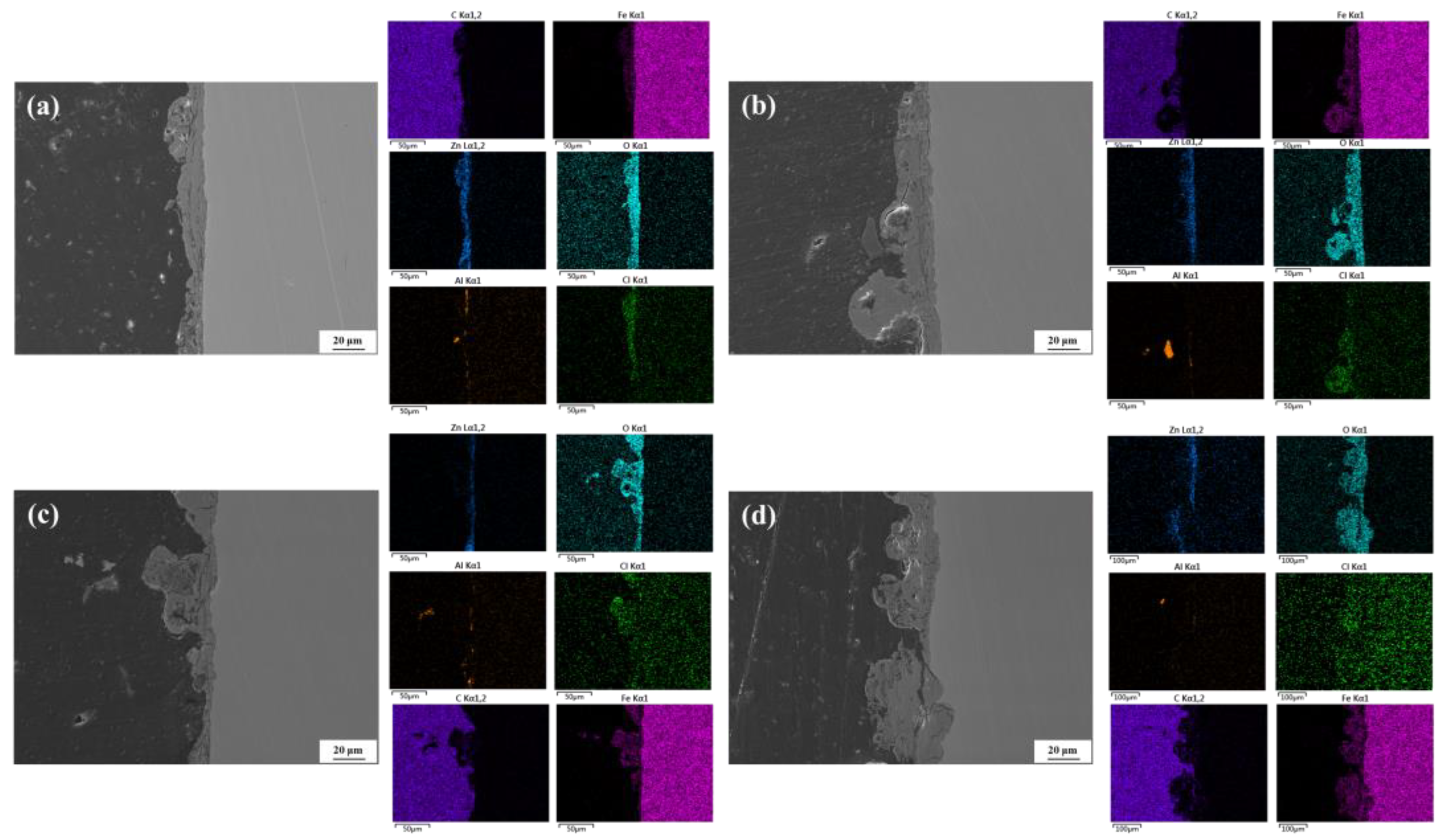
3.4. Microscopic Morphology of Corrosion
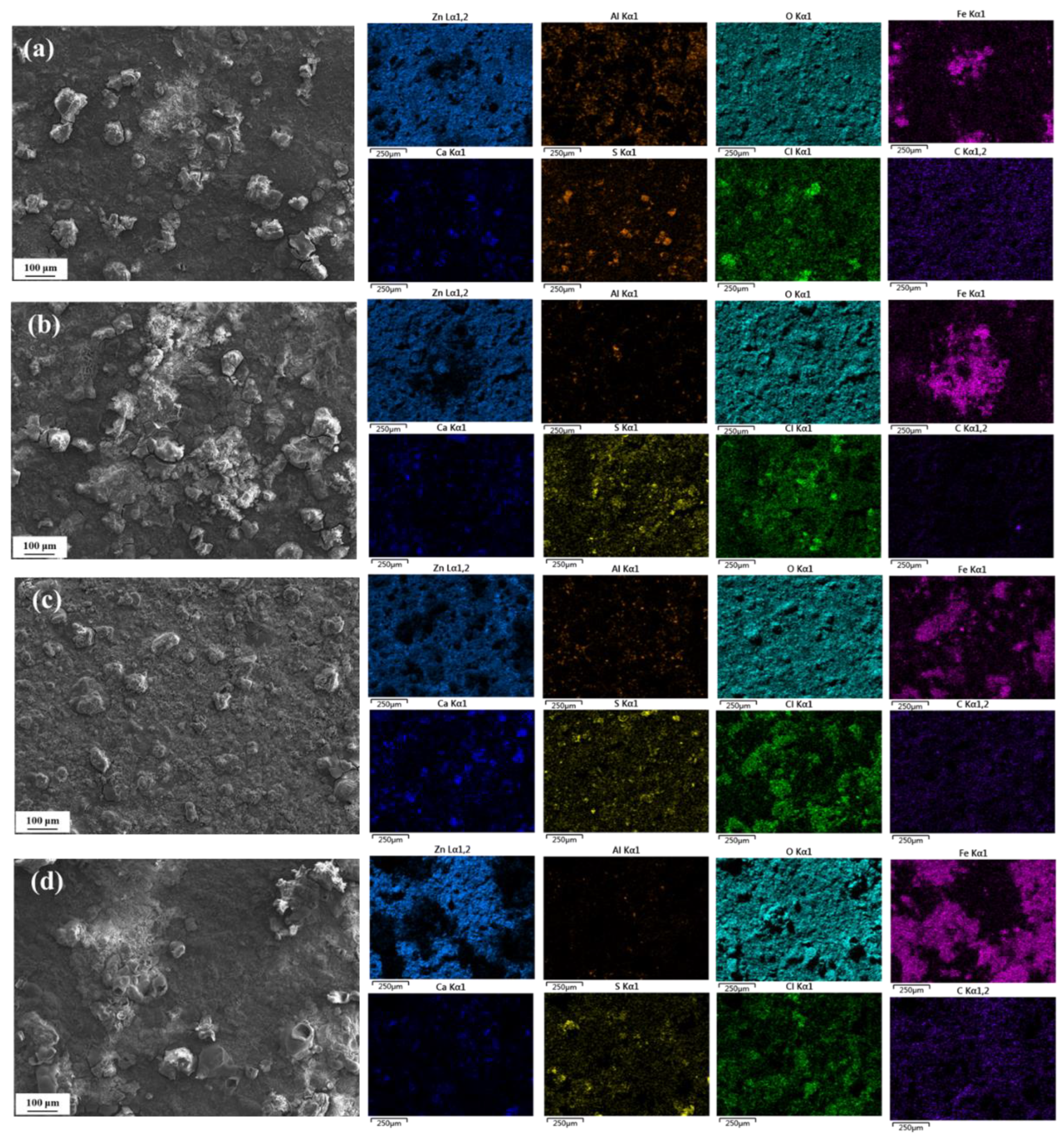
3.4.1. Surface Morphology
3.4.2. Cross-Sectional Morphology
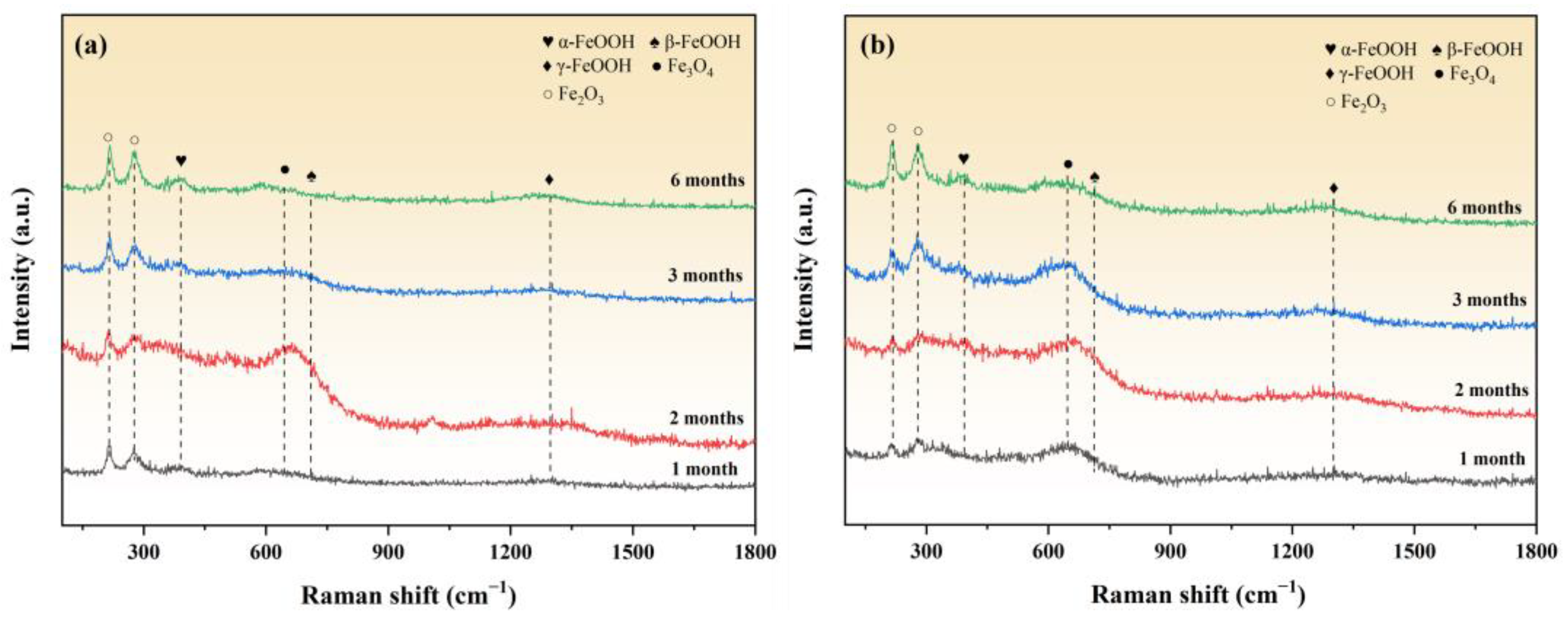
3.5. Corrosion Product Analysis
3.5.1. XRD Analysis
