Laser-Prepared ZnO-Ag Nanoparticles with High Light-Enhanced Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
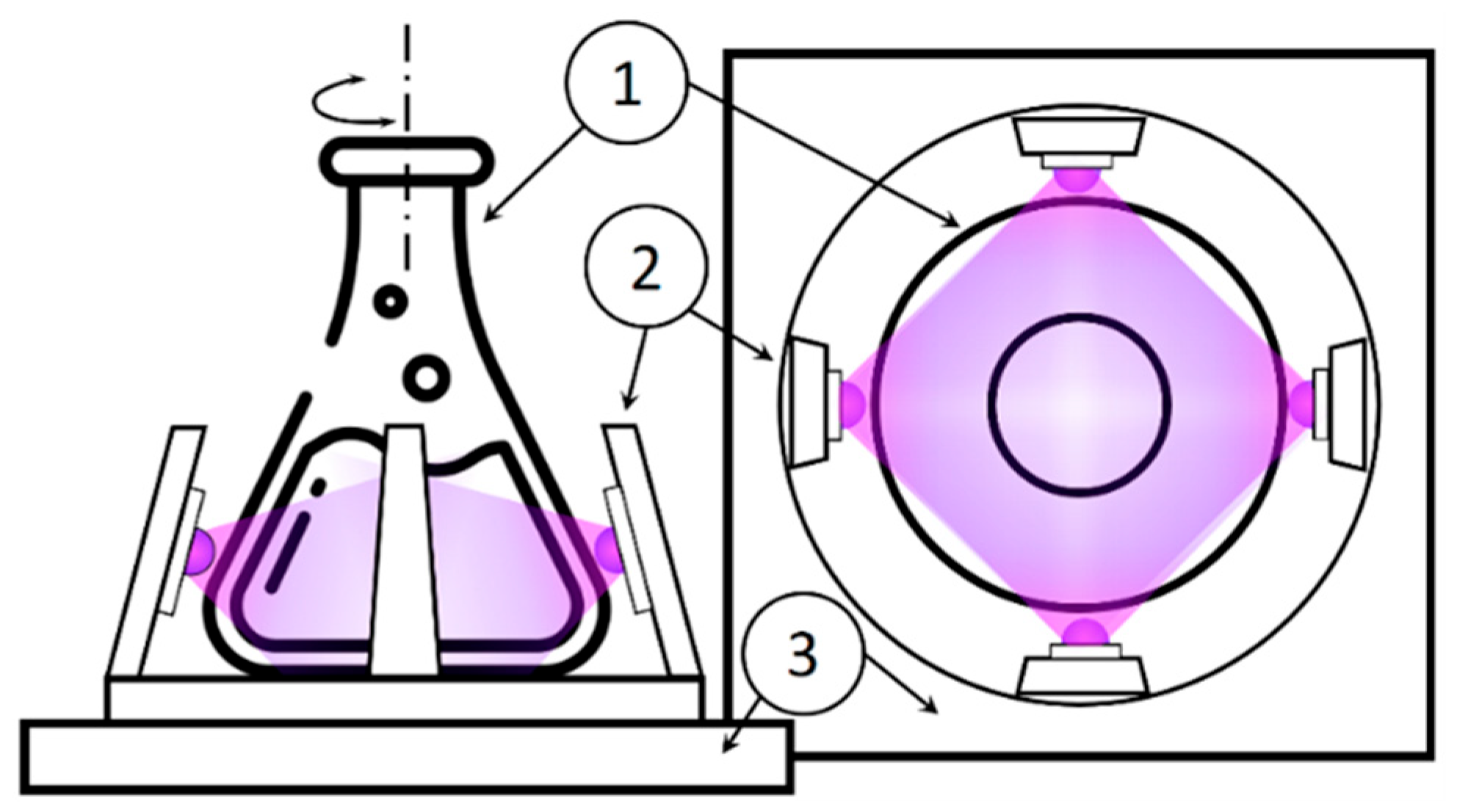
2.1. PLA Synthesis of ZnO and ZnO-1Ag NPs
2.2. Characterization Methods
2.3. Antibacterial Activity of NPs and of Irradiation
3. Results and Discussion
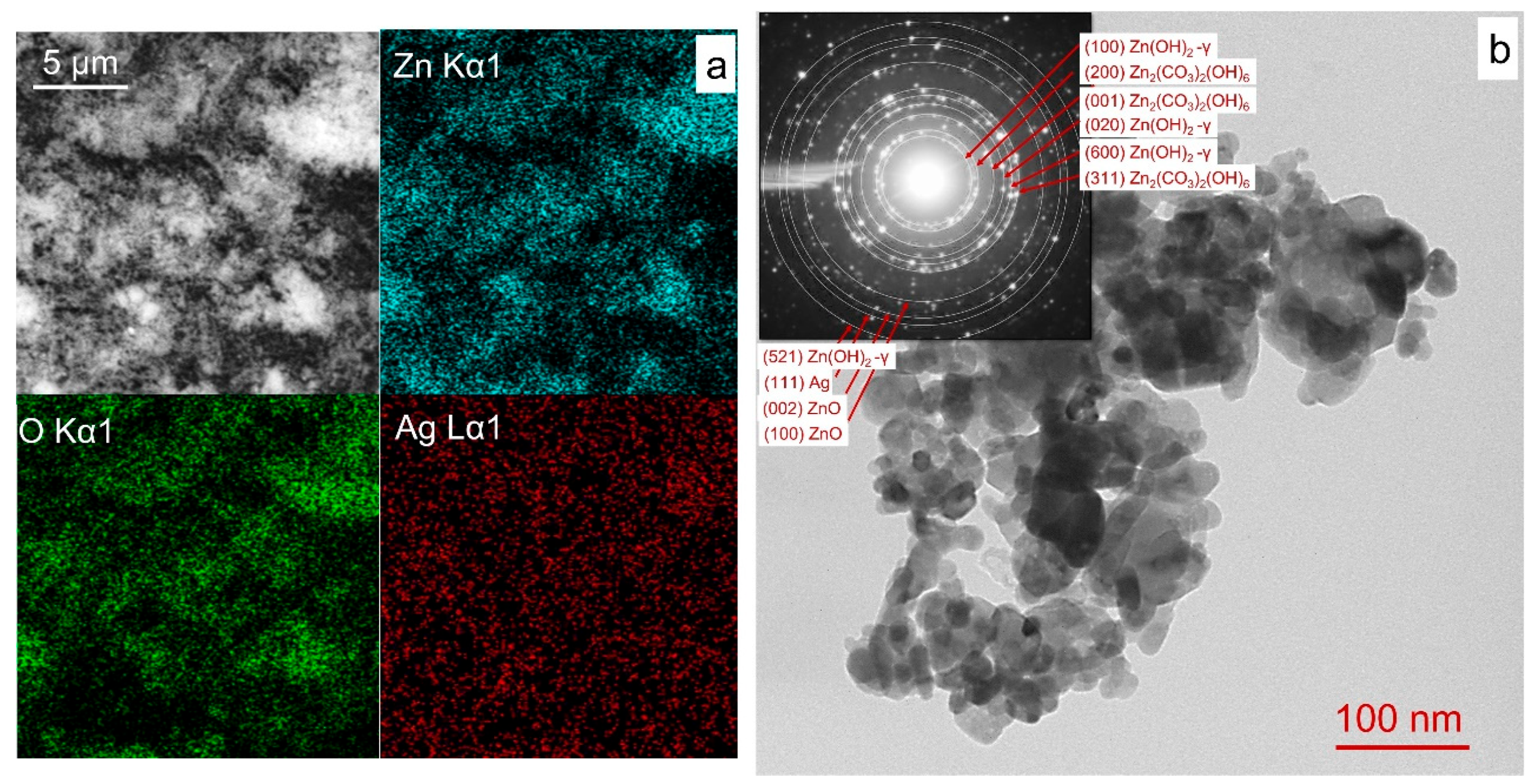
3.1. Materials Characterization
3.2. Bacterial Inactivation
3.2.1. Evolution of Bacteria in PHFM
- (i)
- (ii)
- The exponential phase, in which rapid population growth occurs (the number of bacteria doubles at regular intervals). The exponential phase of the evolution of S. aureus bacteria in our case lasted from 4 to 8 h.
- (iii)
- The stationary phase, where the number of new cells in each time interval is equal to the number of cells that die in the same time interval [68]. This was observed over the next 24 h with a bacterial concentration of 107 CFU/mL.
- (iv)
- The death phase occurs when nutrients in the growth medium are depleted, leading to a decline in the bacterial population. In our experiment, this phase was observed beginning on the second day of Staphylococcus aureus evolution.
