From Salivary Dysfunction to Prosthetic Challenges in Xerostomia and Denture Retention with Oral Gels
Abstract
1. Introduction
2. Physiology and Dysregulation of Salivary Secretion
2.1. Salivary Gland Function and Saliva Composition
2.2. Etiology of Salivary Gland Dysfunction
2.3. Radiation-Induced Salivary Dysfunction
3. Functional Components of Saliva and the Basis for Oral Gels
3.1. Salivary Components and Their Functional Roles
3.2. Mucins: Structure, Glycosylation, and Role in Oral Lubrication
4. Adsorption of Salivary Pellicle
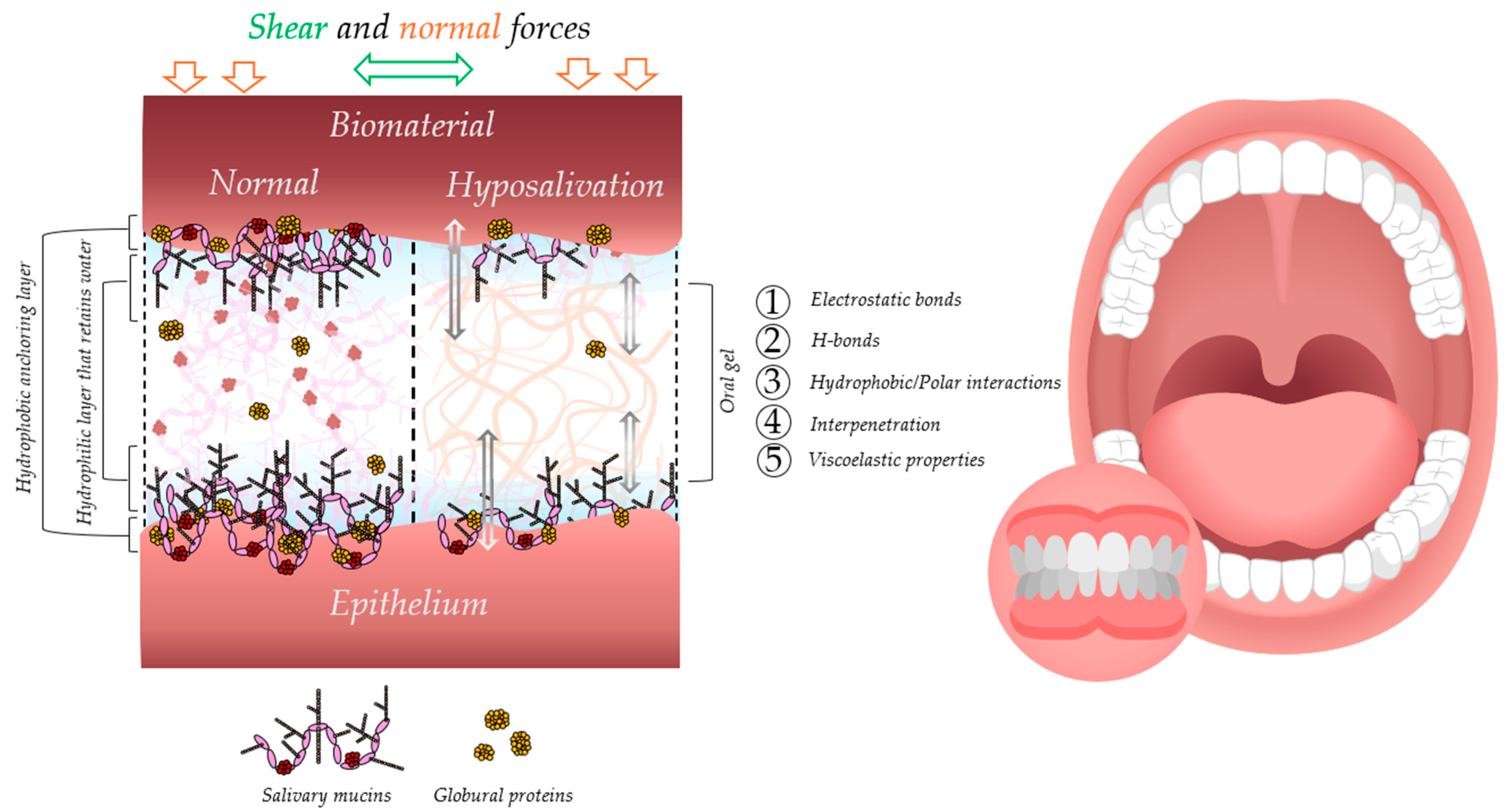
5. Mucoadhesion
5.1. Theories and Mechanisms of Mucoadhesion
5.2. Evaluation Methods for Mucoadhesive Performance
6. Impact of Lubrication on Oral Biomechanics and Usage of Prosthetic Devices
6.1. Role of Lubrication in Maintaining Oral Biomechanical Balance
6.2. Consequences of Reduced Lubrication for Denture Wearers
7. Oral Lubrication, Tribology, and Rheology
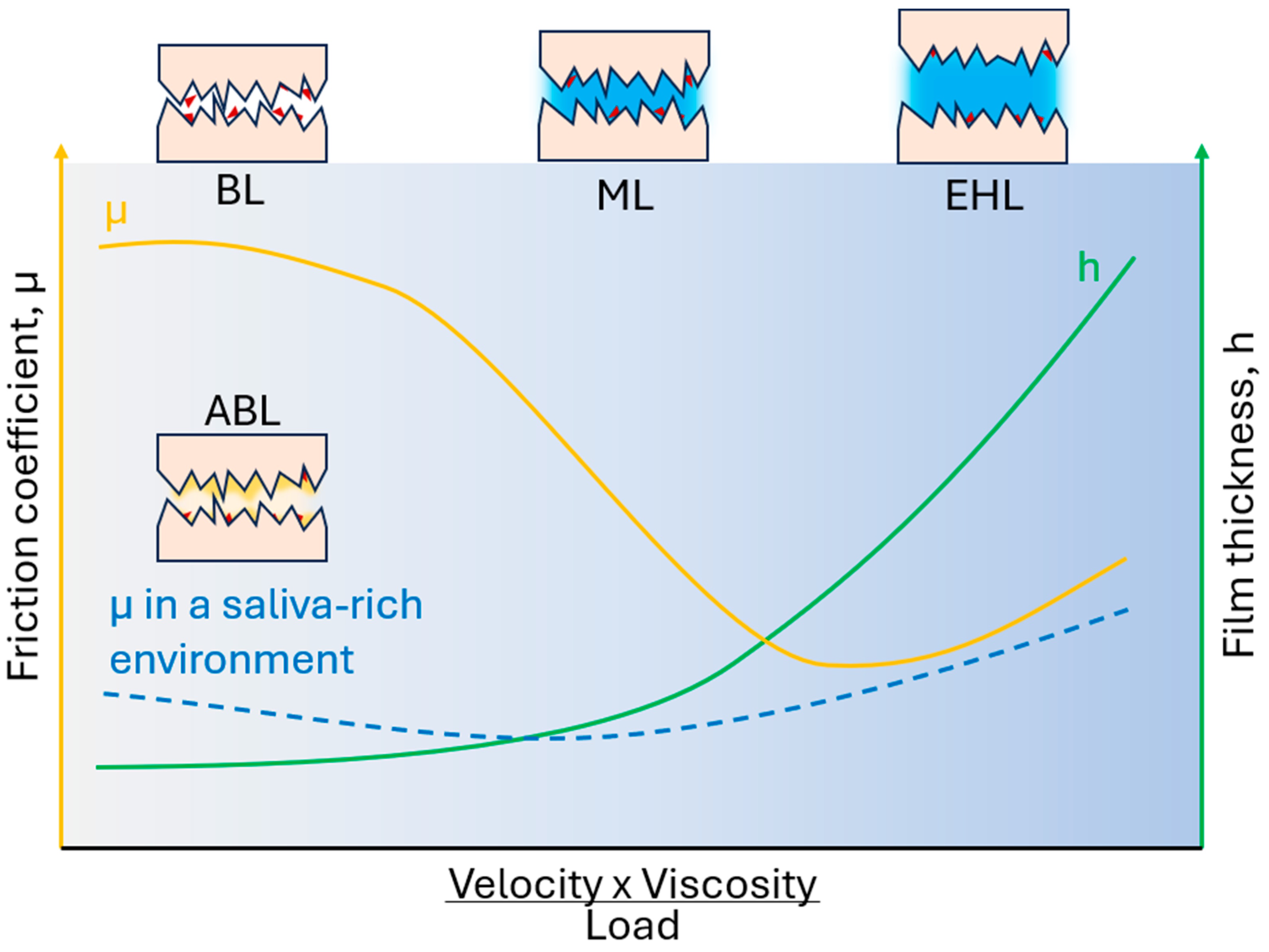
7.1. Rheological and Tribological Principles in Oral Lubrication
7.2. Biological Interfaces, Salivary Films, and Friction Reduction Mechanisms
7.3. Rheological Evaluation of Commercial Oral Gels
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Humphrey, S.P.; Williamson, R.T. A Review of Saliva: Normal Composition, Flow, and Function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.J.; Aguirre, A.; Tabak, L.A.; Hatton, M.N. Artificial Salivas: Present and Future. J. Dent. Res. 1987, 66, 693–698. [Google Scholar] [CrossRef]
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The Functions of Human Saliva: A Review Sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Bolscher, J.G.M.; Nazmi, K.; Kalay, H.; van’t Hof, W.; Amerongen, A.V.N.; Veerman, E.C.I. Histatins Are the Major Wound-Closure Stimulating Factors in Human Saliva as Identified in a Cell Culture Assay. FASEB J. 2008, 22, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; DÍaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The Salivary Peptide Histatin-1 Promotes Endothelial Cell Adhesion, Migration, and Angiogenesis. FASEB J. 2017, 31, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Critical Review in Oral Biology & Medicine: Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The Power of Saliva: Antimicrobial and Beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef]
- Shakeeb, N.; Varkey, P.; Ajit, A. Human Saliva as a Diagnostic Specimen for Early Detection of Inflammatory Biomarkers by Real-Time RT-PCR. Inflammation 2021, 44, 1713–1723. [Google Scholar] [CrossRef]
- Scully, C. Challenges in Predicting Which Oral Mucosal Potentially Malignant Disease Will Progress to Neoplasia. Oral Dis. 2014, 20, 1–5. [Google Scholar] [CrossRef]
- Kim, Y.J. Xerostomia and Its Cellular Targets. Int. J. Mol. Sci. 2023, 24, 5358. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, A.; Lenér, F.; Marklund, B.; Mossberg, K.; Çevik-Aras, H. Prevalence of Dry Mouth in Adult Patients in Primary Health Care. Acta Odontol. Scand. 2022, 80, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Dibello, V.; Zupo, R.; Sardone, R.; Lozupone, M.; Castellana, F.; Dibello, A.; Daniele, A.; De Pergola, G.; Bortone, I.; Lampignano, L.; et al. Oral Frailty and Its Determinants in Older Age: A Systematic Review. Lancet Healthy Longev. 2021, 2, e507–e520. [Google Scholar] [CrossRef]
- Niedermeier, W.; Huber, M.; Fischer, D.; Beier, K.; Müller, N.; Schuler, R.; Brinninger, A.; Fartasch, M.; Diepgen, T.; Matthaeus, C.; et al. Significance of Saliva for the Denture-Wearing Population. Gerodontology 2000, 17, 104–118. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.; Lynch, E. Xerostomia in Complete Denture Wearers: Prevalence, Clinical Findings and Impact on Oral Functions. Gerodontology 2014, 31, 49–55. [Google Scholar] [CrossRef]
- Bergdahl, M. Salivary Flow and Oral Complaints in Adult Dental Patients. Community Dent. Oral Epidemiol. 2000, 28, 59–66. [Google Scholar] [CrossRef]
- Lakhyani, R.; Wagdargi, S.S. Saliva and Its Importance in Complete Denture Prosthodontics. Natl. J. Integr. Res. Med. 2012, 3, 139–146. [Google Scholar]
- Lal, Q.; Godil, A.; Shaikh, M.; Musani, S.; Dugal, R.; Kirad, A. Wettability of Two Different Artificial Saliva Substitutes on Injection Moulded Heat Polymerized Acrylic Resin and CAD-CAM Acrylic Resin: An In Vitro Study. Dent. 3000 2023, 11. [Google Scholar] [CrossRef]
