Preparation of Cu-Containing Substances via an Ultrasonic-Assisted Solvothermal Approach and Their Catalytic Effects on the Thermal Decomposition of Ammonium Perchlorate
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Cu-Containing Substances
2.2.1. Preparation of Cu3(BTC)2
2.2.2. Preparation of Cu Powder
2.2.3. Preparation of Cu@AC
2.3. Characterization
3. Results and Discussion
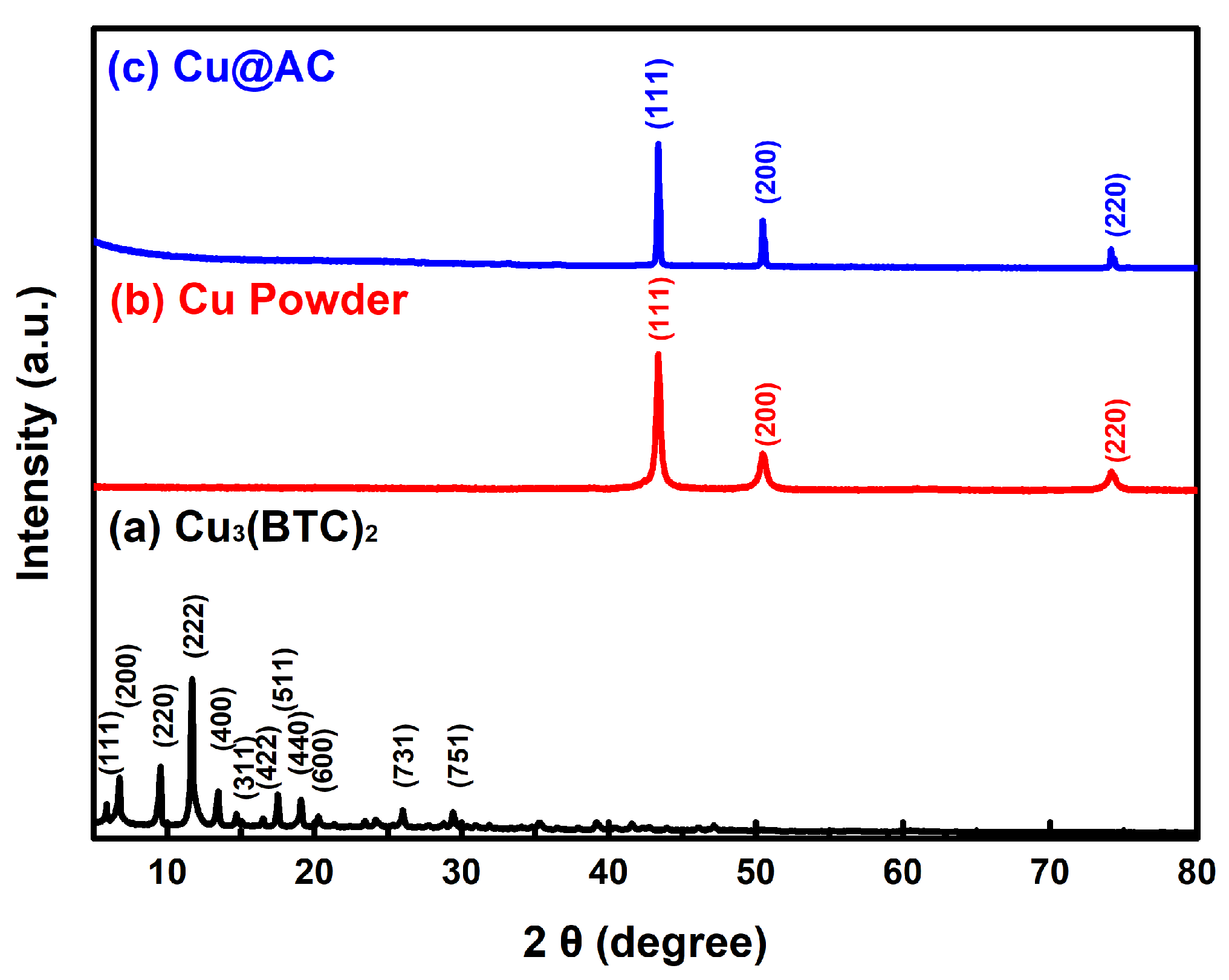
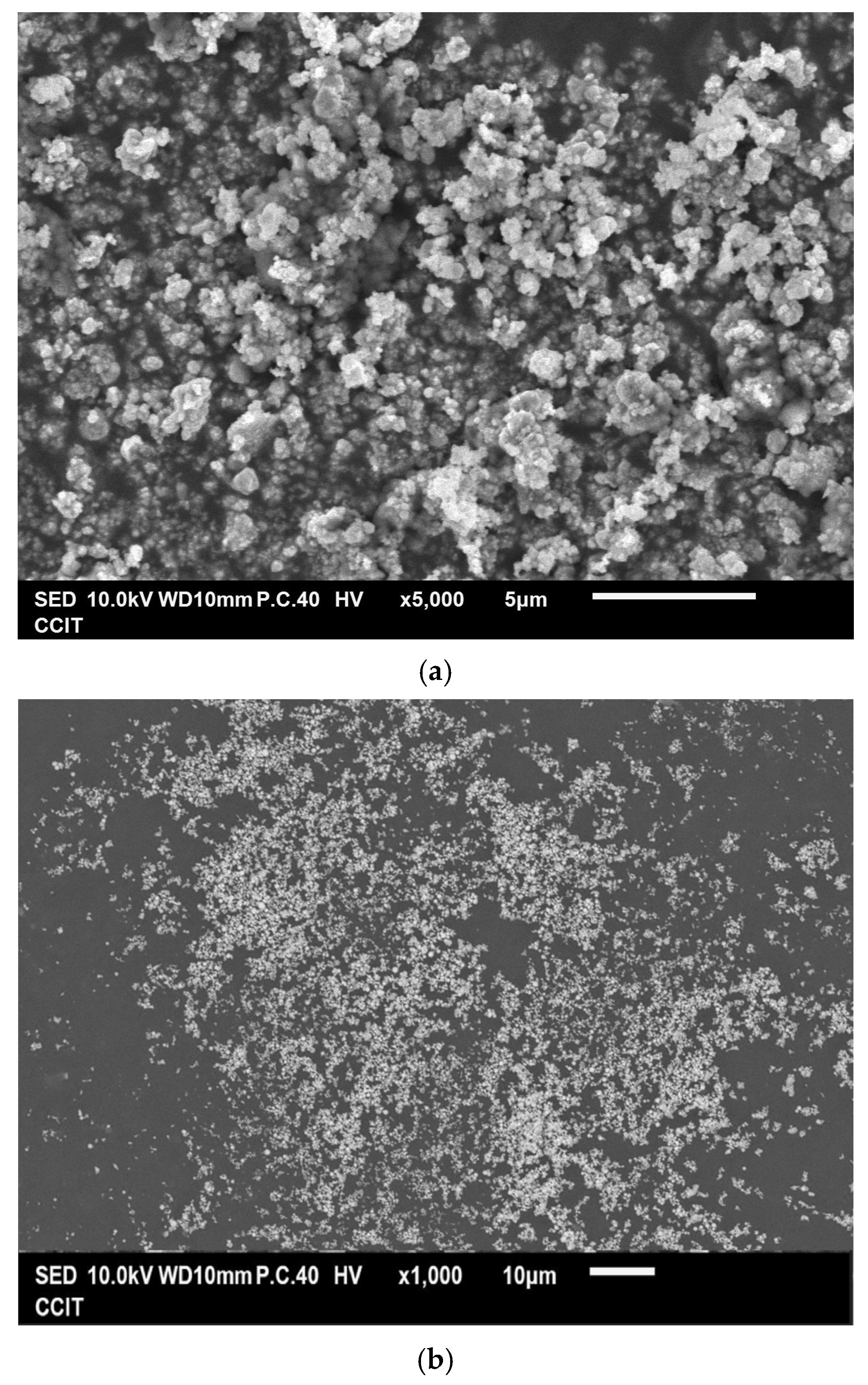
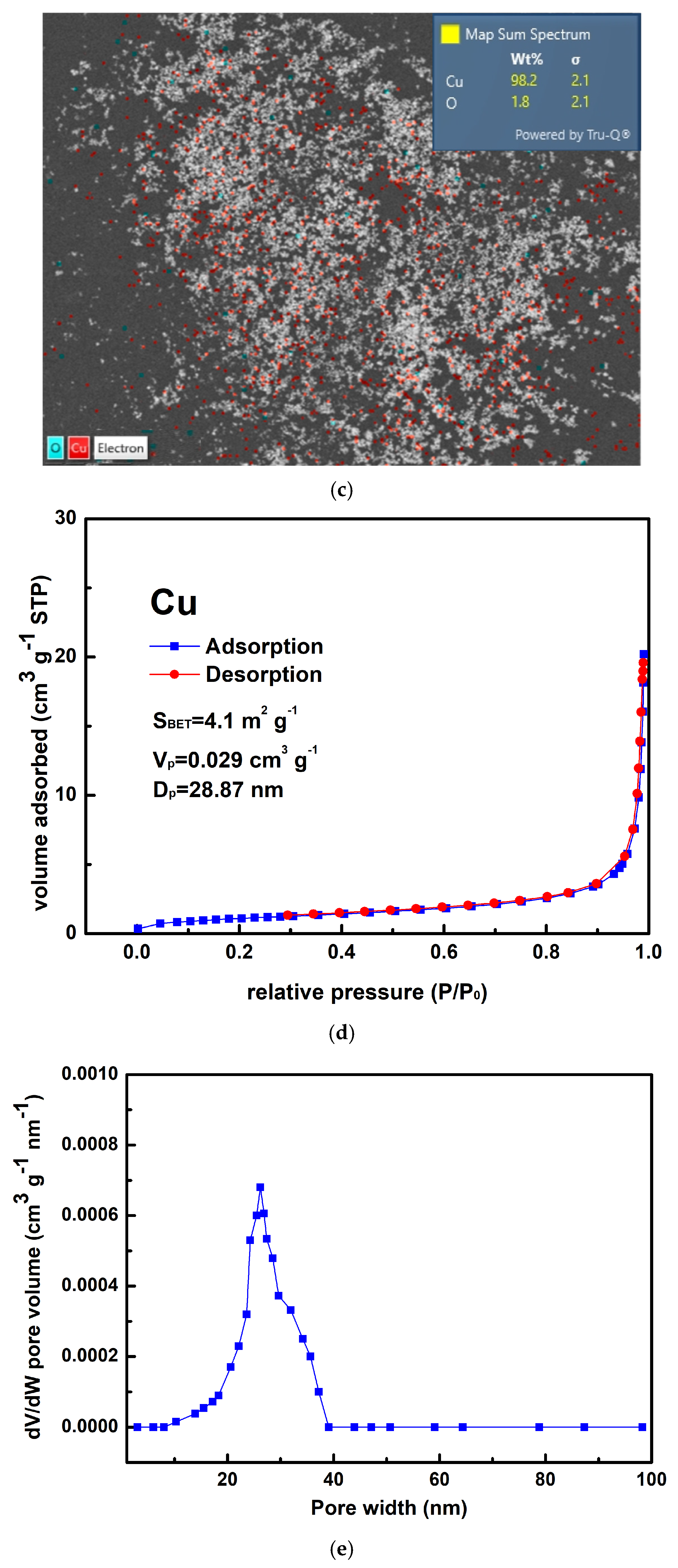
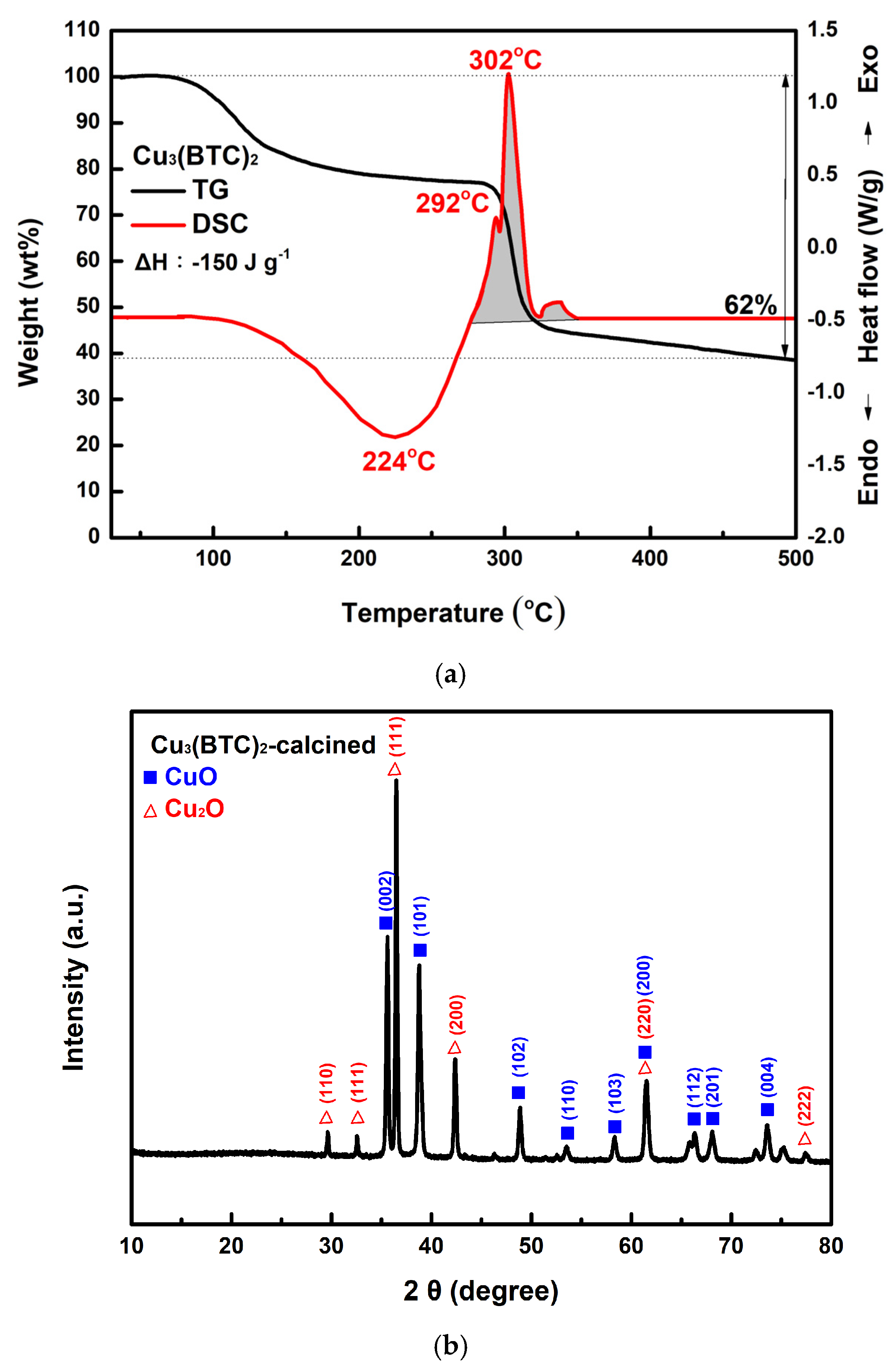