3.5.2. Raman Analysis
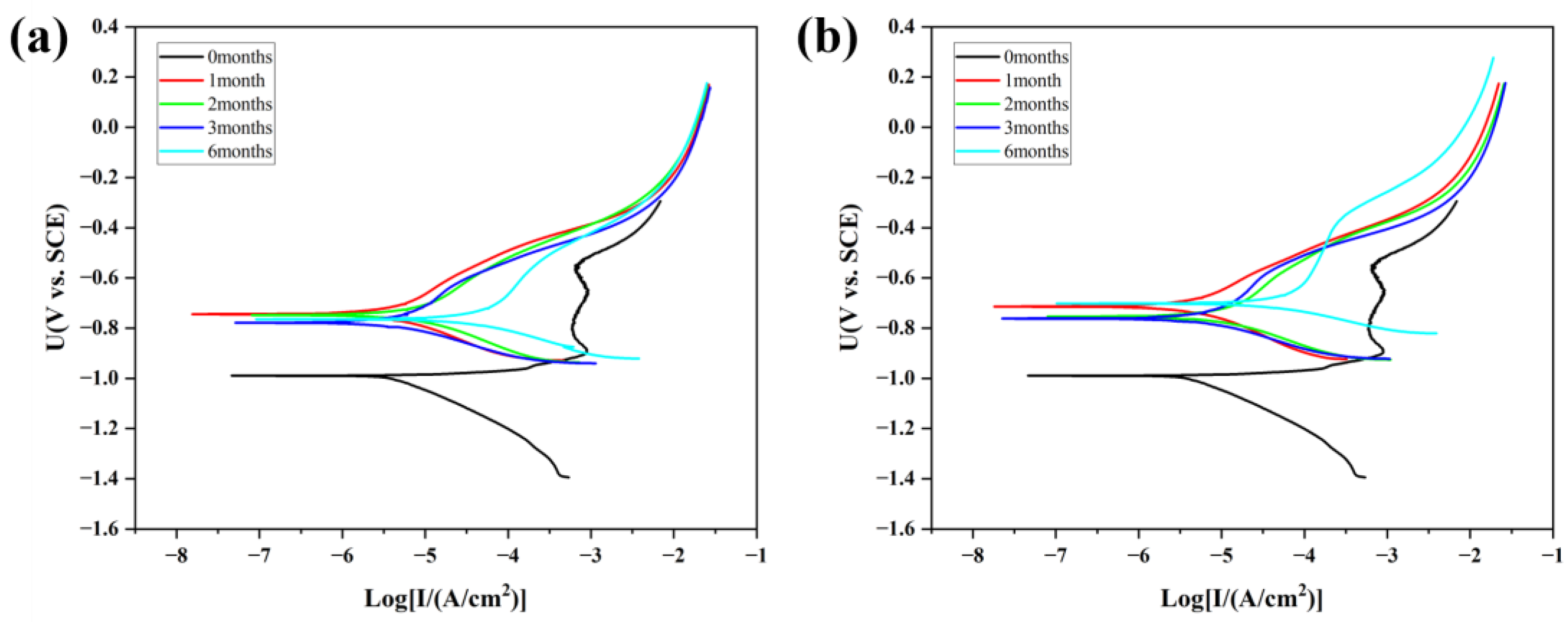
3.6. Electrochemical Testing
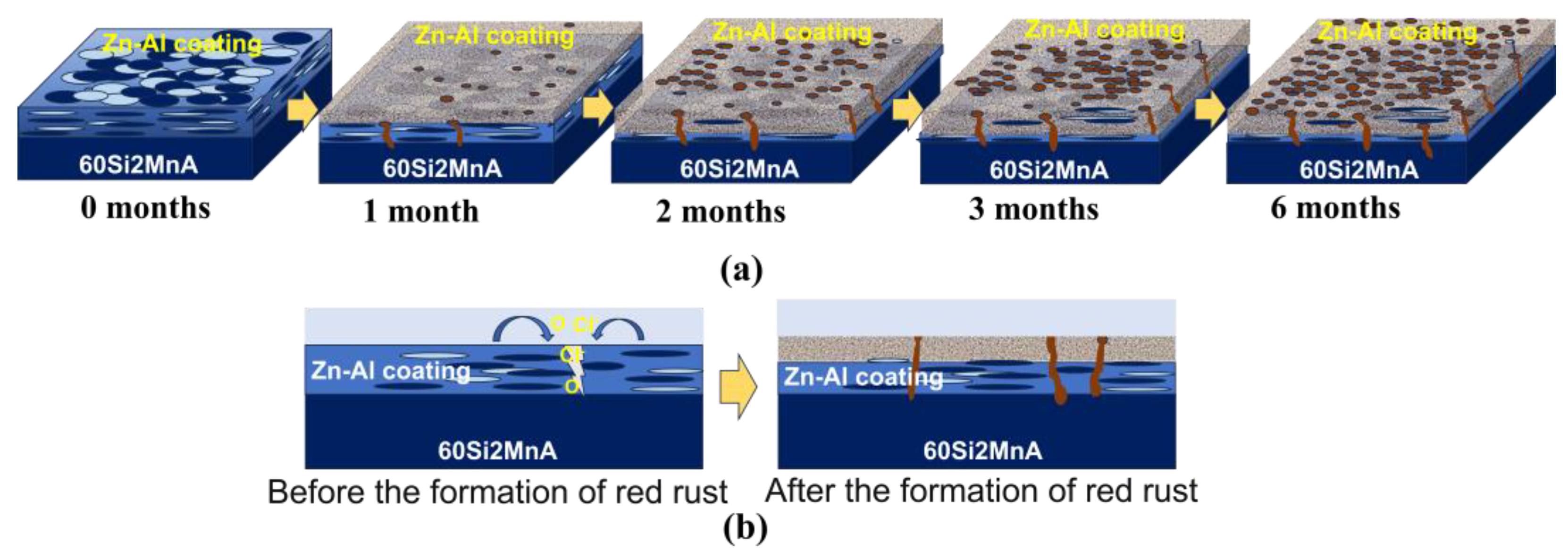
3.7. Analysis of Corrosion Mechanism
4. Conclusions
- (1)
- The weight gain of spring steel samples with Zn-Al coatings increases with prolonged corrosion time under both 20 °C/75% RH and 40 °C/75% RH conditions, with the weight gain rate being higher at 40 °C than at 20 °C. Combined with the macroscopic morphology, the consumption of the Zn-Al coating gradually increases over time, and its protective effect on the internal substrate weakens. At 40 °C, the coating essentially loses its protective effectiveness after six months of corrosion. This indicates that temperature can serve as an equivalent acceleration factor to enhance the efficiency of testing for samples with Zn-Al coatings.
