The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Protocol
2.3. Eligibility Criteria
- Studies involving the examination of metal-modified glass-ionomer cements;
- Studies evaluating fluoride release;
- In vitro studies;
- Full-text articles
- Studies in English;
- Studies not focusing on glass-ionomers modified with metal/metal particles;
- Measurement of other properties than fluoride release;
- Non-English paper;
- Systematic review papers;
- Review articles;
- No full text accessible;
- Duplicated publications.
2.4. Information Sources, Search Strategy, and Study Selection
2.5. Data Collection Process and Data Items
2.6. Risk of Bias and Quality Assessment
2.7. Quality Assessment
- Is it clear in the study what is the ‘cause’ and what is the ‘effect’?
- Were the participants included in any similar comparisons?
- Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? Was there a control group?
- Were there multiple measurements of the outcome both before and after the intervention/exposure?
- Was a follow-up completed, and if not, were differences between groups in terms of their follow-up adequately described and analyzed? Were the outcomes of participants included in any comparisons measured in the same way?
- Were the outcomes measured in a reliable way?
- Was an appropriate statistical analysis used?
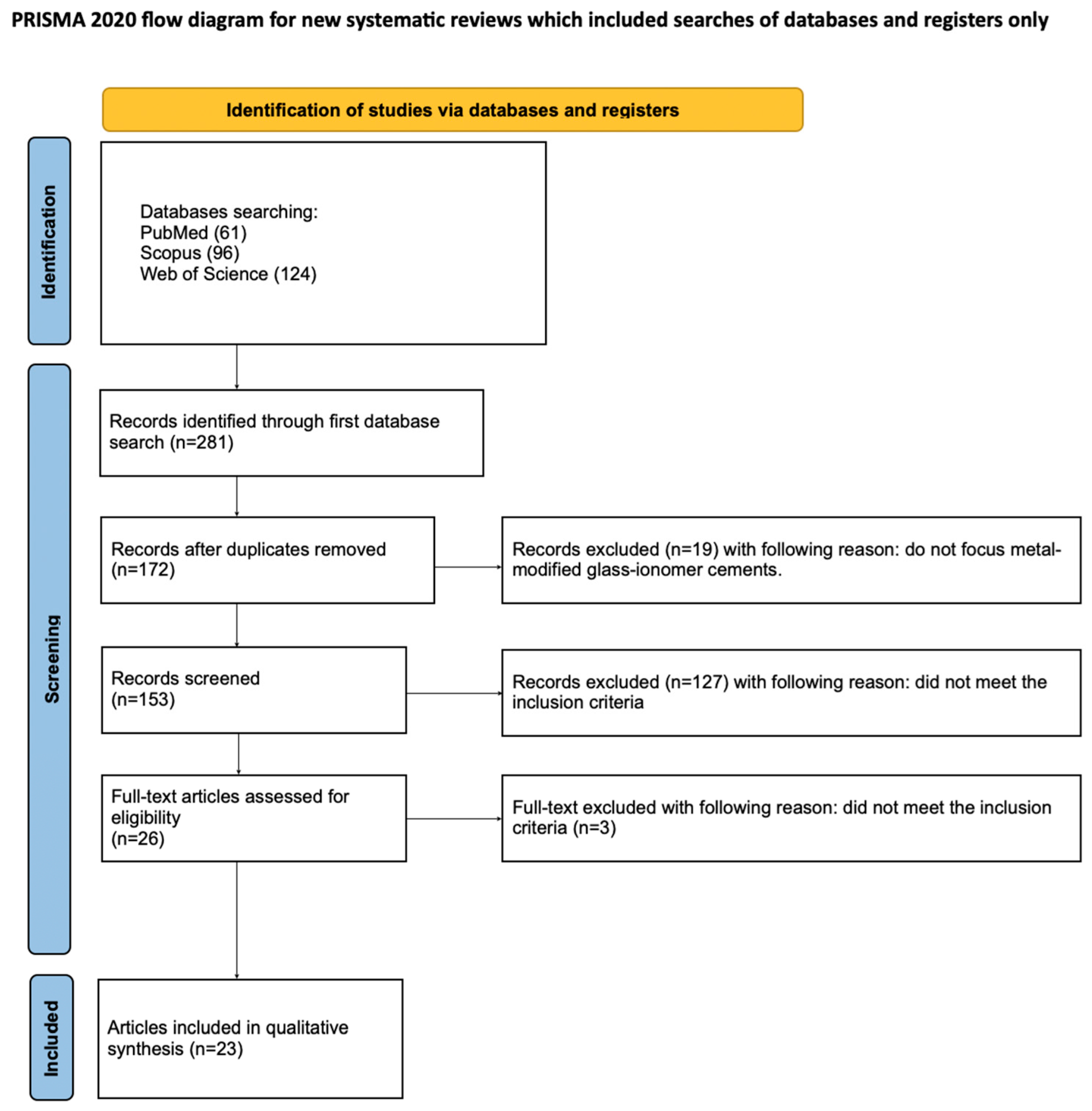
3. Results
3.1. Study Selection
3.2. General Characteristics of the Included Studies
3.3. Main Study Outcomes
3.3.1. Influence of Silver Additions on Fluoride Release
3.3.2. Influence of Zinc Oxide and Its Compounds
3.3.3. Influence of Strontium Additions
3.3.4. Influence of Titanium Dioxide
3.3.5. Influence of Zirconium Dioxide
3.3.6. Comparative Summary
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenes-Alvarado, A.; Cury, J.A.; Brenes-Alvarado, A.; Cury, J.A. Fluoride Release from Glass Ionomer Cement and Resin-modified Glass Ionomer Cement Materials under Conditions Mimicking the Caries Process. Oper. Dent. 2021, 46, 457–466. [Google Scholar] [CrossRef]
- el Mallakh, B.F.; Sarkar, N.K. Fluoride release from glass-ionomer cements in de-ionized water and artificial saliva. Dent. Mater. 1990, 6, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Perez, D.; Vargas-Coronado, R.; Cervantes-Uc, J.M.; Rodriguez-Fuentes, N.; Aparicio, C.; Covarrubias, C.; Alvarez-Perez, M.; Garcia-Perez, V.; Martinez-Hernandez, M.; Cauich-Rodriguez, J.V. Antibacterial activity of a glass ionomer cement doped with copper nanoparticles. Dent. Mater. J. 2020, 39, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.D.; Hu, J.C.-C. How Fluoride Protects Dental Enamel from Demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134–141. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef]
- ten Cate, J.M.; van Loveren, C. Fluoride mechanisms. Dent. Clin. N. Am. 1999, 43, 713–742. [Google Scholar] [CrossRef]
- Marquis, R.E. Antimicrobial actions of fluoride for oral bacteria. Can. J. Microbiol. 1995, 41, 955–964. [Google Scholar] [CrossRef]
- Di Lauro, A.; Di Duca, F.; Montuori, P.; Dal Piva AMde, O.; Tribst, J.P.M.; Borges, A.L.S.; Ausiello, P. Fluoride and Calcium Release from Alkasite and Glass Ionomer Restorative Dental Materials: In Vitro Study. J. Funct. Biomater. 2023, 14, 109. [Google Scholar] [CrossRef]
- Aliberti, A.; Di Duca, F.; Triassi, M.; Montuori, P.; Scippa, S.; Piscopo, M.; Ausiello, P. The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study. Polymers 2025, 17, 640. [Google Scholar] [CrossRef]
- Piszko, P.J.; Kulus, M.; Piszko, A.; Kiryk, J.; Kiryk, S.; Kensy, J.; Małyszek, A.; Michalak, M.; Dobrzyński, W.; Matys, J.; et al. The Influence of Calcium Ions and pH on Fluoride Release from Commercial Fluoride Gels in an In Vitro Study. Gels 2025, 11, 486. [Google Scholar] [CrossRef]
- Morales-Valenzuela, A.A.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Garcia-Contreras, R.; Hegazy-Hassan, W.; Toral-Rizo, V.H.; Salmerón-Valdés, E.N. Enhancement of fluoride release in glass ionomer cements modified with titanium dioxide nanoparticles. Medicine 2022, 101, e31434. [Google Scholar] [CrossRef] [PubMed]
- Tokarczuk, D.; Tokarczuk, O.; Kiryk, J.; Kensy, J.; Szablińska, M.; Dyl, T.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Fluoride Release by Restorative Materials after the Application of Surface Coating Agents: A Systematic Review. Appl. Sci. 2024, 14, 4956. [Google Scholar] [CrossRef]
- Tiwari, S.; Nandlal, B. Effect of nano-filled surface coating agent on fluoride release from conventional glass ionomer cement: An in vitro trial. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 91–95. [Google Scholar] [CrossRef]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In Vitro Assessment of Long-Term Fluoride Ion Release from Nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef]
- Shalaby, H.A.; Soliman, N.K.; Al-Saudi, K.W. Antibacterial and preventive effects of newly developed modified nano-chitosan/glass-ionomer restoration on simulated initial enamel caries lesions: An in vitro study. Dent. Med. Probl. 2024, 61, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Billington, R.W.; Pearson, G.J. A long term study of fluoride release from metal-containing conventional and resin-modified glass-ionomer cements. J. Oral Rehabil. 2001, 28, 41–47. [Google Scholar] [CrossRef]
- Karantakis, P.; Helvatjoglou-Antoniades, M.; Theodoridou-Pahini, S.; Papadogiannis, Y. Fluoride release from three glass ionomers, a compomer, and a composite resin in water, artificial saliva, and lactic acid. Oper. Dent. 2000, 25, 20–25. [Google Scholar]
- Mitra, S.B.; Kedrowski, B.L. Long-term mechanical properties of glass ionomers. Dent. Mater. 1994, 10, 78–82. [Google Scholar] [CrossRef]
- Sarkar, N.K. Metal–matrix interface in reinforced glass ionomers. Dent. Mater. 1999, 15, 421–425. [Google Scholar] [CrossRef]
- Walls, A.W.G.; Adamson, J.; McCabe, J.F.; Murray, J.J. The properties of a glass polyalkenoate (ionomer) cement incorporating sintered metallic particles. Dent. Mater. 1987, 3, 113–116. [Google Scholar] [CrossRef]
- Lubojanski, A.; Dobrzynski, M.; Nowak, N.; Rewak-Soroczynska, J.; Sztyler, K.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Wiglusz, K.; et al. Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry. Nanomaterials 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Cvjeticanin, M.; Ramic, B.; Milanović, M.; Veljović, D.; Andjelkovic, A.; Maletic, S.; Jevrosimov, I.; Bajkin, B.; Guduric, V. Cell viability assessment and ion release profiles of GICs modified with TiO2- and Mg-doped hydroxyapatite nanoparticles. J. Dent. 2024, 145, 105015. [Google Scholar] [CrossRef]
