Stoichiometry of Bulk Nb1−βSnβ Superconductors Synthesised by Arc Melting
Abstract
1. Introduction
2. Materials and Methods
- A pulse tube cryocooler with base temperature below 3 K and cooling power 0.5–1 W at 4.2 K;
- A high-stability 20 bit magnet power supply;
- A 5 Tesla magnet;
- A variable temperature sample space of 25 mm ID, 100 mm long isothermal region;
- A cryocooler for both the magnet and the sample space so that neither liquid nitrogen nor liquid helium was required.
3. Results
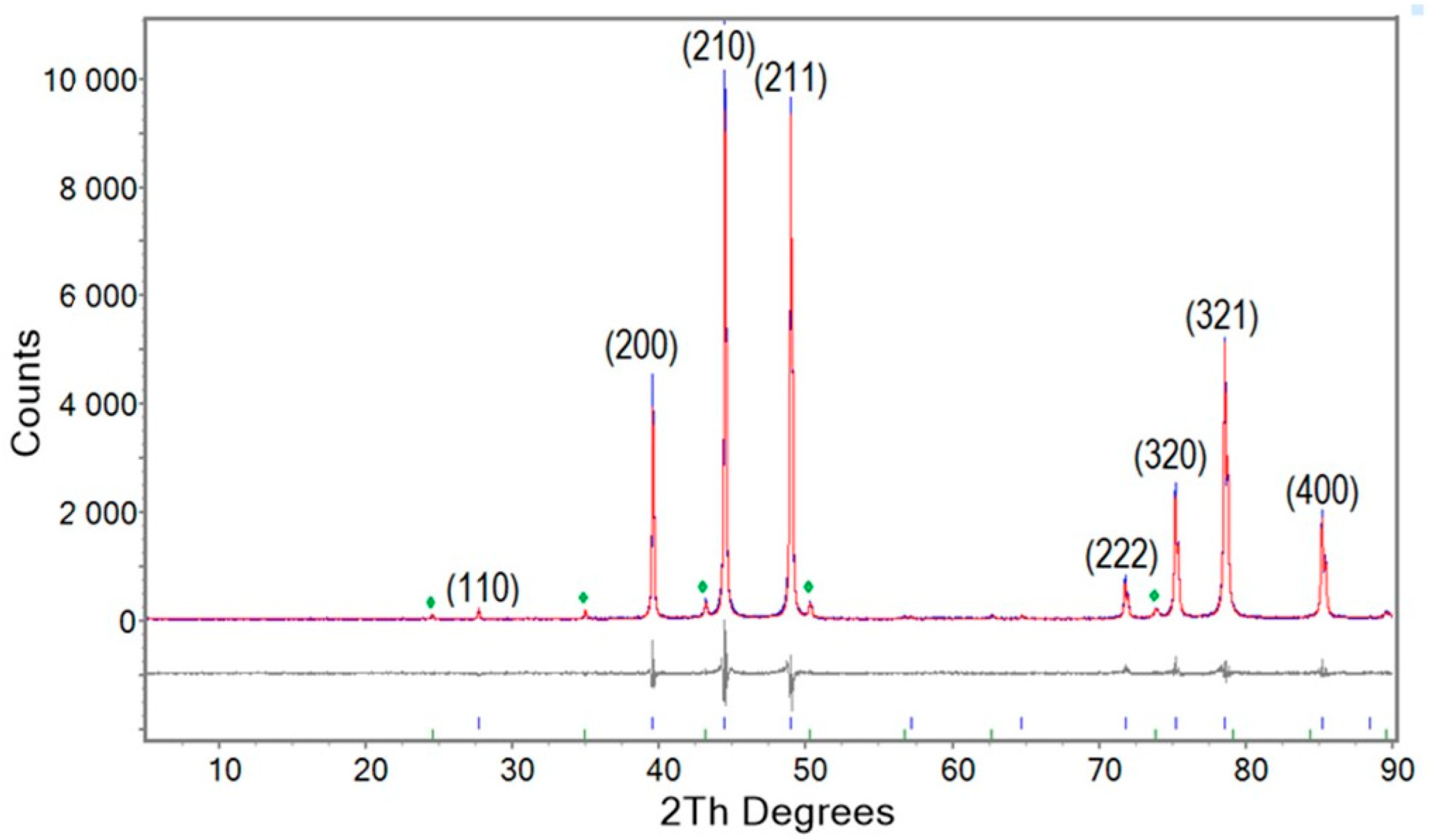
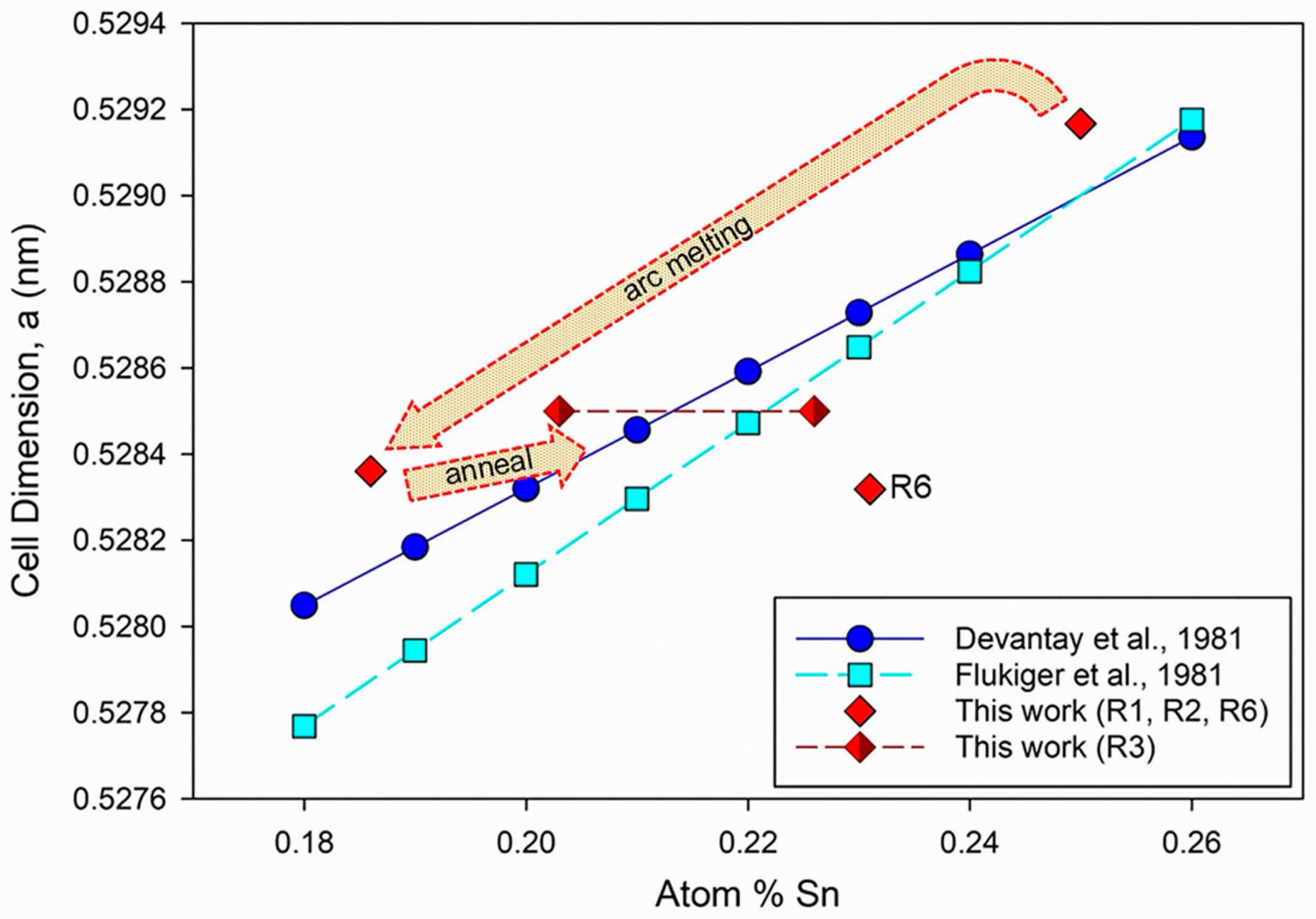
3.1. Crystallography and Phase Relations
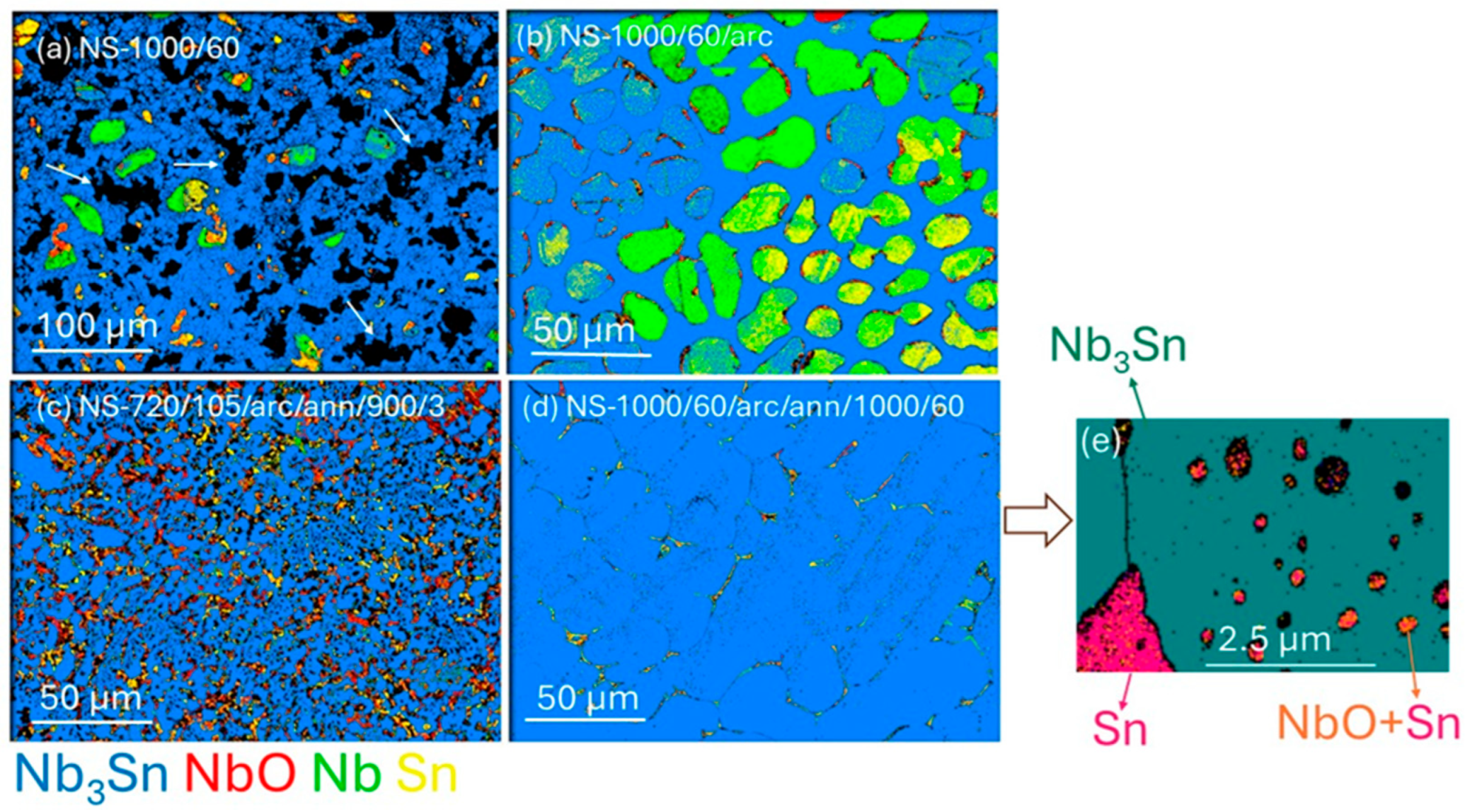
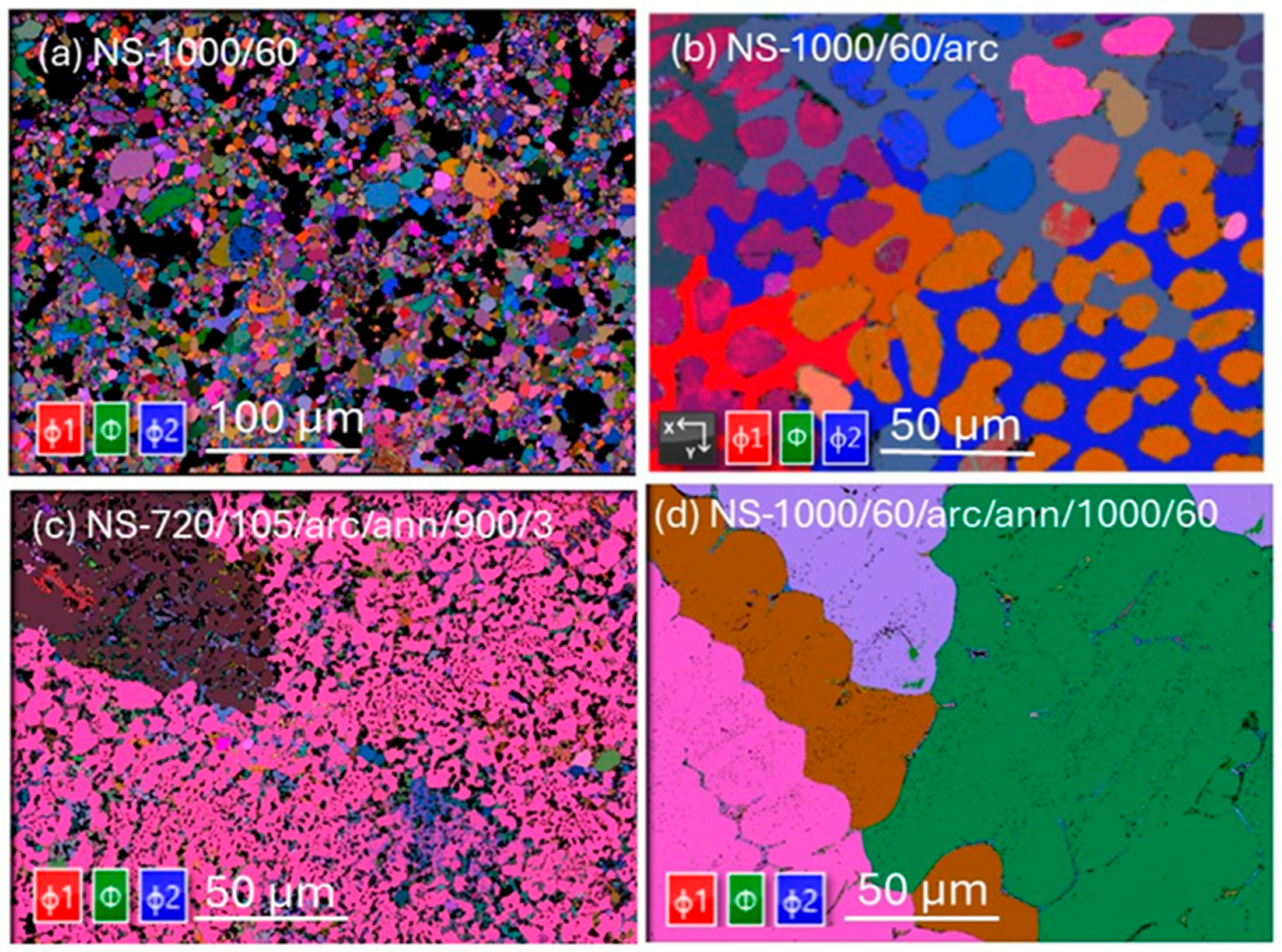
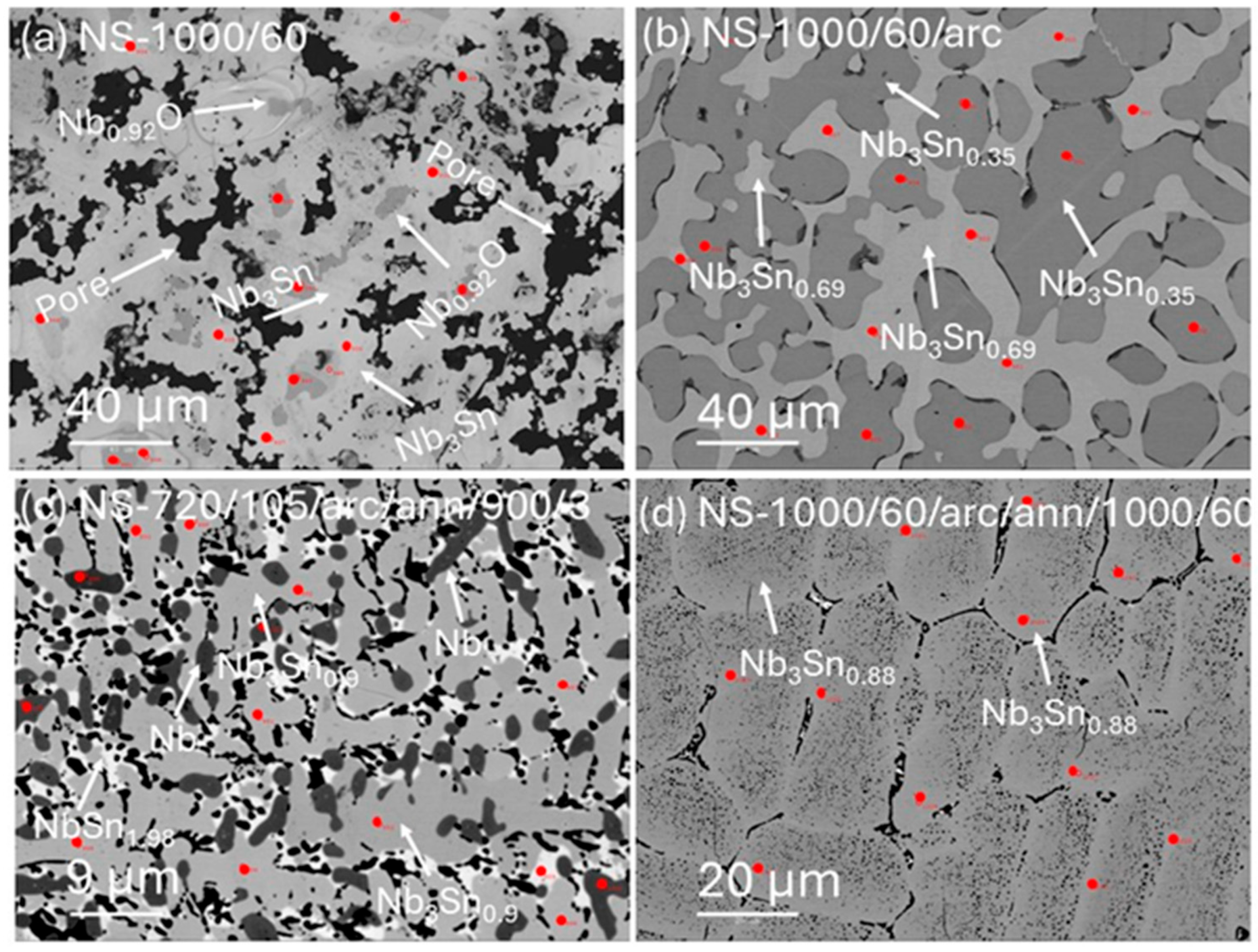
3.2. Microstructures and Grain Compositions
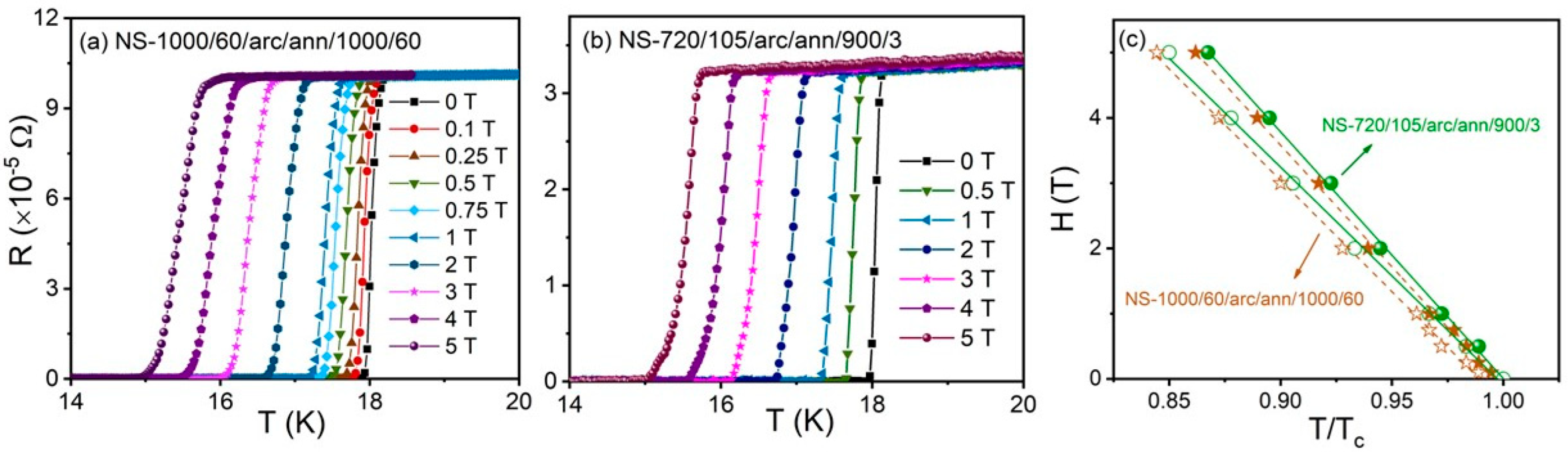
3.3. Electrical and Magnetic Properties
4. Discussion
4.1. Structure and Composition
4.2. Superconductivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthias, B.T.; Geballe, T.H.; Geller, S.; Corenzwit, E. Superconductivity of Nb3Sn. Phys. Rev. 1954, 95, 1435. [Google Scholar] [CrossRef]
- Miyazaki, T.; Matsukura, N.; Miyatake, T.; Shimada, M.; Takabatake, K.; Itoh, K.; Kiyoshi, T.; Sato, A.; Inoue, K.; Wada, H. Development of Bronze-Processed Nb3Sn Superconductors for 1 GHz NMR Magnets. In Advances in Cryogenic Engineering Materials; Balachandran, U.B., Gubser, D.G., Hartwig, K.T., Reed, R.P., Warnes, W.H., Bardos, V.A., Eds.; Springer: Boston, MA, USA, 1998; pp. 935–941. [Google Scholar]
- Tommasini, D. Nb3Sn Accelerator Dipole Magnet Needs for a Future Circular Collider. In Nb3Sn Accelerator Magnets: Designs, Technologies and Performance; Schoerling, D., Zlobin, A.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 427–439. [Google Scholar]
