Influence of Al2O3 Additive on the Synthesis Kinetics of 1.13 nm Tobermorite, and Its Crystallinity and Morphology
Abstract
1. Introduction
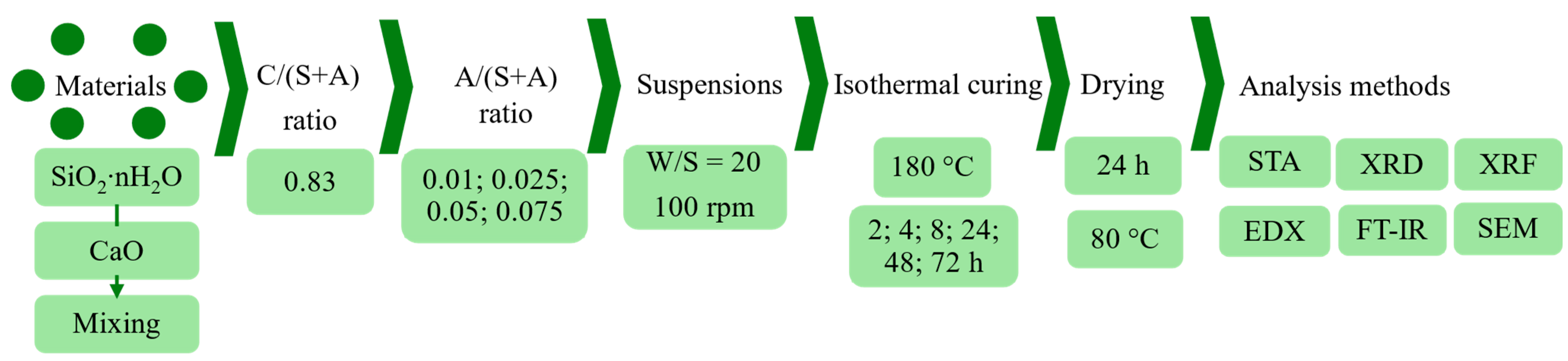
2. Materials and Methods
2.1. Materials and Raw Mixture Preparation
2.2. Instrumental Analysis
3. Results
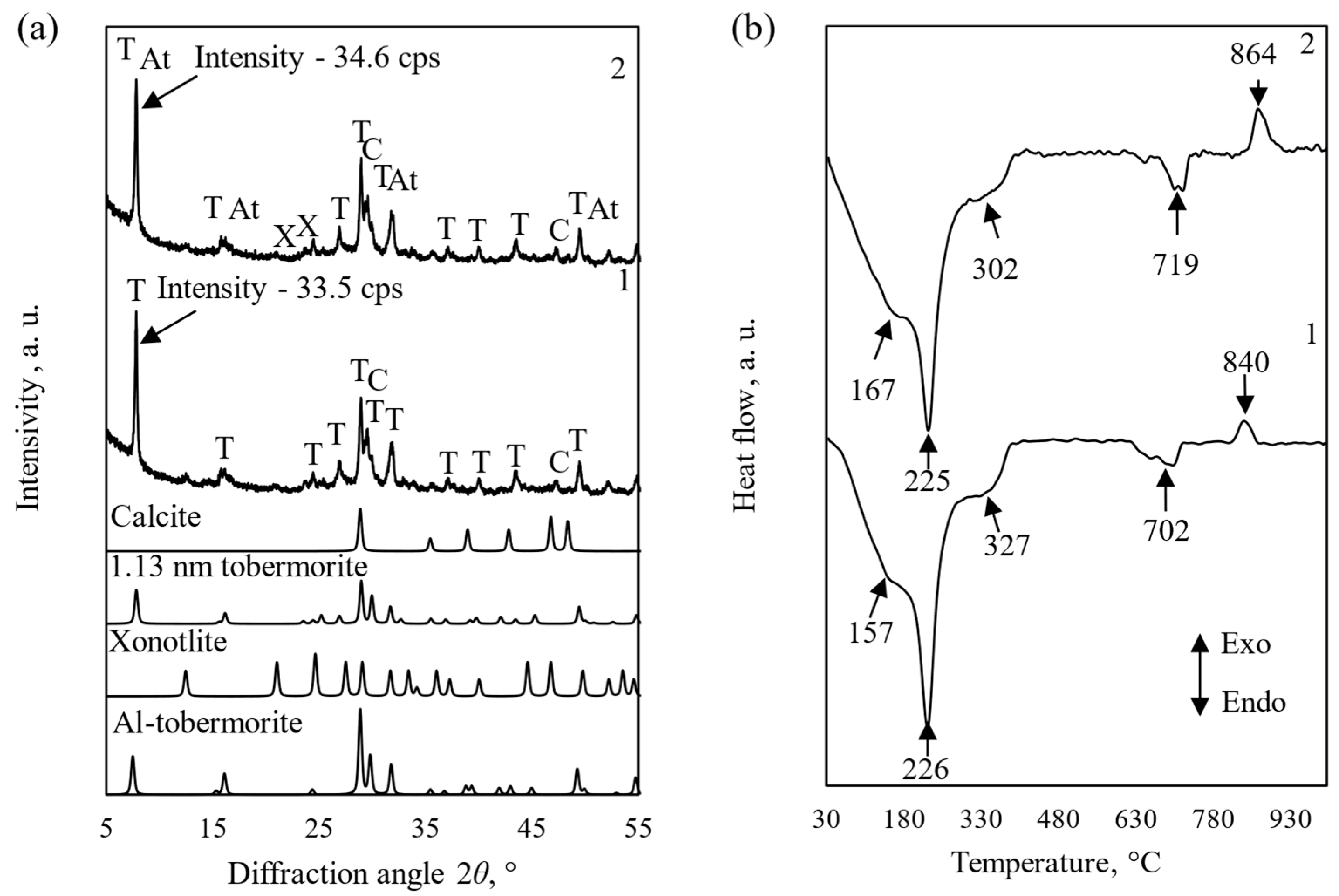