- Taqa, A.A.; Nazhat, M.N.; Basshi, T.Y.; Al_jader, G.H. Evaluation of Physical and Chemical Properties of Saliva on Retention of Complete Denture (In Vitro Study). Int. J. Dent. Res. 2018, 3, 50–54. [Google Scholar] [CrossRef]
- Narita, T.; Qi, B.; Murakami, M.; Sugiya, H. Pilocarpine Induces the Residual Secretion of Salivary Fluid in Perfused Submandibular Glands of Rats. PLoS ONE 2019, 14, e0221832. [Google Scholar] [CrossRef]
- Yasuda, H.; Niki, H. Review of the Pharmacological Properties and Clinical Usefulness of Muscarinic Agonists for Xerostomia in Patients with Sjögren’s Syndrome. Clin. Drug Investig. 2002, 22, 67–73. [Google Scholar] [CrossRef]
- Rocchi, C.; Barazzuol, L.; Coppes, R.P. The Evolving Definition of Salivary Gland Stem Cells. NPJ Regen. Med. 2021, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.Y. The Meaning, the Sense and the Significance: Translating the Science of Mesenchymal Stem Cells into Medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.A.; Brunsting, J.F.; Weirenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Rescue of Salivary Gland Function after Stem Cell Transplantation in Irradiated Glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef]
- Song, W.; Liu, H.; Su, Y.; Zhao, Q.; Wang, X.; Cheng, P.; Wang, H. Current Developments and Opportunities of Pluripotent Stem Cells-Based Therapies for Salivary Gland Hypofunction. Front. Cell Dev. Biol. 2024, 12, 1346996. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early Responses to Adenoviral-Mediated Transfer of the Aquaporin-1 CDNA for Radiation-Induced Salivary Hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef]
- Rocchi, C.; Emmerson, E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020, 26, 649–669. [Google Scholar] [CrossRef]
- Darvell, B.W.; Clark, R.K.F. The Physical Mechanisms of Complete Denture Retention. Br. Dent. J. 2000, 189, 248–252. [Google Scholar] [CrossRef]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef]
- Sotres, J.; Pettersson, T.; Lindh, L.; Arnebrant, T. NanoWear of Salivary Films vs. Substratum Wettability. J. Dent. Res. 2012, 91, 973–978. [Google Scholar] [CrossRef]
- Engel, A.S.; Kranz, H.T.; Schneider, M.; Tietze, J.P.; Piwowarcyk, A.; Kuzius, T.; Arnold, W.; Naumova, E.A. Biofilm Formation on Different Dental Restorative Materials in the Oral Cavity. BMC Oral Health 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral Multispecies Biofilm Development and the Key Role of Cell-Cell Distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Chibly, A.; Aure, M.; Patel, V.; Hoffman, M.P. Salivary Gland Function, Development, and Regeneration. Physiol. Rev. 2022, 102, 1495–1552. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary Secretion in Health and Disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Proctor, G.B. The Physiology of Salivary Secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Song, E.A.C.; Chung, S.H.; Kim, J.H. Molecular Mechanisms of Saliva Secretion and Hyposecretion. Eur. J. Oral Sci. 2024, 132, e12969. [Google Scholar] [CrossRef]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary Hypofunction: An Update on Aetiology, Diagnosis and Therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, V.; Jensen, S.B.; Smith, D.K.; Bohlke, K.; Bauman, J.; Brennan, M.T.; Coppes, R.P.; Jessen, N.; Malhotra, N.K.; Murphy, B.; et al. Salivary Gland Hypofunction and/or Xerostomia Induced by Nonsurgical Cancer Therapies: ISOO/MASCC/ASCO Guideline. J. Clin. Oncol. 2021, 39, 2825–2843. [Google Scholar] [CrossRef]
- Murray Thomson, W.; Poulton, R.; Mark Broadbent, J.; Al-Kubaisy, S. Xerostomia and Medications among 32-Year-Olds. Acta Odontol. Scand. 2006, 64, 249–254. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Xiao, F. Neuromyotonia as an Unusual Neurological Complication of Primary Sjögren’s Syndrome: Case Report and Literature Review. Clin. Rheumatol. 2017, 36, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, C.; Eisbruch, A. Organ-Sparing Radiation Therapy for Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2011, 8, 639–648. [Google Scholar] [CrossRef]
- Glennon, S.G.; Huedo-Medina, T.; Rawal, S.; Hoffman, H.J.; Litt, M.D.; Duffy, V.B. Chronic Cigarette Smoking Associates Directly and Indirectly with Self-Reported Olfactory Alterations: Analysis of the 2011–2014 National Health and Nutrition Examination Survey. Nicotine Tob. Res. 2019, 21, 818–827. [Google Scholar] [CrossRef]