- Mitchell, N.; Breschi, M.; Tronza, V. The use of Nb3Sn in fusion: Lessons learned from the ITER production including options for management of performance degradation. Supercond. Sci. Technol. 2020, 33, 054007. [Google Scholar] [CrossRef]
- Godeke, A. A review of the properties of Nb3Sn and their variation with A15 composition, morphology and strain state. Supercond. Sci. Technol. 2006, 19, R68–R80. [Google Scholar] [CrossRef]
- Sun, W.; Cheng, J.; Chen, S. Preparation and Superconducting Properties of Nb3Sn by Mechanical Alloying. J. Low Temp. Phys. 2021, 205, 100–111. [Google Scholar] [CrossRef]
- Flükiger, R.; Uglietti, D.; Senatore, C.; Buta, F. Microstructure, composition and critical current density of superconducting Nb3Sn wires. Cryogenics 2008, 48, 293–307. [Google Scholar] [CrossRef]
- Devantay, H.; Jorda, J.L.; Decroux, M.; Muller, J.; Flükiger, R. The physical and structural properties of superconducting A15-type Nb-Sn alloys. J. Mater. Sci. 1981, 16, 2145–2153. [Google Scholar] [CrossRef]
- Flükiger, R.; Schauer, W.; Specking, W.; Schmidt, B.; Springer, E. Low-Temperature Phase-Transformation in Nb3Sn Multifilamentary Wires and the Strain Dependence of their Critical Current-Density. IEEE Trans. Magn. 1981, 17, 2285–2288. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Q.; Wang, H.; Dai, Y. Microstructure evolution of Nb3Sn superconductors during diffusion treatment by bronze route. Rare Met. 2012, 31, 446–450. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Q.; Wang, C.G.; Chen, J.Y.; Li, Z.; Shi, Y.G.; Liu, X.H.; Feng, Y.; Yan, G.; Li, J.F.; et al. Investigation on the Recrystallization and Nb3Sn Microstructure of Nb Alloys with Large Deformation During Heat Treatment. IEEE Trans. Appl. Supercond. 2024, 34, 5. [Google Scholar] [CrossRef]
