External Barrier and Internal Attack: Synergistic Effect of Microcapsule Fire Extinguishing Agent and Fine Water Mist on Suppressing Lithium-Ion Battery Fire
Abstract
1. Introduction
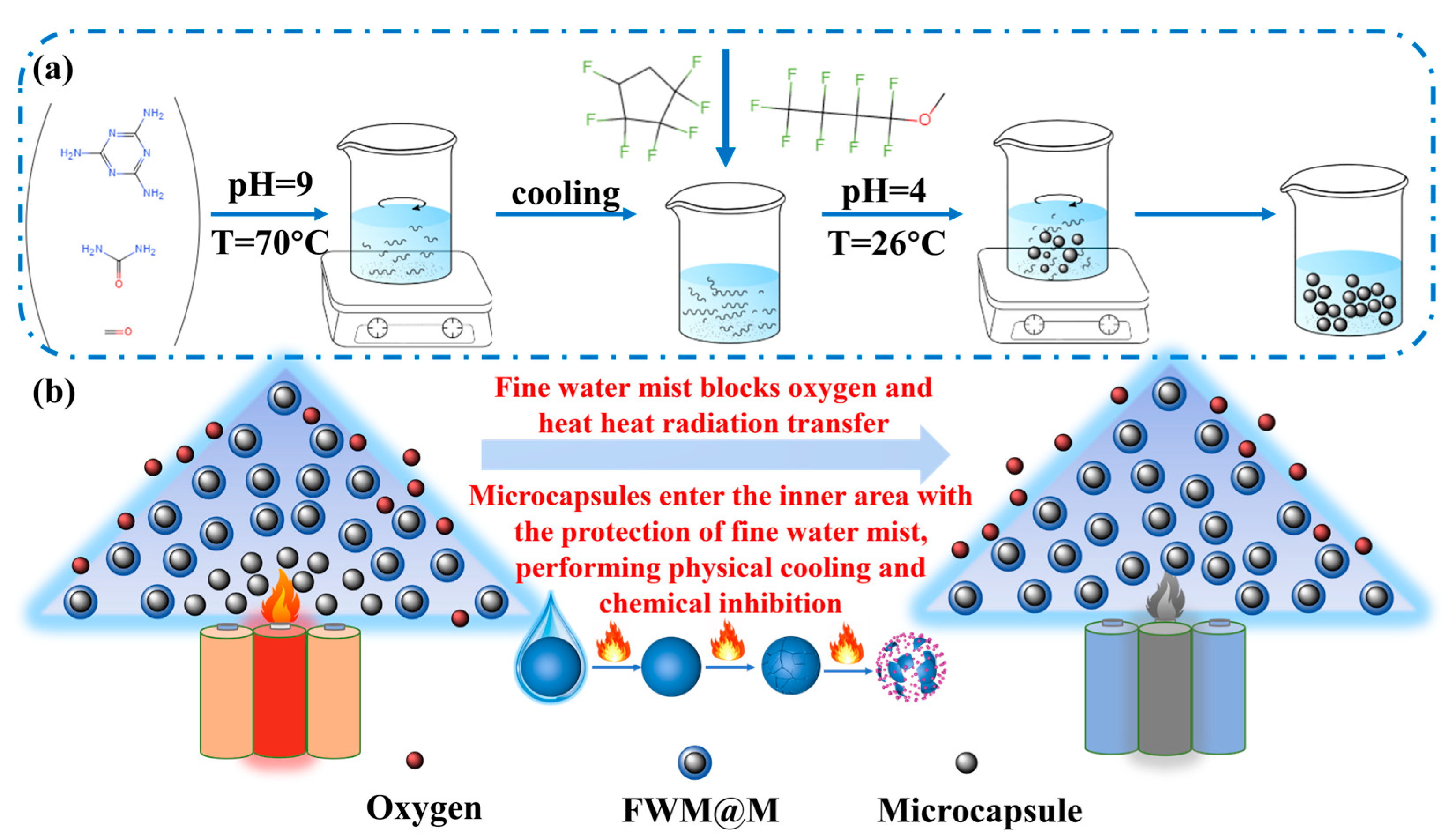
2. Materials Preparation and Characterization Methods
3. Results
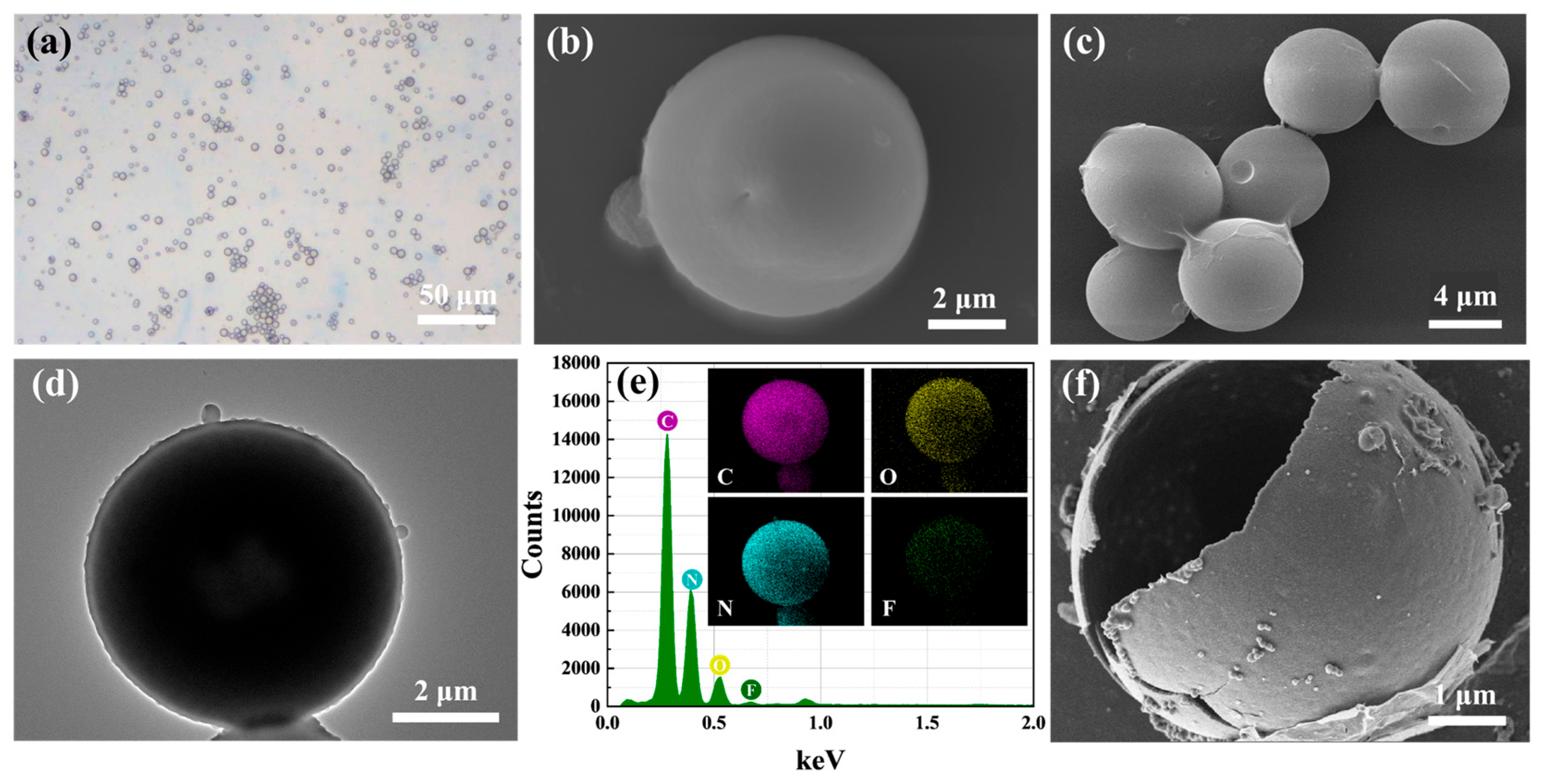
3.1. Materials Characterization
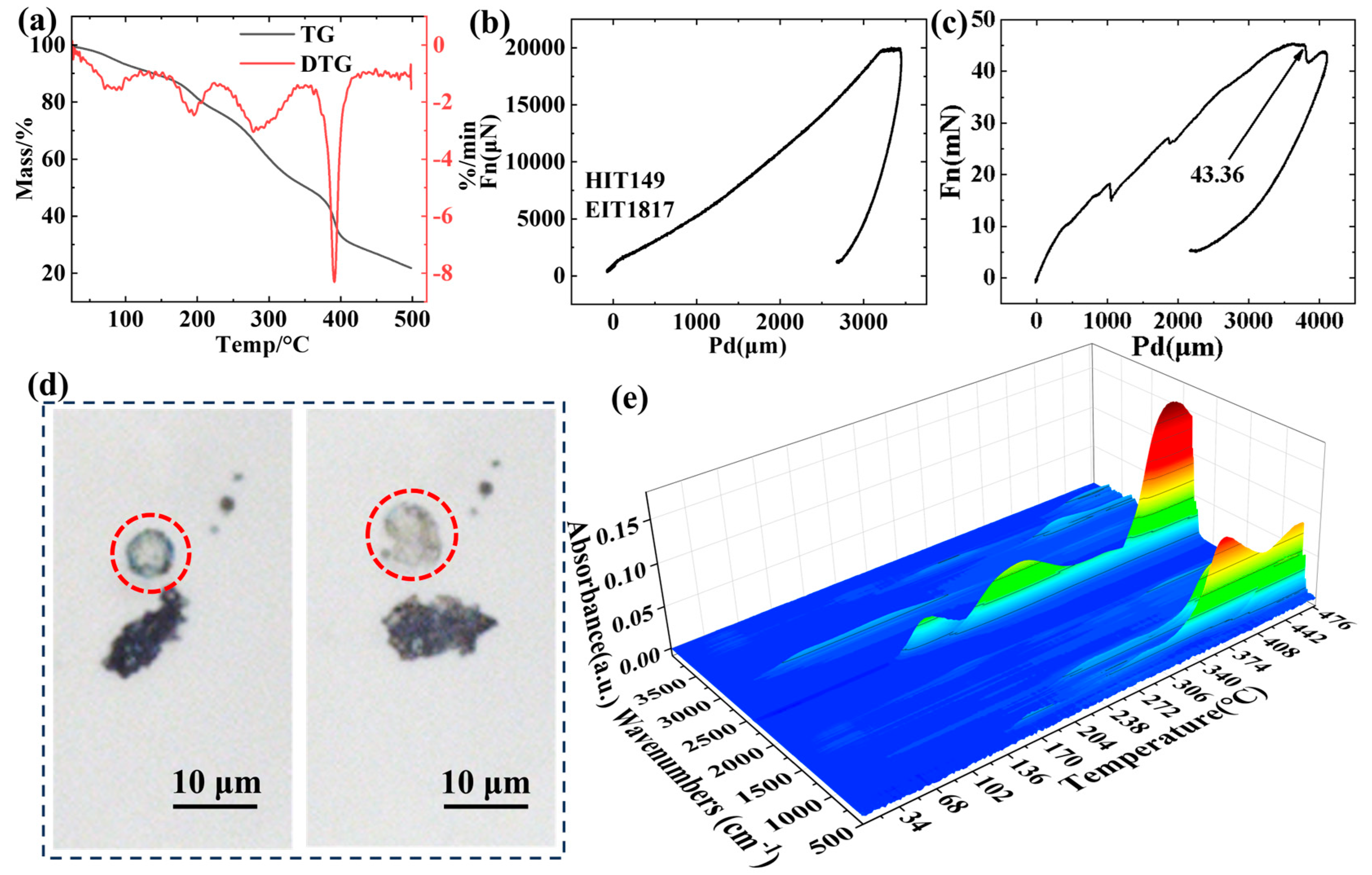
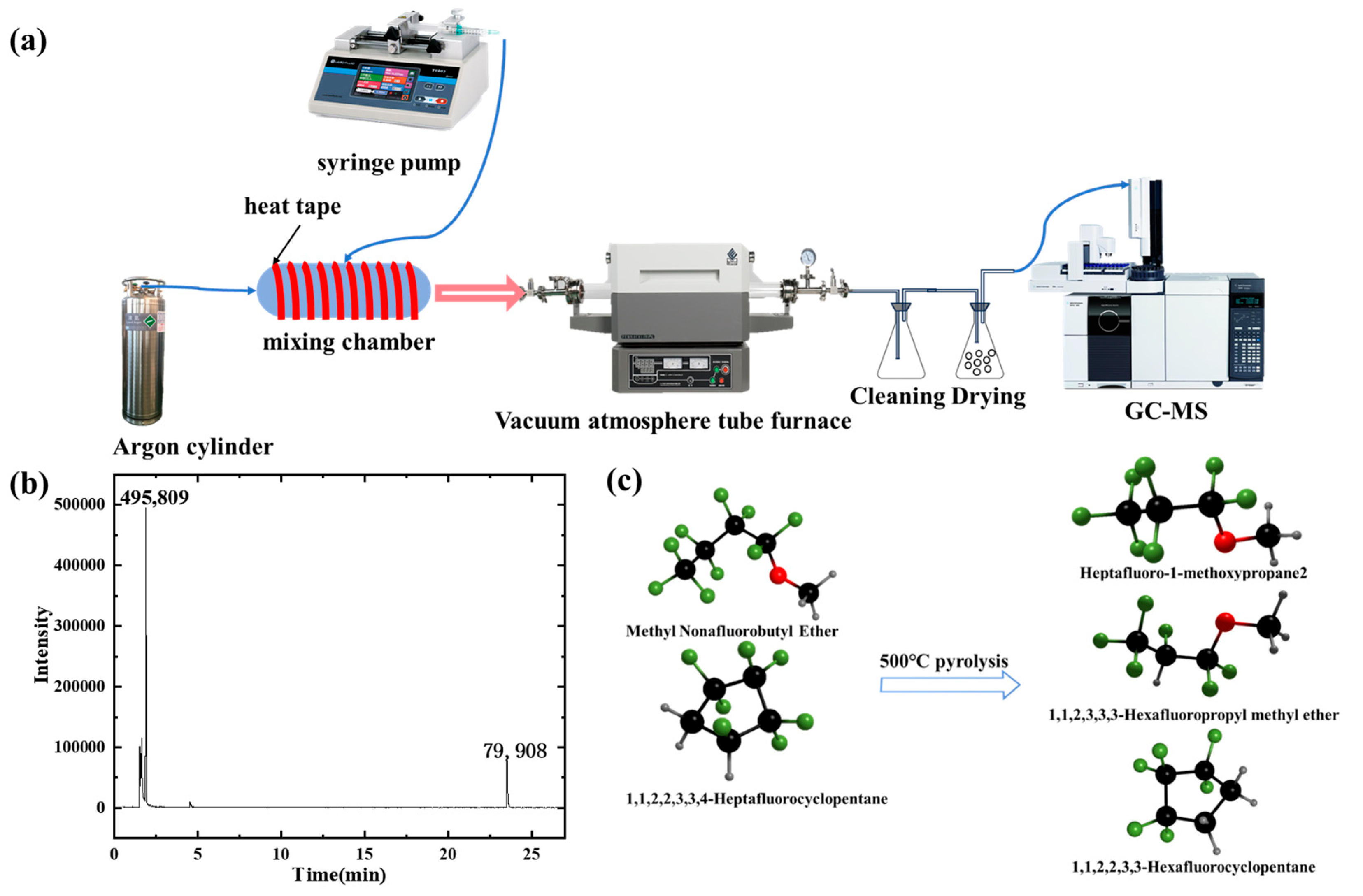
3.2. High-Temperature Pyrolysis Characteristics of Core Material
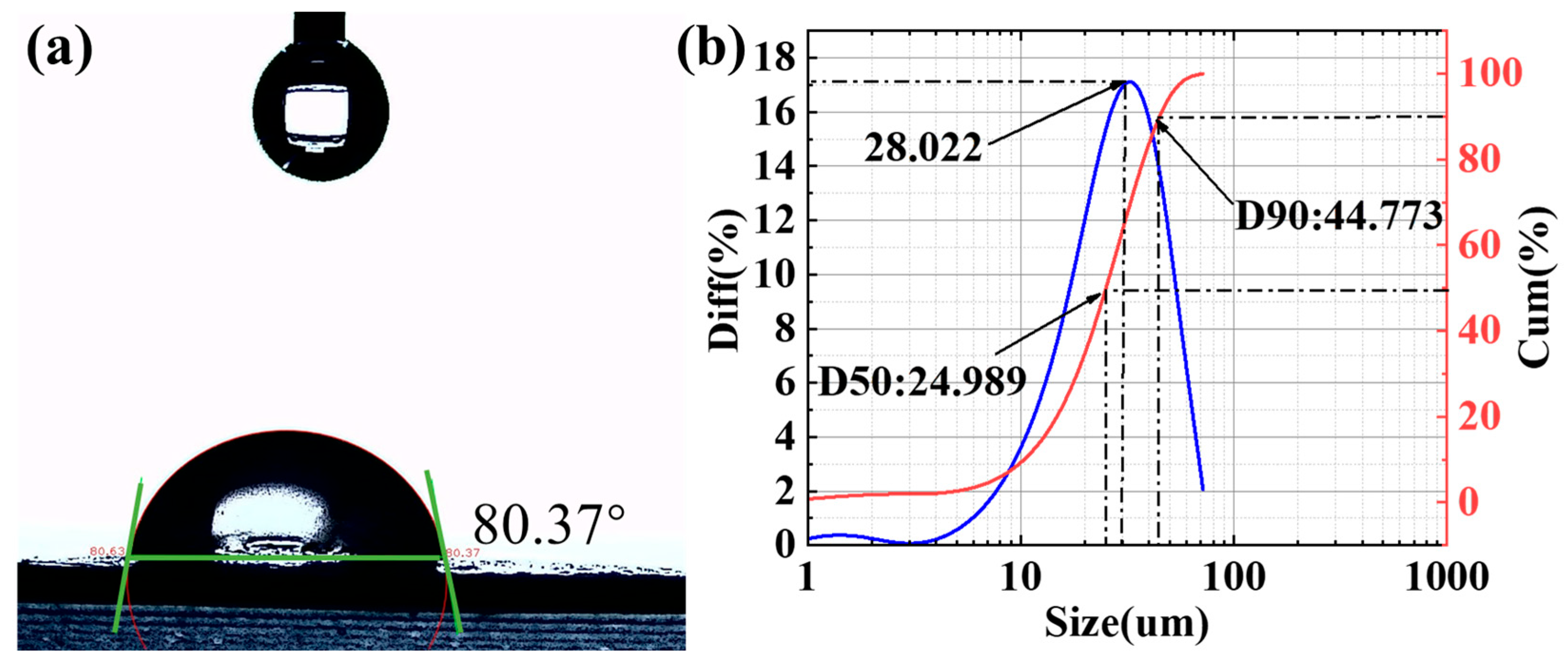
3.3. Contact Angle and Particle Size Analysis
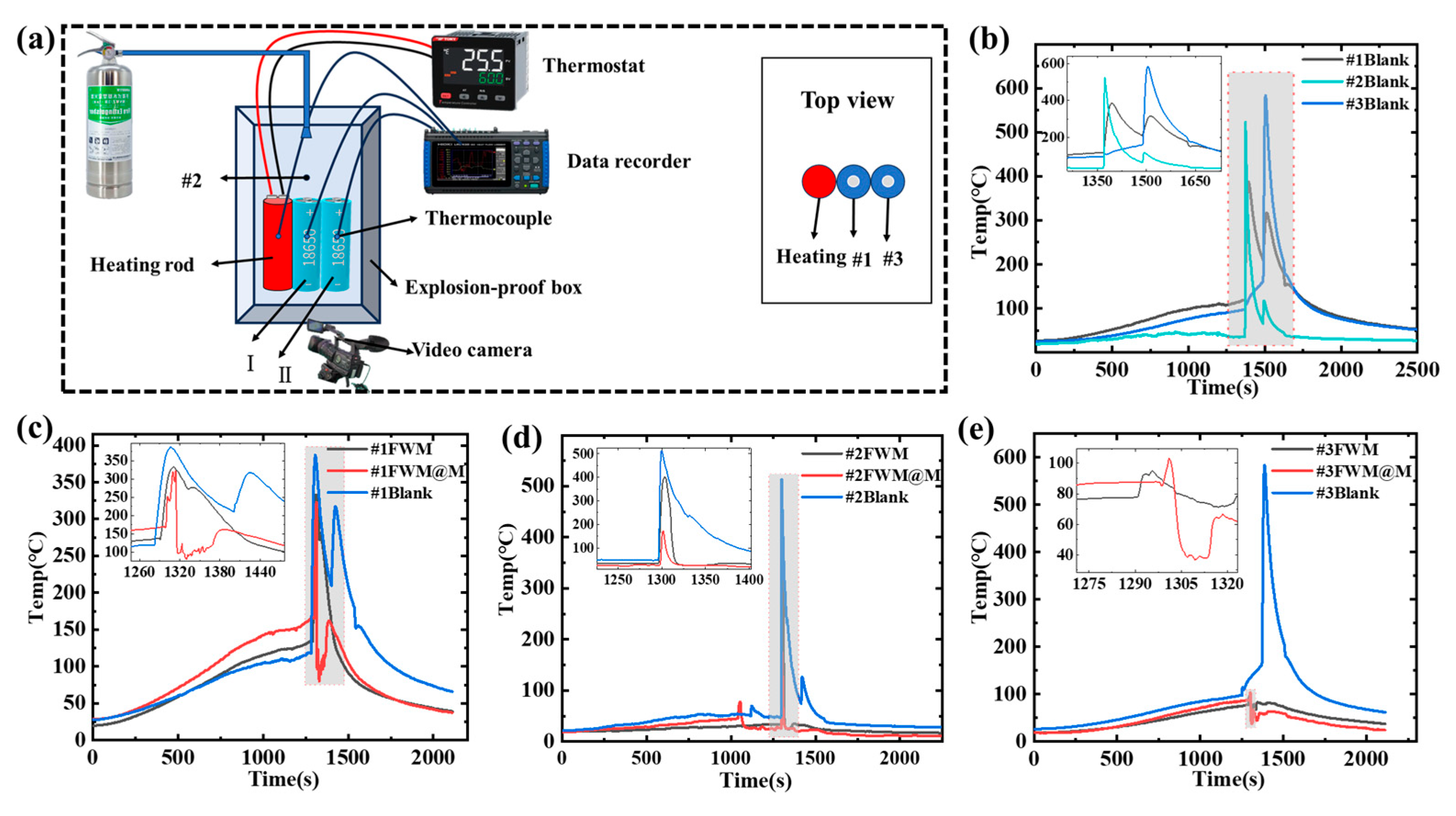
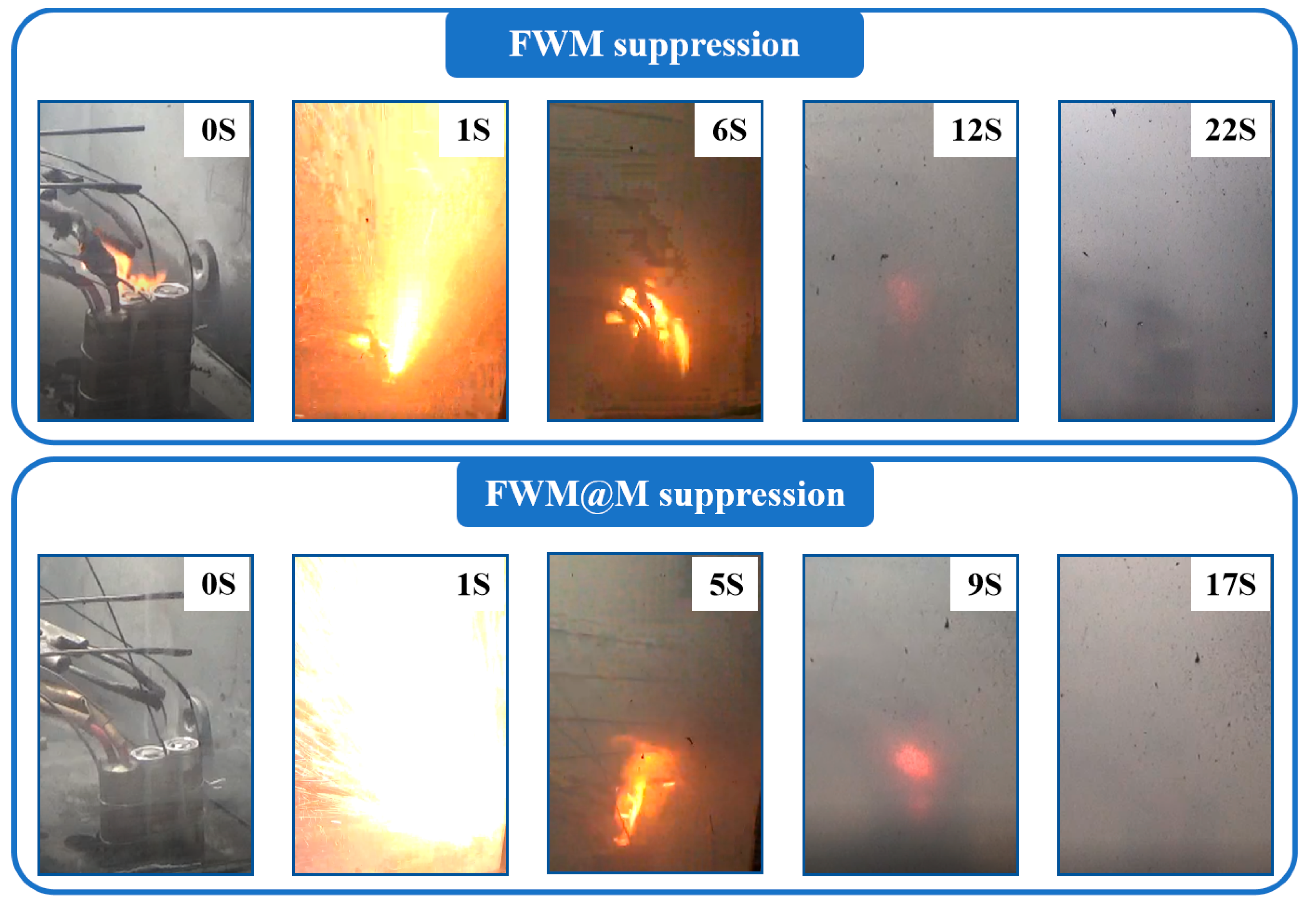
3.4. Lithium-Ion Battery Fire Suppression Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Kwon, M.; Yoon Choi, J.; Choi, S. Full-scale fire testing of battery electric vehicles. Appl. Energy 2023, 332, 120497. [Google Scholar] [CrossRef]
- Ping, P.; Wang, Q.; Huang, P.; Li, K.; Sun, J.; Kong, D.; Chen, C. Study of the fire behavior of high-energy lithium-ion batteries with full-scale burning test. J. Power Sources 2015, 285, 80–89. [Google Scholar] [CrossRef]
- Chen, H.; Fang, Y.; Liu, X.; Jiang, X.; Zhong, F.; Yang, H.; Ai, X.; Cao, Y. A controllable thermal-sensitivity separator with an organic–inorganic hybrid interlayer for high-safety lithium-ion batteries. Mater. Chem. Front. 2021, 5, 2313–2319. [Google Scholar] [CrossRef]
- Isak, B.; Pugdahl, K.; Karlsson, P.; Tankisi, H.; Finnerup, N.B.; Furtula, J.; Johnsen, B.; Sunde, N.; Jakobsen, J.; Fuglsang-Frederiksen, A. Quantitative sensory testing and structural assessment of sensory nerve fibres in amyotrophic lateral sclerosis. J. Neurol. Sci. 2017, 373, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Trypsianis, G.; Papazoglou, D.; Maltezos, E.; Papanas, N. Clinical Tools for Peripheral Neuropathy to Exclude Cardiovascular Autonomic Neuropathy in Type 2 Diabetes Mellitus. Diabetes Ther. 2020, 11, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Sachau, J.; Otto, J.C.; Kirchhofer, V.; Larsen, J.B.; Kennes, L.N.; Hüllemann, P.; Arendt-Nielsen, L.; Baron, R. Development of a bedside tool-kit for assessing sensitization in patients with chronic osteoarthritis knee pain or chronic knee pain after total knee replacement. PAIN 2022, 163, 308–318. [Google Scholar] [CrossRef]
- Panchal, A.A.; Pennebaker, T.N.T.; Sebti, E.; Li, Y.; Li, Y.; Clément, R.J.; Canepa, P. Compatibility of Halide Bilayer Separators for All-Solid-State Batteries. ACS Energy Lett. 2024, 9, 5935–5944. [Google Scholar] [CrossRef]
- Lashari, N.u.R.; Mehmood, A.; Hussain, A.; Raza, W.; Ahmed, I.; Mushtaq, M.A.; Wei, L.; Cai, X.; Liu, D. Complexion-induced Cu2+ coordinated phase separation PBI membrane for lithium metal batteries. J. Power Sources 2024, 624, 235590. [Google Scholar] [CrossRef]
- Andersson, R.; Borodin, O.; Johansson, P. Dynamic Structure Discovery Applied to the Ion Transport in the Ubiquitous Lithium-ion Battery Electrolyte LP30. J. Electrochem. Soc. 2022, 169, 100540. [Google Scholar] [CrossRef]
- Jalees, S.; Hussain, A.; Iqbal, R.; Raza, W.; Ahmad, A.; Saleem, A.; Majeed, M.K.; Faheem, M.; Ahmad, N.; Rehman, L.N.U.; et al. Functional PBI membrane based on polyimide covalent organic framework for durable lithium metal battery. J. Energy Storage 2024, 101, 113985. [Google Scholar] [CrossRef]
- Hussain, A.; Mehmood, A.; Raza, W.; Faheem, M.; Saleem, A.; Kashif Majeed, M.; Iqbal, R.; Aziz, M.A. Highly Stretchable Polyurethane Porous Membranes with Adjustable Morphology for Advanced Lithium Metal Batteries. Chem. Asian J. 2024, 19, e202400245. [Google Scholar] [CrossRef] [PubMed]
- Chae, O.B.; Adiraju, V.A.K.; Lucht, B.L. Lithium Cyano Tris (2,2,2-trifluoroethyl) Borate as a Multifunctional Electrolyte Additive for High-Performance Lithium Metal Batteries. ACS Energy Lett. 2021, 6, 3851–3857. [Google Scholar] [CrossRef]
