Polyethyleneimine Modified Expanded Vermiculite-Supported Nano Zero-Valent Iron for Cr(VI) Removal from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Preparation of nZVI@PEI/EVMT
2.2.1. Synthesis of PEI Modified EVMT (PEI/EVMT)
2.2.2. Synthesis of nZVI@PEI/EVMT
2.3. Characterization Techniques
2.4. Adsorption Process
2.5. Kinetics Experiments
2.6. Adsorption Isotherm Experiments
3. Results and Discussion
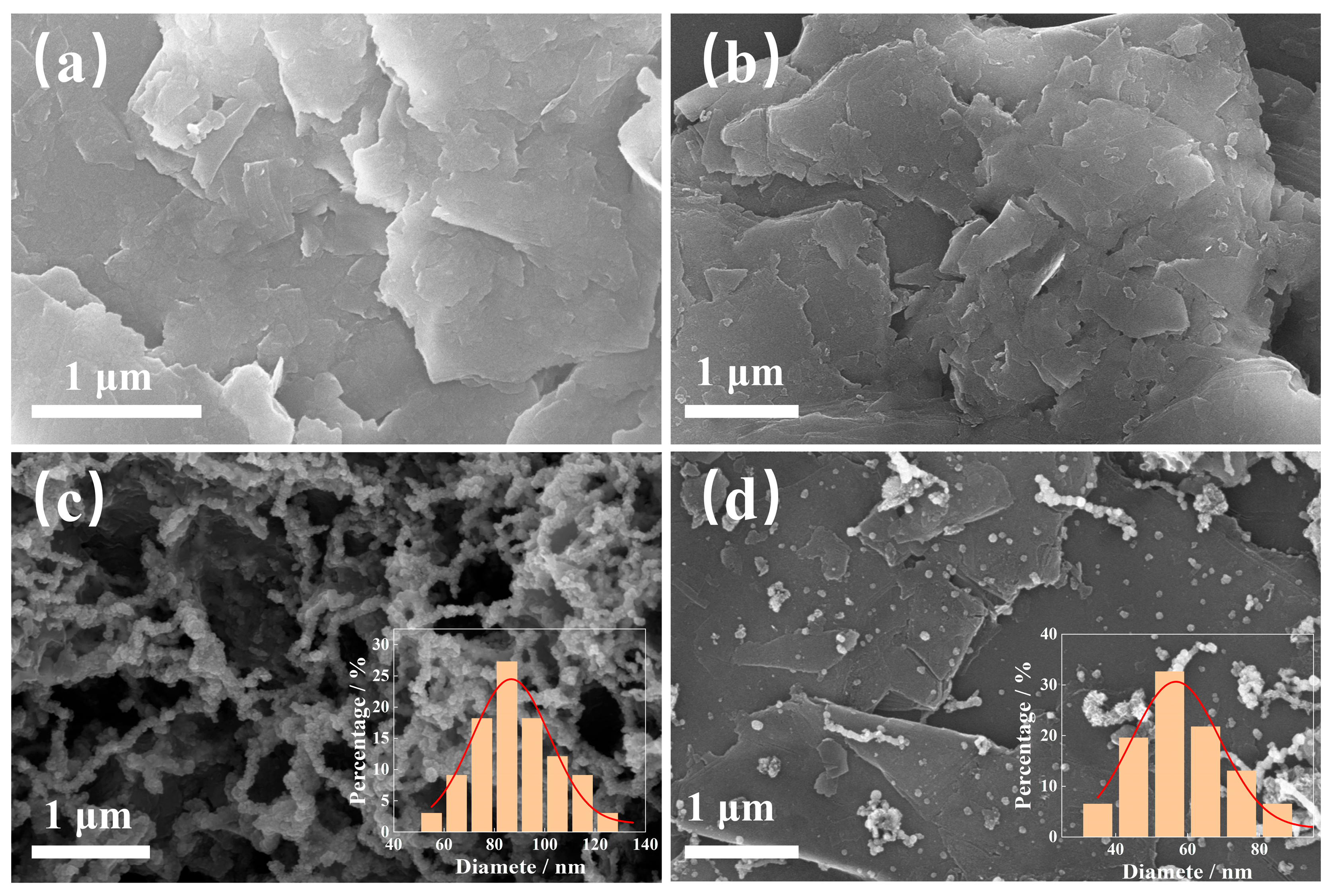
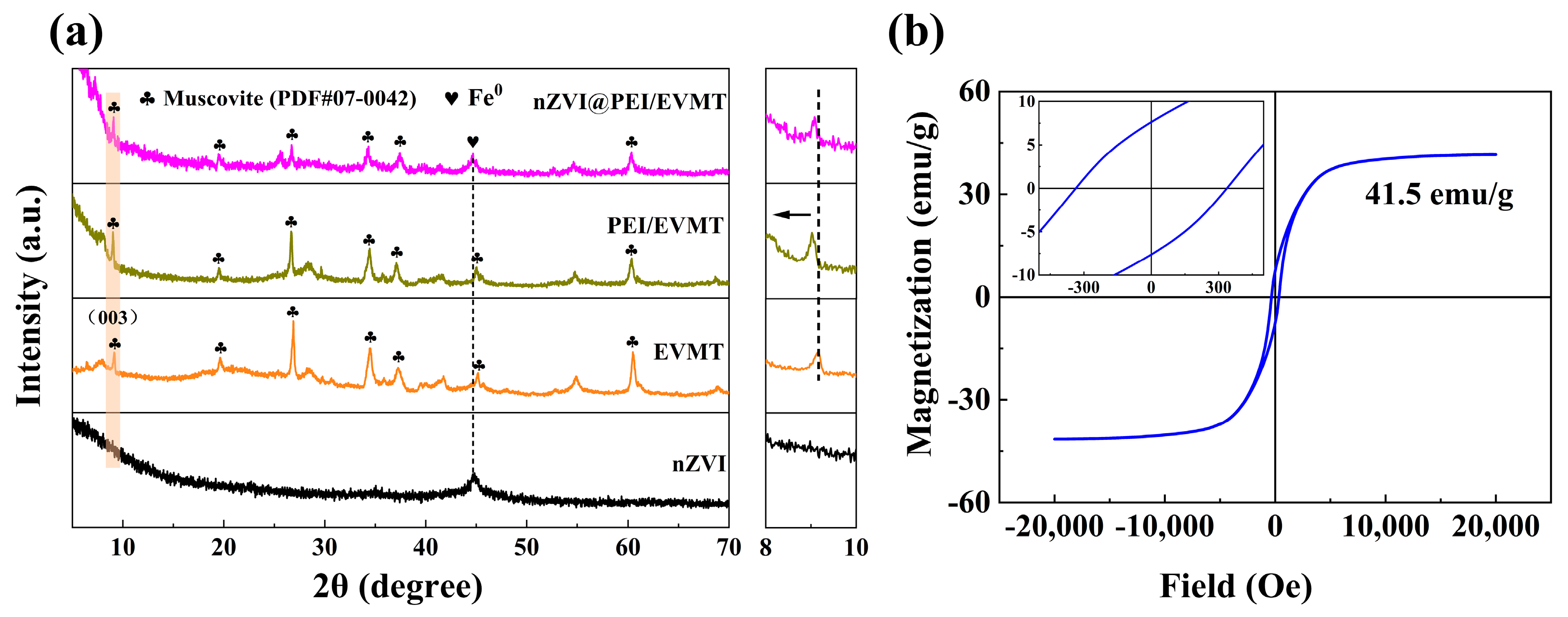
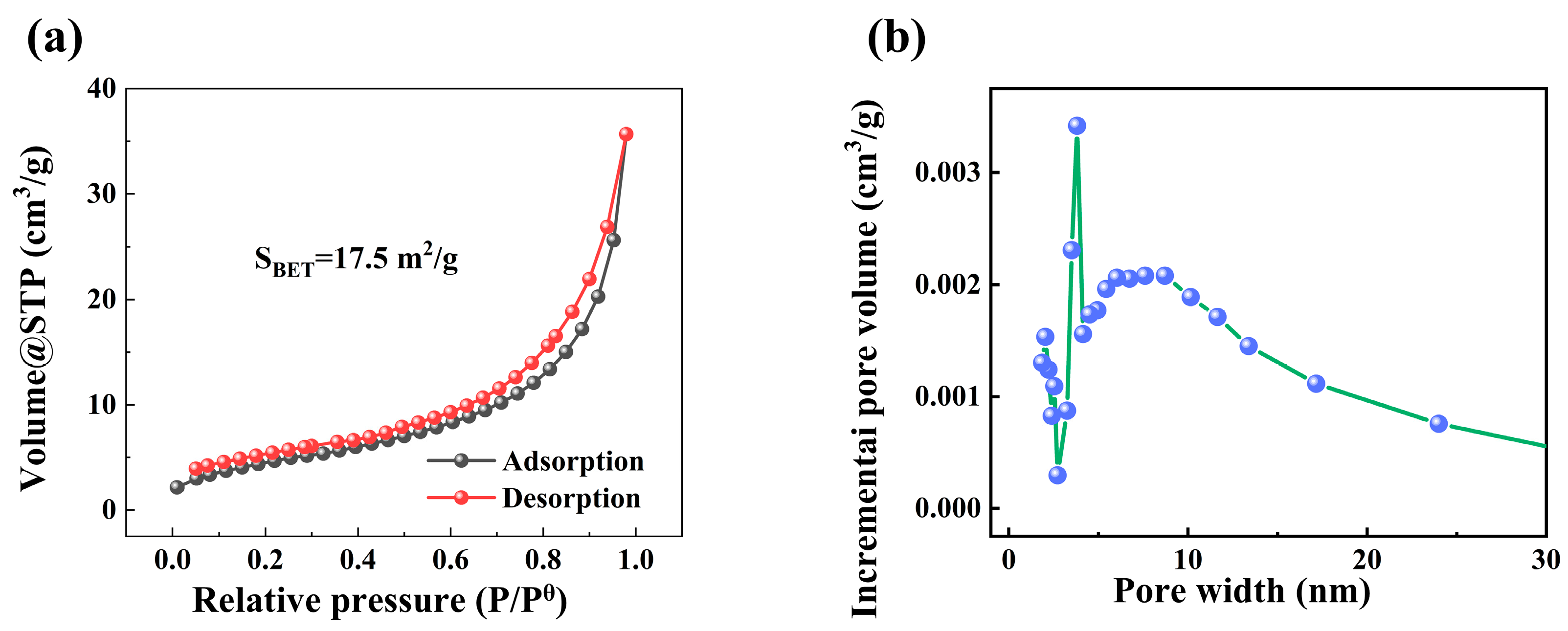
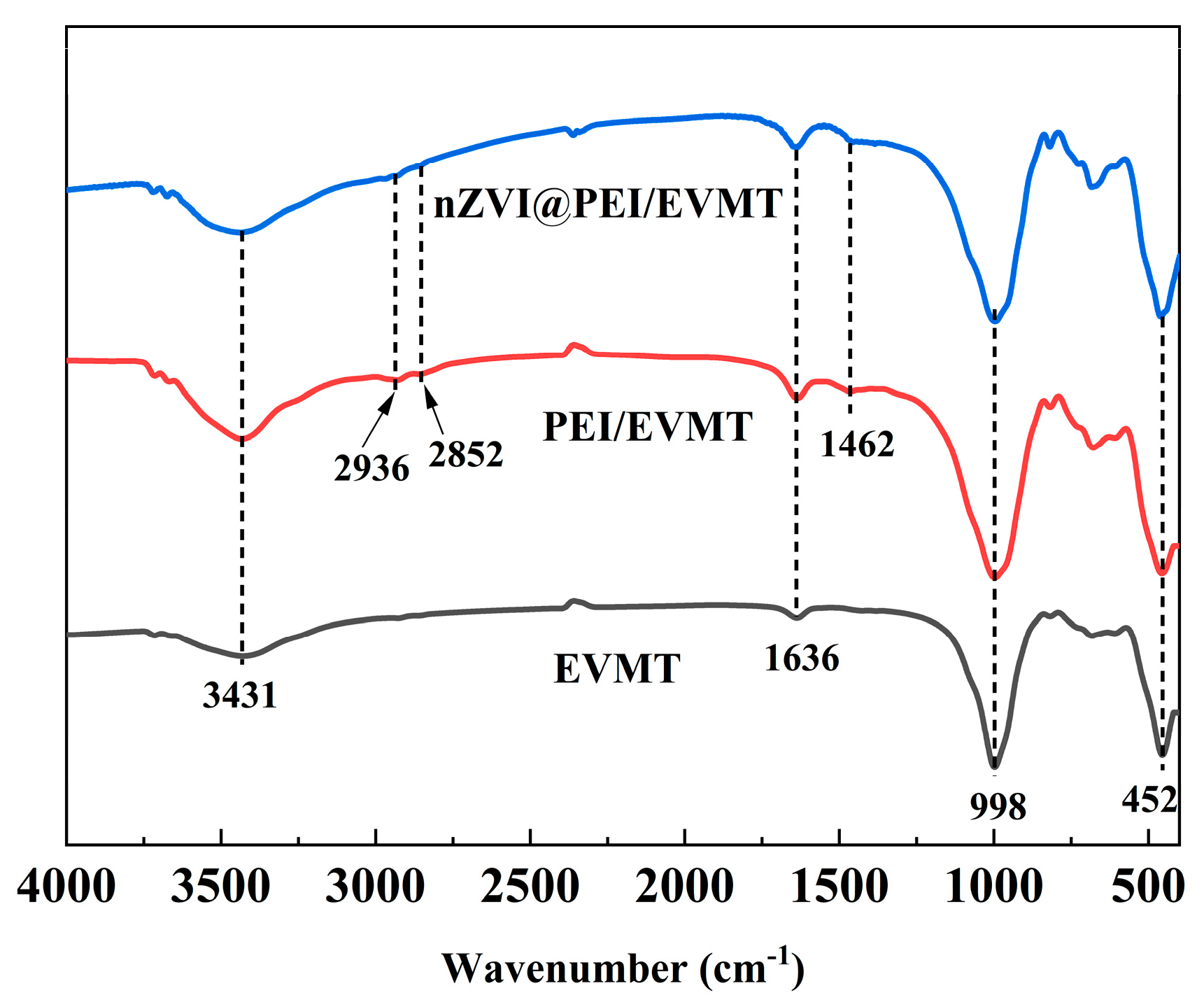
3.1. Characterization of Materials
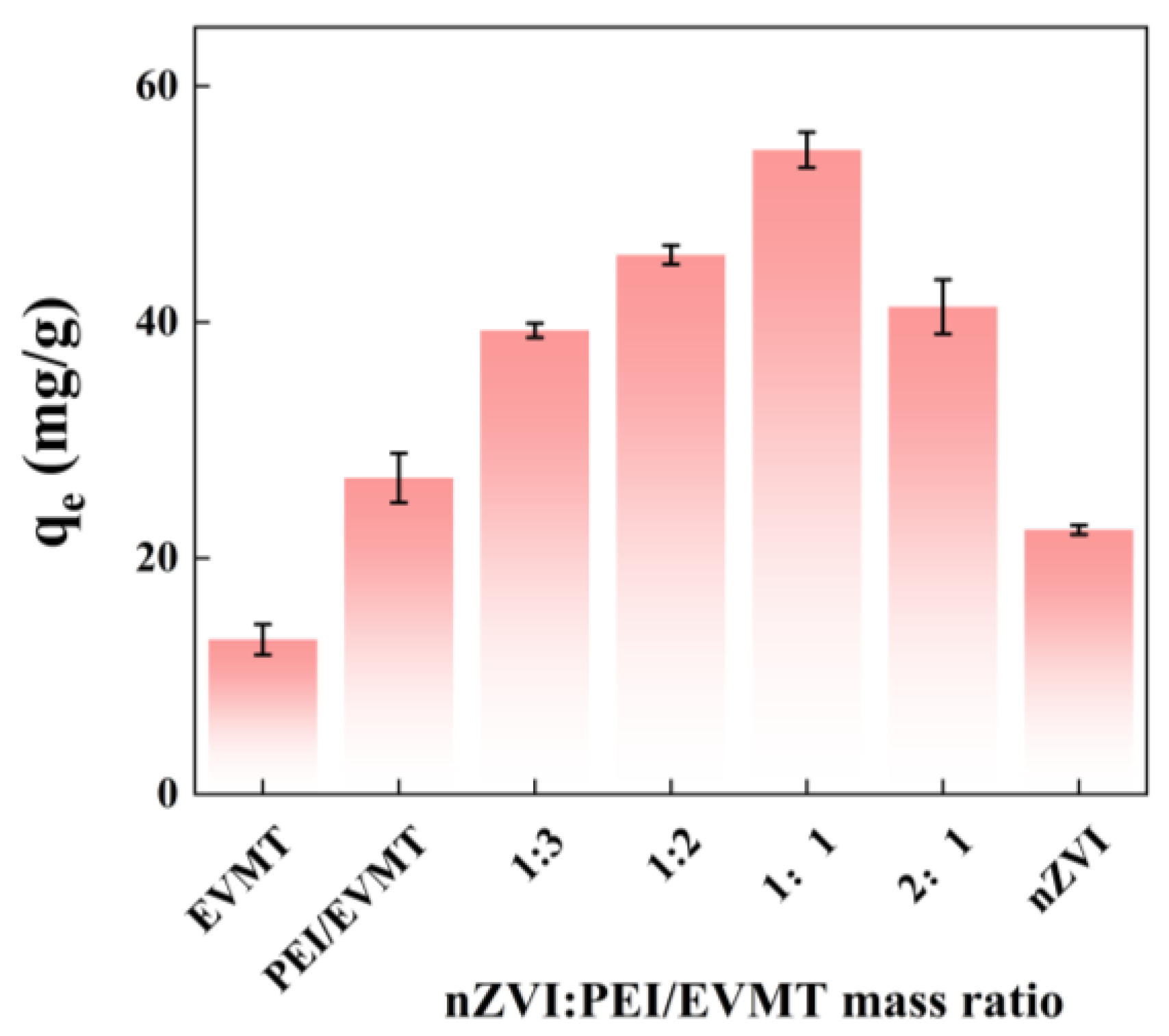
3.2. Effect of nZVI@PEI/EVMT Mass Ratio
3.3. The Effect of Initial pH on Adsorption
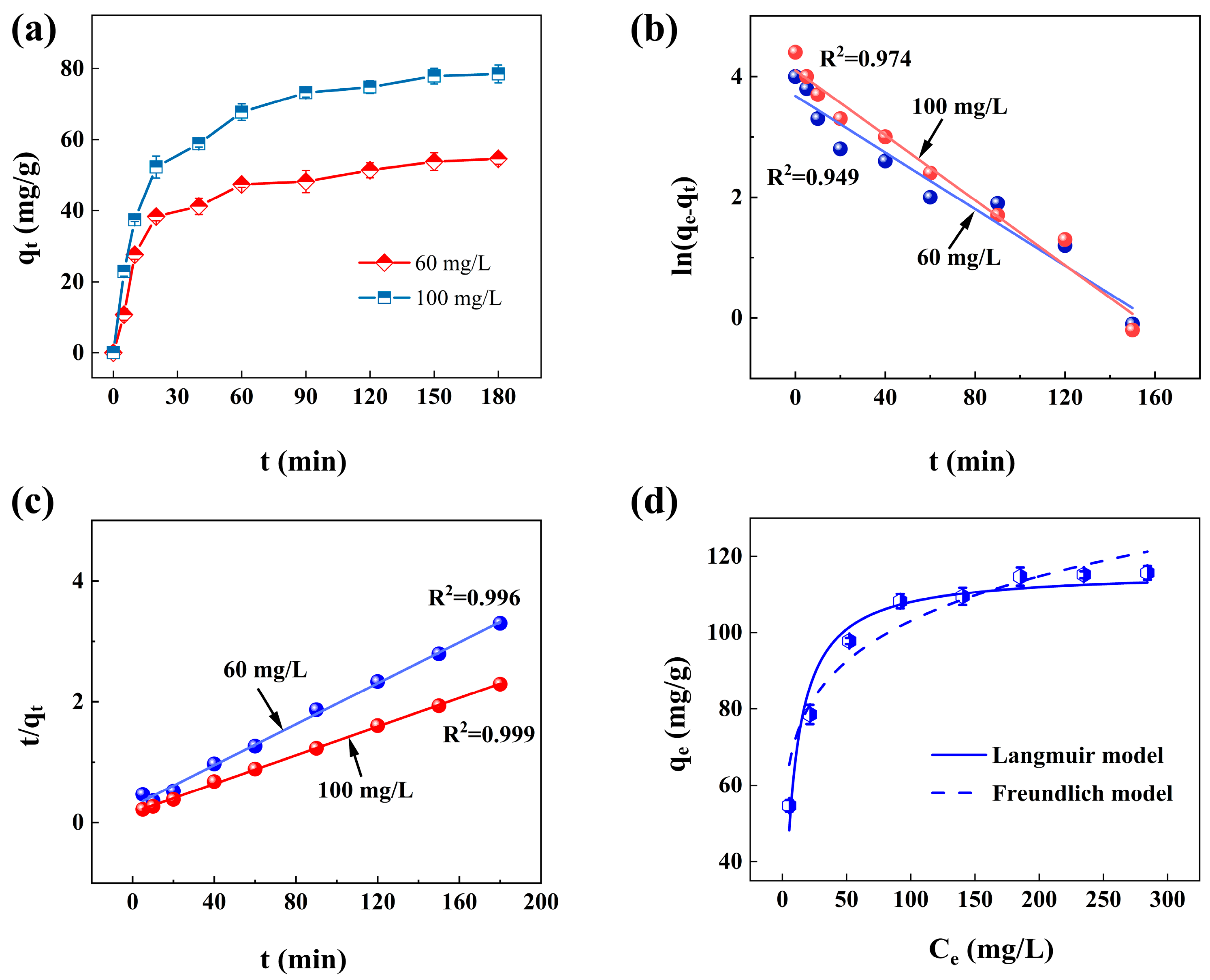
3.4. Adsorption Kinetics
3.5. Adsorption Isotherms
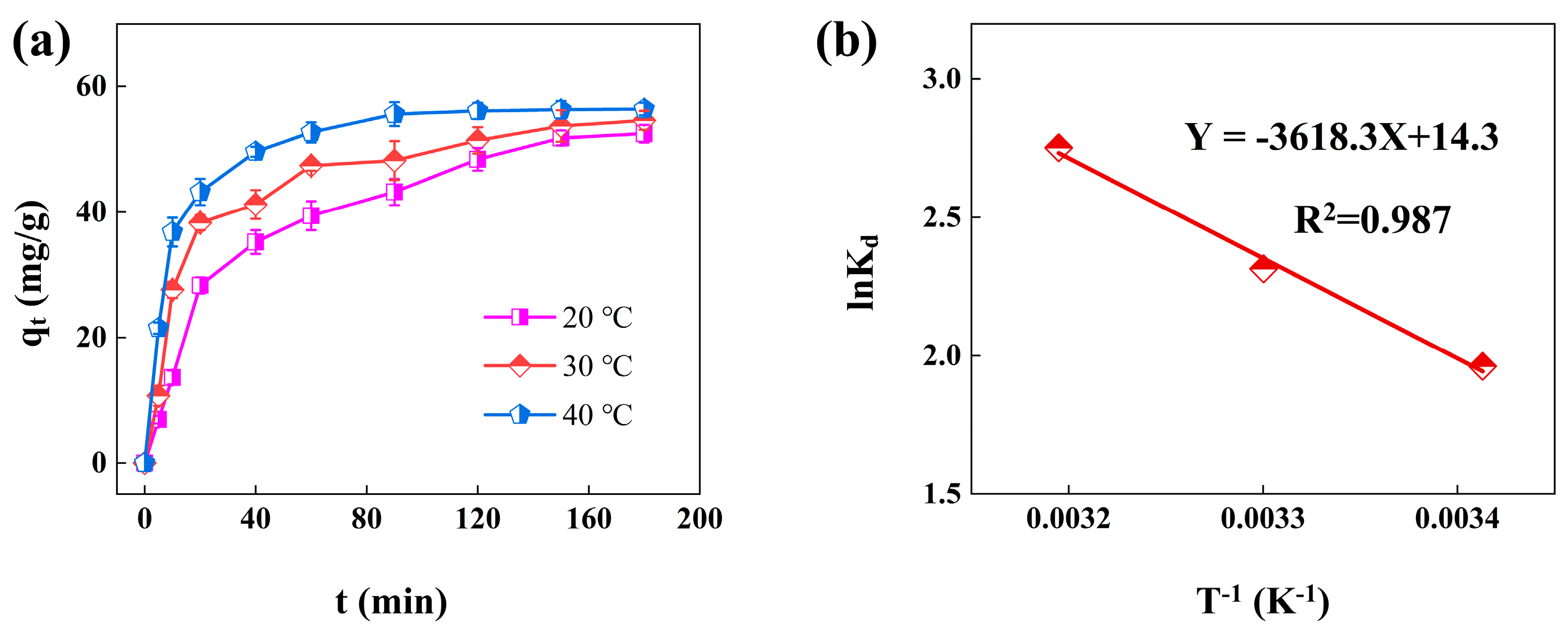
3.6. Adsorption Thermodynamics
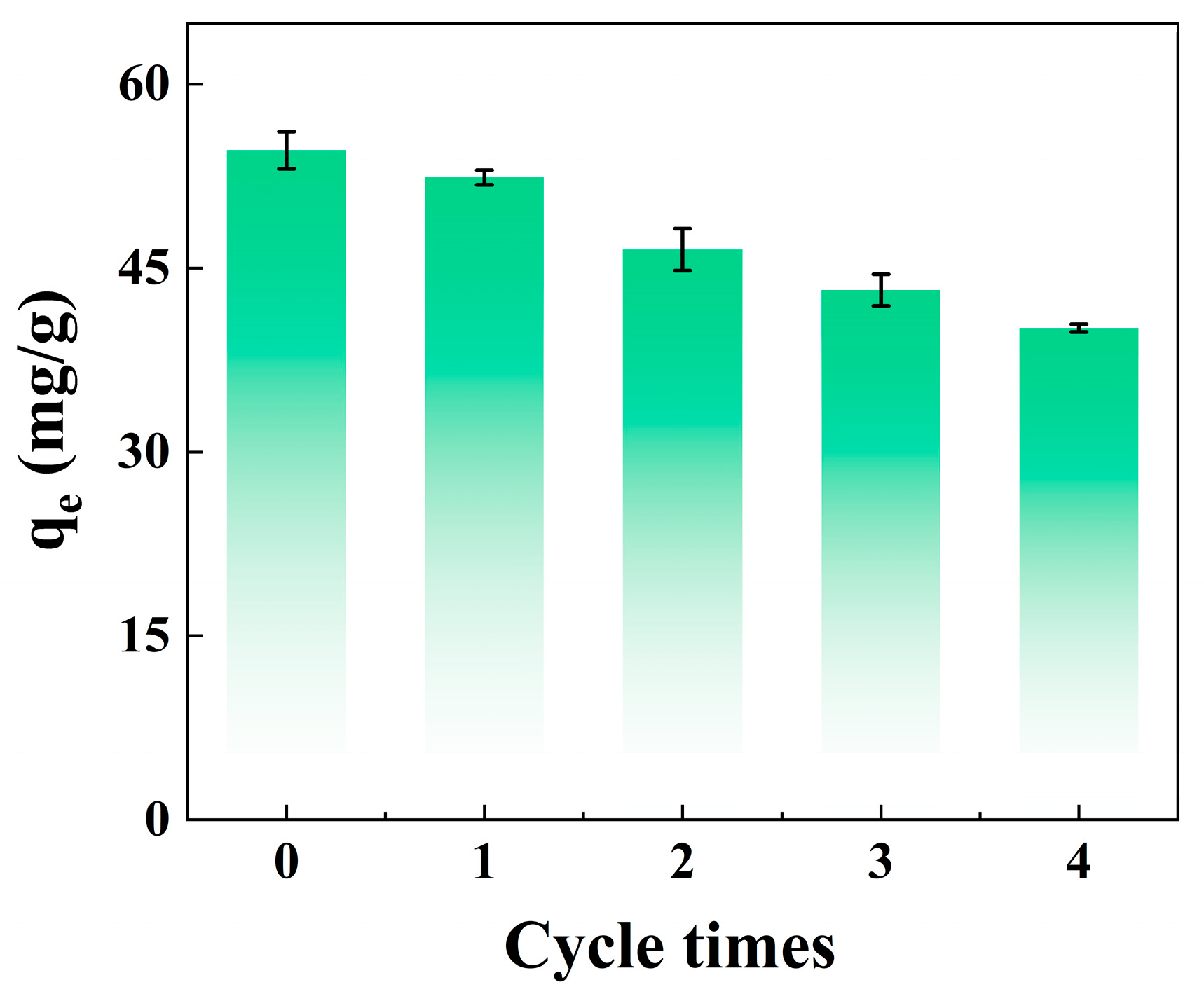