- Gloria D’costa, V.; Singhal, D.K.; Acharya, S. Efficacy of GC Gold Label 9 and GC Miracle Mix ® Restorations Efficacy of GC Gold Label 9 and GC Miracle Mix® Restorations using Atraumatic Restorative Treatment (ART) in Rural Settings: A Randomized Controlled Trial. J. Clin. Pediatr. Dent. 2020, 44, 148–153. [Google Scholar] [CrossRef]
- Sztyler, K.; Wiglusz, R.J.; Dobrzynski, M. Review on Preformed Crowns in Pediatric Dentistry—The Composition and Application. Materials 2022, 15, 2081. [Google Scholar] [CrossRef]
- Williams, J.A.; Billington, R.W.; Pearson, G. Silver and fluoride ion release from metal-reinforced glass-ionomer filling materials. J. Oral Rehabil. 1997, 24, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Almuhaiza, M. Glass-ionomer cements in restorative dentistry: A critical appraisal. J. Contemp. Dent. Pract. 2016, 17, 331–336. [Google Scholar] [CrossRef]
- Zheng, L.; Li, K.; Ning, C.; Sun, J. Study on antibacterial and fluoride-releasing properties of a novel composite resin with fluorine-doped nano-zirconia fillers. J. Dent. 2021, 113, 103772. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef]
- Gu, Y.W.; Yap, A.U.J.; Cheang, P.; Khor, K.A. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC). Biomaterials 2005, 26, 713–720. [Google Scholar] [CrossRef]
- Alaohali, A.; Brauer, D.S.; Gentleman, E.; Sharpe, P.T. A modified glass ionomer cement to mediate dentine repair. Dent. Mater. 2021, 37, 1307–1315. [Google Scholar] [CrossRef]
- Guo, T.; Yang, M.; Wang, D.; Zheng, J.; Gao, S.S. Antibiofilm and mechanical properties of silver nanowire-modified glass ionomer cement. J. Dent. 2023, 135, 104569. [Google Scholar] [CrossRef]
- Tjan, A.H.L.; Morgan, D.L. Metal-reinforced glass ionomers: Their flexural and bond strengths to tooth substrates. J. Prosthet. Dent. 1988, 59, 137–141. [Google Scholar] [CrossRef]
- Chungk, H. The properties of metal-reinforced glass ionomer materials. J. Oral Rehabil. 1993, 20, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Biglar, N.; Chaychi Raghimi, E.; Sadighian, S.; Karamitanha, F.; Zajkani, E.; Nourian, A. Effect of incorporating silica-hydroxyapatite-silver hybrid nanoparticles into the resin-modified glass ionomer on the adhesive remnant index score and shear bond strength of orthodontic metal brackets: An in vitro study. Int. Orthod. 2023, 21, 100761. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Mielczarek, A.; Jackowska, T.; Mielnik-Błaszczak, M.; Turska-Szybka, A.; Opydo-Szymaczek, J.; Jurczak, A.; Kaczmarek, U. Fluoride agents in the prevention and treatment of dental caries and erosion in children, adolescents and adults—Recommendations of Polish Experts. Update of recommendations: Individual fluoride prevention in children and adolescents—Recommendations of Polish Experts. Nowa Stomatol. 2022, 27. [Google Scholar] [CrossRef]
- Kosior, P.; Dobrzyński, M.; Korczyński, M.; Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Piesiak-Pańczyszyn, D.; Fita, K.; Janeczek, M. Long-term release of fluoride from fissure sealants—In vitro study. J. Trace Elem. Med. Biol. 2017, 41, 107–110. [Google Scholar] [CrossRef]
- Iranparvar, P.; Ghasemi, A.; Iranparvar, P. Adhesion of glass ionomer cements to primary dentin using a universal adhesive. Dent. Med. Probl. 2024, 61, 93–98. [Google Scholar] [CrossRef]
- Garcez, R.M.V.D.B.; Buzalaf, M.A.R.; De Araújo, P.A. Fluoride release of six restorative materials in water and pH-cycling solutions. J. Appl. Oral Sci. 2007, 15, 406. [Google Scholar] [CrossRef]
- Bahadure, R.N.; Pandey, R.K.; Kumar, R.; Gopal, K.; Singh, R.K. An estimation of fluoride release from various dental restorative materials at different pH: In vitro study. J. Indian Soc. Pedod. Prev. Dent. 2012, 30, 122–126. [Google Scholar] [CrossRef]
- Nigam, A.G.; Jaiswal, J.; Murthy, R.; Pandey, R. Estimation of Fluoride Release from Various Dental Materials in Different Media—An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2009, 2, 1. [Google Scholar] [CrossRef]
- Kumari, P.D.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, C.R. Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review. J. Oral Biol. Craniofac. Res. 2019, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Jingarwar, M.M.; Pathak, A.; Bajwa, N.K.; Sidhu, H.S. Quantitative Assessment of Fluoride Release and Recharge Ability of Different Restorative Materials in Different Media: An In Vitro Study. J. Clin. Diagn. Res. 2014, 8, ZC31. [Google Scholar] [CrossRef]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Oleniacz-Trawińska, M.; Kotela, A.; Kensy, J.; Kiryk, S.; Dobrzyński, W.; Kiryk, J.; Gerber, H.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Affecting Fluoride Release from Compomer Restorative Materials: A Systematic Review. Materials 2025, 18, 1627. [Google Scholar] [CrossRef] [PubMed]
- Morawska-Wilk, A.; Kensy, J.; Kiryk, S.; Kotela, A.; Kiryk, J.; Michalak, M.; Grychowska, N.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review. Nanomaterials 2025, 15, 651. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamashita, S.; Mendonca, M.; Brueckner, S.; Achong-Bowe, R.; Thompson, J.; Kuriki, N.; Mizuhira, M.; Benjamin, Y.; Duncan, H.F.; et al. Ultrastructural evaluation of adverse effects on dentine formation from systemic fluoride application in an experimental mouse model. Int. Endod. J. 2025, 58, 128–140. [Google Scholar] [CrossRef]
- Forsten, L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 1998, 19, 503–508. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Kanjevac, T.; Milovanovic, M.; Volarevic, V.; Lukic, M.L.; Arsenijevic, N.; Markovic, D.; Zdravkovic, N.; Tesic, Z.; Lukic, A. Cytotoxic Effects of Glass Ionomer Cements on Human Dental Pulp Stem Cells Correlate with Fluoride Release. Med. Chem. 2012, 8, 40–45. [Google Scholar] [CrossRef]
- Guida, A.; Towler, M.R.; Wall, J.G.; Hill, R.G.; Eramo, S. Preliminary work on the antibacterial effect of strontium in glass ionomer cements. J. Mater. Sci. Lett. 2003, 22, 1401–1403. [Google Scholar] [CrossRef]
- Pardi, M.; Ribeiro, K.L.G.; Marques, M.B.E.S.; Costa, C.R.; de Brito Silva, E.B.; Schiavon, M.A.; dos Reis, A.C.; Lepri, C.P.; de Castro, D.T. Incorporation of AgVO3 into Glass Ionomer Cement: Ionic Release. Pesqui. Bras. Odontopediatria Clin. Integr. 2025, 25, e240015. [Google Scholar] [CrossRef]
- Guo, T.; Wang, D.; Gao, S.S. The antibiofilm effect and mechanism of silver nanowire-modified glass ionomer cement against multi-species oral biofilm. BMC Oral Health 2025, 25, 160. [Google Scholar] [CrossRef]