- Saleem, A.; Zhu, H.; Majeed, M.K.; Iqbal, R.; Jabar, B.; Hussain, A.; Ashfaq, M.Z.; Ahmad, M.; Rauf, S.; Mwizerwa, J.P.; et al. Manganese and Cobalt-Free Ultrahigh-Ni-Rich Single-Crystal Cathode for High-Performance Lithium Batteries. ACS Appl. Mater. Interfaces 2023, 15, 20843–20853. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.; Jo, J.; Yu, S.; Shin, S.; Hur, K.; Koo, B.; Kim, K.H.; Hwang, J. Multilayered separators with core-shell structured nanocellulose-SiO2 nanocomposites for lithium-ion batteries. Carbohydr. Polym. 2025, 362, 123677. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, Q.; Liu, M.; Han, Z.; Li, C.; Sun, C.; Chen, B.; Zhong, X.; Qiu, L.; Zhou, G. Stabilized Solid Electrolyte Interphase Induced by Ultrathin Boron Nitride Membranes for Safe Lithium Metal Batteries. Nano Lett. 2021, 21, 8447–8454. [Google Scholar] [CrossRef] [PubMed]
- Yeon, D.; Lee, Y.; Ryou, M.-H.; Lee, Y.M. New flame-retardant composite separators based on metal hydroxides for lithium-ion batteries. Electrochim. Acta 2015, 157, 282–289. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, X.; Wu, B.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Bis (2,2,2-trifluoroethyl) methylphosphonate: An Novel Flame-retardant Additive for Safe Lithium-ion Battery. Electrochim. Acta 2014, 129, 300–304. [Google Scholar] [CrossRef]
- Russo, P.; Di Bari, C.; Mazzaro, M.; De Rosa, A.; Morriello, I. Effective Fire Extinguishing Systems for Lithium-ion Battery. Chem. Eng. Trans. 2018, 67, 727–732. [Google Scholar] [CrossRef]
- Tang, W.; Yuan, L.; Thomas, R.; Soles, J. Comparison of Fire Suppression Techniques on Lithium-Ion Battery Pack Fires. Min. Metall. Explor. 2023, 40, 1081–1087. [Google Scholar] [CrossRef]
- Xu, J.; Guo, P.; Duan, Q.; Yu, X.; Zhang, L.; Liu, Y.; Wang, Q. Experimental study of the effectiveness of three kinds of extinguishing agents on suppressing lithium-ion battery fires. Appl. Therm. Eng. 2020, 171, 115076. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, F.; Fu, Y.; Cheng, Y.; Yang, H.; Lu, S. A comparative study on the thermal runaway inhibition of 18650 lithium-ion batteries by different fire extinguishing agents. iScience 2021, 24, 102854. [Google Scholar] [CrossRef]