3.7. Materials Reusability
4. Adsorption Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, Z.; Peng, C.; Deng, L.; Liu, S. Effective Adsorption and Sensitive Detection of Cr(VI) by Chitosan/Cellulose Nanocrystals Grafted with Carbon Dots Composite Hydrogel. Polymers 2021, 13, 3788. [Google Scholar] [CrossRef]
- Pang, F.; Wei, C.; Zhang, Z.; Wang, W.; Wang, Z. The migration and immobilization for heavy metal chromium ions in the hydration products of calcium sulfoaluminate cement and their leaching behavior. J. Clean. Prod. 2022, 365, 132778. [Google Scholar] [CrossRef]
- An, Q.; Li, X.Q.; Nan, H.Y.; Yu, Y.; Jiang, J.N. The potential adsorption mechanism of the biochars with different modification processes to Cr(VI). Environ. Sci. Pollut. Res. 2018, 25, 31346–31357. [Google Scholar] [CrossRef]
- Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Naidu, R.; Rahman, M.M. A comprehensive review of the current progress of chromium removal methods from aqueous solution. Toxics 2023, 11, 252. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2019, 217, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.D.; Hu, Q.; Xiao, Y.Y.; Cheng, X.; Liu, H.; Wang, N.; Qiu, B.; Guo, Z.H. Triple layered core-shell ZVI@carbon@polyaniline composite enhanced electron utilization in Cr (VI) reduction. J. Mater. Chem. 2018, A6, 11119–11128. [Google Scholar] [CrossRef]
- Liu, Y.; Sohi, S.P.; Liu, S.; Guan, J.; Zhou, J.; Chen, J. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 2019, 235, 276–281. [Google Scholar] [CrossRef]
- Shao, X.; Yu, J.; Chang, J.; Huang, Z.; Jiang, Y.; Deng, S. Effect of vermiculite modified with nano-iron-based material on stabilization of lead in lead contaminated soil. Environ. Sci. Pollut. R. 2023, 30, 83821–83833. [Google Scholar] [CrossRef] [PubMed]