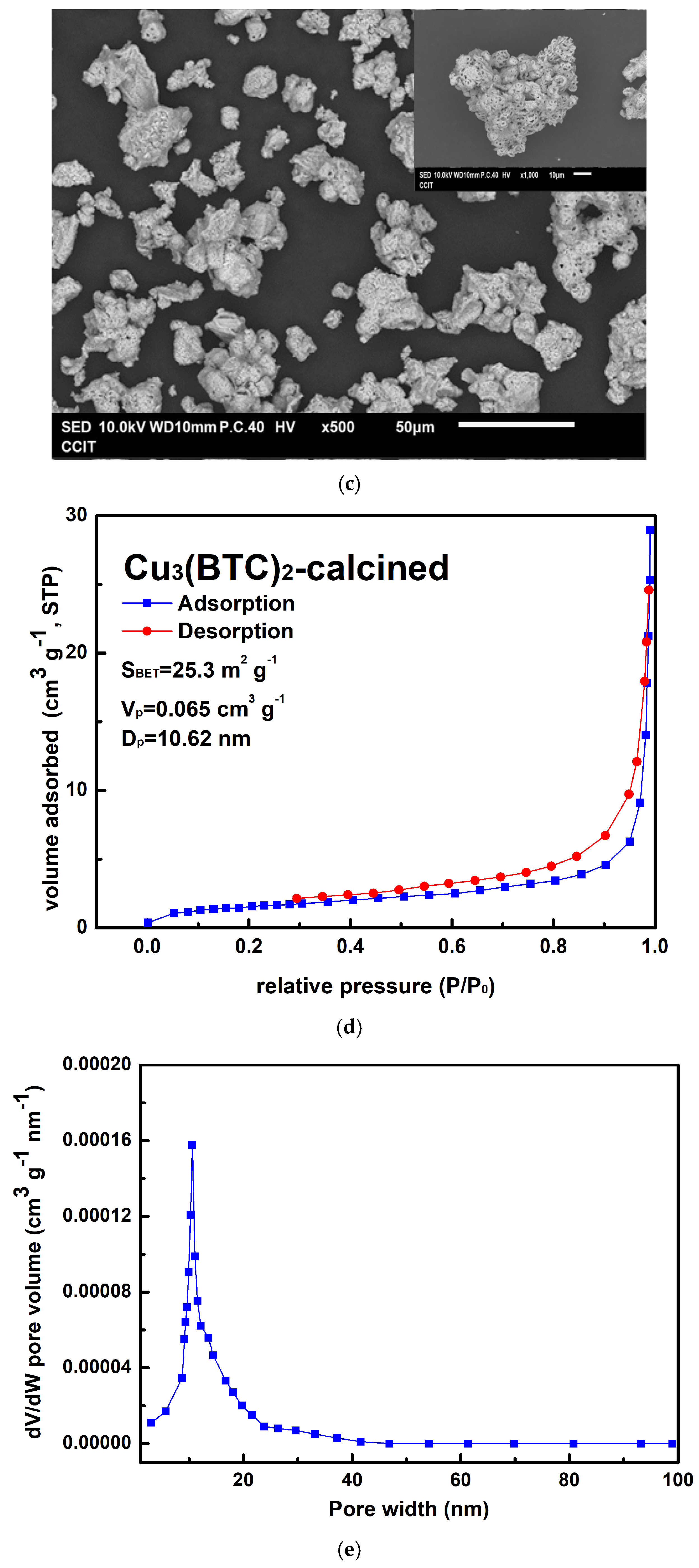
3.1. Characterization of the Resultant Products
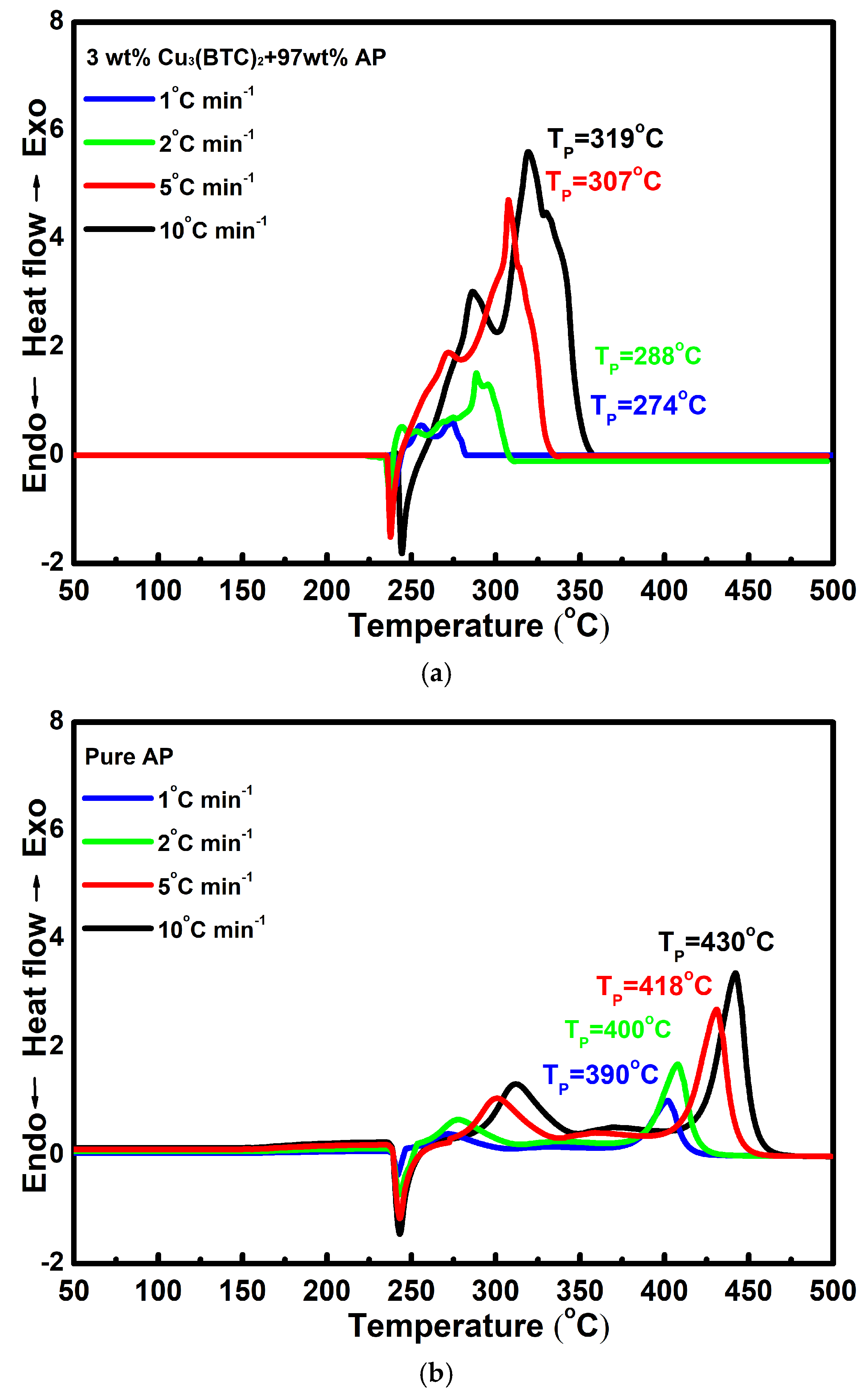
3.2. Test of Catalytic Effect
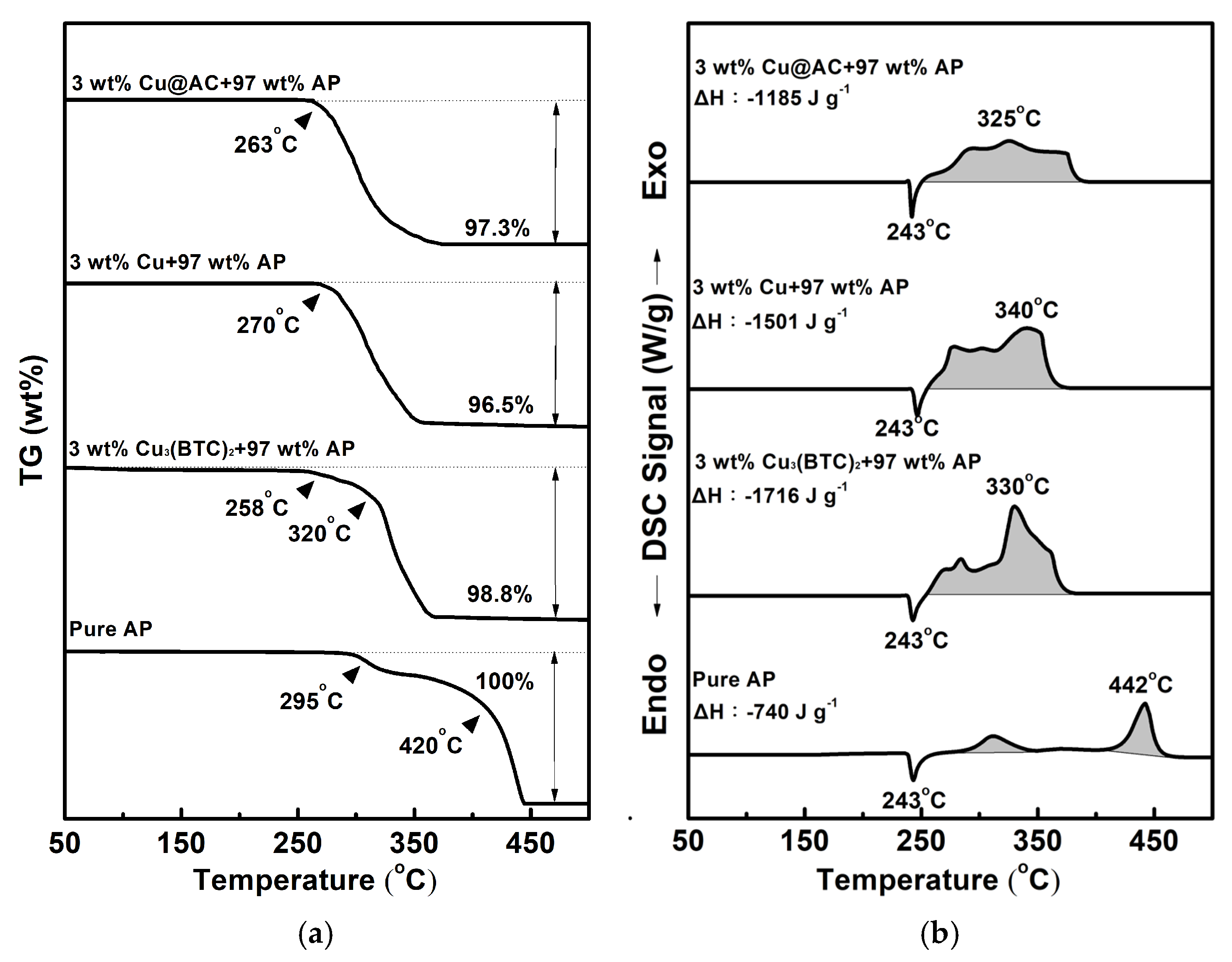
3.3. Thermal Behavior of Cu3(BTC)2 and Properties of the Resulting Residue After Thermolysis