- Diep, M.T.; Jensen, J.L.; Skudutyte-Rysstad, R.; Young, A.; Sødal, A.T.T.; Petrovski, B.É.; Hove, L.H. Xerostomia and Hyposalivation among a 65-Yr-Old Population Living in Oslo, Norway. Eur. J. Oral Sci. 2021, 129, e12757. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Wang, E.A.; Fazel, N. Etiology, Evaluation, and Management of Xerostomia. Clin. Dermatol. 2017, 35, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Y.; Hoseini, E.G.; Atoof, F.; Mottaghi, R. Xerostomia (Dry Mouth) in Patients with COVID-19: A Case Series. Future Virol. 2021, 16, 315–319. [Google Scholar] [CrossRef]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 Infection of the Oral Cavity and Saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef]
- Weng, P.L.; Aure, M.H.; Maruyama, T.; Ovitt, C.E. Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity. Cell Rep. 2018, 24, 1464–1470.e3. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L. Xerostomia in Patients on Chronic Hemodialysis. Nat. Rev. Nephrol. 2012, 8, 176–182. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, M.B.; Erdogan, B.; Bardakcı, M.; Algın, E.; Gulbagcı, B.; Hacibekiroglu, I.; Hamdard, J.; Olmez, O.F.; Akkus, H.; Oksuzoglu, B.; et al. Major and Minor Salivary Gland Cancers: A Multicenter Retrospective Study. Head Neck 2023, 45, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.A.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Dirix, P.; Nuyts, S.; Van Den Bogaert, W. Radiation-Induced Xerostomia in Patients with Head and Neck Cancer: A Literature Review. Cancer 2006, 107, 2525–2534. [Google Scholar] [CrossRef]
- Li, Y.; Taylor, J.M.G.; Ten Haken, R.K.; Eisbruch, A. The Impact of Dose on Parotid Salivary Recovery in Head and Neck Cancer Patients Treated with Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 660–669. [Google Scholar] [CrossRef]
- Meyer, S.; Chibly, A.M.; Burd, R.; Limesand, K.H. Insulin-Like Growth Factor-1-Mediated DNA Repair in Irradiated Salivary Glands Is Sirtuin-1 Dependent. J. Dent. Res. 2017, 96, 225–232. [Google Scholar] [CrossRef]
- Mitchell, G.C.; Fillinger, J.L.; Sittadjody, S.; Avila, J.L.; Burd, R.; Limesand, K.H. IGF1 Activates Cell Cycle Arrest Following Irradiation by Reducing Binding of ΔNp63 to the P21 Promoter. Cell Death Dis. 2010, 1, e50. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. JNCI Monogr. 2019, 2019, lgz016. [Google Scholar] [CrossRef]
- Diwanji, N.; Bergmann, A. An Unexpected Friend—ROS in Apoptosis-Induced Compensatory Proliferation: Implications for Regeneration and Cancer. Semin. Cell Dev. Biol. 2018, 80, 74–82. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Calcium Signalling in Salivary Gland Physiology and Dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef]
- Vinke, J.; Kaper, H.J.; Vissink, A.; Sharma, P.K. Dry Mouth: Saliva Substitutes Which Adsorb and Modify Existing Salivary Condition Films Improve Oral Lubrication. Clin. Oral Investig. 2020, 24, 4019–4030. [Google Scholar] [CrossRef]
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the Management of Dry Mouth: Topical Therapies. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Mouly, S.; Salom, M.; Tillet, Y.; Coudert, A.C.; Oberli, F.; Preshaw, P.M.; Desjonquères, S.; Bergmann, J.F. Management of Xerostomia in Older Patients: A Randomised Controlled Trial Evaluating the Efficacy of a New Oral Lubricant Solution. Drugs Aging 2007, 24, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Bozorgi, S.; Vladescu, S.; Forte, A.E.; Myant, C.; Potineni, R.V.; Reddyhoff, T.; Baier, S.K. A Study of Saliva Lubrication Using a Compliant Oral Mimic. Food Hydrocoll. 2019, 92, 10–18. [Google Scholar] [CrossRef]
- Saitoh, E.; Taniguchi, M.; Ochiai, A.; Kato, T.; Imai, A.; Isemura, S. Bioactive Peptides Hidden in Human Salivary Proteins. J. Oral Biosci. 2017, 59, 71–79. [Google Scholar] [CrossRef]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative Human Salivary and Plasma Proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Ince, D.; Lucas, T.M.; Malaker, S.A. Current Strategies for Characterization of Mucin-Domain Glycoproteins. Curr. Opin. Chem. Biol. 2022, 69, 102174. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.H.; Mei, M.L.; Chu, C.H. Acquired Salivary Pellicle and Oral Diseases: A Literature Review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef]
