The Effects of Calcination Process Parameters on RHA Reactivity and Mortar Mechanical Properties
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Method
3. Results and Discussion
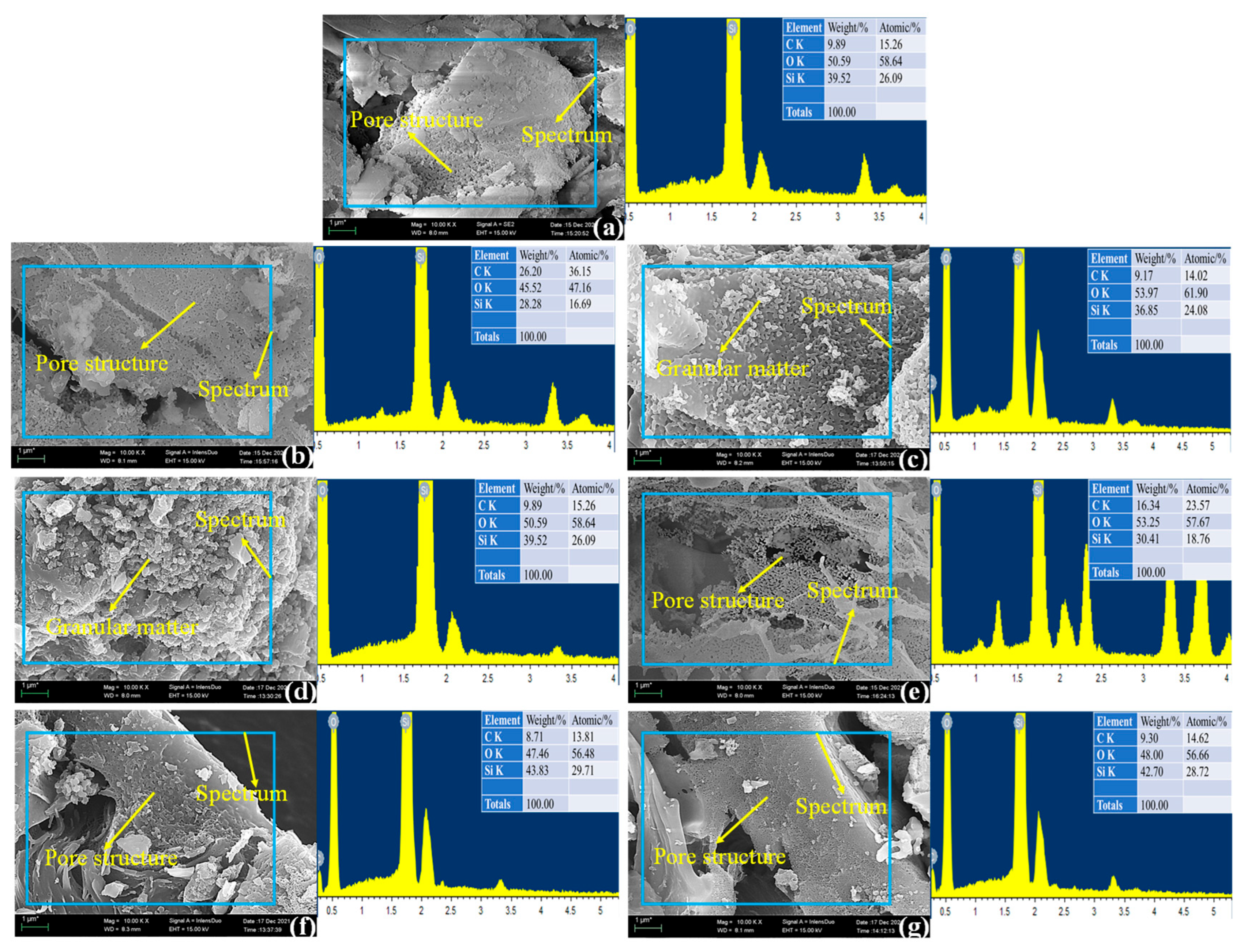
3.1. RHA Micro-Morphological Analysis
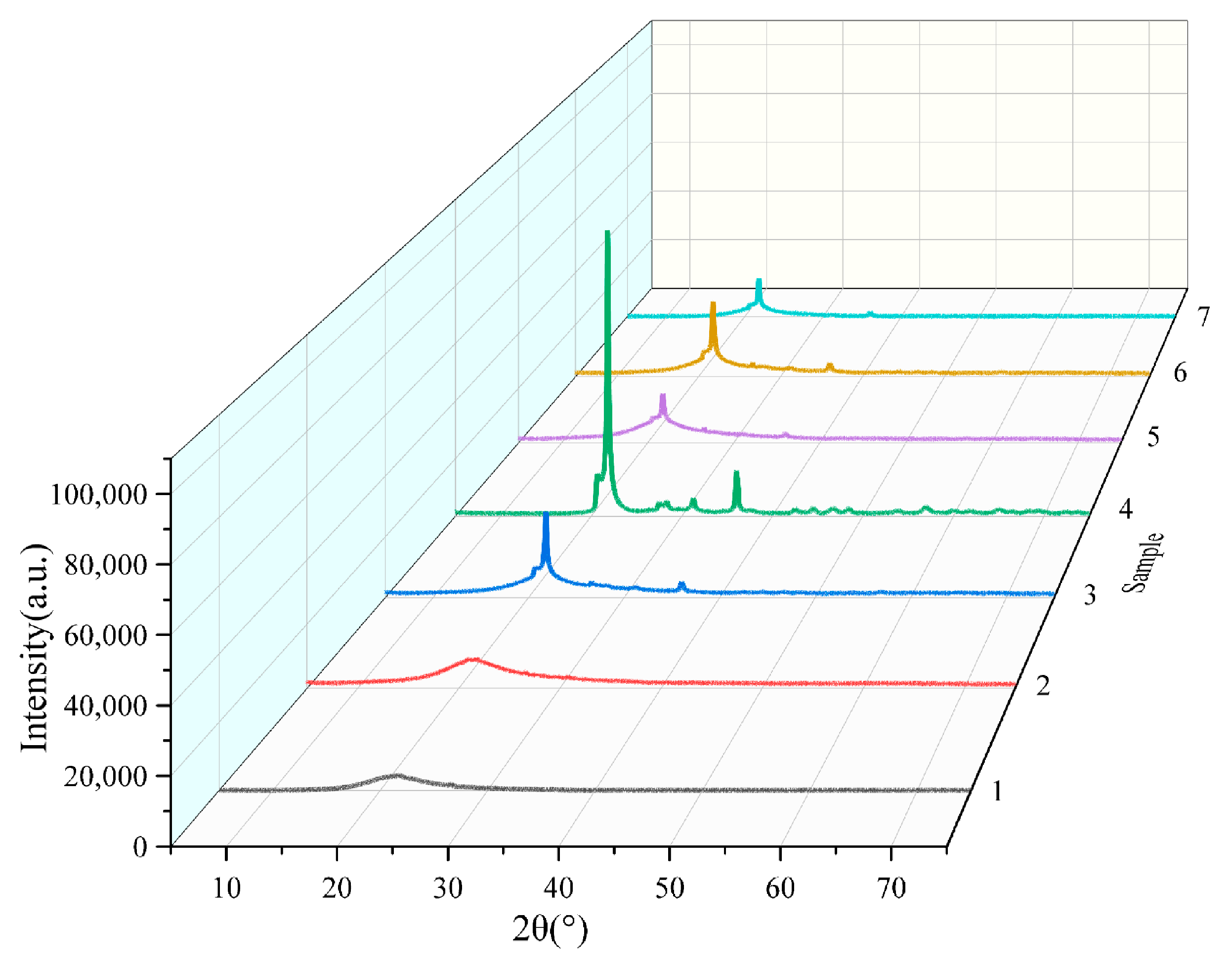
3.2. RHA Composition Analysis
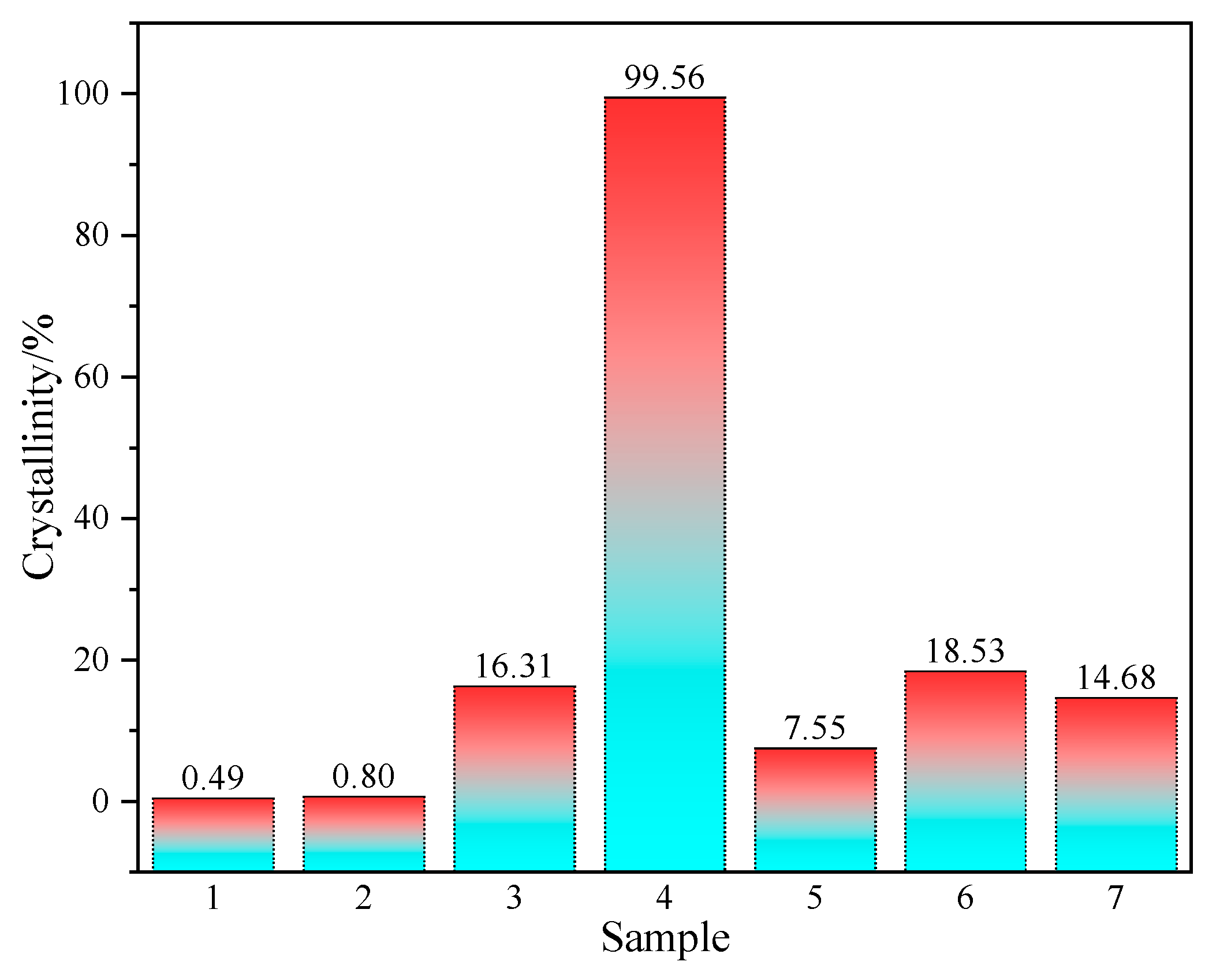
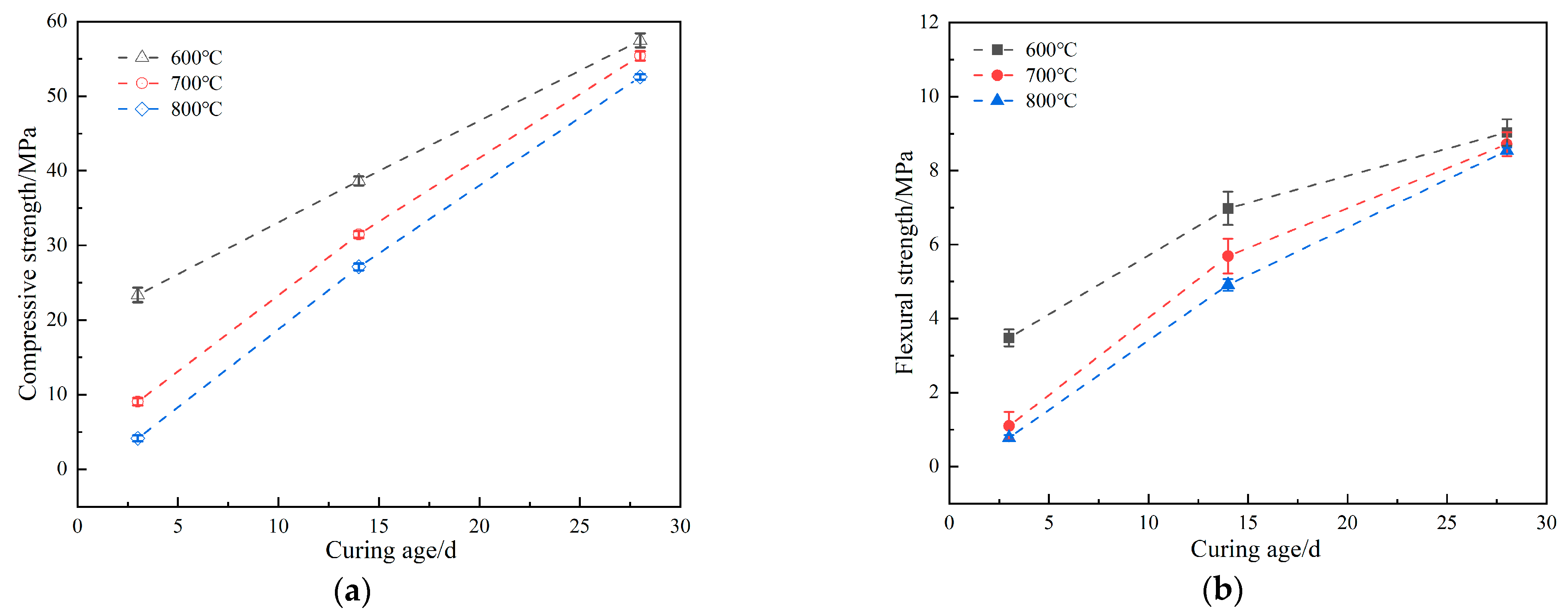
3.3. Effect of Crystallinity of RHA on Mechanical Properties of Mortar
4. Conclusions
- (1)
- When the calcination temperature was at 600–700 °C, the fragmented flakes on the surface of rice husk ash gradually became concave, and their roughness gradually increased into a honeycomb structure with cross-linking, and the XRD results showed that the silica in the rice husk ash was amorphous, and the silica content was as high as 95%, which provided a high activity.
- (2)
- When the calcination temperature was 800 °C, more granular material appeared on the surface of rice husk ash, and the crystallinity was 16.3%. But at the calcination temperature of 900 °C, the cross-linked honeycomb structure of the rice husk ash disappeared and became irregular sphere-like, with a crystallinity of 99.6%, indicating that the crystalline transformation of SiO2 occurred when the calcination temperature reached 800 °C, and all of the amorphous SiO2 was transformed into crystalline SiO2 when it reached 900 °C, and its activity was lost.
- (3)
- The subcooled treatment with distilled water altered the microstructure of the rice husk ash, resulting in a crystallinity decrease from 16.3% under natural cooling to 7.5%. However, modifications in the heating rate or isothermal duration had little effect on the micro-morphology and crystallinity of the rice husk ash, indicating that the influence of calcination process parameters on the activity of rice husk ash followed this order: calcination temperature, cooling treatment, heating rate, and constant temperature time.