- (2)
- According to the XRD and Raman results, the main phases of the corrosion products include ZnO, Zn(OH)2, Zn5(CO3)2(OH)6, Fe3O4, Fe2O3, FeOOH, etc., and the presence of Al corrosion products cannot be ruled out. As the corrosion time progresses, the characteristic peaks of Zn corrosion products gradually weaken, while those of Fe corrosion products gradually strengthen.
- (3)
- Based on the electrochemical test results, the corrosion potential of samples after corrosion under different conditions increases compared to uncorroded samples, indicating reduced corrosion sensitivity. In the first three months of corrosion, even though obvious pitting occurs on the sample surface, the self-corrosion current density slightly decreases compared to before corrosion due to the protective effect of the remaining coating and corrosion products, leading to improved corrosion resistance. After six months of corrosion and under the combined influence of Cl− and solid particles deposited on the surface, the integrity of the coating and the substrate corrosion product layer deteriorates, and the self-corrosion current density increases nearly tenfold, resulting in a significant decline in corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavares, S.; Pardal, J.; Landim, R.; Barbosa, C.; dos Santos, F.; Pimenta, A. Failure during fabrication of clamps of Mn-alloyed steel for springs in heavy vehicles. Eng. Fail. Anal. 2020, 109, 104244. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, C.; Wang, W.; Wu, L.; Cao, P. Influence of corrosion on seismic performance of steel beam-to-column connections. Eng. Struct. 2024, 312, 118284. [Google Scholar] [CrossRef]
- Zeng, Y.; Qin, Z.; Su, C.; Li, M.; Sun, C.; Yuan, X.; Ren, P.; Zhang, X. Effect of rainfall pH value on fretting corrosion characteristics of 60Si2Mn steel for high-speed railway track fastener clips. Eng. Fail. Anal. 2023, 144, 106954. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Abadchi, M.R.; Mirzaee, M.; Tabar, F.A.; Ramezanzadeh, B. Recent advances and future perspectives for carbon nanostructures reinforced organic coating for anti-corrosion application. Surf. Interfaces 2021, 23, 100994. [Google Scholar] [CrossRef]
- Habib, S.; Shakoor, R.; Kahraman, R. A focused review on smart carriers tailored for corrosion protection: Developments, applications, and challenges. Prog. Org. Coat. 2021, 154, 106218. [Google Scholar] [CrossRef]
- Seshadri, A.; Shirvan, K. Development of hydrothermal corrosion model and BWR metal coating for CVD SiC in light water reactors. J. Nucl. Mater. 2023, 576, 154252. [Google Scholar] [CrossRef]
- Su, F.; Zhang, P.; Wei, D.; Chen, X.; Ding, F.; Wang, B. Corrosion behavior of hot-dip Al–Zn coating doped with Si, RE, and Mg during exposure to sodium chloride containing environments. Mater. Corros. 2018, 69, 714–724. [Google Scholar] [CrossRef]
- Chang, J.-K.; Lin, C.-S.; Wang, W.-R.; Jian, S.-Y. High temperature deformation behaviors of hot dip 55 wt% Al-Zn coated steel. Appl. Surf. Sci. 2020, 511, 145550. [Google Scholar] [CrossRef]
- Han, X.; Sakairi, M. Role of hygroscopic chloride salts on corrosion performance and hydrogen absorption of steel under cyclic wet-dry conditions. Corros. Sci. 2024, 231, 111993. [Google Scholar] [CrossRef]
- Zou, Z.; Zeng, J.; Liu, Z.; Mo, Y.; Liang, G.; Wang, L.; Guo, Z. Study on the corrosion electrochemistry behavior and wear resistance of the arc thermal sprayed Zn–Al alloy coating. J. Mater. Res. Technol. 2023, 24, 8414–8428. [Google Scholar] [CrossRef]
- Katayama, H.; Kuroda, S. Long-term atmospheric corrosion properties of thermally sprayed Zn, Al and Zn–Al coatings exposed in a coastal area. Corros. Sci. 2013, 76, 35–41. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Cui, S.; Wang, S.; Cheng, B. Influence of Al contents on the long-term corrosion behaviors of cold-sprayed Zn-xAl coatings. Surf. Coat. Technol. 2024, 476, 130201. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Chen, H.; Li, S.; Wu, H.; Gao, X.; Du, L.; Song, L. Corrosion Behavior of Corrosion-Resistant Spring Steel Used in High Speed Railway. Appl. Sci. 2021, 11, 8668. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Tiwari, S.; Kango, S. Atmospheric corrosion of materials and their effects on mechanical properties: A brief review. Mater. Today: Proc. 2021, 44, 4677–4681. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS) — Surface texture: Profile method — Terms, definitions and surface texture parameters. International Organization for Standardization: Geneva, Switzerland, 1997.