- Raghimi, E.C.; Biglar, N.; Sadighian, S.; Karamitanha, F.; Nouri, A.; Nourian, A. Compressive strength and fluoride release profile of a glass ionomer cement reinforced with silver-hydroxyapatite-silica hybrid nanoparticles: An in vitro study. Int. Orthod. 2024, 22, 100871. [Google Scholar] [CrossRef]
- Saad Bin Qasim, S.; Bmuajdad, A. The effect of mesoporous silica doped with silver nanoparticles on glass ionomer cements; physiochemical, mechanical and ion release analysis. BMC Oral Health 2024, 24, 1269. [Google Scholar] [CrossRef]
- Potiprapanpong, W.; Naruphontjirakul, P.; Khamsuk, C.; Channasanon, S.; Toneluck, A.; Tanodekaew, S.; Monmaturapoj, N.; Young, A.M.; Panpisut, P. Assessment of Mechanical/Chemical Properties and Cytotoxicity of Resin-Modified Glass Ionomer Cements Containing Sr/F-Bioactive Glass Nanoparticles and Methacrylate Functionalized Polyacids. Int. J. Mol. Sci. 2023, 24, 10231. [Google Scholar] [CrossRef]
- AlMatar, D.; AlSanousi, S.; Ahmed, J.; Saad Bin Qasim, S. The In-Vitro Effect of Silver and Zinc Oxide Nanoparticles on Fluoride Release and Microhardness of a Resin-Modified Glass Ionomer Cement. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1507–1516. [Google Scholar] [CrossRef]
- Thongsri, O.; Srisuwan, S.; Thaitalay, P.; Dangwiriyakul, R.; Chanlek, N.; Talabnin, C.; Rattanachan, S.T. Fluoride release and uptake characteristics of the sol-gel derived glass ionomer cement modified with fluoride containing strontium-based bioactive glass nanoparticles. J. Solgel Sci. Technol. 2023, 105, 857–870. [Google Scholar] [CrossRef]
- Gunay, A.; Celenk, S.; Adiguzel, O.; Cangul, S.; Ozcan, N.; Cakmakoglu, E.E. Comparison of Antibacterial Activity, Cytotoxicity, and Fluoride Release of Glass Ionomer Restorative Dental Cements in Dentistry. Med. Sci. Monit. 2022, 28, e939065. [Google Scholar] [CrossRef] [PubMed]
- Wassel, M.O.; Allam, G.G. Anti-Bacterial Effect, Fluoride Release, and Compressive Strength of a Glass Ionomer Containing Silver and Titanium Nanoparticles. Indian J. Dent. Res. 2022, 33, 75–79. [Google Scholar] [CrossRef]
- Alshehri, T.D.; Kotha, S.B.; Abed, F.M.; Barry, M.J.; Alasmari, A.; Mallineni, S.K. Effect of the Addition of Varying Concentrations of Silver Nanoparticles on the Fluoride Uptake and Recharge of Glass Ionomer Cement. Nanomaterials 2022, 12, 1971. [Google Scholar] [CrossRef]
- Kohno, T.; Liu, Y.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Evaluation of ion release and the recharge ability of glass-ionomer cement containing BioUnion filler using an in vitro saliva-drop setting assembly. Dent. Mater. 2021, 37, 882–893. [Google Scholar] [CrossRef]
- Malekhoseini, Z.; Rezvani, M.B.; Niakan, M.; Atai, M.; Mohammadi Bassir, M.; Alizade, H.S.; Siabani, S. Effect of zinc oxide nanoparticles on physical and antimicrobial properties of resin-modified glass ionomer cement. Dent. Res. J. 2021, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Bahammam, S.; Nathanson, D.; Fan Medina, Y. Assessing the release of the fluoride ion in four restorative glass ionomer cements. Fluoride 2020, 53, 611–620. [Google Scholar]
- Putri, L.K.; Rianti, D.; Harijanto, E. Release of fluoride to the addition of nanoparticle zinc oxide with glass ionomer cements. Int. J. Pharm. Res. 2020, 12, 1530–1533. [Google Scholar] [CrossRef]
- Karimi, M.; Hesaraki, S.; Alizadeh, M.; Kazemzadeh, A. Effect of synthetic amorphous calcium phosphate nanoparticles on the physicochemical and biological properties of resin-modified glass ionomer cements. Mater. Sci. Eng. C 2019, 98, 227–240. [Google Scholar] [CrossRef]
- Cibim, D.D.; Saito, M.T.; Giovani, P.A.; Borges, A.F.S.; Pecorari, V.G.A.; Gomes, O.P.; Lisboa-Filho, P.N.; Nociti-Junior, F.H.; Puppin-Rontani, R.M.; Kantovitz, K.R. Novel nanotechnology of TiO2 improves physical-chemical and biological properties of glass ionomer cement. Int. J. Biomater. 2017, 2017, 7123919. [Google Scholar] [CrossRef]
- Saxena, S.; Tiwari, S. Energy dispersive X-ray microanalysis, fluoride release, and antimicrobial properties of glass ionomer cements indicated for atraumatic restorative treatment. J. Int. Soc. Prev. Community Dent. 2016, 6, 366–372. [Google Scholar] [CrossRef]
- Shahid, S.; Hassan, U.; Billington, R.W.; Hill, R.G.; Anderson, P. Glass ionomer cements: Effect of strontium substitution on esthetics, radiopacity and fluoride release. Dent. Mater. 2014, 30, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Selimović-Dragaš, M.; Hasić-Branković, L.; Korać, F.; Đapo, N.; Huseinbegović, A.; Kobašlija, S.; Lekić, M.; Hatibović-Kofman, Š. In vitro fluoride release from a different kind of conventional and resin modified glass-ionomer cements. Bosn. J. Basic Med. Sci. 2013, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [CrossRef]
- Osinaga, P.W.; Helena Grande, R.M.; Ballester, R.Y.; Regina Simionato, M.L.; Âlia Regina Delgado Rodrigues, C.M.; Muench, A. Zinc sulfate addition to glass-ionomer-based cements: Influence on physical and antibacterial properties, zinc and fluoride release. Dent. Mater. 2003, 19, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Helvatjoglu-Antoniades, M.; Karantakis, P.; Papadogiannis, Y.; Kapetanios, H. Fluoride release from restorative materials and a luting cement. J. Prosthet. Dent. 2001, 86, 156–164. [Google Scholar] [CrossRef]
- Hattab, F.N.; Amin, W.M. Fluoride release from glass ionomer restorative materials and the effects of surface coating. Biomaterials 2001, 22, 1449–1458. [Google Scholar] [CrossRef]
- Yip, H.K.; Lam, W.T.C.; Smales, R.J. Fluoride release, weight loss and erosive wear of modern aesthetic restoratives. Br. Dent. J. 1999, 187, 265–270. [Google Scholar] [CrossRef]
- Wandera, A.; Spencer, P.; Bohaty, B. In vitro comparative fluoride release, and weight and volume change in light-curing and self-curing glass ionomer materials. Pediatr. Dent. 1996, 18, 210–214. [Google Scholar]

| Study | Aim of the Study | Material and Methods | Results | Conclusions |
|---|---|---|---|---|
| Pardi [54] | Assessment of the surface characteristics and ion release behavior of glass ionomer cement (GIC) modified with nanostructured silver vanadate (AgVO3). | GIC samples prepared with AgVO3 concentrations of 0% (control), 1%, 2.5%, and 5%. Fluoride release measured via ion-selective electrode; surface distribution assessed by SEM/EDS; silver and vanadium ion release quantified using ICP-MS. | Fluoride release peaked at day 7 in all groups and then declined by day 28. 1% and 2.5% AgVO3-modified samples showed higher fluoride release than unmodified samples. Silver release increased notably in the 2.5% and 5% groups. The highest vanadium release occurred at 5% concentration. | The introduction of AgVO3 altered both the surface characteristics and the ion release profile of glass ionomer cement (GIC). The addition of AgVO3 at concentrations of 1% and 2.5% initially promoted an increase in fluoride release. |