- Chaudhury, N.M.A.; Shirlaw, P.; Pramanik, R.; Carpenter, G.H.; Proctor, G.B. Changes in Saliva Rheological Properties and Mucin Glycosylation in Dry Mouth. J. Dent. Res. 2015, 94, 1660–1667. [Google Scholar] [CrossRef]
- Alliende, C.; Kwon, Y.J.; Brito, M.; Molina, C.; Aguilera, S.; Pérez, P.; Leyton, L.; Quest, A.F.G.; Mandel, U.; Veerman, E.; et al. Reduced Sulfation of MUC5B Is Linked to Xerostomia in Patients with Sjögren Syndrome. Ann. Rheum. Dis. 2008, 67, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Keimel, R.; Gugatschka, M.; Kolb, D.; Leitinger, G.; Roblegg, E. Investigation of Changes in Saliva in Radiotherapy-Induced Head Neck Cancer Patients. Int. J. Environ. Res. Public Health 2021, 18, 1629. [Google Scholar] [CrossRef] [PubMed]
- Ohyabu, N.; Kakiya, K.; Yokoi, Y.; Hinou, H.; Nishimura, S.I. Convergent Solid-Phase Synthesis of Macromolecular MUC1 Models Truly Mimicking Serum Glycoprotein Biomarkers of Interstitial Lung Diseases. J. Am. Chem. Soc. 2016, 138, 8392–8395. [Google Scholar] [CrossRef] [PubMed]
- Cherian, R.M.; Jin, C.; Liu, J.; Karlsson, N.G.; Holgersson, J. Recombinant Mucin-Type Fusion Proteins with a Galα1,3Gal Substitution as Clostridium Difficile Toxin A Inhibitors. Infect. Immun. 2016, 84, 2842–2852. [Google Scholar] [CrossRef]
- Becker, T.; Dziadek, S.; Wittrock, S.; Kunz, H. Synthetic Glycopeptides from the Mucin Family as Potential Tools in Cancer Immunotherapy. Curr. Cancer Drug Targets 2006, 6, 491–517. [Google Scholar] [CrossRef]
- Kwan, C.S.; Cerullo, A.R.; Braunschweig, A.B. Design and Synthesis of Mucin-Inspired Glycopolymers. ChemPlusChem 2020, 85, 2704–2721. [Google Scholar] [CrossRef]
- Schlatterer, R.; Marczynski, M.; Hermann, B.; Lieleg, O.; Balzer, B.N. Unfolding of von Willebrand Factor Type D Like Domains Promotes Mucin Adhesion. Nano Lett. 2025, 11, 37. [Google Scholar] [CrossRef]
- An, J.; Jin, C.; Dėdinaitė, A.; Holgersson, J.; Karlsson, N.G.; Claesson, P.M. Influence of Glycosylation on Interfacial Properties of Recombinant Mucins: Adsorption, Surface Forces, and Friction. Langmuir 2017, 33, 4386–4395. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Bradley, M.; Patrojanasophon, P. Maleimide-Functionalized Carboxymethyl Cellulose: A Novel Mucoadhesive Polymer for Transmucosal Drug Delivery. Carbohydr. Polym. 2022, 288, 119368. [Google Scholar] [CrossRef]
- Martin-Alarcon, L.; Govedarica, A.; Ewoldt, R.H.; Bryant, S.L.; Jay, G.D.; Schmidt, T.A.; Trifkovic, M. Scale-Dependent Rheology of Synovial Fluid Lubricating Macromolecules. Small 2024, 20, 2306207. [Google Scholar] [CrossRef]
- Weiand, E.; Koenig, P.H.; Rodriguez-Ropero, F.; Roiter, Y.; Angioletti-Uberti, S.; Dini, D.; Ewen, J.P. Boundary Lubrication Performance of Polyelectrolyte-Surfactant Complexes on Biomimetic Surfaces. Langmuir 2024, 40, 7933–7946. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Karalus, W.; Sidorenko, J.; Dąbrowski, J.R.; Kalska-Szostko, B. Biotribological Properties of Dentures Lubricated with Artificial Saliva. J. Frict. Wear 2016, 37, 544–551. [Google Scholar] [CrossRef]
- Riquelme, N.; Laguna, L.; Tárrega, A.; Robert, P.; Arancibia, C. Oral Behavior of Emulsified Systems with Different Particle Size and Thickening Agents under Simulated Conditions. Food Res. Int. 2021, 147, 110558. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Łysik, D.; Klekotka, M. Effect of Saliva and Mucin-Based Saliva Substitutes on Fretting Processes of 316 Austenitic Stainless Steel. Metals 2019, 9, 178. [Google Scholar] [CrossRef]
- Mystkowska, J.; Lysik, D.; Germaniuk, M.; Niemirowicz-Laskowska, K.; Bucki, R. The Influence of PH and Temperature on Stability of Artificial Saliva Based on Porcine Gastric Mucin. In Proceedings of the 15th International Conference Mechatronic Systems and Materials, MSM 2020, Bialystok, Poland, 1–3 July 2020. [Google Scholar]
- Schweigel, H.; Wicht, M.; Schwendicke, F. Salivary and Pellicle Proteome: A Datamining Analysis. Sci. Rep. 2016, 6, 38882. [Google Scholar] [CrossRef] [PubMed]
- Vukosavljevic, D.; Custodio, W.; Buzalaf, M.A.R.; Hara, A.T.; Siqueira, W.L. Acquired Pellicle as a Modulator for Dental Erosion. Arch. Oral Biol. 2014, 59, 631–638. [Google Scholar] [CrossRef]