- Banno, N. Low-temperature superconductors: Nb3Sn, Nb3Al, and NbTi. Superconductivity 2023, 6, 100047. [Google Scholar] [CrossRef]
- Goldacker, W.; Ahrens, R.; Nindel, M.; Obst, B.; Meingast, C. HIP Sythesized Nb3Sn Bulk Materials with Extraordinary Homogeneity. IEEE Trans. Appl. Supercond. 1993, 3, 1322–1325. [Google Scholar] [CrossRef]
- Zhou, J.; Jo, Y.; Hawn Sung, Z.; Zhou, H.; Lee, P.J.; Larbalestier, D.C. Evidence that the upper critical field of Nb3Sn is independent of whether it is cubic or tetragonal. Appl. Phys. Lett. 2011, 99, 122507. [Google Scholar] [CrossRef]
- Jain, D.; Sudarsan, V.; Tyagi, A.K. Synthesis of Metallic Materials by Arc Melting Technique. In Handbook on Synthesis Strategies for Advanced Materials: Volume-I: Techniques and Fundamentals; Tyagi, A.K., Ningthoujam, R.S., Eds.; Springer: Singapore, 2021; pp. 197–213. [Google Scholar]
- Kirschen, M.; Hay, T.; Echterhof, T. Process Improvements for Direct Reduced Iron Melting in the Electric Arc Furnace with Emphasis on Slag Operation. Processes 2021, 9, 402. [Google Scholar] [CrossRef]
- Singh, R. (Ed.) Chapter 5—Production of Steel. In Applied Welding Engineering, 2nd ed.; Butterworth-Heinemann: Waltham, MA, USA, 2016; pp. 37–55. [Google Scholar]
- Konushkin, S.V.; Kolmakov, A.G.; Nasakina, E.O.; Sergienko, K.V.; Sevost’yanov, M.A. Production of a Ti-20 at % Nb-5 at % Ta Alloy in an Argon Arc Furnace. Russ. Metall. 2022, 2022, 569–574. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Song, K.X. Improving the mechanical properties of WC/Cu composites through vacuum consumable arc melting. Mater. Lett. 2025, 384, 137966. [Google Scholar] [CrossRef]
- Gonçalves, V.R.M.; Lisboa, P.N.; Afonso, C.R.M. Unravelling microstructure of novel as-cast in-situ a Ti and β Ti-Nb alloy matrix composites with NbC addition. Mater. Lett. 2023, 349, 134794. [Google Scholar] [CrossRef]