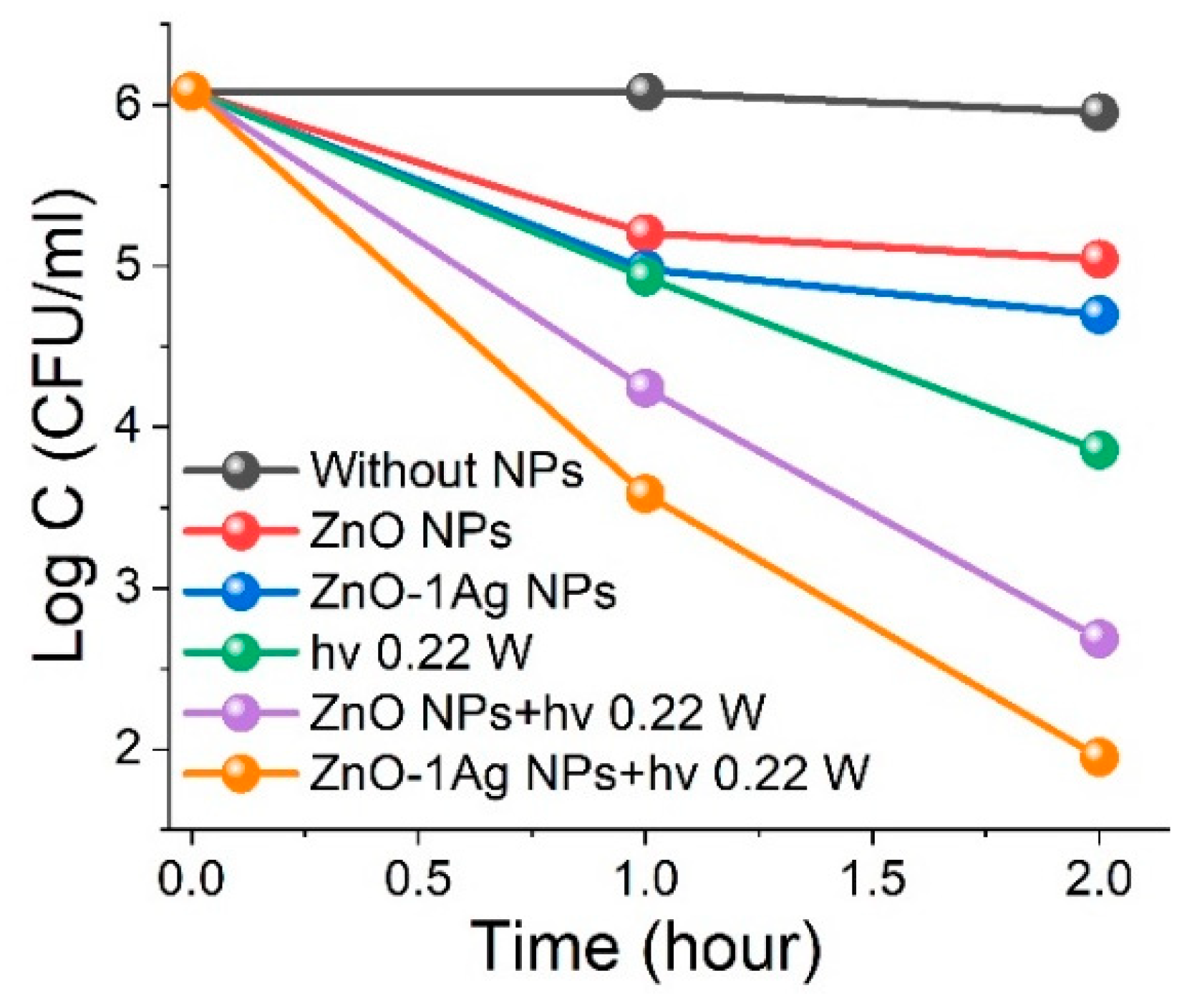
3.2.2. Influence of ZnO NP Concentration and LED 375 Irradiation on Bacterial Inactivation
3.2.3. Effects of Wavelength and Power of LED Irradiation
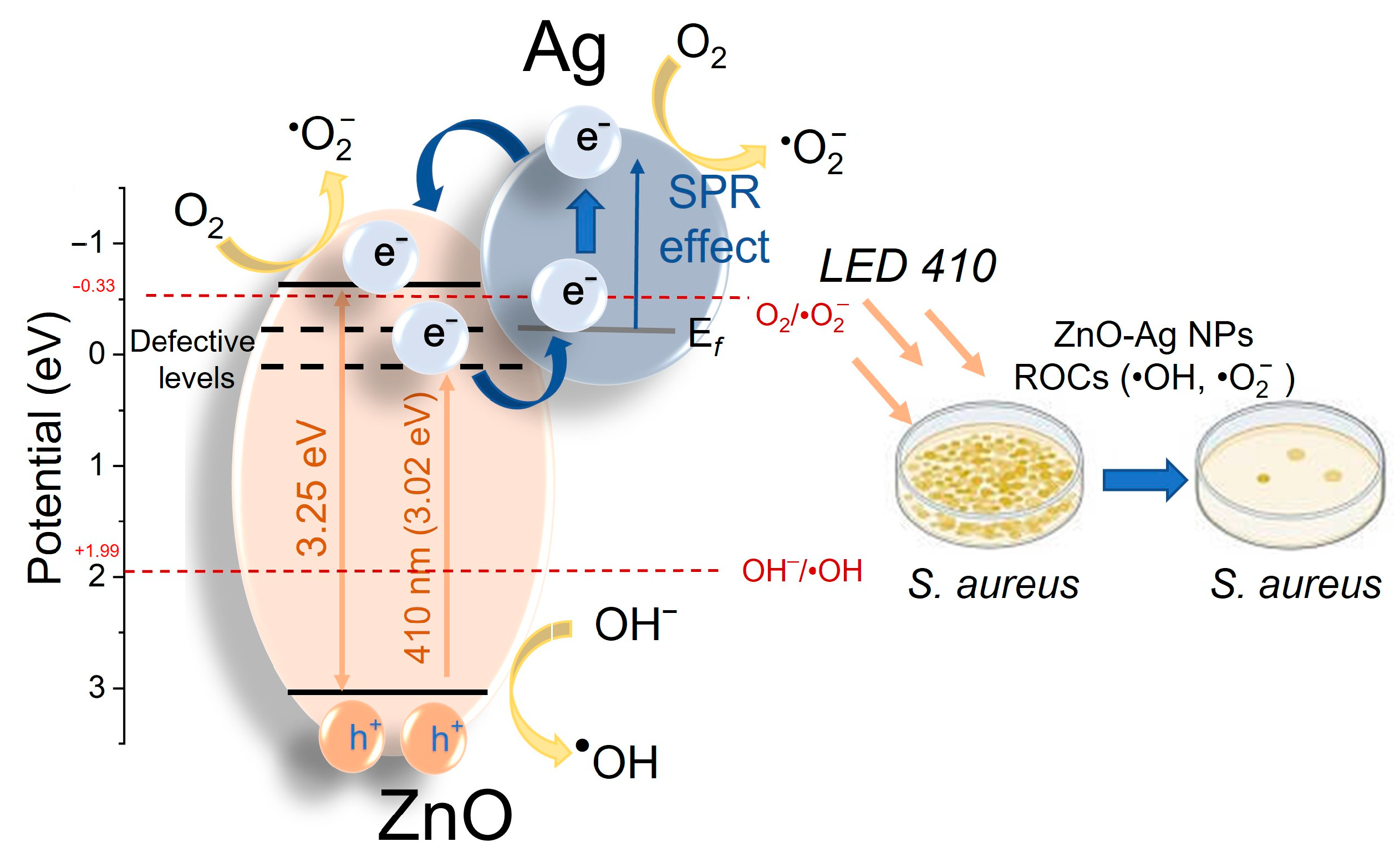
3.2.4. Effect of Ag Dopant on Antibacterial Activity of ZnO NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargas-Berrons, K.; Bernal-Jacome, L.; de Leon-Martinez, L.D.; Flores-Ramirez, R. Emerging pollutants (EPs) in Latin América: A critical review of under-studied EPs, case of study -Nonylphenol-. Sci. Total Environ. 2020, 726, 138493. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Favaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. Recent development of novel nanocomposites for photocatalysis mediated remediation of phenolic derivatives: A comprehensive review. J. Ind. Eng. Chem. 2023, 127, 18–35. [Google Scholar] [CrossRef]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Gwimbi, P.; George, M.; Ramphalile, M. Bacterial contamination of drinking water sources in rural villages of Mohale Basin, Lesotho: Exposures through neighbourhood sanitation and hygiene practices. Environ. Health Prev. Med. 2019, 24, 33. [Google Scholar] [CrossRef]
- Li, X.; Li, G.; Huang, H.; Wan, P.; Lu, Y.; Li, Z.; Xie, L.; Xiong, W.; Zeng, Z. The occurrence and contamination of optrA-positive methicillin-resistant Staphylococcus aureus from duck farms in Guangdong, China. J. Glob. Antimicrob. Resist. 2023, 35, 86–92. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, K.; Nair, K.M.; Pillai, S.C. Photocatalytic degradation of antibiotic-resistant genes and bacteria using 2D nanomaterials: What is known and what are the challenges? Curr. Opin. Green Sustain. Chem. 2021, 30, 100471. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.N. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Bhosale, A.; Kadam, J.; Gade, T.; Sonawane, K.; Garadkar, K. Efficient photodegradation of methyl orange and bactericidal activity of Ag doped ZnO nanoparticles. J. Indian Chem. Soc. 2023, 100, 100920. [Google Scholar] [CrossRef]
- Zuarez-Chamba, M.; Rajendran, S.; Herrera-Robledo, M.; Priya, A.K.; Navas-Cárdenas, C. Bi-based photocatalysts for bacterial inactivation in water: Inactivation mechanisms, challenges, and strategies to improve the photocatalytic activity. Environ. Res. 2022, 209, 112834. [Google Scholar] [CrossRef] [PubMed]
- Talreja, N.; Chauhan, D.; Ashfaq, M. Photo-Antibacterial Activity of Two-Dimensional (2D)-Based Hybrid Materials: Effective Treatment Strategy for Controlling Bacterial Infection. Antibiotics 2023, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, Y.; Zheng, Y.; Liu, X.; Tan, L.; Wu, S. Two-dimensional antibacterial materials. Prog. Mater. Sci. 2022, 130, 100976. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2021, 11, 622534. [Google Scholar] [CrossRef]
- Xie, Y.; Qub, X.; Li, J.; Li, D.; Wei, W.; Hui, D.; Zhang, Q.; Meng, F.; Yin, H.; Xu, X.; et al. Ultrafast physical bacterial inactivation and photocatalytic self-cleaning of ZnO nanoarrays for rapid and sustainable bactericidal applications. Sci. Total Environ. 2020, 738, 139714. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Jiang, H.; Wang, X. Progress on photocatalytic semiconductor hybrids for bacterial inactivation. Mater. Horiz. 2021, 8, 2964. [Google Scholar] [CrossRef]