- (4)
- The compressive and flexural strengths of the rice husk ash cement mortar decreased with the increase in the calcined rice husk temperature, corresponding to a rise in crystallinity. When compared with the rice husk ash calcined at 800 °C, the rice husk ash calcined at 600 °C significantly improved the early mechanical properties of the cement mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antiohos, S.K.; Tapali, J.G.; Zervaki, M.; Sousa-Coutinho, J.; Tsimas, S.; Papadakis, V.G. Low embodied energy cement containing untreated RHA: A strength development and durability study. Constr. Build. Mater. 2013, 49, 455–463. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes–A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Trejo, D. Hydration and phase formation of blended cementitious systems incorporating chemically transformed rice husk ash. Cem. Concr. Compos. 2015, 59, 100–106. [Google Scholar] [CrossRef]
- Zunino, F.; Lopez, M. Decoupling the physical and chemical effects of supplementary cementitious materials on strength and permeability: A multi-level approach. Cem. Concr. Compos. 2016, 65, 19–28. [Google Scholar] [CrossRef]
- Alex, J.; Dhanalakshmi, J.; Ambedkar, B. Experimental investigation on rice husk ash as cement replacement on concrete production. Constr. Build. Mater. 2016, 127, 353–362. [Google Scholar] [CrossRef]
- Shen, J.; Liu, X.; Zhu, S.; Zhang, H.; Tan, J. Effects of calcination parameters on the silica phase of original and leached rice husk ash. Mater. Lett. 2011, 65, 1179–1183. [Google Scholar] [CrossRef]