| Guo [55] | Assessing antibiofilm properties and mechanical/biochemical performance of glass ionomer cement modified with silver nanowires (AgNWs). | 6 GIC groups were tested: control (no nanosilver), four AgNW concentrations (0.05–0.5 wt%), and 0.5 wt% AgNP (positive control). Analyses included fluoride release (IC: ICS-6000), biofilm formation, lactic acid production, mechanical properties, color stability, and cytotoxicity. | Fluoride release and lactic acid levels showed no significant differences across samples. AgNW-containing GIC reduced biofilm adherence versus conventional GIC. | Fluoride ion release remained stable across all GIC materials, with no significant differences between groups or time intervals. |
| Raghimi [56] | Assessment of fluoride release and mechanical performance of glass ionomer cement containing hybrid nanoparticles composed of silver hydroxyapatite and silica. | 60 cylindrical samples were prepared using BracePaste composite, pure RMGI (GC Fuji II LC), and RMGI with Ag/HA/Si hybrid nanoparticles (0.1–2 wt%). Fluoride release was measured via an ion-selective electrode, and compressive strength (MPa) via a universal testing machine. | Resin-modified glass ionomer containing 1% (0.21 ± 0.07 mg/mL) and 2% (0.45 ± 0.22 mg/mL) hybrid nanoparticles exhibited a significantly higher fluoride release compared to the control group (0.09 ± 0.03 mg/mL). Ag/HA/Si hybrid nanoparticle addition did not significantly affect compressive strength. | Fluoride release increased with higher Ag/HA/Si hybrid nanoparticle concentrations in RMGIC. This enhancement suggests improved potential for dental applications. |
| Qasim [57] | To assess how mesoporous silica with silver nanoparticles affects properties and ion release in conventional glass ionomer cements. | Conventional GICs were modified with mesoporous silica containing silver nanoparticles (1–5%). Fluoride release was measured via HPLC (Prominence Shimadzu), and other ions (Al, Ca, Na, P, Ag) via ICP-OES. Additional tests included NanoCT, FTIR, surface microhardness, water sorption, and solubility. | Control specimens showed the highest fluoride release, followed by the 5%, 3%, and 1% groups. Significant differences existed between control and 5% specimens during weeks 1–4, with no significant differences within groups. Modified GICs demonstrated similar microhardness values to conventional ones. | Mesoporous silica with silver nanoparticles reduced fluoride release in glass ionomer cement compared to conventional material, though release increased with higher additive content. |
| Potiprapanpong [58] | To analyze the mechanical behavior, chemical composition, and biocompatibility of RMGICs enhanced with strontium/fluoride bioactive glass nanoparticles and polyacids functionalized with methacrylate groups. | RMGICs were mixed with HEMA and spherical Sr/F-bioactive glass nanoparticles. Fluoride release was measured over 4 weeks via ion-selective electrode (Orion) in deionized water (37 °C). Al, Ca, P, and Sr concentrations were assessed after 4 weeks using ICP-OES (Optima 8300). Setting reaction, BFS, BFM, and cytotoxicity were also evaluated. | All RMGICs showed time-dependent increases in cumulative fluoride release. Materials with 5% HEMA and either 10% or 5% Sr/F-BGNPs had similar highest release values (137.5 ± 6.1 ppm and 136.6 ± 2.2 ppm, respectively). HEMA addition increased average cumulative fluoride release by 89.11%, while higher Sr/F-BGNPs concentrations had minimal effect. | HEMA addition and higher concentrations (10%) of spherical Sr/F-bioactive glass nanoparticles enhanced cumulative fluoride release. Modified RMGIC formulations released more fluoride than commercial material. |
| AlMatar [59] | Evaluation of the effect of adding SNP (silver) and ZnONP (zinc oxide) to the RMGIC structure on fluorine release. | The samples were prepared by adding to RMGIC 1. 5 wt% SNP 2. 5 wt% ZnONP 3. 5 wt% SNP and ZnONP (1:1). The samples were cured and examined: under SEM, spectroscope (FTIR), nanotomography, Vickers hardness test, and HPLC. | ZnONP and ZnONP+SNP increased sample roughness (SEM/nanotomography). SNP and ZnONP+SNP decreased hardness. SNP and SNP+ZnONP samples showed increased F release at specific intervals versus control. | Adding silver or zinc nanoparticles to RMGIC in combination with SNP increases the release of F from the material. |
| Thongsri [60] | To investigate whether the addition of strontium-containing bioactive glass (BGF) to sol-gel GI affects the absorption and release of fluoride from the material. | Bioactive glass containing strontium (BGF) was added to sol-gel GI (SCGI) in amounts of 0, 1, 3, and 6 wt%. The release and absorption of F in the samples was assessed using: FISE, SEM-EDS, and XPS. Cytotoxicity, setting time, and resistance to compression were also tested. | BGF >1% increases setting time and reduces compressive strength; only BGF 1% improves CS. SCGI and SCGI+BGF1% show higher F uptake/rerelease than commercial GI, which has greater initial F release. SCGI+BGF toxicity is comparable to commercial GI. | The addition of bioactive glass containing strontium in an appropriate amount to GI can improve its F-releasing and absorption properties and biomechanical properties without affecting toxicity. |
| Gunay [61] | Evaluation of four different glass ionomers regarding their F release, antibacterial properties, and cytotoxicity. | 200 samples of 4 glass-ionomers (Riva Silver with silver, Equia Forte HT glass hybrid, ChemFil Rock with zinc, and KetacTM Molar Easymix) were tested for: antibacterial properties (bacterial growth assessment), cytotoxicity (WST-1 analysis with mouse fibroblasts), and F release (ion-selective electrode, Thermo Orion 720 A+, measured on days 1, 2, 3, 7, 14, 21, 28). | Ketac TM Molar released the most fluoride. ChemFil Rock released the least fluoride. GI with silver released more fluoride than GI with zinc, but less than classic GI-Ketac. | The best GI in terms of fluoride release is GI without added metal ions and glass. |