- Park, C.; Hong, J.-H.; Kim, B.-Y.; An, S.; Yoon, S.S. Supersonically sprayed copper oxide titania nanowires for antibacterial activities and water purification. Appl. Surf. Sci. 2023, 611, 155513. [Google Scholar] [CrossRef]
- Liu, J.; Rojas-Andrade, M.D.; Chata, G.; Peng, Y.; Roseman, G.; Lu, J.-E.; Millhauser, G.L.; Saltikov, C.; Chen, S. Photo-enhanced antibacterial activity of ZnO/graphene quantum dot nanocomposites. Nanoscale 2018, 10, 158–166. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Usgodaarachchi, L.; Jayanetti, M.; Liyanaarachchi, C.; Kandanapitiye, M.; Vigneswaran, S. Efficient Visible-Light Photocatalysis and Antibacterial Activity of TiO2-Fe3C-Fe-Fe3O4/Graphitic Carbon Composites Fabricated by Catalytic Graphitization of Sucrose Using Natural Ilmenite. ACS Omega 2022, 7, 25403–25421. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Erasmus, E.E.; Mogale, R.; Marogoa, N.; Jayiya, A.; Visser, H.G. Using visible light to activate antiviral and antimicrobial properties of TiO2 nanoparticles in paints and coatings: Focus on new developments for frequent-touch surfaces in hospitals. J. Coat. Technol. Res. 2023, 20, 789–817. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Bacova, J.; Monfort, O.; Dworniczek, E.; Paluch, E.; Hanif, M.B.; Rauf, S.; Motlochova, M.; Capek, J.; Hensel, K.; et al. Critical comparison of aerogel TiO2 and P25 nanopowders: Cytotoxic properties, photocatalytic activity and photoinduced antimicrobial/antibiofilm performance. Appl. Surf. Sci. 2022, 579, 152145. [Google Scholar] [CrossRef]
- Moon, K.-S.; Choi, E.-J.; Bae, J.-M.; Park, Y.-B.; Oh, S. Visible Light-Enhanced Antibacterial and Osteogenic Functionality of Au and Pt Nanoparticles Deposited on TiO2 Nanotubes. Materials 2020, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. 2006, 78, 595–604. [Google Scholar] [CrossRef]
- Jha, S.; Rani, R.; Sing, S. Biogenic Zinc Oxide Nanoparticles and Their Biomedical Applications: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Abutahaa, N.; Hezamb, A.; Almekhlafia, F.A.; Saeed, A.M.N.; Namrathae, K.; Byrappa, K. Rational design of Ag-ZnO-Fe3O4 nanocomposite with promising antimicrobial activity under LED light illumination. Appl. Surf. Sci. 2020, 527, 146893. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Do, T.V.; Ngo, T.D.; Nguyen, T.A.; Lu, L.T.; Vu, Q.T.; Thia, L.P.; Tran, D.L. Photocurable acrylate epoxy/ZnO–Ag nanocomposite coating: Fabrication, mechanical and antibacterial properties. RSC Adv. 2022, 12, 23346. [Google Scholar] [CrossRef]
- Loka, C.; Lee, K.-S. Enhanced Visible-Light-Driven Photocatalysis of Ag/Ag2O/ZnO Nanocomposite Heterostructures. Nanomater. 2022, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, Y.; Zhang, J.; Lin, Z.; Zheng, J.; Huang, F. Schottky or Ohmic Metal–Semiconductor Contact: Influence on Photocatalytic Efficiency of Ag/ZnO and Pt/ZnO Model Systems. ChemSusChem 2014, 7, 101–104. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, L.; Wang, Y.; He, W. Plasmon excitation facilitating generation of electrons and reactive oxygen species for broad spectrum photocatalytic activity. Appl. Surf. Sci. 2022, 584, 152655. [Google Scholar] [CrossRef]