- Luo, W.-t.; Zhu, S.-b.; Gong, J.-h.; Zhou, Z. Research and Development of Fire Extinguishing Technology for Power Lithium Batteries. Procedia Eng. 2018, 211, 531–537. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, L.; Duan, Q.; Wang, S.; Duan, P.; Wei, Z.; Zhang, L.; Jia, Z.; Jin, K.; Wang, Q. Experimental study on exploration of optimum extinguishing agent for 243 Ah lithium iron phosphate battery fires. Process Saf. Environ. Prot. 2023, 177, 138–151. [Google Scholar] [CrossRef]
- Xu, C.; Ouyang, M.; Lu, L.; Liu, X.; Wang, S.; Feng, X. Preliminary Study on the Mechanism of Lithium Ion Battery Pack under Water Immersion. ECS Trans. 2017, 77, 209. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Q.; Li, K.; Ping, P.; Sun, J. The combustion behavior of large scale lithium titanate battery. Sci. Rep. 2015, 5, 7788. [Google Scholar] [CrossRef]
- Shuang, W.; Zhi-ming, D.U.; Ze-lin, Z.; Zhi-yue, H.A.N. Research progress on safety of lithium-ion batteries. Chin. J. Eng. 2018, 40, 901–909. [Google Scholar] [CrossRef]
- Spinner, N.S.; Field, C.R.; Hammond, M.H.; Williams, B.A.; Myers, K.M.; Lubrano, A.L.; Rose-Pehrsson, S.L.; Tuttle, S.G. Physical and chemical analysis of lithium-ion battery cell-to-cell failure events inside custom fire chamber. J. Power Sources 2015, 279, 713–721. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, G.; Duan, Q.; Chen, M.; Li, Y.; Wu, K.; Liu, B.; Peng, P.; Sun, J. The Efficiency of Heptafluoropropane Fire Extinguishing Agent on Suppressing the Lithium Titanate Battery Fire. Fire Technol. 2016, 52, 387–396. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, P.; Duan, Q.; Zhao, C.; Wang, Q. Experimental investigation on the cooling and suppression effects of liquid nitrogen on the thermal runaway of lithium ion battery. J. Power Sources 2021, 495, 229795. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, K.; Sun, J.; Wang, Q. A Review of Fire-Extinguishing Agents and Fire Suppression Strategies for Lithium-Ion Batteries Fire. Fire Technol. 2024, 60, 817–858. [Google Scholar] [CrossRef]