| Wassel [62] | Evaluation of how the addition of Ti Ag ions to GI will affect its antibacterial, mechanical, and fluoride-releasing properties. | 10 samples were prepared per parameter for conventional GI (control), GI + 5%wt Ag-NP, and GI + 5%wt TiO2-NP. Tests included: antibacterial properties (inhibition zones), fluoride release (selective electrode Orion Research, Inc., measured at 24 h, 14 and 28 days), and compressive strength (load at fracture, MPa). | GI with Ag and TiO2 produced larger growth inhibition zones than conventional GI samples. GI with Ag released the most F, GI with TiO2 the least over 28 days. CS values were significantly higher for TiO2 samples than for GI with Ag and conventional GI. | The inclusion of Ag and Ti ions in the GI structure improves the tested material parameters. |
| Alshehri [63] | Evaluation of the effect of adding silver to GI on fluoride ion release and recharging parameters. | 60 samples in 6 groups were tested: conventional GIC, GIC-Ag 0.1%, GIC-Ag 0.2%, conventional GIC + F, GIC-Ag 0.1% + F, and GIC-Ag 0.2% + F. Groups 4-6 received fluoride loading from 1450 ppm paste. Fluoride uptake and recharging were measured using FISE (HI4110, Hanna Instruments) on days 1, 2, 7, 15, and 30. | Conventional GICs released the most fluoride in both non-loaded (group 1) and F-loaded (group 4) categories. Non-loaded GICs released more fluoride than their F-loaded counterparts. | Adding Ag ions to GI did not improve the remineralizing properties of the material. |
| Kohno [64] | To investigate the possibility of loading and releasing Zn2+ and F− from GIC containing BioUnion filler. | Artificial saliva was dripped onto the GIC containing BioUnion filler and periodically replaced with acetic acid. The release/loading of Zn2+ and F− ions was checked by measuring their concentrations. | The concentration of released Zn2+ and F− was higher in acid than in artificial saliva. However, in none of the GICs was the concentration of released F- sufficient to inhibit bacterial biofilm. | GIC containing BioUnion filler releases Zn2+ and F− ions and can also be recharged by applying a tooth gel containing Zn2+ and F−. |
| Malekhoseini [65] | Evaluation of the effect of different concentrations of ZnO nanoparticles in RMGI on mechanical and antibacterial properties. | 100 glass ionomer samples with ZnO concentrations of 0%, 1%, 2%, 3%, or 4% were prepared. The concentration of Zn and F ions released from them was measured using a spectrophotometer or IPC, respectively. | The lowest level of fluoride was released from the glass ionomer containing 3% ZnO, and the highest from the RMGIC containing 2% ZnO. | Adding 2% ZnO nanoparticles to RMGI significantly increases fluoride release and antibacterial activity without affecting mechanical parameters. |
| Bahammam [66] | To investigate the fluoride release over a 9-day period from four glass ionomer cements. | Disks were made of ChemFil ROCK, Fuji IX, Riva self-cure, and Ketac Nano materials. They were immersed in distilled water, and fluoride release was measured using energy dispersive spectrometry. | The first-day fluoride release of Fuji IX was significantly higher than that of Ketac Nano, Riva self-cure, and ChemFil ROCK. | Among glass ionomer restorative cements, there is a wide range of fluoride ion release, with the highest level of release on the first day observed with Fuji IX cement, followed by Ketac Nano, Riva self-cure, and ChemFil ROCK. |
| Putri [67] | To investigate the effect of ZnO addition to GIC on the release of fluoride ions. | In group I, 1 g of ZnO was added to 9 g of glass ionomer powder, and in group II, 1.5 g of ZnO was added to 8.5 g of powder. Samples were prepared by mixing them with glass ionomer liquid. They were immersed in distilled water for 24 h, and then the level of released fluorine was tested. | Adding 1 g of zinc oxide nanoparticles can significantly increase the fluoride release of GIC. Meanwhile, adding 2 g of zinc oxide nanoparticles cannot increase the fluoride release of GIC. | Adding 1g of ZnO to the glass ionomer increases the fluoride release. |
| Karimi [68] | To find the optimal dose of ACP to add to RMGIC to activate alkaline phosphatase (ALP) and osteogenic differentiation of human mesenchymal stem cells (hMSC) without compromising GIC properties. | A GIC powder consisting of melt-derived strontium fluoro-aluminosilicate glass (SFAG) and synthetic ACP nanoparticles was created. This was combined with a commercial glass ionomer liquid, and a sample was made. The amount of fluoride released was then checked after 28 days of soaking in distilled water. | Addition of ACP to GIC up to 5.0% does not significantly reduce the release of F−. | ACP nanoparticles improved ALP activity and hMSC differentiation at the cost of negligible changes in fluoride release rate and compressive strength. |
| Cibim [69] | Evaluation of the effect of TiO2 addition to GIC on its physicochemical and biological properties. | Samples were prepared from GIC powder with TiO2 added in different proportions mixed with GIC liquid. They were stored in a demineralizing and remineralizing solution, changing it every 24 h. The amount of released fluoride was checked after 15 days. | All TiO2 groups released more fluoride compared to the control group, except KM + 3% TiO2. | The addition of 5% TiO2 to GIC increased the non-collagenous composition of the ECM improved microhardness and fluoride release capacity without affecting the surface roughness. |
| Saxena [70] | Comparison of powder composition, fluoride release, and antimicrobial properties of atraumatic zirconia restorative material with conventional GIC. | Zirconomer and Fuji IX samples were prepared and stored in artificial saliva. Fluoride content in the sample was tested after 24 h, 3, 7, 15, and 30 days. | In each of the measurements, Zirconomer showed significantly higher amounts of released fluoride. | Zirconomer has greater antibacterial activity against Streptococcus mutans and Lactobacillus casei, and releases more fluoride. However, it does not have antifungal activity against Candida albicans. |
| Shahid [71] | To evaluate the effect of replacing CaO and CaF2 with SrO and SrF2 in glass ionomer powder on the aesthetics and ion release of the finished GIC. | Samples were prepared from ionic glasses with different contents of Sr, Ca, and F. They were stored in acetic acid at pH 4.0 for 7 days at 37 °C. Then the contents of F−, Sr2+, Ca2+, and Al3+ ions in the solutions were examined. | The release of fluoride ions and all cations is linear with time, indicating diffusion control. This is consistent with the pH of the acidic medium and its changes. | Replacing calcium with strontium increases fluoride release by GIC. |