3.4. EGA for Thermal Reaction of 3 wt% Cu3(BTC)2+97 wt% AP
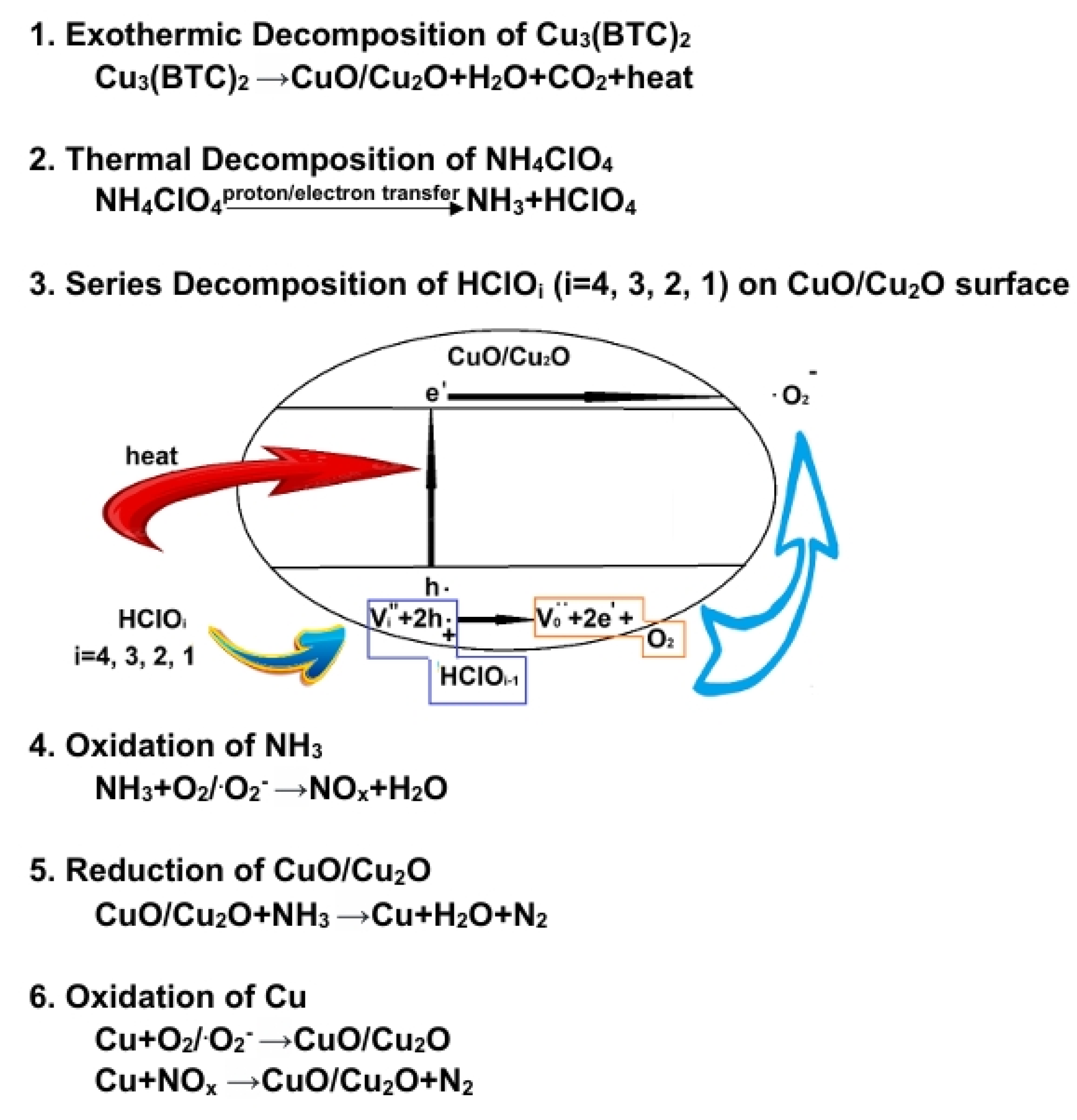
3.5. Possible Catalytic Mechanism
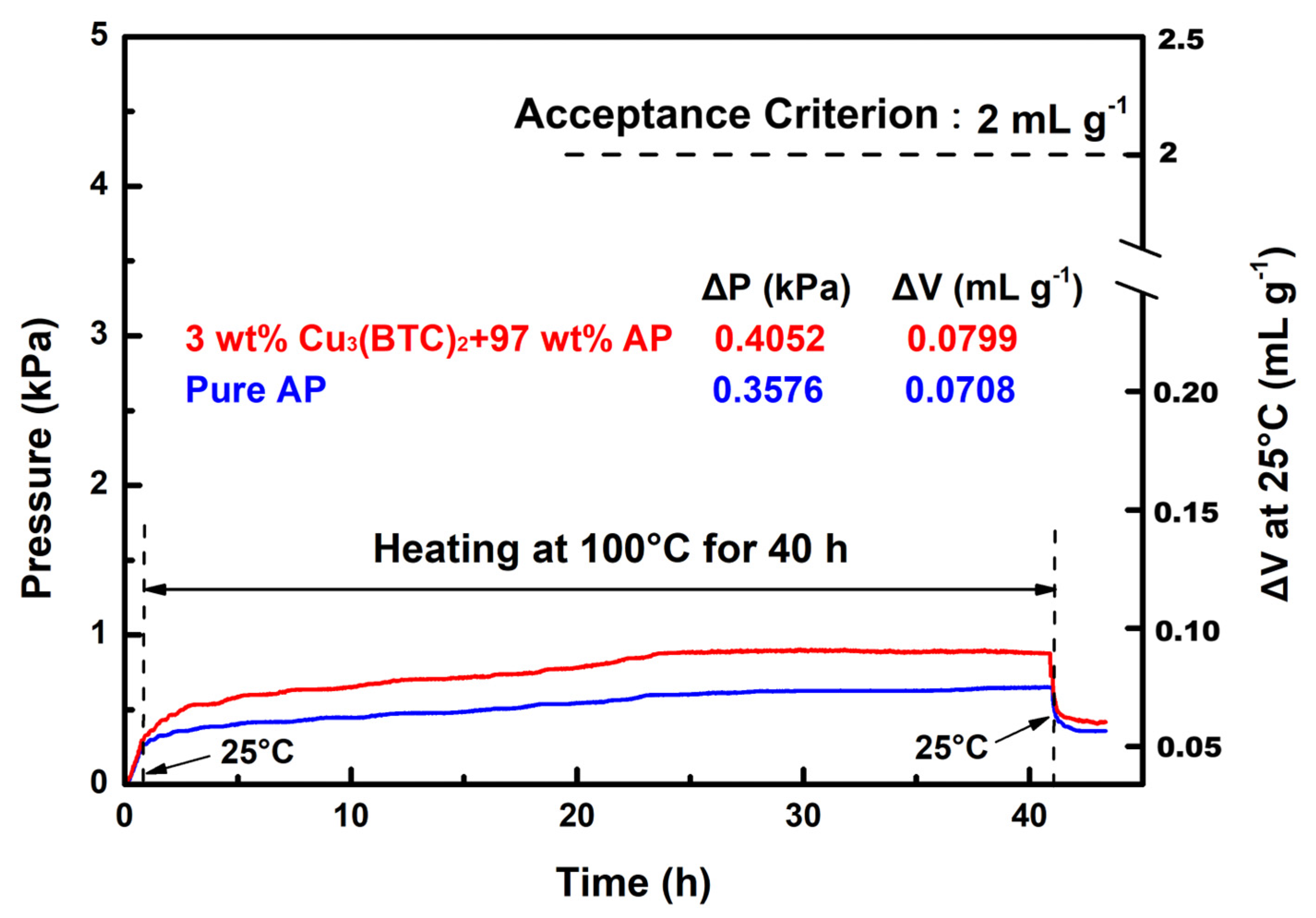
3.6. Compatibility Between AP and Cu3BTC2
4. Conclusions
- (1)
- A one-pot, ultrasonic-assisted solvothermal method was successfully employed to prepare three copper-containing substances: Cu3(BTC)2, fine copper powder, and Cu@AC. These materials exhibited significant catalytic effects on the thermal decomposition of AP, reducing the initiation temperature and enhancing heat release.