- Yu, H.-Z.; Liu, X. An Efficacy Evaluation of Water Mist Protection Against Solid Combustible Fires in Open Environment. Fire Technol. 2019, 55, 343–361. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Li, H.; Sun, J.; Wang, Q. Experimental study on a novel safety strategy of lithium-ion battery integrating fire suppression and rapid cooling. J. Energy Storage 2020, 28, 101185. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Duan, Q.; Chen, M.; Xu, J.; Zhao, C.; Sun, J.; Wang, Q. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Gao, J.; He, Z.; Yao, S.; Zhou, X.; Zhang, H. In situ extinguishing mechanism and performance of self-portable microcapsule fire extinguishing agent for lithium-ion batteries. J. Energy Storage 2024, 93, 112393. [Google Scholar] [CrossRef]
- Kruzel, M.; Bohdal, T.; Dutkowski, K. External Condensation of HFE 7000 and HFE 7100 Refrigerants in Shell and Tube Heat Exchangers. Materials 2021, 14, 6825. [Google Scholar] [CrossRef]
- Lu, D.; Chao, M.; Zhou, X. Theoretical Studies on the Reactions of 1,1,2,2,3,3,4-Heptafluorocyclopentane with Hydroxyl and Hydrogen Free Radicals. Chin. J. Chem. 2014, 32, 897–908. [Google Scholar] [CrossRef]
- Adiga, K.C.; Hatcher, R.F.; Sheinson, R.S.; Williams, F.W.; Ayers, S. A computational and experimental study of ultra fine water mist as a total flooding agent. Fire Saf. J. 2007, 42, 150–160. [Google Scholar] [CrossRef]
- Joseph, P.; Nichols, E.; Novozhilov, V. A comparative study of the effects of chemical additives on the suppression efficiency of water mist. Fire Saf. J. 2013, 58, 221–225. [Google Scholar] [CrossRef]
- Rahim, R.; Abdul Raman, A.A. Carbon dioxide emission reduction through cleaner production strategies in a recycled plastic resins producing plant. J. Clean. Prod. 2017, 141, 1067–1073. [Google Scholar] [CrossRef]