- Huang, H.; Gao, X.; Wang, H.; Ye, H. Influence of rice husk ash on strength and permeability of ultra-high performance concrete. Constr. Build. Mater. 2017, 149, 621–628. [Google Scholar] [CrossRef]
- Agwa, I.S.; Omar, O.M.; Tayeh, B.A.; Abdelsalam, B.A. Effects of using rice straw and cotton stalk ashes on the properties of lightweight self-compacting concrete. Constr. Build. Mater. 2020, 235, 117541. [Google Scholar] [CrossRef]
- Rashid, M.; Molla, M.K.; Ahmed, T.U. Durability of mortar in presence of Rice Husk Ash. World Acad. Sci. Eng. Technol. 2010, 43, 736–739. [Google Scholar]
- Monteiro, P.J.M.; Mehta, P.K. Concrete: Microstructure, Properties and Materials; McGraw-Hill Publishing: New York, NY, USA, 2006. [Google Scholar]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Carbonation and Hydration of Calcium Hydroxide and Calcium Silicate Binders with Rice Husk Ash. In Proceedings of the 2nd International RILEM Symposium, Quebec, ON, Canada, 11–13 September 2006; Rilem Publications SARL: Bagneux, France, 2006. [Google Scholar]
- Kang, S.-H.; Hong, S.-G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2019, 115, 389–400. [Google Scholar] [CrossRef]
- Nair, D.G.; Fraaij, A.; Klaassen, A.A.K.; Kentgens, A.P.M. A structural investigation relating to the pozzolanic activity of rice husk ashes. Cem. Concr. Res. 2008, 38, 861–869. [Google Scholar] [CrossRef]
- Shatat, M.R. Hydration behavior and mechanical properties of blended cement containing various amounts of rice husk ash in presence of metakaolin. Arab. J. Chem. 2016, 9, S1869–S1874. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Xu, J.; Wu, Q.; Zhai, M. Influence of rice husk ash on the waterproof properties of ultrafine fly ash based geopolymer. Constr. Build. Mater. 2019, 208, 394–401. [Google Scholar] [CrossRef]