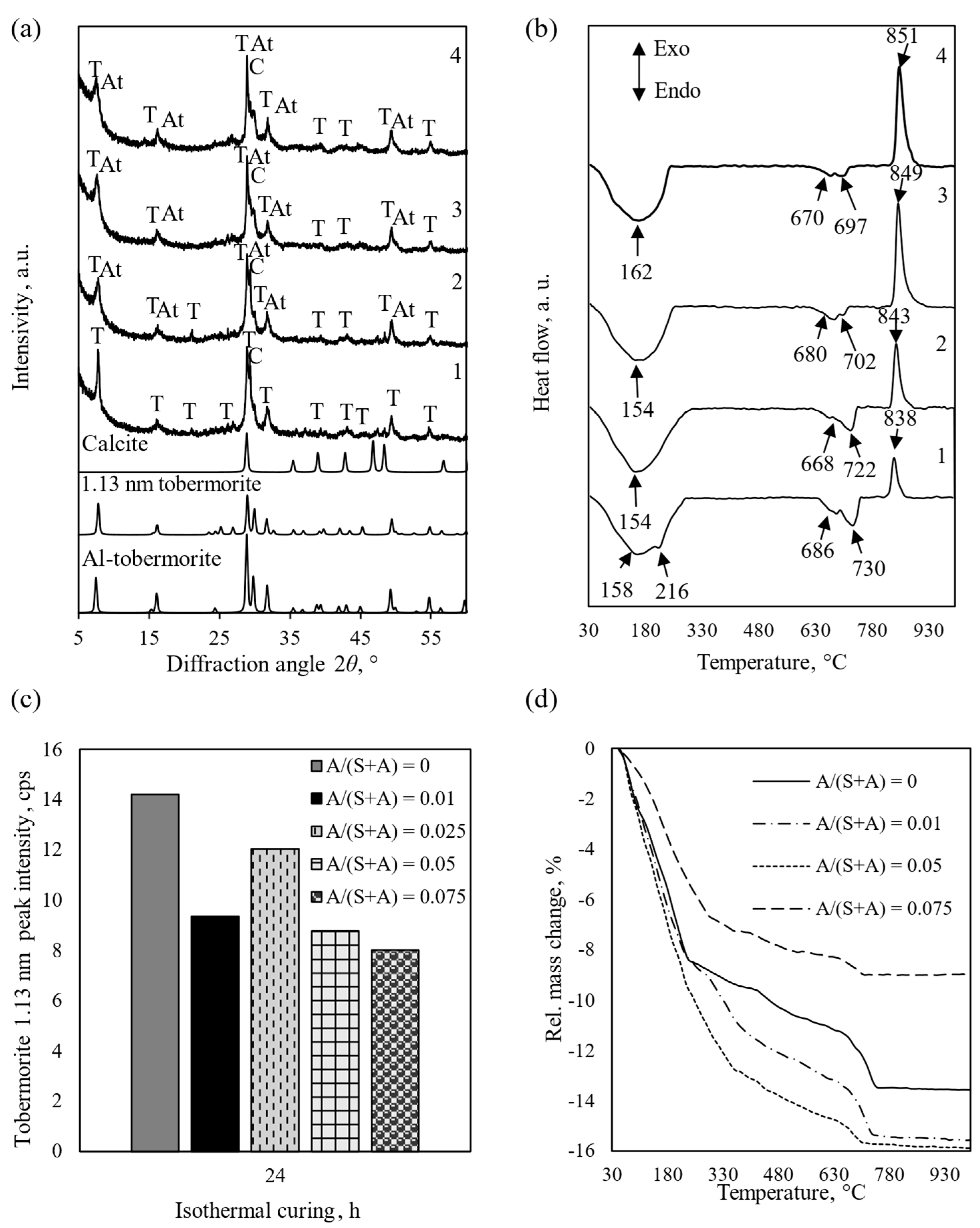
3.1. Influence of Al2O3 on the Mineralogical Composition of Synthesis Products