| Selimovic-Dragas [72] | To determine the amount of fluoride released from GIC and RMGIC and its influence on the cytotoxicity of these materials. | Samples were prepared from GC Fuji IX GP Fast, GC FUJI Triage, Ketac Silver, GC Fuji II LC, GC Fuji Plus, and Vitrebond and stored in distilled water. Fluoride measurements were taken after 8 and 24 h. | After both 8 and 24 h, Ketac Silver released the least fluoride of all materials tested. | Silver-doped GIC released less fluoride than conventional and resin-modified glass ionomer materials. |
| Xu [73] | To assess compressive strength, fluoride release, and recharge of 15 dental materials and explore correlations between these properties. | 15 fluoride-releasing materials (various glass ionomers, compomers, cermets, and composites) were tested. Cylindrical specimens were light-cured per manufacturer instructions. Compressive strength was measured at 24 h (Instron). Fluoride release was monitored daily for 21 days, with rechargeability tested after 3 months using 2% NaF foam. | Miracle Mix had the highest fluoride release (398 mg/cm2/21 days), exceeding Ketac-Silver (318 mg/cm2) especially in the first 4 days, but both showed lower strength than conventional GICs. Resin-modified GIs (led by Photac-Fil) had better strength than conventional GICs. Compomers/composites showed the highest strength and lowest fluoride release, except for Solitaire (422 mg/cm2). | Materials combining strong fluoride release with good mechanical properties remain limited. Resin-modified glass ionomers provide the best balance for high-caries-risk patients. |
| Osinaga [74] | Evaluating how the addition of zinc sulfate (ZnSO4) to GIC and RMGIC affects its physical, antibacterial, and F and Zn release properties. | Ketac-Fil (conventional GIC) and Vitremer (RMGIC) were modified with 0%, 5%, or 10% ZnSO4. 72 samples stored in artificial saliva were tested for F and Zn release over 30 days (days 16–30 with recharging) using ion-selective electrodes. Solubility, flexural strength, and antibacterial properties were also evaluated. | F release peaked on day 1, then decreased and stabilized, with a temporary spike after day 15 recharging. 10% ZnSO4 samples released most Zn (0.9 ± 0.5 and 7.5 ± 0.4 ppm) and were higher in Vitremer than Ketac-Fil. Zn release was highest in the first 24 h, minimal thereafter, with no post-recharge increase. Higher ZnSO4 increased solubility without affecting flexural strength. 10% ZnSO4 showed best antibacterial effect. | The addition of ZnSO4 to GICs improved antibacterial properties against S. mutans without negatively impacting their physical characteristics or fluoride release patterns. |
| Helvatjoglu-Antoniades [75] | Assessment of the amount of fluoride released from different restorative materials. | 9 materials were tested: four GICs (Miracle-Mix, Fuji Type III, Fuji II LC, and Ketac-Silver), Ketac-Cem luting cement, Compoglass Flow compomer, two sealants (Fissurit F, Helioseal F), and Tetric composite. Seven samples per material were placed in 7mL double-distilled water at 37 °C. Fluoride release was measured via ion-selective electrode at 4 h to 112 days. | All materials showed high initial fluoride release (first 24 h), followed by substantial decrease and gradual prolonged release. Glass ionomers released more fluoride than composites. Release ranking: Miracle Mix > Fuji III/Ketac Cem > Fuji II LC > Ketac Silver/Compoglass Flow > Fissurit F/Helioseal F > Tetric. 50% of cumulative release occurred in the first week. | Fluoride release was observed in all materials over the entire 16-week period. Glass ionomers and compomers exhibited higher fluoride release levels compared to sealants and composite resins. |
| Hattab [76] | To investigate the fluoride release from GIC, comparing release in deionized water and artificial saliva. | 54 samples each of Ketac-Fil, Fuji II, and Ketac-Silver were divided into uncoated, varnish-coated, and resin-coated groups. Each was tested in deionized water, artificial saliva (pH 5.5), or hydroxyapatite suspension (50 mL, 37 °C). Fluoride release was measured via specific electrode over 28 days at intervals from 1 h to weekly. | Conventional GICs released 4x more fluoride than Ketac-Silver. All showed high initial release, stabilizing after 2 weeks. Artificial saliva reduced release versus water; coatings decreased release 27.5–79.9%. Hydroxyapatite absorbed nearly all fluoride. Only small percentages (1.0–3.8%) of total fluoride were released over 28 days. | GIC released less fluoride in artificial saliva than in deionized water, and surface coatings reduced its fluoride release. |
| Author | Type of GIC Cement | Type of Additive/Metal | Amount/Concentration of Additive (%) | Fluoride Measurement Method | Storage Environment | Total Fluoride (ppm) | Total Fluoride (ppm) | Mechanical Parameters—Numerical Values |
|---|---|---|---|---|---|---|---|---|
| Pardi [54] | Riva Self Cure (conventional GIC) | AgVO3-nanostructured silver vanadate | 0, 1, 2.5 and 5 wt% | FISE (Fluoride Ion-Selective Electrode) | deionized water | Riva Self Cure 0% Day 1: 10 ± 1 ppm Day 7: 15 ± 1 ppm Day 14: 10 ± 3 ppm Day 21: 5.9 ± 0.7 ppm Day 28: 4 ± 1 ppm Riva Self Cure + 1% AgVO3 Day 1: 9.5 ± 0.9 ppm Day 7: 20 ± 2 ppm Day 14: 10 ± 1 ppm Day 21: 6 ± 1 ppm Day 28: 4.8 ± 0.3 ppm Riva Self Cure + 2.5% AgVO3 Day 1: 9.7 ± 0.7 ppm Day 7: 20 ± 2 ppm Day 14: 10.4 ± 0.8 ppm Day 21: 6.2 ± 0.8 ppm Day 28: 4.9 ± 0.4 ppm Riva Self Cure + 5% AgVO3 Day 1: 9.2 ± 0.7 ppm Day 7: 19 ± 2 ppm Day 14: 12 ± 2 ppm Day 21: 5.2 ± 0.7 ppm Day 28: 4.9 ± 0.7 ppm | Ag+ and V4+/V5+ | N/A |
| Guo [55] | Ketac Molar Easymix (conventional GIC) | AgNW and AgNP silver nanowire and silver nanoparticles | GIC (0), AgNW-GIC (0.05, 0.1, 0.3, 0.5), AgNP-GIC (0.5) wt% | Ion chromatograph (IC) | deionized water | No numeric data | N/A |
The compressive strength ± SD:
|
| Raghimi [56] | Fuji II LC (RMGIC) | Ag/HA/Si silver hydroxyapatite-silica hybrid na noparticles | 0, 0.1, 0.5, 1 and 2 wt% | FISE | distilled water | 0% GI (mean ± SD) Day 1: 0.11 ± 0.35 ppm Day 2: 0.11 ± 0.35 ppm Day 3: 0.86 ± 0.35 ppm Day 7: 0.11 ± 0.10 ppm Day 14: 0.08 ± 0.10 ppm Day 28: 0.05 ± 0.15 ppm 0.1% GI (mean ± SD) Day 1: 0.08 ± 0.25 ppm Day 2: 0.10 ± 0.22 ppm Day 3: 0.11 ± 0.15 ppm Day 7: 0.11 ± 0.10 ppm Day 14: 0.10 ± 0.10 ppm Day 28: 0.08 ± 0.30 ppm 0.5% GI (mean ± SD) Day 1: 0.10 ± 0.02 ppm Day 2: 0.12 ± 0.01 ppm Day 3: 0.12 ± 0.00 ppm Day 7: 0.15 ± 0.10 ppm Day 14: 0.17 ± 0.10 ppm Day 28: 0.17 ± 0.57 ppm 1% GI (mean ± SD) Day 1: 0.15 ± 0.06 ppm Day 2: 0.13 ± 0.04 ppm Day 3: 0.18 ± 0.01 ppm Day 7: 0.23 ± 0.03 ppm Day 14: 0.27 ± 0.01 ppm Day 28: 0.32 ± 0.02 ppm 2% GI (mean ± SD) Day 1: 0.22 ± 0.02 ppm Day 2: 0.34 ± 0.04 ppm Day 3: 0.35 ± 0.05 ppm Day 7: 0.52 ± 0.02 ppm Day 14: 0.64 ± 0.09 ppm Day 28: 0.63 ± 0.45 ppm | N/A | The compressive strength (mean ± SD):