- Mohseni, E.; Kazemi, M.J.; Koushkbaghi, M.; Zehtab, B.; Behforouz, B. Evaluation of mechanical and durability properties of fiber-reinforced lightweight geopolymer composites based on rice husk ash and nano-alumina. Constr. Build. Mater. 2019, 209, 532–540. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Y.; Wang, D. Effect of Rice Husk Ash on High-Temperature Mechanical Properties and Microstructure of Concrete. Kem. U Ind. 2017, 66, 157–164. [Google Scholar] [CrossRef]
- Rego, J.H.S.; Nepomuceno, A.A.; Figueiredo, E.P.; Hasparyk, N.P.; Borges, L.D. Effect of Particle Size of Residual Rice-Husk Ash in Consumption of Ca(OH)2. J. Mater. Civ. Eng. 2015, 27, 04014178. [Google Scholar] [CrossRef]
- Suomie, R.W.; Mishra, B.P.; Das, S. Performance of rice husk ash (RHA) and recycled coarse aggregate (RCA) for sustainable concrete: A review. Next Mater. 2025, 8, 100778. [Google Scholar] [CrossRef]
- Su, Q.; Xu, J. Influence of waste glass and rice husk ash on the dynamic compressive properties and variable-angle shear characteristics of concrete after high-temperature treatment: Experimental and numerical study. Structures 2024, 69, 107419. [Google Scholar] [CrossRef]
- JGJ/T 70-2009; Standard for Test Methods of Basic Properties of Construction Mortar. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- Bui, D.D. Rice Husk Ash a Mineral Admixture for High Performance Concrete; Delft University Press: Delft, The Netherlands, 2001. [Google Scholar]
- Vieira, A.P.; Toledo Filho, R.D.; Tavares, L.M.; Cordeiro, G.C. Effect of particle size, porous structure and content of rice husk ash on the hydration process and compressive strength evolution of concrete. Constr. Build. Mater. 2020, 236, 117553. [Google Scholar] [CrossRef]
- Xu, W.; Lo, T.Y.; Memon, S.A. Microstructure and reactivity of rich husk ash. Constr. Build. Mater. 2012, 29, 541–547. [Google Scholar] [CrossRef]