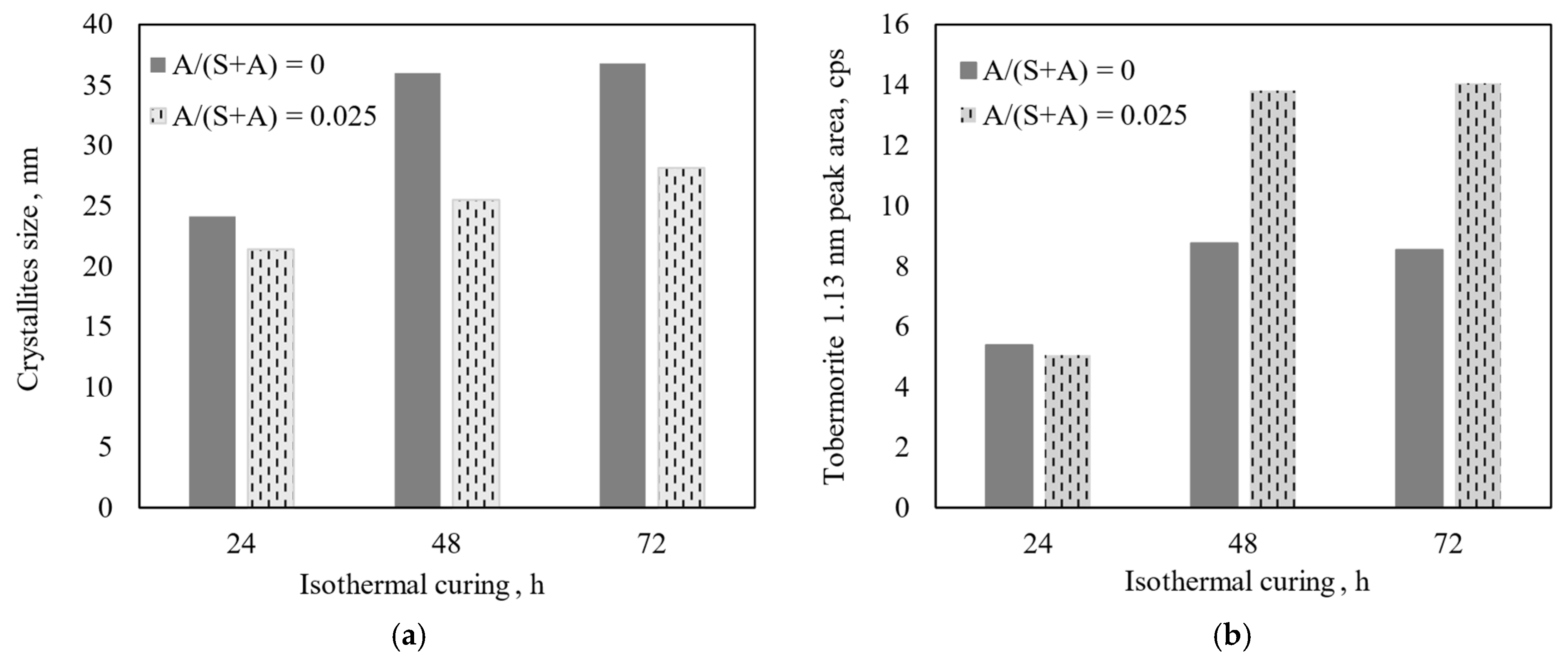
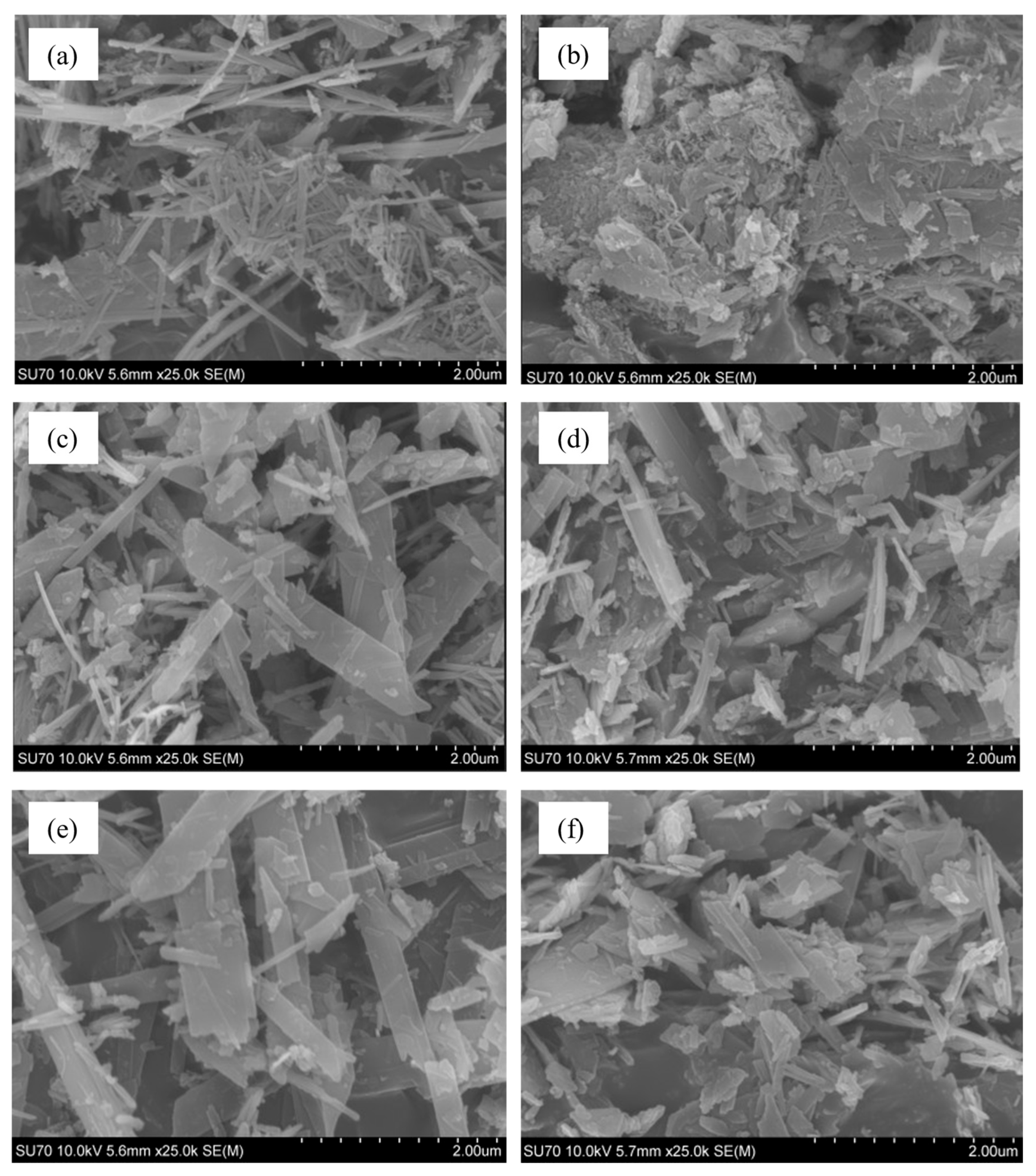
3.2. Properties and Morphology of Pure and Al-Substituted Tobermorite
4. Conclusions
- 1.