- (2)
- The observed catalytic activity can be attributed to these substances’ appropriate specific surface area and porous nature, which provide numerous active sites for redox reactions. The heat generated from the interactions between the copper-containing substances and AP facilitates proton transfer. Furthermore, the resulting composites of metallic copper and copper oxides serve as effective media for electron transfer. These characteristics collectively promote AP’s thermal decomposition into reactive gaseous species. Notably, the regeneration of metallic copper and copper oxides ensures the maintenance of catalytic efficiency during the pyrolysis of AP.
- (3)
- Cu3(BTC)2 demonstrated the most effective catalytic performance among the three substances evaluated. At a concentration of 3 wt%, it reduced the thermal decomposition temperature of AP by approximately 112 °C and significantly increased heat release by a factor of ~2.5. The Ea for the thermal decomposition reaction was approximately 128 kJ mol−1, approximately 80 kJ mol−1 lower than that of pure AP. This standardized VST confirmed the favorable compatibility between AP and Cu3(BTC)2. These findings suggest that Cu3(BTC)2 has considerable potential as an additive for AP-based high-energy materials.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics, 1st ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 209–330. [Google Scholar]
- Schmidt, E.W. Encyclopedia of Oxidizers, 1st ed.; De Gruyter: Berlin, Germany, 2022; pp. 3383–3880. [Google Scholar]
- Strawson, J. Ammonium Perchloride. In Encyclopedia of Toxicology; Wexler, P., Ed.; Academic Press: Cambridge, UK, 2005; pp. 105–107. [Google Scholar]
- Yan, Q.-L.; Zhao, F.-Q.; Kuo, K.K.; Zhang, X.-H.; Zeman, S.; DeLuca, L.T. Catalytic effects of nano additives on decomposition and combustion of RDX-, HMX-, and AP-based energetic compositions. Prog. Energy Combus. Sci. 2016, 57, 75–136. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Thermal decomposition of ammonium perchlorate. Thermochim. Acta 2006, 443, 1–36. [Google Scholar] [CrossRef]
- Liu, L.; Li, F.; Tan, L.; Ming, L.; Yi, Y. Effects of Nanometer Ni, Cu, Al and NiCu Powders on the Thermal Decomposition of Ammonium Perchlorate. Propellants Explos. Pyrotech. 2004, 29, 34–38. [Google Scholar] [CrossRef]
- Gao, J.-m.; Wang, L.; Yu, H.-j.; Xiao, A.-g.; Ding, W.-b. Recent Research Progress in Burning Rate Catalysts. Propellants Explos. Pyrotech. 2011, 36, 404–409. [Google Scholar] [CrossRef]
- Tong, R.; Zhou, Y.; Wang, L.; Yu, H.; Ren, F.; Saleem, M.; Amer, W.A. Recent research progress in the synthesis and properties of burning rate catalysts based on ferrocene-containing polymers and derivatives. J. Organomet. Chem. 2014, 755, 16–32. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Zhao, W.; Fang, H.; Wu, C.; Wang, D. Enhanced decomposition of laminated ammonium perchlorate composite. Sci. Rep. 2021, 11, 22622. [Google Scholar] [CrossRef]
- Zao, M.; Jiang, X.; Lu, L.; Wang, X. Nano or micro? A mechanism on thermal decomposition of ammonium perchlorate catalyzed by cobalt oxalate. J. Hazard. Mater. 2012, 225–226, 124–130. [Google Scholar]
- Vaka, P.R.; Rathi, N.; Ramakrishna, P.A. Experimental investigation of erosion rate of insulation materials using hybrid rockets. FirePhysChem 2021, 1, 222–230. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Shkineva, T.K.; Vatsadze, I.A.; Kormanov, A.V.; Kozeev, A.M.; Suponitsky, K.Y.; Pivnika, A.N.; Sheremetev, A.B. Novel energetic CNO oxidizer: Pernitro-substituted pyrazolyl-furazan framework. FirePhysChem 2021, 1, 83–89. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Chen, S.; Wang, W.; Xu, K. In situ synthesis and catalytic decomposition mechanism of CuFe2O4/g-C3N4 nanocomposite on AP and RDX. J. Anal. Appl. Pyrol. 2021, 160, 105372. [Google Scholar]
- Duan, H.; Lin, X.; Liu, G.; Xu, L.; Li, F. Synthesis of Ni nanoparticles and their catalytic effect on the decomposition of ammonium perchlorate. J. Mater. Process. Technol. 2008, 208, 494–498. [Google Scholar] [CrossRef]
- Chandrababu, P.; Thankaraian, J.; Nair, V.S.; Raghavan, R. Decomposition of ammonium perchlorate: Exploring catalytic activity of nanocomposites based on nano Cu/Cu2O dispersed on graphitic carbon nitride. Thermovhim. Acta 2020, 691, 178720. [Google Scholar] [CrossRef]
- Rodríguez-Pesina, M.; García-Domínguez, J.; García-Hernández, F.; Flores-Vélez, L.M.; Domínguez, O. The Thermal Decomposition of Ammonium Perchlorate-Aluminum Propellants in Presence of Metallic Zinc Particles. Mater. Sci. Appl. 2017, 8, 436–447. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X. Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate. Catalysts 2025, 15, 354. [Google Scholar] [CrossRef]
- Dubey, R.; Chawla, M.; Siril, P.F.; Singh, G. Bi-metallic nanocomposites of Mn with very high catalytic activity for burning rate enhancement of composite solid propellants. Thermovhim. Acta 2013, 572, 30–38. [Google Scholar] [CrossRef]
- Ma, Z.; Li, F.; Bai, H. Effect of Fe2O3 in Fe2O3/AP Composite Particles on Thermal Decomposition of AP and on Burning Rate of the Composite Propellant. Propellants Explos. Pyrotech. 2006, 31, 447–451. [Google Scholar] [CrossRef]