- Yoshida, A.; Kashiwa, K.; Hashizume, S.; Naito, H. Inhibition of counterflow methane/air diffusion flame by water mist with varying mist diameter. Fire Saf. J. 2015, 71, 217–225. [Google Scholar] [CrossRef]
- Zhang, T.; Han, Z.; Du, Z.; Zhang, Z.; Liu, K. Application of thermal mechanism to evaluate the effectiveness of the extinguishment of CH4/air cup-burner flame by water mist with additives. Int. J. Hydrog. Energy 2016, 41, 15078–15088. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, C.; Zhou, Y.; Zhang, R.; Zhang, J.; Liu, Y.; Qian, X. The extinguishment mechanisms of a micelle encapsulator F-500 on lithium-ion battery fires. J. Energy Storage 2022, 55, 105186. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; He, T.; You, T.; Parker, T.; Wang, Q. Suppression behavior of water mist containing compound additives on lithium-ion batteries fire. Process Saf. Environ. Prot. 2022, 161, 476–487. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, J.; Wang, Z.; Wang, J.; Bai, W. Inhibition of thermal runaway in lithium-ion batteries by fine water mist containing a low-conductivity compound additive. J. Clean. Prod. 2022, 340, 130841. [Google Scholar] [CrossRef]
- Lee, D.H.; Kwon, S.; Kim, Y.E.; Kim, N.Y.; Joo, J.B. Double-Layered Polymer Microcapsule Containing Non-Flammable Agent for Initial Fire Suppression. Materials 2022, 15, 7831. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, T.; Yuan, S.; Chang, C.; Wang, K.; Song, Z.; Qian, X. Patent-based technological developments and surfactants application of lithium-ion batteries fire-extinguishing agent. J. Energy Chem. 2024, 88, 39–63. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Du, J.; Tian, J.; Li, Y.; Zhao, Y.; Wu, H.; Zhong, Y.; Cao, Y.-C.; Cheng, S. Fabrication of a microcapsule extinguishing agent with a core–shell structure for lithium-ion battery fire safety. Mater. Adv. 2021, 2, 4634–4642. [Google Scholar] [CrossRef]