- The Al2O3 additive first aids in the formation of 1.13 nm tobermorite during the hydrothermal synthesis, but later begins to inhibit the recrystallization of C-S-H(I), and pure, high crystallinity 1.13 nm tobermorite is more easily formed in mixtures without the alumina additive.
- 2.
- Additives containing aluminum ions influence the morphology of 1.13 nm tobermorite. The product synthesized within 24 h from the CaO–SiO2 mixture without Al2O3 is dominated by long (up to 12 nm), thin fibers. By prolonging the synthesis to 48–70 h, they take on the shape of rectangular parallelepiped crystals. When alumina is added, the morphology changes—after 24 h agglomerates dominate, the surface of which is partially covered with crystal plates. By extending the curing time, amorphous aggregates are absent and the crystal shape becomes increasingly square.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSH | Calcium silicate hydrate |
| C-S-H(I) | Semi-crystalline calcium silicate hydrate with a variable CaO/SiO2 molar ratio from 0.7 to 1.1 |
| LOI | Losses on ignition |
| XRD | X-Ray diffraction |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray |
| A/(S + A) | Al2O3/(SiO2 + Al2O3) |
| C/(S + A) | CaO/(SiO2 + Al2O3) |
References
- Guo, W.; Shun, C.; Liang, F.; Jin, L.; Ji, C.; Zhang, P.; Fei, B. Ultra-light-weight, anti-flammable and water-proof cellulosic aerogels for thermal insulation applications. Int. J. Biol. Macromol. 2023, 246, 125343. [Google Scholar] [CrossRef] [PubMed]
- Popov, T.A.; Emberlin, J.; Church, M.K.; Aberg, N.; Josling, P. Powder cellulose in allergic rhinitis management: Relevance of vitro findings to real-life safety. Int. Arch. Allergy Immunol. 2019, 179, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Engineering ToolBox, Insulation Materials—Temperature Ranges. 2005. Available online: https://www.engineeringtoolbox.com/insulation-temperatures-d_922.html (accessed on 7 August 2024).
- Mahltig, B.; Kyosev, Y. Inorganic and composite fibers. Production, properties, and applications. Chapter 7—Glass Fibers. In Inorganic and Composite Fibers: Production, Properties, and Applications; Martynova, E., Cebull, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 131–163. [Google Scholar]
- Eske, J. How to Remove Fiberglass in Skin. Medical News Today. Available online: https://www.medicalnewstoday.com/articles/fiberglass-in-skin (accessed on 23 July 2024).
- Zhang, J.; Xu, X.; Cheng, F.; Ramakrishna, S. Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics. Materials 2022, 15, 7256. [Google Scholar] [CrossRef] [PubMed]
- Tingley, D.D.; Hathway, A.; Davison, B.; Allwood, D. The environmental impact of phenolic foam insulation boards. Proc. Inst. Civil Eng. Constr. Mater. 2017, 170, 91–103. [Google Scholar] [CrossRef]
- Ko, H.; Lee, H.S.; Lim, H.M. Effects of additives in colloidal silica based inorganic-hybrid binder for mineral wool insulation boards. J. Asian Ceram. Soc. 2020, 8, 1285–1295. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Liu, W.; Qiu, R.; Wei, H.; Zong, K.; Cai, X. A review of recent progress on the silica aerogel monoliths: Synthesis, reinforcement, and applications. J. Mater. Sci. 2021, 56, 10812–10833. [Google Scholar] [CrossRef]
- Lei, J.; Zheng, S.; Han, Z.; Niu, Y.; Pan, D.; Liu, H.; Liu, C.; Shen, C. A Brief Review on the Preparation and Application of Silica Aerogel. Eng. Sci. 2024, 30, 1214. [Google Scholar] [CrossRef]
- Qi, F.; Zhu, G.; Chen, Y.; Zhu, Y.; Li, S.; Zhang, J.; Li, H. Mechanism of One-Step Hydrothermal Process to Prepare Tobermorite Thermal Insulation Materials during Recovery of Silicon-Rich Lye. Ind. Eng. Chem. Res. 2024, 63, 12875–12886. [Google Scholar] [CrossRef]
- Ogur, E.; Botti, R.; Bortolotti, M.; Colombo, P.; Vakifahmetoglu, C. Synthesis and additive manufacturing of calcium silicate hydrate scaffolds. J. Mater. Res. Technol. 2021, 11, 1142–1151. [Google Scholar] [CrossRef]
- Liu, J.; Pan, X.; Guo, Y.; Zou, Z.; Wang, Z.; Yu, H. Crystallization mechanism and physical properties of xonotlite intensified by inorganic and organic additives based on direct hydrothermal synthesis. J. Non-Cryst. Solids 2024, 640, 123121. [Google Scholar] [CrossRef]
- EN 16977:2020; Thermal Insulation Products for Buildings—Factory Made Calcium silicate (CS) Products—Specification. European Committee for Standardization: Brussels, Belgium, 2020; pp. 1–34.