- Wong, R.S.; Bennick, A. The Primary Structure of a Salivary Calcium-Binding Proline-Rich Phosphoprotein (Protein C), a Possible Precursor of a Related Salivary Protein A. J. Biol. Chem. 1980, 255, 5943–5948. [Google Scholar] [CrossRef]
- Zentner, A.; Heaney, T.G. An In Vitro Investigation of the Role of High Molecular Weight Human Salivary Sulphated Glycoprotein in Periodontal Wound Healing. J. Periodontol. 1995, 66, 944–955. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; Van Der Mei, H.C. Biofilm Formation on Dental Restorative and Implant Materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Mystkowska, J. Biocorrosion of Dental Alloys Due to Desulfotomaculum Nigrificans Bacteria. Acta Bioeng. Biomech. 2016, 18, 87–96. [Google Scholar] [CrossRef]
- Sundararaj, D.; Venkatachalapathy, S.; Tandon, A.; Pereira, A. Critical Evaluation of Incidence and Prevalence of White Spot Lesions during Fixed Orthodontic Appliance Treatment: A Meta-Analysis. J. Int. Soc. Prev. Community Dent. 2015, 5, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ambikathanaya, U.K.; Swamy, K.N.R.; Gujjari, A.K.; Tejaswi, S.; Shetty, S.; Ravi, M.B. Effect of Acrylic Removable Partial Denture in Caries Prevalence among Diabetic and Non-Diabetic Patients. J. Pharm. Bioallied Sci. 2022, 14, S917–S922. [Google Scholar] [CrossRef]
- Martínez-Hernández, M.; Reyes-Grajeda, J.P.; Hannig, M.; Almaguer-Flores, A. Salivary Pellicle Modulates Biofilm Formation on Titanium Surfaces. Clin. Oral. Investig. 2023, 27, 6135–6145. [Google Scholar] [CrossRef]
- Hannig, M. Ultrastructural Investigation of Pellicle Morphogenesis at Two Different Intraoral Sites during a 24-h Period. Clin. Oral Investig. 1999, 3, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Tang, Y.; Zeng, Q.; Zheng, J. On Adhesion Mechanism of Salivary Pellicle-PDMS Interface. Biosurf. Biotribol. 2019, 5, 93–96. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What Interactions Drive the Salivary Mucosal Pellicle Formation? Colloids Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M. Transmission Electron Microscopic Study of In Vivo Pellicle Formation on Dental Restorative Materials. Eur. J. Oral Sci. 1997, 105, 422–433. [Google Scholar] [CrossRef]
- Juriaanse, A.C.; Booij, M.; Arends, J.; Ten Bosch, J.J. The Adsorption in Vitro of Purified Salivary Proteins on Bovine Dental Enamel. Arch. Oral Biol. 1981, 26, 91–96. [Google Scholar] [CrossRef]
- Fischer, N.G.; Aparicio, C. The Salivary Pellicle on Dental Biomaterials. Colloids Surf. B Biointerfaces 2021, 200, 111570. [Google Scholar] [CrossRef]
- Hirsh, S.L.; McKenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M.M.M. The Vroman Effect: Competitive Protein Exchange with Dynamic Multilayer Protein Aggregates. Colloids Surf. B Biointerfaces 2013, 103, 395–404. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The Blood Compatibility Challenge. Part 2: Protein Adsorption Phenomena Governing Blood Reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Yakubov, G.E.; Macakova, L.; Wilson, S.; Windust, J.H.C.; Stokes, J.R. Aqueous Lubrication by Fractionated Salivary Proteins: Synergistic Interaction of Mucin Polymer Brush with Low Molecular Weight Macromolecules. Tribol. Int. 2015, 89, 34–45. [Google Scholar] [CrossRef]
- Boyd, H.; Gonzalez-Martinez, J.F.; Welbourn, R.J.L.; Gutfreund, P.; Klechikov, A.; Robertsson, C.; Wickström, C.; Arnebrant, T.; Barker, R.; Sotres, J. A Comparison between the Structures of Reconstituted Salivary Pellicles and Oral Mucin (MUC5B) Films. J. Colloid Interface Sci. 2021, 584, 660–668. [Google Scholar] [CrossRef]
- Nagasawa, D.; Azuma, T.; Noguchi, H.; Uosaki, K.; Takai, M. Role of Interfacial Water in Protein Adsorption onto Polymer Brushes as Studied by SFG Spectroscopy and QCM. J. Phys. Chem. C 2015, 119, 17193–17201. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Alaei, S.; Omidian, H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur. J. Pharm. Sci. 2021, 159, 105727. [Google Scholar] [CrossRef]
- Hombach, J.; Bernkop-Schnürch, A. Mucoadhesive Drug Delivery Systems. Handb. Exp. Pharmacol. 2010, 197, 251–266. [Google Scholar]