- Pan, C.; Guo, M.; Han, W.; Wang, Z.; Wang, C. Study of corrosion evolution of carbon steel exposed to an industrial atmosphere. Corros. Eng. Sci. Technol. 2019, 54, 241–248. [Google Scholar] [CrossRef]
- Dang, L.; Gao, Z.; Wang, X.; Wei, C.; Han, L.; Chen, J. Wavelet Analysis of Electrochemical Noise Measurements During Atmospheric Corrosion of Mild Steel in the Simulated Marine Environment. Int. J. Electrochem. Sci. 2020, 15, 4326–4337. [Google Scholar] [CrossRef]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Hao, T.; Ye, H.; He, Y.; Wei, J.; Li, Q.; Dai, B.; Wu, J.; Yang, B.; Lin, Z.; Chai, L.; et al. Effect of in-situ oxidation on the phase composition and magnetic properties of Fe3O4: Implications for zinc hydrometallurgy. Inorg. Chem. Commun. 2022, 144, 109863. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, S.; Wang, Y.; Taleb, W.; Sun, J.; Zhang, L.; Barker, R.; Neville, A. The formation of FeCO3 and Fe3O4 on carbon steel and their protective capabilities against CO2 corrosion at elevated temperature and pressure. Corros. Sci. 2019, 157, 392–405. [Google Scholar] [CrossRef]
- Chung, S.; Lin, A.; Chang, J.; Shih, H. EXAFS study of atmospheric corrosion products on zinc at the initial stage. Corros. Sci. 2000, 42, 1599–1610. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Liu, M.; Song, G.-L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Guo, M.; Tang, J.; Peng, C.; Li, X.; Wang, C.; Pan, C.; Wang, Z. Effects of salts and its mixing ratio on the corrosion behavior of 316 stainless steel exposed to a simulated salt-lake atmospheric environment. Mater. Chem. Phys. 2022, 276, 125380. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Shang, T.; Liu, W.; Jiang, G.; Liu, C.; Cheng, X.; Zhang, X.; Li, X. Influence mechanism of different elements and alloy phases on the corrosion resistance of Zn-Al-Mg coated steel in the atmospheric environment: A review. Corros. Commun. 2024, 13, 49–59. [Google Scholar] [CrossRef]
- Liang, M.; Melchers, R.; Chaves, I. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Neufeld, A.; Cole, I.; Bond, A.; Furman, S. The initiation mechanism of corrosion of zinc by sodium chloride particle deposition. Corros. Sci. 2002, 44, 555–572. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Tariq, N.U.H.; Wang, J.; Cui, X.; Xiong, T. Microstructure and Corrosion Behavior of Cold-Sprayed Zn-Al Composite Coating. Coatings 2020, 10, 931. [Google Scholar] [CrossRef]
- Rosalbino, F.; Scavino, G.; Macciò, D.; Saccone, A. Influence of the alloying component on the corrosion behaviour of zinc in neutral aerated sodium chloride solution. Corros. Sci. 2014, 89, 286–294. [Google Scholar] [CrossRef]



| Specimen Type | Ecorr (V) | Icorr (A·cm−2) |
|---|---|---|
| Uncorroded | −1.029 | 7.23 × 10−6 |
| 20 °C/75% RH 1 month | −0.745 | 3.42 × 10−6 |