- Galvankova, L.; Masilko, J.; Solny, T.; Stepankova, E. Tobermorite synthesis under hydrothermal conditions. Procedia Eng. 2016, 151, 100–107. [Google Scholar] [CrossRef]
- Jimenez, I.; Perez, G.; Guerrero, A.; Ruiz, B. Mineral phases synthesized by hydrothermal treatment from biomass ashes. Int. J. Miner. Process. 2017, 158, 8–12. [Google Scholar] [CrossRef]
- Smalakys, G.; Siauciunas, R. The synthesis of 1.13 nm tobermorite from carbonated opoka. J. Therm. Anal. Calorim. 2018, 134, 493–502. [Google Scholar]
- Liu, F.; Cao, J.X.; Zhu, B. Effect of anion impurity on preparing xonotlite whiskers via hydrothermal synthesis. Adv. Mater. Res. Trans. 2011, 148–149, 1755–1758. [Google Scholar] [CrossRef]
- Pugovkina, Y.; Kutugin, V.; Ostroumova, A.; Rymanova, I. High temperature and heat insulated calcium silicate materials. Key Eng. Mater. Trans. 2016, 683, 209–214. [Google Scholar] [CrossRef]
- Akbayrak, S.; Ozkar, S. Inverse relation between the catalytic activity and catalyst concentration for the ruthenium(0) nanoparticles supported on xonotlite nanowire in hydrogen generation from the hydrolysis of sodium borohydride. J. Mol. Catal. A Chem. 2016, 424, 254–260. [Google Scholar] [CrossRef]
- Shaw, S.; Henderson, C.M.B.; Komanschek, B.U. Dehydration/recrystallization mechanisms, energetics, and kinetics of hydrated calcium silicate minerals: An in situ TGA/DSC and synchrotron radiation SAXS/WAXS study. Chem. Geol. 2000, 167, 141–159. [Google Scholar] [CrossRef]
- Biagoni, C.; Bonaccorsi, E.; Lezzerini, M.; Merlino, S. Thermal behaviour of Al-rich tobermorite. Eur. J. Mineral. 2016, 28, 23–32. [Google Scholar] [CrossRef]
- Małecki, M.; Kurdowski, W.; Walczak, P. Influence of gypsum and limestone, used as mineral additives, on autoclaved aerated concrete properties. ce/papers 2018, 2, 231–234. [Google Scholar] [CrossRef]
- Paradiso, P.; Santos, R.L.; Horta, R.B.; Lopes, J.; Ferreira, P.; Colaço, R. Formation of nanocrystalline tobermorite in calcium silicate binders with low C/S ratio. Acta Mater. 2018, 152, 7–15. [Google Scholar] [CrossRef]
- Grangeon, S.; Claret, F.; Roosz, C.; Sato, T.; Gaboreau, S.; Linard, Y. Structure of nanocrystalline calcium silicate hydrates: Insights from X-ray diffraction, synchrotron X-ray absorption and nuclear magnetic resonance. J. Appl. Crystallogr. 2016, 49, 771–783. [Google Scholar] [CrossRef]
- Siauciunas, R.; Smalakys, G.; Eisinas, A.; Prichockiene, E. Synthesis of high crystallinity 1.13 nm tobermorite and xonotlite from natural rocks, their properties and application for heat-resistant products. Materials 2022, 15, 3474. [Google Scholar] [CrossRef]
- Jansen, D.; Lothenbach, B.; Yan, Y.; Schreiner, J. Synthesis, structural characterization, and thermodynamic properties of 11 Å Al-tobermorite. ce/papers 2023, 6, 234–237. [Google Scholar] [CrossRef]
- Smalakys, G.; Siauciunas, R. Peculiarities of xonotlite synthesis from the raw materials with different SiO2 activities. J. Therm. Anal. Calorim. 2020, 142, 1671–1679. [Google Scholar] [CrossRef]
- Mao, F.; Ai, H. A Study on the Hydrothermal Synthesis of Calcium Silicate Products by Calcination of Full-Component Waste Concrete. Sustainability 2023, 15, 16341. [Google Scholar] [CrossRef]