- Smart, J.D. The Basics and Underlying Mechanisms of Mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive Polymeric Platforms for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A Food Perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Abruzzo, A.; Vitali, B.; Lombardi, F.; Guerrini, L.; Cinque, B.; Parolin, C.; Bigucci, F.; Cerchiara, T.; Arbizzani, C.; Gallucci, M.C.; et al. Mucoadhesive Buccal Films for Local Delivery of Lactobacillus Brevis. Pharmaceutics 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Abruzzo, A.; Musazzi, U.M.; Cilurzo, F.; Nicoletta, F.P.; Dalena, F.; Parolin, C.; Vitali, B.; Cerchiara, T.; Luppi, B.; et al. Freeze-Dried Matrices Based on Polyanion Polymers for Chlorhexidine Local Release in the Buccal and Vaginal Cavities. J. Pharm. Sci. 2019, 108, 2447–2457. [Google Scholar] [CrossRef]
- Abruzzo, A.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Bilayered Buccal Films as Child-Appropriate Dosage Form for Systemic Administration of Propranolol. Int. J. Pharm. 2017, 531, 257–265. [Google Scholar] [CrossRef]
- Szekalska, M.; Wróblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole. Mar. Drugs 2019, 17, 692. [Google Scholar] [CrossRef] [PubMed]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and Evaluation of Chitosan-Based Buccal Bioadhesive Films of Zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal Administration of Mucoadhesive Blend Films Saturated with Propranolol Loaded Nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef]
- Fernandes, F.P.; Fortes, A.C.; Da Cruz Fonseca, S.G.; Breitkreutz, J.; Ferraz, H.G. Manufacture and Characterization of Mucoadhesive Buccal Films Based on Pectin and Gellan Gum Containing Triamcinolone Acetonide. Int. J. Polym. Sci. 2018, 2018, 2403802. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Vimal, P.; Attimarad, M.; Harsha, S. Development and Evaluation of Palonosetron Loaded Mucoadhesive Buccal Films. J. Drug Deliv. Sci. Technol. 2018, 47, 351–358. [Google Scholar] [CrossRef]
- dos Santos Garcia, V.A.; Borges, J.G.; Osiro, D.; Vanin, F.M.; de Carvalho, R.A. Orally Disintegrating Films Based on Gelatin and Pregelatinized Starch: New Carriers of Active Compounds from Acerola. Food Hydrocoll. 2020, 101, 105518. [Google Scholar] [CrossRef]
- Ryu, J.H.; Choi, J.S.; Park, E.; Eom, M.R.; Jo, S.; Lee, M.S.; Kwon, S.K.; Lee, H. Chitosan Oral Patches Inspired by Mussel Adhesion. J. Control. Release 2020, 317, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological Analysis and Mucoadhesion: A 30 Year-Old and Still Active Combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef]
- Peck, C.C. Biomechanics of Occlusion—Implications for Oral Rehabilitation. J. Oral Rehabil. 2016, 43, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.W. The History of Prosthetic Dentistry. J. Prosthet. Dent. 1959, 9, 841–846. [Google Scholar] [CrossRef]
- Kydd, W.L.; Daly, C.H. The Biologic and Mechanical Effects of Stress on Oral Mucosa. J. Prosthet. Dent. 1982, 47, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Maruo, Y.; Nishigawa, G.; Irie, M.; Oka, M.; Hara, T.; Suzuki, K.; Minagi, S. Stress Distribution Prevents Ischaemia and Bone Resorption in Residual Ridge. Arch. Oral Biol. 2010, 55, 873–878. [Google Scholar] [CrossRef]
- Imai, Y.; Sato, T.; Mori, S.; Okamoto, M. A Histomorphometric Analysis on Bone Dynamics in Denture Supporting Tissue under Continuous Pressure. J. Oral Rehabil. 2002, 29, 72–79. [Google Scholar] [CrossRef]
- Mori, S.; Sato, T.; Hara, T.; Nakashima, K.; Minagi, S. Effect of Continuous Pressure on Histopathological Changes in Denture-Supporting Tissues. J. Oral Rehabil. 1997, 24, 37–46. [Google Scholar] [CrossRef]
- Chen, J.; Ahmad, R.; Li, W.; Swain, M.; Li, Q. Biomechanics of Oral Mucosa. J. R. Soc. Interface 2015, 12, 20150325. [Google Scholar] [CrossRef]
- Kumakura, S.; Sakurai, K.; Tahara, Y.; Nakagawa, K. Relationship between Buccal Mucosa Ridging and Viscoelastic Behaviour of Oral Mucosa. J. Oral Rehabil. 2011, 38, 429–433. [Google Scholar] [CrossRef]
- Stokes, I.A.F.; Laible, J.P.; Gardner-Morse, M.G.; Costi, J.J.; Iatridis, J.C. Refinement of Elastic, Poroelastic, and Osmotic Tissue Properties of Intervertebral Disks to Analyze Behavior in Compression. Ann. Biomed. Eng. 2011, 39, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kindler, S.; Seebauer, C.; Mksoud, M.; Samietz, S.; Kocher, T.; Holtfreter, B.; Lucas, C.; Völzke, H.; Metelmann, H.R.; Rau, A.; et al. Impact of Dental Restorations and Removable Prostheses on Potentially Malignant Oral Mucosal Disorders in the General Population. J. Prosthet. Dent. 2023, 129, 89–95. [Google Scholar] [CrossRef]
- Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3029. [Google Scholar] [CrossRef] [PubMed]
- Jainkittivong, A.; Aneksuk, V.; Langlais, R.P. Oral Mucosal Lesions in Denture Wearers. Gerodontology 2010, 27, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Martori, E.; Ayuso-Montero, R.; Martinez-Gomis, J.; Viñas, M.; Peraire, M. Risk Factors for Denture-Related Oral Mucosal Lesions in a Geriatric Population. J. Prosthet. Dent. 2014, 111, 273–279. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Zhang, L. Evaluation of Friction in Different Oral Restoration Materials and Its Influencing Factors. Mater. Express 2020, 10, 1746–1752. [Google Scholar] [CrossRef]
- Prinz, J.F.; de Wijk, R.A.; Huntjens, L. Load Dependency of the Coefficient of Friction of Oral Mucosa. Food Hydrocoll. 2007, 21, 402–408. [Google Scholar] [CrossRef]
- Suchatlampong, C.; Davies, E.; von Fraunhofer, J.A. Frictional Characteristics of Resilient Lining Materials. Dent. Mater. 1986, 2, 135–138. [Google Scholar] [CrossRef]
- Waters, M.G.J.; Jagger, R.G.; Polyzois, G.L. Wettability of Silicone Rubber Maxillofacial Prosthetic Materials. J. Prosthet. Dent. 1999, 81, 439–443. [Google Scholar] [CrossRef]
- Aspinall, S.R.; Parker, J.K.; Khutoryanskiy, V.V. Oral Care Product Formulations, Properties and Challenges. Colloids Surf. B Biointerfaces 2021, 200, 111567. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Z.R. Friction and Wear Behavior of Human Teeth under Various Wear Conditions. Tribol. Int. 2007, 40, 278–284. [Google Scholar] [CrossRef]
- Zheng, Y.; Bashandeh, K.; Shakil, A.; Jha, S.; Polycarpou, A.A. Review of Dental Tribology: Current Status and Challenges. Tribol. Int. 2022, 166, 107354. [Google Scholar] [CrossRef]
- Mystkowska, J.; Car, H.; Dabrowski, J.R.; Romanowska, J.; Klekotka, M.; Milewska, A.J. Artificial Mucin-Based Saliva Preparations—Physicochemical and Tribological Properties. Oral Health Prev. Dent. 2018, 16, 183–193. [Google Scholar] [CrossRef]
- Andrysewicz, E.; Mystkowska, J.; Kolmas, J.; Jałbrzykowski, M.; Olchowik, R.; Dabrowski, J.R. Influence of Artificial Saliva Compositions on Tribological Characteristics of Ti-6Al-4V Implant Alloy. Acta Bioeng. Biomech. 2012, 14, 71–79. [Google Scholar] [CrossRef]
- Mair, L.H. Wear in Dentistry-Current Terminology. J. Dent. 1992, 20, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, P.; Debels, E.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. How to Simulate Wear? Overview of Existing Methods. Dent. Mater. 2006, 22, 693–701. [Google Scholar] [CrossRef]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear Characteristics of Metallic Biomaterials: A Review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Walczak, M.; Drozd, K. Tribological Characteristics of Dental Metal Biomaterials. Curr. Issues Pharm. Med. Sci. 2016, 29, 158–162. [Google Scholar] [CrossRef]
- Saha, S.; Roy, S. Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review. Materials 2023, 16, 161. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. Wear and Corrosion Interactions on Titanium in Oral Environment: Literature Review. J. Bio-Tribo-Corros. 2015, 1, 13. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, W.H. Aqueous Boundary Lubrication: Molecular Mechanisms, Design Strategy, and Terra Incognita. Curr. Opin. Colloid Interface Sci. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Briscoe, W.H.; Titmuss, S.; Tiberg, F.; Thomas, R.K.; McGillivray, D.J.; Klein, J. Boundary Lubrication under Water. Nature 2006, 444, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Andablo-Reyes, E.; Bryant, M.; Dowson, D.; Neville, A. Lubrication of Soft Oral Surfaces. Curr. Opin. Colloid Interface Sci. 2019, 39, 61–75. [Google Scholar] [CrossRef]
- Yu, S.; Zhong, M.; Xu, W. In Vitro Oral Simulation Based on Soft Contact: The Importance of Viscoelastic Response of the Upper Jaw Substitutes. J. Texture Stud. 2023, 54, 54–66. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Wang, X. In Situ Oral Lubrication and Smoothness Sensory Perception Influenced by Tongue Surface Roughness. J. Sci. Food Agric. 2022, 102, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Reeh, E.S.; Aguirre, A.; Sakaguchi, R.L.; Rudney, J.D.; Levine, M.J.; Douglas, W.H. Hard Tissue Lubrication by Salivary Fluids. Clin. Mater. 1990, 6, 151–161. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A. Comparative Investigation of Tribological Behavior of Hybrid Dental Restorative Composite Materials. Ceram. Int. 2022, 48, 6698–6706. [Google Scholar] [CrossRef]