| 20 °C/75% RH 2 months | −0.740 | 6.10 × 10−6 |
| 20 °C/75% RH 3 months | −0.766 | 4.66 × 10−6 |
| 20 °C/75% RH 6 months | −0.768 | 3.49 × 10−5 |
| 40 °C/75% RH 1 month | −0.713 | 3.81 × 10−6 |
| 40 °C/75% RH 2 months | −0.754 | 7.73 × 10−6 |
| 40 °C/75% RH 3 months | −0.760 | 4.45 × 10−6 |
| 40 °C/75% RH 6 months | −0.704 | 4.24 × 10−5 |
| Sample | Rs/ (Ω·cm2) | Rf/ (Ω·cm2) | Qf | Rt/ (Ω·cm2) | Qdl | ||
|---|---|---|---|---|---|---|---|
| Y0/(Ω−1·cm2·sn) | nf | Y0/(Ω−1·cm2·sn) | ndl | ||||
| Uncorroded | 35.58 | 1195.00 | 4.86 × 10−4 | 0.52 | 1805.30 | 2.54 × 10−2 | 0.68 |
| 20 °C 1 month | 26.06 | 916.90 | 0.71 × 10−3 | 0.46 | 9097.90 | 8.27 × 10−4 | 0.83 |
| 20 °C 2 months | 28.01 | 489.30 | 2.40 × 10−3 | 0.34 | 3299.13 | 1.55 × 10−3 | 0.79 |
| 20 °C 3 months | 24.33 | 47.34 | 2.00 × 10−3 | 0.47 | 7475.49 | 4.85 × 10−3 | 0.64 |
| 20 °C 6 months | 26.99 | 11.43 | 6.74 × 10−3 | 0.42 | 873.27 | 2.44 × 10−2 | 0.54 |
| 40 °C 1 month | 27.29 | 629.17 | 1.67 × 10−4 | 0.58 | 8136.17 | 1.52 × 10−3 | 0.33 |
| 40 °C 2 months | 37.74 | 258.03 | 1.67 × 10−3 | 0.42 | 1928.07 | 6.09 × 10−3 | 0.57 |
| 40 °C 3 months | 22.95 | 61.28 | 1.29 × 10−3 | 0.34 | 4438.70 | 5.87 × 10−3 | 0.64 |
| 40 °C 6 months | 30.42 | 10.86 | 6.84 × 10−3 | 0.50 | 481.64 | 2.72 × 10−2 | 0.63 |
| Specimen Type | Rp/(Ω·cm2) | Specimen Type | Rp/(Ω·cm2) |
|---|---|---|---|
| Uncorroded | 3000.30 | Uncorroded | 3000.30 |
| 20 °C/75% RH 1 month | 10,014.80 | 40 °C/75% RH 1 month | 8765.34 |
| 20 °C/75% RH 2 months | 3788.43 | 40 °C/75% RH 2 months | 2186.10 |
| 20 °C/75% RH 3 months | 7522.83 | 40 °C/75% RH 3 months | 4499.98 |
| 20 °C/75% RH 6 months | 884.70 | 40 °C/75% RH 6 months | 492.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xiao, H.; Liu, B.; Chen, S.; Jiao, X.; Song, S.; Zhang, W.; Jin, Y. Investigation of Corrosion Resistance of 60Si2MnA Spring Steel Coated with Zn-Al in Atmospheric Environments. Materials 2025, 18, 3215. https://doi.org/10.3390/ma18143215
Wang Y, Xiao H, Liu B, Chen S, Jiao X, Song S, Zhang W, Jin Y. Investigation of Corrosion Resistance of 60Si2MnA Spring Steel Coated with Zn-Al in Atmospheric Environments. Materials. 2025; 18(14):3215. https://doi.org/10.3390/ma18143215
Chicago/Turabian StyleWang, Yurong, Hui Xiao, Baolong Liu, Shilong Chen, Xiaofei Jiao, Shuwei Song, Wenyue Zhang, and Ying Jin. 2025. "Investigation of Corrosion Resistance of 60Si2MnA Spring Steel Coated with Zn-Al in Atmospheric Environments" Materials 18, no. 14: 3215. https://doi.org/10.3390/ma18143215
APA StyleWang, Y., Xiao, H., Liu, B., Chen, S., Jiao, X., Song, S., Zhang, W., & Jin, Y. (2025). Investigation of Corrosion Resistance of 60Si2MnA Spring Steel Coated with Zn-Al in Atmospheric Environments. Materials, 18(14), 3215. https://doi.org/10.3390/ma18143215