- Bora, T.; Sathe, P.; Laxman, K.; Dobretsov, S.; Dutta, J. Defect engineered visible light active ZnO nanorods for photocatalytic treatment of water. Catal. Today. 2017, 284, 11–18. [Google Scholar] [CrossRef]
- Guo, H.-L.; Zhu, Q.; Wu, X.-L.; Jiang, Y.-F.; Xiea, X.; Xu, A.-W. Oxygen deficient ZnO1−x nanosheets with high visible light photocatalytic activity. Nanoscale 2015, 7, 7216–7223. [Google Scholar] [CrossRef]
- Kim, S.; Son, N.; Park, S.-M.; Lee, C.-T.; Pandey, S.; Kang, M. Facile Fabrication of Oxygen-Defective ZnO Nanoplates for Enhanced Photocatalytic Degradation of Methylene Blue and In Vitro Antibacterial Activity. Catalysts 2023, 13, 567. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Pandey, S.; Jeong, D.; Lee, C.T.; Do, J.Y.; Park, S.M.; Kang, M. Effective Antibacterial/Photocatalytic Activity of ZnO Nanomaterials Synthesized under Low Temperature and Alkaline Conditions. Nanomaterials 2022, 12, 4417. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef] [PubMed]
- Fakhrutdinova, E.; Reutova, O.; Maliy, L.; Kharlamova, T.; Vodyankina, O.; Svetlichnyi, V. Laser-based Synthesis of TiO2-Pt Photocatalysts for Hydrogen Generation. Materials 2022, 15, 7413. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.D.; Shabalina, A.V.; Gerasimova, M.A.; Nemoykina, A.L.; Vodyankina, O.V.; Svetlichnyi, V.A. Highly defective dark nano titanium dioxide: Preparation via pulsed laser ablation and application. Materials 2020, 13, 2054. [Google Scholar] [CrossRef]
- Gavrilenko, E.A.; Goncharova, D.A.; Lapin, I.N.; Gerasimova, M.A.; Svetlichnyi, V.A. Photocatalytic activity of zinc oxide nanoparticles prepared by laser ablation in a decomposition reaction of rhodamine B. Russ. Phys. J. 2020, 63, 1429–1437. [Google Scholar] [CrossRef]
- Gavrilenko, E.A.; Goncharova, D.A.; Lapin, I.N.; Nemoykina, A.L.; Svetlichnyi, V.A.; Aljulaih, A.A.; Mintcheva, N.; Kulinich, S.A. Comparative study of physicochemical and antibacterial properties of ZnO nanoparticles prepared by laser ablation of Zn target in water and air. Materials 2019, 12, 186. [Google Scholar] [CrossRef]
- Asif, N.; Amir, M.; Fatma, T. Recent advances in the synthesis, characterization and biomedical applications of zinc oxide nanoparticles. Bioprocess Biosyst Eng. 2023, 46, 1377–1398. [Google Scholar] [CrossRef]
- Ishak, M.Q.H.; Shankar, P.; Turabayev, M.E.; Kondo, T.; Honda, M.; Gurbatov, S.O.; Okamura, Y.; Iwamori, S.; Kulinich, S.A. Biodegradable Polymer Nanosheets Incorporated with Zn-Containing Nanoparticles for Biomedical Applications. Materials 2022, 15, 8101. [Google Scholar] [CrossRef]
- Tarasenka, N.; Kornev, V.; Ramanenka, A.; Li, R.; Tarasenko, N. Photoluminescent neodymium-doped ZnO nanocrystals prepared by laser ablation in solution for NIR-II fluorescence bioimaging. Helion 2022, 8, e09554. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.D.; Volokitina, A.V.; Goncharova, D.A.; Kharlamova, T.S.; Kulinich, S.A.; Svetlichnyi, V.A. Composite plasmonic nanostructures of Ag@ZnO generated by laser ablation and their photocatalytic destruction of rhodamine, tetracycline and phenol molecules. Materials 2024, 17, 527. [Google Scholar] [CrossRef]