- Ye, G.; Huang, H.; Van Tuan, N. Rice Husk Ash. In Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials: State-of-the-Art Report of the RILEM Technical Committee 238-SCM, Working Group 4; Springer: Berlin/Heidelberg, Germany, 2018; pp. 283–302. [Google Scholar]
- Rodríguez de Sensale, G.; Rodríguez Viacava, I. A study on blended Portland cements containing residual rice husk ash and limestone filler. Constr. Build. Mater. 2018, 166, 873–888. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Zhang, Z.; Han, K.; Hu, X.; Jiang, F. Action mechanism of rice husk ash and the effect on main performances of cement-based materials: A review. Constr. Build. Mater. 2021, 288, 123068. [Google Scholar] [CrossRef]
- Zareei, S.A.; Ameri, F.; Dorostkar, F.; Ahmadi, M. Rice husk ash as a partial replacement of cement in high strength concrete containing micro silica: Evaluating durability and mechanical properties. Case Stud. Constr. Mater. 2017, 7, 73–81. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Majeed, J. Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J. Mater. Sci. 2006, 41, 7926–7933. [Google Scholar] [CrossRef]
- Feng, Q.; Yamamichi, H.; Shoya, M.; Sugita, S. Study on the pozzolanic properties of rice husk ash by hydrochloric acid pretreatment. Cem. Concr. Res. 2004, 34, 521–526. [Google Scholar] [CrossRef]


| Composition wt% | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | P2O5 | TiO2 | K2O | Na2O | SO3 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 62.4 | 21.35 | 4.76 | 3.06 | 3.13 | 0.49 | — | 0.21 | 0.54 | 2.52 | 1.54 |
| No. | Starting Temperature/°C | Maximum Temperature/°C | Constant Temperature Time/h | Temperature Rise Rate (°C/h) | Cooling Method |
|---|---|---|---|---|---|
| 1 | room temperature | 600 | 2 | 300 | natural cooling |