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO—V2.42—A Fast and Easy to Use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef]
- Polichetti, M.; Galluzzi, A.; Kumar, R.; Goyal, A. Equation for Calculation of Critical Current Density Using the Bean’s Model with Self-Consistent Magnetic Units to Prevent Unit Conversion Errors. Materials 2025, 18, 269. [Google Scholar] [CrossRef]
- Xu, X.C. A review and prospects for Nb3Sn superconductor development. Supercond. Sci. Technol. 2017, 30, 093001. [Google Scholar] [CrossRef]
- Jewell, M.C.; Godeke, A.; Lee, P.J.; Larbalestier, D.C. The upper critical field of stoichiometric and off-stoichiometric bulk, binary Nb3Sn. In Proceedings of the Joint Cryogenic Engineering Conference/International Cryogenic Materials Conference (CEC-ICMC), Anchorage, AK, USA, 22–26 September 2003; pp. 474–481. [Google Scholar]
- Ding, H.; Gao, Y. Analysis of the strain dependence of the superconducting critical properties of single-crystal and polycrystalline Nb3Sn. Supercond. Sci. Technol. 2021, 34, 075006. [Google Scholar] [CrossRef]
- Sumption, M.D.; Bhartiya, S.; Kovacks, C.; Peng, X.; Gregory, E.; Tomsic, M.J.; Collings, E.W. Critical current density and stability of Tube Type Nb3Sn conductors. Cryogenics 2012, 52, 91–99. [Google Scholar] [CrossRef]
- Xu, X.C.; Sumption, M.D.; Peng, X. Internally Oxidized Nb3Sn Strands with Fine Grain Size and High Critical Current Density. Adv. Mater. 2015, 27, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.; Yang, S.T.; Shen, J.K.; Zhang, J.S.; Liu, F.H. Coexistence of superconductivity and charge density wave instability in A15-Nb3Sn. Phys. Chem. Chem. Phys. 2023, 25, 32452–32459. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Eremeev, G.V.; Zuazo, J.R.; Lee, J.Y.; Luo, B.; Martinello, M.; Romanenko, A.; Posen, S. Selective Thermal Evolution of a Native Oxide Layer in Nb and Nb3Sn-Coated SRF Grade Nb: An In Situ XPS Study. J. Phys. Chem. C 2023, 127, 19705–19716. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Baumgartner, T.; Löffler, S.; Stöger-Pollach, M.; Hopkins, S.C.; Ballarino, A.; Eisterer, M.; Bernardi, J. Analysis of inhomogeneities in Nb3Sn wires by combined SEM and SHPM and their impact on Jc and Tc. Supercond. Sci. Technol. 2023, 36, 045008. [Google Scholar] [CrossRef]
- Arul, K.T.; Chang, H.W.; Shiu, H.W.; Dong, C.L.; Pong, W.F. A review of energy materials studied by in situ/operando synchrotron x-ray spectro-microscopy. J. Phys. D Appl. Phys. 2021, 54, 343001. [Google Scholar] [CrossRef]
- Magdy, M. A Brief Account of the Technology Significance of Synchrotron Radiation: A State-of-the-art Angle-Resolved Photoemission Spectroscopy (ARPES). Curr. Anal. Chem. 2025, 21, 1–9. [Google Scholar] [CrossRef]
- Qin, L.; Du, W.J.; Cipiccia, S.; Bodey, A.J.; Rau, C.; Mi, J.W. Synchrotron X-ray operando study and multiphysics modelling of the solidification dynamics of intermetallic phases under electromagnetic pulses. Acta Mater. 2024, 265, 119593. [Google Scholar] [CrossRef]
- Foner, S.; Schwartz, B. Superconductor Materials Science: Metallurgy, Fabrication, and Applications; Springer: New York, NY, USA, 1981. [Google Scholar]
- Welch, D.O.; Dienes, G.J.; Lazareth, O.W.; Hatcher, R.D. Defects and diffusion mechanisms in Nb3Sn. J. Phys. Chem. Solids 1984, 45, 1225–1242. [Google Scholar] [CrossRef]
- Besson, R.; Guyot, S.; Legris, A. Atomic-scale study of diffusion in A15 Nb3Sn. Phys. Rev. B 2007, 75, 054105. [Google Scholar] [CrossRef]