- Heng, B.; Xiao, T.; Hu, X.; Yuan, M.; Tao, W.; Huang, W.; Tang, Y. Catalytic activity of Cu2O micro-particles with different morphologies in the thermal decomposition of ammonium perchlorate. Thermovhim. Acta 2011, 542, 135–139. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Abazari, R.; Zavi, A. Pure CuCr2O4 nanoparticles: Synthesis, characterization and their morphological and size effects on the catalytic thermal decomposition of ammonium perchlorate. Solid State Sci. 2014, 37, 72–79. [Google Scholar] [CrossRef]
- Han, X.; Zhou, L.; Cao, S.; Zhang, L.; Xiang, G.; Chen, J.-F. Exploring the Roles of ZIF-67 as an Energetic Additive in the Thermal Decomposition of Ammonium Perchlorate. Energy Fuels 2021, 35, 4447–4456. [Google Scholar] [CrossRef]
- Feng, C.; Ye, B.; Cheng, W.; Shi, S.; Zhao, F.; An, C.; Wang, J. Microflower-like Fe-Co-MOF with enhanced catalytic performance for decomposition of ammonium perchlorate and combustion of ammonium perchlorate-based composite propellants. Case Stud. Therm. Eng. 2024, 56, 104281. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Kaliaguine, S.; Gobara, M.; Elbasuney, S.; Boto, D.C. Microwave-Assisted Synthesis of the Flexible Iron-based MIL-88B Metal-Organic Framework for Advanced Energetic Systems. J. Inorg. Organomet. Polym. 2022, 32, 2538–2556. [Google Scholar] [CrossRef]
- Tan, B.; Yang, X.; Dou, J.; Duan, B.; Lu, X.; Liu, N. Research progress of EMOFs-based burning rate catalysts for solid propellants. Front. Chem. 2022, 10, 1032163. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Hao, W.; Jin, B.; Zhang, J.; Guo, J.; Luo, L.; Zhang, Q.; Peng, R. Novel energetic coordination compound [Cu(AT)4]Cl2 for catalytic thermal decomposition of ammonium perchlorate. J. Solid State Chem. 2021, 304, 122622. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, S.; Yang, H.; Li, S.; Xie, W.; Zhao, Y.; Zhang, W.; Chen, Y.; Fan, X. Application of 3D energetic metal-organic frameworks containing Cu as the combustion catalyst to composite solid propellant. Combust. Flame 2021, 225, 57–64. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, M.; Zhao, F.; Li, H.; Zuo, Y.; Li, R. Catalytic Performances of rGO-MFe2O4 (M=Ni, Co and Zn) for Pyrolysis of Ammonium Perchlorate. In Proceedings of the 2021 International Conference on Development and Application of Carbon Nanomaterials in Energetic Materials, Wuhan, China, 19–20 August 2021; Gany, A., Fu, X., Eds.; Springer: Singapore, 2022; pp. 227–234. [Google Scholar]
- Liang, T.; Song, R.; Chen, C.; Alomar, T.S.; Xiao, F.; AlMasoud, N.; El-Bahy, Z.M.; Yang, Y.; Algadi, H.; Sun, L. Graphene oxide–supported Cu/Co nano-catalysts for thermal decomposition of ammonium perchlorate composites. Adv. Compos. Hybrid Mater. 2023, 6, 188. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, X.; Li, J.; Xiao, L.; Gao, H.; Zhao, F.; Ma, H. Progress on the application of graphene-based composites toward energetic materials: A review. Def. Technol. 2024, 31, 95–116. [Google Scholar] [CrossRef]
- Li, S.; Niu, Z.; Jiao, Y.; Jin, P.; Yang, D.; Bai, C.; Liu, J.; Li, G.; Luo, Y. Preparation of different morphology Cu/GO nanocomposites and their catalytic performance for thermal decomposition of ammonium perchlorate. RSC Adv. 2022, 12, 22806–22814. [Google Scholar] [CrossRef]
- Soleiman-Beigi, M.; Mohammadi, M.; Kohzadi, H. An overview on copper in industrial chemistry: From ancient pigment to modern catalysis. Coord. Chem. Rev. 2025, 529, 216438. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Velidandi, A.; Rao, G.V.S.N. Nano-sized copper particles: Chemical synthesis, characterization, and their size and surface charge dependent antibacterial potential. Results Chem. 2024, 7, 101258. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal–organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef]
- Fievet, F.; Fievet-Vincent, F.; Lagier, J.-P.; Dumont, B.; Figlarz, M. Controlled nucleation and growth of micrometer-size copper particles prepared by the polyol process. J. Mater. Chem. 1993, 3, 627–632. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Zhuang, C.; Zhang, M.; Gholami, P.; Khataee, A. Sonochemical synthesis of photocatalysts and their applications. J. Mater. Sci. Technol. 2022, 123, 243–256. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic synthesis of the microporous metal-organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. Mater. Int. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of Copper Nanoparticles in Ethylene Glycol by Chemical Reduction with Vanadium (+2) Salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef]
- Zhu, H.-t.; Zhang, C.-y.; Yin, Y.-s. Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradiation. J. Cryst. Growth 2004, 270, 722–728. [Google Scholar] [CrossRef]
- Li, J.-S.; Chen, J.-J.; Hwang, C.-C.; Lu, K.-T.; Yeh, T.-F. Study on Thermal Characteristics of TNT Based Melt-Cast Explosives. Propellants Explos. Pyrotech. 2019, 44, 1270–1281. [Google Scholar] [CrossRef]
- MIL-STD-1751 A; Department of Defense Test Method Standard: Safety and Performance Tests for the Qualification of Explosives (High explosives, Propellants, and Pyrotechnics). Department of Defense: Washington, DC, USA, 2001.