|
| Qasim [57] | Riva Selfcure (conventional GIC) | MSAgNP mesoporous silica with silver nanoparticles | 0, 1, 3 and 5 wt% | High-Performance Liquid Chromatography (HPLC) | distilled water | No numeric data | Al3+, Ca 2+, Na+, P3−, Ag+ | Glass ionomer cements modified with mesoporous silica and silver nanoparticles exhibited microhardness similar to conventional GICs. |
| AlMatar [59] | RMGI (Fuji PLUS) | AgNP ZnONP AgNP+ZnONP | 5 wt | High-Performance Liquid Chromatography (HPLC) | distilled water | At day 2:
| N/A | After 14 days: Vickers microhardness:
|
| Gunay [61] |
| silver-alloy calcium-aluminium-zinc-fluoro-phosphorus-silicate glass | No data | FISE | distilled water | Riva Silver: Day 1 = 3.21 ± 1.96 mg/L Day 2 = 5.47 ± 1.75 mg/L Day 3 = 5.81 ± 1.99 mg/L Day 7 = 9.66 ± 2.53 mg/L Day 14 = 13.34 ± 3.08 mg/L Day 21 = 16.44 ± 4.09 mg/L Day 28 = 18.00 ± 4.09 mg/L ChemFil Rock: Day 1 = 2.16 ± 0.97 mg/L Day 2 = 2.64 ± 1.36 mg/L Day 3 = 2.16 ± 0.77 mg/L Day 7 = 4.14 ± 0.97 mg/L Day 14 = 4.99 ± 1.58 mg/L Day 21 = 6.35 ± 1.82 mg/L Day 28 = 6.73 ± 1.77 mg/L | N/A | N/A |
| Wassel [62] | Conventional self-cure GIC (Riva, SDI) | AgNP TiO2NP | 5 wt | FISE | deionized water | Cumulative values after 28 days: Control—0.056 ± 078 mg/cm2 AgNP—0.065 ± 0.157 mg/cm2 TiO2NP—0.0470 ± 0.056 mg/cm2 | N/A | Compressive strength: Control: 136.48 ± 13.40 MPa Ag: 144.32 ± 14.95 MPa Ti: 166.31 ± 15.08 MPa Compressive strength values are higher when metals are added to the material. |
| Alshehri [63] | conventional GIC (GC Fuji II) | AgNP | 0.1 and 0.2 | FISE | deionized water. | No numeric data | N/A | N/A |
| Selimovic-Dragas [72] | GIC (Ketac Silver) | Ag | No data | FISE | Distilled water | After 8 h = 0.150 (0.106) ug/g After 24 h = 0.229 (0.133) ug/g | N/A | N/A |
| Xu [73] | Ketac Silver, Miracle Mix, | sintered Ag, AgSnCu alloy | N/A | FISE | 3 mL of deionized water | Ketac Silver: 318 ± 47 µg/cm2 Miracle Mix 398 ± 32 µg/cm2 | N/A | Comprehensive strength- resulted in lower properties than other GIC, compomers or composites. |
| Helvatjoglu-Antoniades [75] |
| Miracle Mix (MM) additive: silver alloy Ketac-Silver (K) additive: sintered silver (Ag) | MM: Silver (>50% m/m), tin (<30% m/m), copper (>10% m/m), the powder consists of calcium alumino fluoro silicate glass (<50% m/m) mixed with silver alloy K: Calcium alumino fluoro silicate glass mixed with sintered silver in a ratio of 0.92:1; 48% silver content in the powder | FISE | 7 mL of double distilled water, 37 °C per sample | Total amount of fluoride (cumulative over 112 days ranked from highest to lowest): MM: 11.7 µg/mm2, F III (Fuji III): 8.3 µg/mm2, KC: 7.1 µg/mm2, F II LC: 4.7 µg/mm2 KS: 3.1 µg/mm2 COM: 2.6 µg/mm2 FS: 0.9 µg/mm2 HL: 0.6 µg/mm2 TE: 0.1 µg/mm2 | not tested | not tested |
| Hattab [76] | GIC: Ketac-Fil (KF) and Fuji II (FJ) Metal-reinforced GIC: Ketac-Silver (KS) | KS: sintered silver (Ag) | KS: 40% of the fluoride-containing glass is replaced by silver with ratio of 0.92:1 | FISE | 50 mL, 37 °C of each solution: deionized water, artificial saliva (pH 5.5), aqueous solution of hydroxyapatite | Cumulative release over 28 days: In deionized water:
FJ: 148 μg/cm2 (36.5% of release in deionized water) KF: 161 μg/cm2 (41.1% of release in deionized water) KS: 49 μg/cm2 (36.9% of release in deionized water) Fluoride uptake by hydroxyapatite from GIC samples after 14 days:
| not tested | not tested |
| Karimi [68] | RMGIC (SFAG-ACP powder and GC Fuji LININGTM LC liquid) | melt-derived strontium fluoro-aluminosilicate glass (SFAG) | 0, 1.5, 3, 5, 10 and 20% | FISE | Distilled water | 0% Day 1 = 98.55 Day 7 = 28.76 Day 14 = 19.39 Day 28 = 16.37 1.5% Day 1 = 96.58 Day 7 = 29.77 Day 14 = 19.33 Day 28 = 16.53 3% Day 1 = 91.04 Day 7 = 28.74 Day 14 = 19.01 Day 28 = 16.33 5% Day 1 = 80.59 Day 7 = 22.76 Day 14 = 13.37 Day 28 = 12.96 10% Day 1 = 61.02 Day 7 = 16.67 Day 14 = 11.75 Day 28 = 9.78 20% Day 1 = 33.87 Day 7 = 13.07 Day 14 = 9.76 Day 28 = 7.69 | Ca2+, PO43− | Comprehensive strength: 0% Day 0 = 111.05 Day 1 = 113.12 Day 3 = 117.22 Day 7 = 116.04 Day 14 = 223.02 Day 28 = 300.68 1.5% Day 0 = 115.22 Day 1 = 113.06 Day 3 = 118.08 Day 7 = 118.14 Day 14 = 200.88 Day 28 = 280.98 3% Day 0 = 113.35 Day 1 = 116.66 Day 3 = 116.27 Day 7 = 117.32 Day 14 = 200.17 Day 28 = 280.42 5% Day 0 = 114.44 Day 1 = 110.83 Day 3 = 115.43 Day 7 = 116.19 Day 14 = 188.49 Day 28 = 272.23 10% Day 0 = 107.09 Day 1 = 101.98 Day 3 = 105.35 Day 7 = 110.64 Day 14 = 145.36 Day 28 = 204.25 20% Day 0 = 103.39 Day 1 = 104.77 Day 3 = 105.29 Day 7 = 107.33 Day 14 = 134.07 Day 28 = 192.24 |
| Thongsri [60] | Synthesized sol-gel glass ionomer (SGIC) | bioactive glass powder with SrF2 | 0, 1, 3, 6 wt | FISE | distilled water | No numeric data | Ca, Al, Si | No numeric data. Compressive strength higher with 1% BGF added than SGIC without BGF. |
| Potiprapanpong [58] | Experimental RMGIC | HEMA (2-hydroxyethyl methacrylate) and Sr/F-BGNPs (bioactive glass nanoparticles) | HEMA: 0 or 5 wt%, Sr/F-BGNPs: 5 or 10 wt% | FISE (Orion) | deionized water | The highest cumulative fluoride release (at 4 weeks) was observed for H5S10 (137.5 ± 6.1 ppm). The cumulative fluoride release values for the other materials were as follows: H5S5 (136.6 ± 2.2 ppm), H0S10 (72.6 ± 3.0 ppm) and H0S5 (73.0 ± 9.4 ppm). | Al, Ca, P, and Sr | Biaxial Flexural Strength (BFS ± SD):
|
| Shahid [71] | GIC | SrO and SrF2 | G1 = 0% SrF2, 0% SrO G2 = 2% SrF2, 3% SrO G3 = 2% SrF2, 1.5% SrO G4 = 2% SrF2, 0.5% SrO G5 = 1% SrF2, 0% SrO G6 = 1% SrF2, 1.5% SrO G7 = 1.5% SrF2, 1% SrO G8 = 0.5% SrF2, 2% SrO G9 = 0.25% SrF2, 2.25% SrO G10 = 0% SrF2, 2.5% SrO | FISE | Acetic acid at pH 4 | G1 = 0.56 mequiv/g G2 = 0.93 mequiv/g G3 = 1.11 mequiv/g G4 = 0.75 mequiv/g G5 = 0.47 mequiv/g G6 = 0.8 mequiv/g G7 = 2.61 mequiv/g G8 = 0.29 mequiv/g G9 = 0.21 mequiv/g | Sr2+, Ca2+ and Al3+ | N/A |
| Saxena [70] | GIC (Zirconomer) | ZrO2 | No data | FISE | Artificial saliva | Day 1 = 29.38 ppm Day 3 = 31.69 ppm Day 7 = 35.65 ppm Day 15 = 25.58 ppm Day 30 = 9.46 Control (Fuji IX) Day 1 = 13.72 ppm Day 3 = 15.08 ppm Day 7 = 15.46 ppm Day 15 = 7.39 ppm Day 30 = 2.53 ppm | N/A | N/A |