- Heald, S.M.; Tarantini, C.; Lee, P.J.; Brown, M.D.; Sung, Z.; Ghosh, A.K.; Larbalestier, D.C. Evidence from EXAFS for Different Ta/Ti Site Occupancy in High Critical Current Density Nb3Sn Superconductor Wires. Sci. Rep. 2018, 8, 4798. [Google Scholar] [CrossRef] [PubMed]
- Sitaraman, N.S.; Kelley, M.M.; Porter, R.D.; Liepe, M.U.; Arias, T.A.; Carlson, J.; Pack, A.R.; Transtrum, M.K.; Sundararaman, R. Effect of the density of states at the Fermi level on defect free energies and superconductivity: A case study of Nb3Sn. Phys. Rev. B 2021, 103, 115106. [Google Scholar] [CrossRef]
- Carlson, J.; Pack, A.; Transtrum, M.K.; Lee, J.; Seidman, D.N.; Liarte, D.B.; Sitaraman, N.S.; Senanian, A.; Kelley, M.M.; Sethna, J.P. Analysis of magnetic vortex dissipation in Sn-segregated boundaries in Nb3Sn superconducting RF cavities. Phys. Rev. B 2021, 103, 024516. [Google Scholar] [CrossRef]
- Xu, X.; Peng, X.; Rochester, J.; Lee, J.-Y.; Sumption, M. High critical current density in internally-oxidized Nb3Sn superconductors and its origin. Scr. Mater. 2020, 186, 317–320. [Google Scholar] [CrossRef]
- Banno, N.; Morita, T.; Yu, Z.; Yagai, T.; Tachikawa, K. Effect of Zn addition and Ti doping position on the diffusion reaction of internal tin Nb3Sn conductors. Supercond. Sci. Technol. 2019, 32, 115017. [Google Scholar] [CrossRef]
- Qu, P.S.; Li, S.L.; Liu, G.B.; Sun, X.G.; Xu, M.; Liu, L.; Chen, Y.L.; Wang, W.T.; Zhang, Y.; Feng, Y.; et al. Critical current density enhancement of Nb3Sn superconducting wires by incorporating oxygen through rapid-heating method. Mater. Lett. 2023, 341, 134157. [Google Scholar] [CrossRef]
- Scanlan, R.M.; Fietz, W.A.; Koch, E.F. Flux pinning centers in superconducting Nb3Sn. J. Appl. Phys. 2008, 46, 2244–2249. [Google Scholar] [CrossRef]
- Talantsev, E.F.; Valova-Zaharevskaya, E.G.; Deryagina, I.L.; Popova, E.N. Characteristic Length for Pinning Force Density in Nb3Sn. Materials 2023, 16, 5185. [Google Scholar] [CrossRef]