- Dhandapani, P.; Devanesan, S.; Narenkumar, J.; Maruthamuthu, S.; AlSalhi, M.S.; Rajasekar, A.; Ahamed, A. Novel synthesis of ZnO by Ice-cube method for photo-inactivation of E. coli. Saudi J. Biol. Sci. 2020, 27, 1130–1138. [Google Scholar] [CrossRef]
- Rosenberg, M.; Visnapuu, M.; Saal, K.; Danilian, D.; Pärna, R.; Ivask, A.; Kisand, V. Preparation and Characterization of Photocatalytically Active Antibacterial Surfaces Covered with Acrylic Matrix Embedded Nano-ZnO and Nano-ZnO/Ag. Nanomaterials 2021, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-M.; Quek, J.-A.; Sin, J.-C. Mechanistic investigation of visible light responsive Ag/ZnO micro/nanoflowers for enhanced photocatalytic performance and antibacterial activity. J. Photochem. Photobiol. A Chem. 2018, 353, 171–184. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S.P. Biogenic mediated Ag/ZnO nanocomposites for photocatalytic and antibacterial activities towards disinfection of water. J. Colloid Interface Sci. 2020, 563, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Brobbey, K.J.; Haapanen, J.; Gunell, M.; Toivakka, M.; Mäkelä, J.M.; Eerola, E.; Ali, R.; Saleem, M.R.; Honkanen, S.; Bobacka, J.; et al. Controlled time release and leaching of silver nanoparticles using a thin immobilizing layer of aluminum oxide. Mater. Today Sustain. 2022, 18, 100133. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.H.; Kim, D.J.; Lee, Y.J.; Jhee, K.H.; Sohn, Y.K.; Jang, E.S. Antimicrobial activity of ZnO nanoplates and its Ag nanocomposites: Insight into an ROS-mediated antibacterial mechanism under UV light. J. Solid State Chem. 2018, 269, 416–424. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Izaak, T.I.; Kharlamova, T.S.; Martynova, D.O.; Lapin, I.N.; Svetlichnyi, V.A. Ag/SiOx Nanocomposite Powders Synthesized from Colloids Obtained by Pulsed Laser Ablation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 80–88. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Q.; Hou, W.; Qin, L. Silver-Based Surface Plasmon Sensors: Fabrication and Applications. Int. J. Mol. Sci. 2023, 24, 4142. [Google Scholar] [CrossRef]
- Hlaing, M.; Gebear-Eigzabher, B.; Roa, A.; Marcano, A.; Radu, D.; Laihout, C.-Y. Absorption and scattering cross-section extinction values of silver nanoparticles. Opt. Mater. 2016, 58, 439–444. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobleya, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Ramya, E.; Rao, M.V.; Rao, D.N. Nonlinear optical properties of Ag-enriched ZnO nanostructures. J. Nonlinear Opt. Phys. Mater. 2019, 28, 1950027. [Google Scholar] [CrossRef]
- Patil, S.S.; Patil, R.H.; Kale, S.B.; Tamboli, M.S.; Ambekar, J.D.; Gade, W.N.; Kolekar, S.S.; Kale, B.B. Nanostructured microspheres of silver@zinc oxide: An excellent impeder of bacterial growth and biofilm. J. Nanopart. Res. 2014, 16, 2717. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Chen, W.; Yao, C.; Gan, J.; Jiang, K.; Hu, Z.; Lin, J.; Xu, N.; Sun, J.; Wu, J. ZnO colloids and ZnO nanoparticles synthesized by pulsed laser ablation of zinc powders in water. Mater. Sci. Semicond. Process. 2020, 109, 104918. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurisic, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Kurudirek, S.V.; Pradel, K.C.; Summers, C.J. Low-temperature hydrothermally grown 100 mm vertically well-aligned ultralong and ultradense ZnO nanorod arrays with improved PL property. J. Alloys Compd. 2017, 702, 700–709. [Google Scholar] [CrossRef]