- Saldia, S.Q.; Bacosa, H.; Vegafria, M.C.; Zoleta, J.; Hiroyoshi, N.; Empig, E.; Calleno, C.; Cantong, W.; Ibarra, E.; Aguilos, M.; et al. Combined Potential of Quarry Waste Fines and Eggshells for the Hydrothermal Synthesis of Tobermorite at Varying Cement Content. Preprints 2023. submitted. [Google Scholar] [CrossRef]
- Diez-Garcia, M.; Gaitero, J.J.; Dolado, J.S.; Aymonier, C. Ultra-Fast Supercritical Hydrothermal Synthesis of Tobermorite under Thermodynamically Metastable Conditions. Angew. Chem. Int. Ed. 2017, 56, 3162–3167. [Google Scholar] [CrossRef]
- Glazev, M.; Bazhin, V. On the recycling and use of microsilica in the oil industry. E3S Web Conf. 2021, 266, 02010. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.H.; Tong, L.X. Preparation and Characterization of Calcium Silicate Slag Based Lightweight Wall Materials. Key Eng. Mater. 2012, 512–515, 110–114. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, C.; Jiao, Y.; Zhang, D.; Kang, D.; Wang, K. Study on Crystal Growth of Tobermorite Synthesized by Calcium Silicate Slag and Silica Fume. Materials 2023, 16, 1288. [Google Scholar] [CrossRef]
- Hurt, A.P.; Coleman, A.A.; Ma, H.; Li, Q.; Coleman, N.J. Calcium Silicate Hydrate Cation-Exchanger from Paper Recycling Ash and Waste Container Glass. Ceramics 2022, 5, 301–317. [Google Scholar] [CrossRef]
- Rahman, H.; Li, Q.; Coleman, N.J. Waste Glass-Derived Tobermorite Carriers for Ag+ and Zn2+ Ions. J. Compos. Sci. 2022, 6, 52. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, X.; Li, Q.; Yu, H. Crystallization and phase transition of tobermorite synthesized by hydrothermal reaction from dicalcium silicate. Int. J. Appl. Ceram. Technol. 2020, 17, 1213–1223. [Google Scholar] [CrossRef]
- Mutisya, S.M.; Miranda, C.R. The surface stability and morphology of tobermorite 11 Å from first principles. Appl. Surf. Sci. 2018, 444, 287–292. [Google Scholar] [CrossRef]
- Liao, W.; Li, W.; Fang, Z.; Lu, C.; Xu, Z. Effect of different aluminum substitution rates on the structure of tobermorite. Materials 2019, 12, 3765. [Google Scholar] [CrossRef]
- Lothenbach, B.; Jansen, D.; Yan, Y.; Schreiner, J. Solubility and characterization of synthesized 11 Å Al-tobermorite. Cem. Concr. Res. 2022, 159, 106871. [Google Scholar] [CrossRef]
- Siauciunas, R.; Smalakys, G.; Dambrauskas, T. Porosity of calcium silicate hydrates synthesized from natural rocks. Materials 2021, 14, 5592. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H. Thermal Behavior and Determination of the Heated Structure of 11Å Anomalous Tobermorite by in situ X-ray Diffraction. Acta Geol. Sin. 2021, 95, 810–829. [Google Scholar] [CrossRef]
- Tajuelo Rodriguez, E.; Garbev, K.; Merz, D.; Black, L.; Richardson, I.G. Thermal stability of C-S-H phases and applicability of Richardson and Groves’ and Richardson C-(A)-S-H(I) models to synthetic C-S-H. Cem. Concr. Res. 2017, 93, 45–56. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Elucidating the accelerated carbonation products of calcium silicates using multi-technique approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Bagheri, M.; Lothenbach, B.; Shakoorioskooie, M.; Scrivener, K. Effect of different ions on dissolution rates of silica and feldspars at high pH. Cem. Concr. Res. 2022, 152, 106644. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, L.; Cao, J.; Li, J. Preparation of ultra-light xonotlite thermal insulation material using carbide slag. Journal of Wuhan University of Technology-Mater. Sci. Ed. 2010, 25, 295–297. [Google Scholar]