- Suenaga, M.; Jansen, W. Chemical compositions at and near the grain boundaries in bronze-processed superconducting Nb3Sn. Appl. Phys. Lett. 1983, 43, 791–793. [Google Scholar] [CrossRef]
- Sandim, M.J.R.; Tytko, D.; Kostka, A.; Choi, P.; Awaji, S.; Watanabe, K.; Raabe, D. Grain boundary segregation in a bronze-route Nb3Sn superconducting wire studied by atom probe tomography. Supercond. Sci. Technol. 2013, 26, 055008. [Google Scholar] [CrossRef]

| Run | Sample ID | Sintering Condition | Arc-Melt | Post Arc-Melt Heat Treatment |
|---|---|---|---|---|
| 1 | NS-1000/60 | 1000 °C/60 h | No | No |
| 2 | NS-1000/60/arc | 1000 °C/60 h | Yes | No |
| 3 | NS-1000/60/arc/ann/1000/60 | 1000 °C/60 h | Yes | 1000 °C/60h |
| 4 | NS-720/105 | 720 °C/105 h | No | No |
| 5 | NS-720/105/arc | 720 °C/105 h | Yes | No |
| 6 | NS-720/105/arc/ann/900/3 | 720 °C/105 h | Yes | 900 °C/3h |
| Run | Sample ID | Nb3Sn (%) | NbO (%) | Nb (%) | Unit Cell * (Å) | Rwp (%) | gof |
|---|---|---|---|---|---|---|---|
| 1 | NS-1000/60 | 94.69 | 3.57 | 1.74 | 5.29167(3) | 13.8 | 1.5 |
| 2 | NS-1000/60/arc | 85.16 | 4.80 | 10.04 | 5.28360(5) | 13.5 | 1.6 |
| 3 | NS-1000/60/arc/ann/1000/60 | 94.88 | 5.12 | - | 5.28510(4) | 12.2 | 1.5 |
| 6 | NS-720/105/arc/ann/900/3 | 85.99 | 3.49 | 10.52 | 5.28318(6) | 12.5 | 1.2 |
| Sample | n ** | Element Atom % | Totals (wt%) | Stoichiometry | |||||
|---|---|---|---|---|---|---|---|---|---|
| Nb | Sn | O | Total | Nb | Sn | O | |||
| NS-1000/60 (R1) | |||||||||
| Nb3Sn1.00 | 21 | 75.0 (2) | 25.0 (2) | - | 100 | 98.7 (4) | 3 | 1.00 (1) | - |
| Nb0.92O | 9 | 47.9 (7) | - | 52.1 (7) * | 100 | 100 | 0.92 | - | 1 |
| Nb | 5 | 100.0 | - | - | 100 | 1 | - | - | |
| NS-1000/60/arc (R2) | |||||||||