- Stumpf, S.; Hostnik, G.; Primoži, M.; Leitgeb, M.; Bren, U. Generation Times of E. coli Prolong with Increasing Tannin Concentration while the Lag Phase Extends Exponentially. Plants 2020, 9, 1680. [Google Scholar] [CrossRef]
- Madigan, M. Chapter 5. Microbial Gtowth and Its Control. In Brock Biology of Microorganisms, 15th ed.; Bender, K., Buckley, D., Sattley, W., Stahl, D., Eds.; Global Edition; Pearson: London, UK, 2018; pp. 176–180. [Google Scholar]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.S.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef]
- Kim, Y.; Suh, H.S.; Cha, H.J.; Kim, S.H.; Jeong, K.S.; Kim, D.H. A case of generalized argyria after ingestion of colloidal silver solution. Am. J. Ind. Med. 2009, 52, 179–269. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Yang, H.; Liu, X.; Huang, Q.; Lu, Z. Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J. Mater. Sci. Mater. Med. 2017, 28, 23. [Google Scholar] [CrossRef]
- Ghosh, S.; Goudar, V.S.; Padmalekha, K.G.; Bhat, S.V.; Indi, S.S.; Vasan, H.N. ZnO/Ag nanohybrid: Synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv. 2012, 2, 930–940. [Google Scholar] [CrossRef]
- Nethercot, A.H., Jr. Prediction of Fermi Energies and Photoelectric Thresholds Based on Electronegativity Concepts. Phys. Rev. Lett. 1974, 33, 1088–1091. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Synthesis, physical, mechanical and antibacterial properties of nanocomposites based on poly(vinyl alcohol)/graphene oxide-silver nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef]
- Yang, X.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-Kim, H.; Meyer, J.N. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Yang, T.C.-K. Photocatalytic inactivation of Escherichia coli and Lactobacillus helveticus by ZnO and TiO2 activated with ultraviolet light. Process Biochem. 2003, 39, 475–481. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Hur, J.; Lee, S.W. Morphologies controlled ZnO for inactivation of multidrug-resistant Pseudomonas aeruginosa in solar light. J. Nanotechnol. 2020, 31, 084002. [Google Scholar] [CrossRef]
- Song, M.S.; Patil, R.P.; Hwang, I.S.; Mahadik, M.A.; Jang, T.-H.; Oh, B.T.; Chae, W.-S.; Choi, S.H.; Lee, H.H.; Jang, J.S. In situ fabrication of Ag decorated porous ZnO photocatalyst via inorganic–organic hybrid transformation for degradation of organic pollutant and bacterial inactivation. Chemosphere 2023, 341, 140057. [Google Scholar] [CrossRef]
- Lin, C.; Dong, Y.; Chen, C.; Chen, Q.-Y.; Li, S.-J.; Du, H.; Qu, L.-L. Study of synergetic effect between BODIPY and ZnO on visible light-enhanced antibacterial activity. J. Photochem. Photobiol. A 2024, 453, 115647. [Google Scholar] [CrossRef]



| Sample | Phase Composition | Lattice Parameters, Å | CSR, nm | Ag Content, wt.% * | BET Surface Area (m2/g) | Band Gap (eV) | |