- Eremenko, L.T.; Zhitomirskaya, N.G. Identification of the vibration frequency of the C-F bond in fluoronitro compounds. Bull. Acad. Sci. USSR Div. Chem. Sci. 1973, 22, 2407–2410. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Zhivulin, V.E. Controlled modification of polyvinylidene fluoride as a way for carbyne synthesis. Polym. Degrad. Stab. 2022, 203, 110054. [Google Scholar] [CrossRef]
- Goel, N.; Kumari, P.; Gunjan; Phillips, A.; Bhagat, S. Recent Advances in Fluorination Reactions via De-Carboxylative and De-Oxygenative Strategies: A Perspective. Chem. Rec. 2025, e202500068. [Google Scholar] [CrossRef]
- Zhivulin, V.E.; Pesin, L.A.; Belenkov, E.A.; Greshnyakov, V.A.; Zlobina, N.; Brzhezinskaya, M. Ageing of chemically modified poly(vinylidene fluoride) film: Evolution of triple carbon-carbon bonds infrared absorption. Polym. Degrad. Stab. 2020, 172, 109059. [Google Scholar] [CrossRef]
| Material | Molecular Weight [g/mol] | Boiling Point [°C] | Melting Point [°C] | Liquid Density [g/mL] | Vapor Pressure [mmHg] | ODP | GWP | Dielectric Constant [KV] | Heat of Vaporization [KJ/kg] |
|---|---|---|---|---|---|---|---|---|---|
| F7A | 196 | 82.5 | 21 | 1.58 | 11.8 | 0.0 | 250 | ≥110 | 144 |
| MNFB | 250 | 60 | −135 | 1.52 | 202 | 0.0 | 297 | ≥25 | 163 |
| Item | Specification |
|---|---|
| Battery Model | 18,650 |
| Battery Type | lithium-ion battery (NCM) |
| Weight | 48.5 ± 0.5 g |
| Nominal voltage | 3.65 V |
| State of Charge (SOC) | 100% |
| End Voltage (cut off) | 2.75 V |
| Max Charge Voltage | 4.20 V |
| Battery Capacity | 3500 mAh |
| Agent | Battery Type | Cost | Reusability | Actual Effect | References |
|---|---|---|---|---|---|
| Liquid nitrogen | 18,650-type | High | No | Liquid nitrogen can effectively cool LIB and suppress TR, without having a significant impact on the cycling performance of LIB. | [30] |
| C6F12O + water mist | LiFePO4 | High | No | The flame of the battery was extinguished by C6F12O within 1 s, but its cooling effect was poor. When used in combination with water mist, it significantly prolonged the propagation time of TR in the battery, up to 183 s. | [34] |