- Coleman, N.J. Synthesis, structure and ion exchange properties of 11Å tobermorites from newsprint recycling residue. Mater. Res. Bull. 2005, 40, 2000–2013. [Google Scholar] [CrossRef]


| Oxides | SiO2 | CaO | Al2O3 | K2O | Na2O | MgO | SO3 | Other | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Content, % | 97.7 | 0.59 | 0.13 | 0.53 | 0.18 | 0.26 | 0.25 | 0.17 | 2.51 |
| Compound | d-Spacing, nm | PDF Number | ||||
|---|---|---|---|---|---|---|
| 1.13 nm tobermorite | 1.130 | 0.3080 | 0.2980 | 0.1842 | 0.2820 | 00-019-1364 |
| Al-substituted tobermorite | 1.180 | 0.3090 | 0.2995 | 0.1848 | 0.2814 | 00-019-0052 |
| C/(S + A) | A/(S + A) | Duration of Hydrothermal Treatment, h | |||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 24 | 48 | 72 | ||
| Heat Flux of Exothermic Effect at 830–850 °C, mW/g·103 | |||||||
| 0.83 | 0 | 2.47 | 1.47 | 1.06 | 0.42 | 0.14 | 0.08 |
| 0.025 | 1.32 | 1.31 | 1.20 | 0.92 | 0.74 | 0.67 | |
| Sample After 24 h Curing at 180 °C | Relative Volume, cm3/g | Diameter, µm, at | Mean Diameter, µm | Specific Surface Area, m2/kg | ||
|---|---|---|---|---|---|---|
| 10% | 50% | 90% | ||||
| A/(S + A) = 0 | 16.2 ± 0.9 | 0.94 | 9.88 | 26.86 | 12.20 | 652.7 |
| A/(S + A) = 0.025 | 13.4 ± 1.2 | 2.60 | 13.66 | 35.99 | 16.97 | 401.0 |
| Product | Oxide Content, wt% | Molar Ratio | |||
|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | C/(S + A) | A/(S + A) | |
| Initial mixture | 43.22 ± 0.05 | 54.40 ± 0.06 | 2.37 ± 0.02 | 0.83 | 0.025 |
| After 24 h synthesis | 45.83 ± 0.06 | 52.66 ± 0.06 | 2.31 ± 0.01 | 0.818 | 0.0243 |
| After 48 h synthesis | 45.04 ± 0.04 | 52.50 ± 0.07 | 2.42 ± 0.03 | 0.804 | 0.0255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siauciunas, R.; Steponaityte, L.; Dzvinka, M.; Kareiva, A. Influence of Al2O3 Additive on the Synthesis Kinetics of 1.13 nm Tobermorite, and Its Crystallinity and Morphology. Materials 2025, 18, 3086. https://doi.org/10.3390/ma18133086
Siauciunas R, Steponaityte L, Dzvinka M, Kareiva A. Influence of Al2O3 Additive on the Synthesis Kinetics of 1.13 nm Tobermorite, and Its Crystallinity and Morphology. Materials. 2025; 18(13):3086. https://doi.org/10.3390/ma18133086
Chicago/Turabian StyleSiauciunas, Raimundas, Liveta Steponaityte, Marius Dzvinka, and Aivaras Kareiva. 2025. "Influence of Al2O3 Additive on the Synthesis Kinetics of 1.13 nm Tobermorite, and Its Crystallinity and Morphology" Materials 18, no. 13: 3086. https://doi.org/10.3390/ma18133086
APA StyleSiauciunas, R., Steponaityte, L., Dzvinka, M., & Kareiva, A. (2025). Influence of Al2O3 Additive on the Synthesis Kinetics of 1.13 nm Tobermorite, and Its Crystallinity and Morphology. Materials, 18(13), 3086. https://doi.org/10.3390/ma18133086