| 2 | room temperature | 700 | 2 | 300 | natural cooling |
| 3 | room temperature | 800 | 2 | 300 | natural cooling |
| 4 | room temperature | 900 | 2 | 300 | natural cooling |
| 5 | room temperature | 800 | 2 | 300 | distilled water subcooling |
| 6 | room temperature | 800 | 2 | 400 | natural cooling |
| 7 | room temperature | 800 | 1 | 300 | natural cooling |
| Mix | w/c | Cement/g | Sand/g | Water/g | Rice Husk Ash/g |
|---|---|---|---|---|---|
| 600 °C | 0.4 | 900 | 2000 | 400 | 100 |
| 700 °C | 0.4 | 900 | 2000 | 400 | 100 |
| 800 °C | 0.4 | 900 | 2000 | 400 | 100 |
| Temperature of Combustion | SiO2 | Al2O3 | Na2O | P2O5 | CaO | MgO |
|---|---|---|---|---|---|---|
| 600 °C | 94.57 | 1.21 | 0.21 | 0.22 | 0.35 | 0.92 |
| 700 °C | 94.74 | 1.41 | 0.22 | 0.21 | 0.33 | 0.85 |
| 800 °C | 94.78 | 1.24 | 0.21 | 0.22 | 0.36 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Li, L.; Quan, L.; Tian, B.; Zhang, P.; Li, S. The Effects of Calcination Process Parameters on RHA Reactivity and Mortar Mechanical Properties. Materials 2025, 18, 3129. https://doi.org/10.3390/ma18133129
Ji J, Li L, Quan L, Tian B, Zhang P, Li S. The Effects of Calcination Process Parameters on RHA Reactivity and Mortar Mechanical Properties. Materials. 2025; 18(13):3129. https://doi.org/10.3390/ma18133129
Chicago/Turabian StyleJi, Jianrui, Lihui Li, Lei Quan, Bo Tian, Panpan Zhang, and Sili Li. 2025. "The Effects of Calcination Process Parameters on RHA Reactivity and Mortar Mechanical Properties" Materials 18, no. 13: 3129. https://doi.org/10.3390/ma18133129
APA StyleJi, J., Li, L., Quan, L., Tian, B., Zhang, P., & Li, S. (2025). The Effects of Calcination Process Parameters on RHA Reactivity and Mortar Mechanical Properties. Materials, 18(13), 3129. https://doi.org/10.3390/ma18133129