| HFC-227ea + water mist | LiFePO4 | Low | No | HFC-227ea failed to extinguish the flame, but it effectively reduced the fire intensity. When used in combination with fine water mist, it extinguished the flame within 120 s. | [34] |
| 3% F-500 + water mist | 18,650-type | Low | No | No re-ignition was observed after the application of 3% F-500 in combination with fine water mist. | [43] |
| Dry powders | 18,650-type | Average | No | The battery fire can be briefly extinguished by the continuous discharge of dry powder for 45 s, but it reignites after 5 s. | [20] |
| F7A + MNFB + water mist | 18,650-type | Low | No | Compared with pure fine water mist, the battery temperature decreases 66 s faster from its peak to a lower temperature, and the peak temperature of the high-temperature material above the battery is reduced by 228.2 °C. | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, Z.; Gao, J.; Guo, Y.; Zhang, H.; Wang, M. External Barrier and Internal Attack: Synergistic Effect of Microcapsule Fire Extinguishing Agent and Fine Water Mist on Suppressing Lithium-Ion Battery Fire. Materials 2025, 18, 3082. https://doi.org/10.3390/ma18133082
Wang X, He Z, Gao J, Guo Y, Zhang H, Wang M. External Barrier and Internal Attack: Synergistic Effect of Microcapsule Fire Extinguishing Agent and Fine Water Mist on Suppressing Lithium-Ion Battery Fire. Materials. 2025; 18(13):3082. https://doi.org/10.3390/ma18133082
Chicago/Turabian StyleWang, Xiangjian, Zhanwen He, Jianjun Gao, Yibo Guo, Haijun Zhang, and Mingchao Wang. 2025. "External Barrier and Internal Attack: Synergistic Effect of Microcapsule Fire Extinguishing Agent and Fine Water Mist on Suppressing Lithium-Ion Battery Fire" Materials 18, no. 13: 3082. https://doi.org/10.3390/ma18133082
APA StyleWang, X., He, Z., Gao, J., Guo, Y., Zhang, H., & Wang, M. (2025). External Barrier and Internal Attack: Synergistic Effect of Microcapsule Fire Extinguishing Agent and Fine Water Mist on Suppressing Lithium-Ion Battery Fire. Materials, 18(13), 3082. https://doi.org/10.3390/ma18133082