|---|---|---|---|---|---|---|---|
| Phase | Content, % | ||||||
| ZnO | ZnO | 100 | a = b = 3.2489 c = 5.2049 | 43 | – | 21 | 3.25 |
| ZnO-1Ag | ZnO | 99 | a = b = 3.2484 c =5.2009 | 37 | 0.98 | 26 | 3.24 |
| Ag | 1 | ||||||
| Antibacterial Material (Synthesis Method) C (g/L) | Bacterium C (CFU/mL) | Experimental Environment/ Light Source Parameters/ Irradiation Time | Residual Survival | Ref. |
|---|---|---|---|---|
| ZnO (sol-gel) 10 | S. aureus 106 | LB culture medium/ Vis LED 600 nm/ 3 h | ZnO + hv—3.57% | [41] |
| E. coli 106 | ZnO + hv—4.28% | |||
| Commercial (Riedel-de Haën) ZnO 2 | E. coli 107 | LB culture medium/ UV lamp 365 nm, 20 W/m2/ 40 min | only hv~106 CFU/mL ZnO + hv~1 CFU/mL | [77] |
| L. helveticus 106 | only hv~5 × 106 CFU/mL ZnO+ hv 2 × 102 CFU/mL | |||
| ZnO of different shapes (co-precipitation) 2 | P. aeruginosa 107 | PBS solution/ Sunlight/ 45 min | only hv~106 CFU/mL ZnO (flower-shaped) + hv—CR ZnO (other shapes) + hv~3—50 CFU/mL | [78] |
| ZnO NPs of different shapes (ice-cube mediated synthesis) 0.5 | E. coli 104 | PBS solution/ Sunlight (India10.07 °N-78.80 °E)/ 30–75 min | ZnO + hv—CR | [51] |
| Ag/ZnO (solvothermal) ~0.01 | E. coli ~105 | PBS solution/ UV light/ 2 h | Ag/ZnO + hv—CR | [79] |
| S. aureus ~105 | Ag/ZnO + hv—CR | |||
| BIN ZnO 0.006–0.01 | E. coli ~105 | Nutrient broth/LED (7W)/2 h | BIN ZnO + hv—CR | [80] |
| S. aureus ~105 | ||||
| BIN ZnO + hv—CR | ||||
| ZnO and ZnO/Ag NPs in matrix | E. coli ~105 | Nutrient medium/low-intensity UVA illumination/2 h | ZnO~102 CFU/cm2 and ZnO/Ag~102 CFU/cm2 | [52] |
| S. aureus ~105 | ||||
| ZnO~10–102 CFU/cm2 and ZnO/Ag~104 CFU/cm2 | ||||
| ZnO (flower- shaped) and Ag/ZnO (co-precipitation) 1 | E. coli ~107 | Sterile saline water/Vis light source/180 min | ZnO–<50% CFU Ag/ZnO–CR | [53] |
| ZnO, ZnO-1Ag (PLA) 0.05 | S. aureus 106 | PBS solution/ LED 375 nm, 0.17 W, 0.38 W and LED 410 nm, 0.22, 0.36 W/ 2 h | only hv 375 nm 0.38 W~103 CFU/mL only hv 410 nm 0.36 W~3 × 103 CFU/mL ZnO + hv 375 nm 0.17 W—CR ZnO + hv 410 nm 0.36 W—CR ZnO + hv 410 nm 0.22 W~2.8 × 102 CFU/mL ZnO-1Ag + hv 410 nm 0.22 W—6 × 101 CFU/mL | The Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volokitina, A.V.; Fakhrutdinova, E.D.; Goncharova, D.A.; Kulinich, S.A.; Svetlichnyi, V.A. Laser-Prepared ZnO-Ag Nanoparticles with High Light-Enhanced Antibacterial Activity. Materials 2025, 18, 3088. https://doi.org/10.3390/ma18133088
Volokitina AV, Fakhrutdinova ED, Goncharova DA, Kulinich SA, Svetlichnyi VA. Laser-Prepared ZnO-Ag Nanoparticles with High Light-Enhanced Antibacterial Activity. Materials. 2025; 18(13):3088. https://doi.org/10.3390/ma18133088
Chicago/Turabian StyleVolokitina, Anastasia V., Elena D. Fakhrutdinova, Daria A. Goncharova, Sergei A. Kulinich, and Valery A. Svetlichnyi. 2025. "Laser-Prepared ZnO-Ag Nanoparticles with High Light-Enhanced Antibacterial Activity" Materials 18, no. 13: 3088. https://doi.org/10.3390/ma18133088
APA StyleVolokitina, A. V., Fakhrutdinova, E. D., Goncharova, D. A., Kulinich, S. A., & Svetlichnyi, V. A. (2025). Laser-Prepared ZnO-Ag Nanoparticles with High Light-Enhanced Antibacterial Activity. Materials, 18(13), 3088. https://doi.org/10.3390/ma18133088