- Pailler-Mattei, C.; Vargiolu, R.; Tupin, S.; Zahouani, H. Ex Vivo Approach to Studying Bio-Adhesive and Tribological Properties of Artificial Salivas for Oral Dryness (Xerostomia). Wear 2015, 332–333, 710–714. [Google Scholar] [CrossRef]
- Zembyla, M.; Liamas, E.; Andablo-Reyes, E.; Gu, K.; Krop, E.M.; Kew, B.; Sarkar, A. Surface Adsorption and Lubrication Properties of Plant and Dairy Proteins: A Comparative Study. Food Hydrocoll. 2021, 111, 106364. [Google Scholar] [CrossRef]
- Kullaa-Mikkonen, A.; Sorvari, T.E. A Scanning Electron Microscopic Study of the Dorsal Surface of the Human Tongue. Cells Tissues Organs 1985, 123, 114–120. [Google Scholar] [CrossRef]
- Cheng, S.; Gandevia, S.C.; Green, M.; Sinkus, R.; Bilston, L.E. Viscoelastic Properties of the Tongue and Soft Palate Using MR Elastography. J. Biomech. 2011, 44, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Alsanei, W.A.; Chen, J. Studies of the Oral Capabilities in Relation to Bolus Manipulations and the Ease of Initiating Bolus Flow. J. Texture Stud. 2014, 45, 1–12. [Google Scholar] [CrossRef]
- Ranc, H.; Elkhyat, A.; Servais, C.; Mac-Mary, S.; Launay, B.; Humbert, P. Friction Coefficient and Wettability of Oral Mucosal Tissue: Changes Induced by a Salivary Layer. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 155–161. [Google Scholar] [CrossRef]
- Harvey, N.M.; Yakubov, G.E.; Stokes, J.R.; Klein, J. Lubrication and Load-Bearing Properties of Human Salivary Pellicles Adsorbed Ex Vivo on Molecularly Smooth Substrata. Biofouling 2012, 28, 843–856. [Google Scholar] [CrossRef]
- Hatton, M.N.; Levine, M.J.; Margarone, J.E.; Aguirre, A. Lubrication and Viscosity Features of Human Saliva and Commercially Available Saliva Substitutes. J. Oral Maxillofac. Surg. 1987, 45, 496–499. [Google Scholar] [CrossRef]
- Reeh, E.S.; Douglas, W.H.; Levine, M.J. Lubrication of Saliva Substitutes at Enamel-to-Enamel Contacts in an Artificial Mouth. J. Prosthet. Dent. 1996, 75, 649–656. [Google Scholar] [CrossRef]
- Bongaerts, J.H.H.; Rossetti, D.; Stokes, J.R. The Lubricating Properties of Human Whole Saliva. Tribol. Lett. 2007, 27, 277–287. [Google Scholar] [CrossRef]
- Yakubov, G.E.; McColl, J.; Bongaerts, J.H.H.; Ramsden, J.J. Viscous Boundary Lubrication of Hydrophobic Surfaces by Mucin. Langmuir 2009, 25, 2313–2321. [Google Scholar] [CrossRef]
- Lee, S.; Müller, M.; Rezwan, K.; Spencer, N.D. Porcine Gastric Mucin (PGM) at the Water/Poly(Dimethylsiloxane) (PDMS) Interface: Influence of PH and Ionic Strength on Its Conformation, Adsorption, and Aqueous Lubrication Properties. Langmuir 2005, 21, 8344–8353. [Google Scholar] [CrossRef]
- Douglas, W.H.; Reeh, E.S.; Ramasubbu, N.; Raj, P.A.; Bhandary, K.K.; Levine, M.J. Statherin: A Major Boundary Lubricant of Human Saliva. Biochem. Biophys. Res. Commun. 1991, 180, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.M.; Carpenter, G.H.; Proctor, G.B.; Klein, J. Normal and Frictional Interactions of Purified Human Statherin Adsorbed on Molecularly-Smooth Solid Substrata. Biofouling 2011, 27, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.R.; Boehm, M.W.; Baier, S.K. Oral Processing, Texture and Mouthfeel: From Rheology to Tribology and Beyond. Curr. Opin. Colloid Interface Sci. 2013, 18, 349–359. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysik, D.; Niewęgłowska, J.; Mystkowska, J. From Salivary Dysfunction to Prosthetic Challenges in Xerostomia and Denture Retention with Oral Gels. Materials 2025, 18, 3141. https://doi.org/10.3390/ma18133141
Łysik D, Niewęgłowska J, Mystkowska J. From Salivary Dysfunction to Prosthetic Challenges in Xerostomia and Denture Retention with Oral Gels. Materials. 2025; 18(13):3141. https://doi.org/10.3390/ma18133141
Chicago/Turabian StyleŁysik, Dawid, Joanna Niewęgłowska, and Joanna Mystkowska. 2025. "From Salivary Dysfunction to Prosthetic Challenges in Xerostomia and Denture Retention with Oral Gels" Materials 18, no. 13: 3141. https://doi.org/10.3390/ma18133141
APA StyleŁysik, D., Niewęgłowska, J., & Mystkowska, J. (2025). From Salivary Dysfunction to Prosthetic Challenges in Xerostomia and Denture Retention with Oral Gels. Materials, 18(13), 3141. https://doi.org/10.3390/ma18133141