| Cibim [69] | GIC + TiO2 nanotubes | TiO2 | 0, 3, 5 and 7% | FISE | Demineralizing and remineralizing solutions | DE solution Control Day 1 = 0.198 (0.05) ppm Day 2 = 0.156 (0.04) ppm Day 3 = 0.145 (0.05) ppm Day 5 = 0.141 (0.05) ppm Day 7 = 0.141 (0.05) ppm Day 9 = 0.124 (0.04) ppm Day 12 = 0.159 (0.04) ppm Day 15 = 0.165 (0.07) ppm 3% Day 1 = 0.298 (0.07) ppm Day 2 = 0.233 (0.06) ppm Day 3 = 0.199 (0.03) ppm Day 5 = 0.179 (0.02) ppm Day 7 = 0.179 (0.03) ppm Day 9 = 0.158 (0.03) ppm Day 12 = 0.168 (0.04) ppm Day 15 = 1.172 (0.04) ppm 5% Day 1 = 0.292 (0.08) ppm Day 2 = 0.256 (0.08) ppm Day 3 = 0.197 (0.06) ppm Day 5 = 0.187 (0.05) ppm Day 7 = 0.212 (0.06) ppm Day 9 = 0.155 (0.05) ppm Day 12 = 0.213 (0.04) ppm Day 15 = 0.213 (0.04) ppm 7% Day 1 = 0.311 (0.08) ppm Day 2 = 0.249 (0.09) ppm Day 3 = 0.191 (0.05) ppm Day 5 = 0.181 (0.05) ppm Day 7 = 0.168 (0.05) ppm Day 9 = 0.154 (0.04) ppm Day 12 = 0.171 (0.04) ppm Day 15 = 0.164 (0.06) ppm RE solution Control Day 1 = 0.049 (0.01) ppm Day 2 = 0.03 (0.01) ppm Day 3 = 0.033 (0.01) ppm Day 5 = 0.027 (0.01) ppm Day 7 = 0.03 (0.01) ppm Day 9 = 0.032 (0.01) ppm Day 12 = 0.031 (0.01) ppm Day 15 = 0.031 (0.01) ppm 3% Day 1 = 0.041 (0.01) ppm Day 2 = 0.029 (0.01) ppm Day 3 = 0.037 (0.01) ppm Day 5 = 0.036 (0.01) ppm Day 7 = 0.033 (0.01) ppm Day 9 = 0.037 (0.01) ppm Day 12 = 0.034 (0.01) ppm Day 15 = 0.039 (0.01) ppm 5% Day 1 = 0.037 (0.01) ppm Day 2 = 0.035 (0.02) ppm Day 3 = 0.036 (0.03) ppm Day 5 = 0.04 (0.01) ppm Day 7 = 0.038 (0.01) ppm Day 9 = 0.042 (0.01) ppm Day 12 = 0.037 (0.01) ppm Day 15 = 0.047 (0.01) ppm 7% Day 1 = 0.067 (0.02) ppm Day 2 = 0.047 (0.02) ppm Day 3 = 0.043ppm Day 5 = 0.041 (0.01) ppm Day 7 = 0.039 (0.01) ppm Day 9 = 0.044 (0.01) ppm Day 12 = 0.045 (0.02) ppm Day 15 = 0.044 (0.02) ppm | N/A | Surface roughness Control = 0.41 ± 0.14 3% = 0.55 ± 0.17 5% = 0.49 ± 0.07 7% = 0.58 ± 0.16 Surface hardness Control = 81.48 ± 9.87 3% = 105.87 ± 12.71 5% = 118.25 ± 4.21 7% = 75.13 ± 6.61 |
| Putri [67] | GIC with ZnO nanoparticles | ZnO | 10 or 15% | Spectrophotometer | Distilled water | Control = 0.417 (0.133) ppm 10% = 0.571 (0.099) ppm 15% = 0.457 (0.144) ppm | N/A | N/A |
| Bahammam [66] | GIC (ChemFil ROCK) | calcium-aluminum-zinc-fluoro-phosphor-silicate glass | No data | FISE (Fisher Scientific Accumet 13-620–629) | Distilled water | 1.68 ± 0.08 μg/cm2 | O, F, Na, Mg, Al, Si, P, S, Ca, Sr, Zn, and Zr | N/A |
| Malekhoseini [65] | RMGI (Fuji II LC + ZnO nanoparticles) | ZnO | 0%, 1%, 2%, 3% or 4% | FISE and potentiometer | Deionized water | 3% = 34.6 ppm 0% = 35 ppm | Zn | Flexural strength: no numeric data Flexural modulus: no numeric data Micro shear bond strength: Control: Day 1 = 10.8 ± 2.2 MPa Day 7 = 12.36 ± 3.6 MPa Day 30 = 16.76 ± 5.82 MPa 2%: Day 1 = 10.96 ± 3.72 MPa Day 7 = 14.63 ± 2.56 MPa Day 30 = 12.1 ± 2.78 MPa |
| Kohno [64] | GIC with BioUnion Filler | Fluorozincsilicate glass | No data | FISE | Artificial saliva + acetate buffer solution (pH 4.5) | No numeric data | Zn | N/A |
| Osinaga [74] | GIC: Ketac-Fil RMGIC: Vitremer | ZnSO4 | 5% ZnSO4, 10% ZnSO4 | FISE | Artificial saliva (1 mL), 100% humid environment, 37 °C | For Ketac-Fil groups:
| Zinc (Zn)—measured by inductively coupled argon plasma emission spectrometry | Flexural strength:
|
| Authors | (1) Is It Clear in the Study What Is the ‘Cause’ and What Is the ‘Effect’? | (2) Were the Participants Included in Any Comparisons Similar? | (3) Were the Participants Included in Any Comparisons Receiving Similar Treatment/Care, Other than the Exposure or Intervention of Interest? | (4) Was There a Control Group? | (5) Were There Multiple Measurements of the Outcome Both Pre and Post the Intervention/Exposure? | (6) Was Follow up Complete and If Not, Were Differences Between Groups in Terms of Their Follow Up Adequately Described and Analyzed? | (7) Were the Outcomes of Participants Included in Any Comparisons Measured in the Same Way? | (8) Were Outcomes Measured in a Reliable Way? | (9) Was Appropriate Statistical Analysis Used? |
|---|---|---|---|---|---|---|---|---|---|
| Pardi [54] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Guo [55] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Raghimi [56] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Qasim [57] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Potiprapanpong [58] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| AlMatar [59] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Thongsri [60] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Gunay [61] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Wassel [62] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Alshehri [63] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Kohno [64] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Malekhoseini [65] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Bahammam [66] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Putri [67] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Karimi [68] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Cibim [69] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Saxena [70] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Shahid [71] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Selimovic-Dragas [72] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Xu [73] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Osinaga [74] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Helvatjoglu-Antoniades [75] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Hattab [76] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimas, S.; Kiryk, S.; Kiryk, J.; Kotela, A.; Kensy, J.; Michalak, M.; Rybak, Z.; Matys, J.; Dobrzyński, M. The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies. Materials 2025, 18, 3187. https://doi.org/10.3390/ma18133187
Klimas S, Kiryk S, Kiryk J, Kotela A, Kensy J, Michalak M, Rybak Z, Matys J, Dobrzyński M. The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies. Materials. 2025; 18(13):3187. https://doi.org/10.3390/ma18133187
Chicago/Turabian StyleKlimas, Sylwia, Sylwia Kiryk, Jan Kiryk, Agnieszka Kotela, Julia Kensy, Mateusz Michalak, Zbigniew Rybak, Jacek Matys, and Maciej Dobrzyński. 2025. "The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies" Materials 18, no. 13: 3187. https://doi.org/10.3390/ma18133187
APA StyleKlimas, S., Kiryk, S., Kiryk, J., Kotela, A., Kensy, J., Michalak, M., Rybak, Z., Matys, J., & Dobrzyński, M. (2025). The Impact of Environmental and Material Factors on Fluoride Release from Metal-Modified Glass Ionomer Cements: A Systematic Review of In Vitro Studies. Materials, 18(13), 3187. https://doi.org/10.3390/ma18133187