- Yang, T.-M.; Lai, J.-T.; Li, W.-H.; Peng, C.-H.; Lu, K.-T. Study on synthesis and characterization of spherical copper(I) 5-nitrotetrazolate (DBX-1). Propellants Explos. Pyrotech. 2023, 48, e202300226. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Shaheen, M.; Khan, M.W.; Siddique, S.; Aftab, S.; Wabaidur, S.M.; Iqbal, M.J. Exploring MOF-199 composites as redox-active materials for hybrid battery-supercapacitor devices. ROC Adv. 2023, 13, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-K.; Hundal, G.; Jang, I.T.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S. Microwave synthesis of hybrid inorganic–organic materials including porous Cu3(BTC)2 from Cu(II)-trimesate mixture. Microporous Mesoporous Mater. 2009, 119, 331–337. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Criado, J.M.; Pérez-Maqueda, L.A. Kissinger kinetic analysis of data obtained under different heating schedules. J. Therm. Anal. Calorim. 2008, 94, 427–432. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Gupta, N.K.; Bae, J.; Kim, K.S. From MOF-199 Microrods to CuO Nanoparticles for Room-Temperature Desulfurization: Regeneration and Repurposing Spent Adsorbents as Sustainable Approaches. ACS Omega 2021, 6, 25631–25641. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall: Hoboken, NJ, USA, 2001; pp. 96–102. [Google Scholar]
- Wang, J.; Zhang, W.; Zheng, Z.; Gao, Y.; Ma, K.; Ye, J.; Yang, Y. Enhanced thermal decomposition properties of ammonium perchlorate through addition of 3DOM core-shell Fe2O3/Co3O4 composite. J. Alloys Compd. 2017, 724, 720–727. [Google Scholar] [CrossRef]
- Li, N.; Cao, M.; Wu, Q.; Hu, C. A facile one-step method to produce Ni/graphene nanocomposites and their application to the thermal decomposition of ammonium perchlorate. CrystEngComm 2012, 14, 428–434. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, D. Effects of different phases of MnO2 nanorods on the catalytic thermal decomposition of ammonium perchlorate. Ceram. Int. 2015, 41, 7054–7058. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Abazari, R. A facile one-step route for production of CuO, NiO, and CuO–NiO nanoparticles and comparison of their catalytic activity for ammonium perchlorate decomposition. RSC Adv. 2015, 5, 96777–96784. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Song, Y.; Li, C.; Cheng, W.; Xu, C.; Yun, N.; An, C. Thermal decomposition reaction mechanism and combustion performance of AlH3/AP energetic composite. Case Stud. Therm. Eng. 2022, 38, 102317. [Google Scholar] [CrossRef]
- Balık, M.; Bulut, V.; Erdogan, I.Y. Optical, structural and phase transition properties of Cu2O, CuO and Cu2O/CuO: Their photoelectrochemical sensor applications. Int. J. Hydrogen Energy 2019, 44, 18744–18755. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Wisz, G.; Rybak-Wilusz, E.; Cholewa, M. Numerical analysis and optimization of Cu2O/TiO2, CuO/TiO2, heterojunction solar cells using SCAPS. J. Phys. Conf. Ser. 2018, 1033, 012002. [Google Scholar] [CrossRef]
- Moakhar, R.S.; Hosseini-Hosseinabad, S.M.; Masudy-Panah, S.; Seza, A.; Jalali, M.; Fallah-Arani, H.; Dabir, F.; Gholipour, S.; Abdi, Y.; Bagheri-Hariri, M.; et al. Photoelectrochemical Water-Splitting Using CuO-Based Electrodes for Hydrogen Production: A Review. Adv. Mater. 2021, 33, 2007285. [Google Scholar] [CrossRef]
- Khiavi, N.D.; Katal, R.; Eshkalak, S.K.; Masudy-Panah, S.; Ramakrishna, S.; Hu, J. Visible Light Driven Heterojunction Photocatalyst of CuO-Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, F.; Qin, Z.; Zhang, M.; Li, N.; Jiang, Z. The effect of oxygen vacancy defects in nano-CuO1-x on its catalytic properties in ammonium perchlorate thermal decomposition. Mater. Today Commun. 2023, 36, 106648. [Google Scholar] [CrossRef]
- Levy, J.B. The Thermal Decomposition of Perchloric Acid Vapor. J. Phys. Chem. 1962, 66, 1092–1097. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 14th ed.; McGraw-Hill: New York, NY, USA, 1992; pp. 6.1–6.123. [Google Scholar]






| Product | Reactant Cu(NO3)2·3H2O Used | Theoretical Production Amount/Content | Actual Amount/Content Obtained * | Yield |
|---|---|---|---|---|
| Cu3(BTC)2 | 1.812 g | 1.512 g | 1.240 ± 0.052 g | 82 ± 3% |
| Cu Powder | 2.416 g | 0.635 g | 0.401 ± 0.033 g | 63 ± 5% |
| Cu@AC | 0.242 g | 6.0 wt% | 4.5 ± 0.3 wt% | 75 ± 5% |
| Additive | Content (wt%) | ΔTp * (°C) | ΔH (J g−1) | Percent Decrease in Ea (%) | Reference |
|---|---|---|---|---|---|
| Cu3(BTC)2 | 3 | 112 | 1716 | 39% | This work |
| ZIF-67 | 5 | 90 | 2282 | 56% | [22] |
| Fe-Co-MOF | 5 | 90 | 1213 | 15% | [23] |
| MIL-88B | - | 71 | 1218 | 24% | [24] |
| Reaction | 260 °C | 400 °C | ||
|---|---|---|---|---|
| ΔrH0(kJ) | ΔrG0(kJ) | ΔrH0(kJ) | ΔrG0(kJ) | |
| I. Oxidation of NH3 | ||||
| 2NH3(g) + 2O2(g) → N2O(g) + 3H2O(g) | −535.90 | −531.19 | −525.91 | −519.96 |
| 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g) | −859.94 | −955.92 | −833.98 | −955.22 |
| 4NH3(g) + 7O2(g) → 4NO2(g) + 6H2O(g) | −1085.7 | −1025.2 | −1055.8 | −979.46 |
| II. Reduction of CuO | ||||
| 3CuO(s) + 2NH3(g) → 3Cu(s) + N2(g) + 3H2O(g) | −334.54 | −367.31 | −327.85 | −428.62 |
| III. Oxidation of Cu | ||||
| 2Cu(s) + O2(g) → 2CuO(s) | −294.78 | −196.02 | −282.92 | −158.22 |
| Cu(s) + N2O(g) → CuO(s) + N2(g) | −238.04 | −228.16 | −237.54 | −225.10 |
| 2Cu(s) + 2NO(g) → 2CuO(s) + N2(g) | −491.36 | −379.40 | −487.92 | −346.56 |
| 4Cu(s) + 2NO2(g) → 4CuO(s) + N2(g) | −673.26 | −540.78 | −659.94 | −492.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-H.; Su, P.-H.; Li, J.-S.; Ke, Y.-J. Preparation of Cu-Containing Substances via an Ultrasonic-Assisted Solvothermal Approach and Their Catalytic Effects on the Thermal Decomposition of Ammonium Perchlorate. Materials 2025, 18, 2928. https://doi.org/10.3390/ma18132928
Peng C-H, Su P-H, Li J-S, Ke Y-J. Preparation of Cu-Containing Substances via an Ultrasonic-Assisted Solvothermal Approach and Their Catalytic Effects on the Thermal Decomposition of Ammonium Perchlorate. Materials. 2025; 18(13):2928. https://doi.org/10.3390/ma18132928
Chicago/Turabian StylePeng, Cheng-Hsiung, Pin-Hsien Su, Jin-Shuh Li, and Yan-Jun Ke. 2025. "Preparation of Cu-Containing Substances via an Ultrasonic-Assisted Solvothermal Approach and Their Catalytic Effects on the Thermal Decomposition of Ammonium Perchlorate" Materials 18, no. 13: 2928. https://doi.org/10.3390/ma18132928
APA StylePeng, C.-H., Su, P.-H., Li, J.-S., & Ke, Y.-J. (2025). Preparation of Cu-Containing Substances via an Ultrasonic-Assisted Solvothermal Approach and Their Catalytic Effects on the Thermal Decomposition of Ammonium Perchlorate. Materials, 18(13), 2928. https://doi.org/10.3390/ma18132928