| Nb3Sn0.69 | 20 | 81.4 (1) | 18.6 (1) | - | 100 | 99.6 (3) | 3 | 0.69 (1) | - |
| Nb3Sn0.35 | 20 | 89.6 (2) | 10.4 (2) | - | 100 | 99.5 (2) | 3 | 0.35 (1) | - |
| NS-1000/60/arc/ann/1000/60 (R3) | |||||||||
| Nb3Sn0.88 | 9 | 77.4 (6) | 22.6 (6) | - | 100 | 99.8 (2) | 3 | 0.88 (3) | - |
| Nb3Sn0.77 | 10 | 79.7 (4) | 20.3 (4) | - | 100 | 99.7 (3) | 3 | 0.77 (2) | - |
| NS-720/105/arc/ann/900/3 (R6) | |||||||||
| Nb3Sn0.90 | 21 | 76.9 (3) | 23.1 (3) | - | 100 | 99.8 (2) | 3 | 0.90 (2) | - |
| Nb1Sn1.98 | 5 | 33.6 (4) | 66.4 (4) | - | 100 | 98.7 (4) | 1 | 1.98 (3) | - |
| Sn (at %) (Calc) [39] | Tc (K) (Calc) [39] | Tc (K) (Experiment) [39] | Sn (at %) (This Work) | Tc (K) (This Work) |
|---|---|---|---|---|
| 18.75 | 9.2 | 6 | 18.60 | 6.78 * |
| 20.83 | 11.3 | 9.5 | 22.59 | 16.26 * |
| 23.44 | 16.1 | 16 | 23.09 | 16.45 * |
| 25.00 | 18.2 | 18 | 25.02 | 16.58 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbazi, M.; Cathey, H.E.; Manshadi, A.D.; Alarco, J.; Mackinnon, I.D.R. Stoichiometry of Bulk Nb1−βSnβ Superconductors Synthesised by Arc Melting. Materials 2025, 18, 3050. https://doi.org/10.3390/ma18133050
Shahbazi M, Cathey HE, Manshadi AD, Alarco J, Mackinnon IDR. Stoichiometry of Bulk Nb1−βSnβ Superconductors Synthesised by Arc Melting. Materials. 2025; 18(13):3050. https://doi.org/10.3390/ma18133050
Chicago/Turabian StyleShahbazi, Mahboobeh, Henrietta E. Cathey, Ali Dehghan Manshadi, Jose Alarco, and Ian D. R. Mackinnon. 2025. "Stoichiometry of Bulk Nb1−βSnβ Superconductors Synthesised by Arc Melting" Materials 18, no. 13: 3050. https://doi.org/10.3390/ma18133050
APA StyleShahbazi, M., Cathey, H. E., Manshadi, A. D., Alarco, J., & Mackinnon, I. D. R. (2025). Stoichiometry of Bulk Nb1−βSnβ Superconductors Synthesised by Arc Melting. Materials, 18(13), 3050. https://doi.org/10.3390/ma18133050