- Dastgeer, G.; Nisar, S.; Rasheed, A.; Akbar, K.; Chavan, V.D.; Kim, D.; Wabaidur, S.M.; Zulfiqar, M.W.; Eom, J. Atomically engineered, high-speed non-volatile flash memory device exhibiting multibit data storage operations. Nano Energy 2024, 119, 109106. [Google Scholar] [CrossRef]
- Dastgeer, G.; Nisar, S.; Zulfiqar, M.W.; Eom, J.; Imran, M.; Akbar, K. A review on recent progress and challenges in high-efficiency perovskite solar cells. Nano Energy 2024, 132, 110401. [Google Scholar] [CrossRef]
- Derdour, K.; Bouchelta, C.; Naser-Eddine, A.K.; Medjram, M.S.; Magri, P. Removal of Cr(VI) from aqueous solutions by using activated carbon supported iron catalysts as efficient adsorbents. World J. Eng. 2018, 15, 3–13. [Google Scholar] [CrossRef]
- Fu, R.; Xu, Z.; Peng, L.; Bi, D. Removal of polybrominated diphenyl ethers by biomass carbon-supported nanoscale zerovalent iron particles: Influencing factors, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2016, 23, 23983–23993. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Y.; Zhang, X.; Chen, Z. Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2011, 171, 612–617. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Du, H.; Li, Q.; Zheng, X.; Qi, R.; Ren, P. Experimental and theoretical study to control the heavy metals in solid waste and sludge during pyrolysis using modified expanded vermiculite. J. Hazard. Mater. 2024, 463, 132885. [Google Scholar] [CrossRef]
- Liu, D.; Deng, S.; Du, R.; Tao, L.; Sun, J.; Yu, G. Efficient and selective removal of copper from aqueous solution by nanosized hydrated zirconium oxides loaded in vermiculite. J. Environ. Chem. Eng. 2020, 8, 104315. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Park, D.; Jin, K.S.; Lee, H. Engineered polymer–clay–copper oxides catalyst for the oxidation and reduction of organic molecules: Synergy of degradation and instinctive interface stability by polymer self-healing function. Surf. Interfaces 2023, 39, 102934. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, X.; Liu, Y.; Xu, B.; Pan, X. Highly stable and covalently functionalized magnetic nanoparticles by polyethyleneimine for Cr(VI) adsorption in aqueous solution. RSC Adv. 2015, 5, 1398–1405. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, B.; Wu, Y.; Liu, Z.; Wang, J.; Xu, J.; Tong, Z.; Mu, X.; Liu, B. Fe3O4@PDA@PEI Core-Shell Microspheres as a Novel Magnetic Sorbent for the Rapid and Broad-Spectrum Separation of Bacteria in Liquid Phase. Materials 2022, 15, 2039. [Google Scholar] [CrossRef]
- Nie, D.; Ma, R.; Zhang, Y.; Wang, W.; Nie, G.; Liu, G.; Liu, W.; Zou, D. Efficient removal of Cr(VI) from wastewater by composite adsorptive membrane modified with polyethyleneimine (PEI). Sep. Purif. Technol. 2024, 346, 127410. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Huang, Y.; Zhu, S.; Yao, Y. Enhanced Hg(II) removal by polyethylenimine-modified fly ash-based tobermorite. Colloid Surface A 2024, 702, 135101. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.K.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr(VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2021, 203, 111891. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Ahmed, I.M.; Bräse, S. Preparation and Characterization of Magnetite Talc (Fe3O4@Talc) Nanocomposite as an Effective Adsorbent for Cr(VI) and Alizarin Red S Dye. Materials 2022, 15, 3401. [Google Scholar] [CrossRef]
- Park, M.H.; Jeong, S.; Lee, G.; Park, H.; Kim, J.Y. Removal of aqueous-phase Pb(II), Cd(II), As(III), and As(V) by nanoscale zero-valent iron supported on exhausted coffee grounds. Waste Manag. 2019, 92, 49–58. [Google Scholar] [CrossRef]
- Cai, X.; Qiu, Y.; Zhou, Y.; Jiao, X. Nanoscale zero-valent iron loaded vermiform expanded graphite for the removal of Cr (VI) from aqueous solution. R. Soc. Open Sci. 2021, 8, 210801. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.; Ge, Z.; Xu, D.; Zhang, K. Self-assembly of vermiculite-polymer composite films with improved mechanical and gas barrier properties. Appl. Clay Sci. 2019, 180, 105198. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, X.; Meng, Z.; Feng, L. Adsorption of ciprofloxacin and tetracycline from wastewater by layered double hydroxides modified vermiculite. J. Porous Mater. 2022, 29, 1299–1308. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Jin, B.; Zhang, B.; Du, H.; Li, Q.; Zheng, X.; Qi, R.; Ren, P. Stabilization of heavy metals in solid waste and sludge pyrolysis by intercalation-exfoliation modified vermiculite. J. Environ. Manag. 2024, 356, 120747. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Yang, G.; Zhao, S. Preparation and research on cationic modified vermiculite with strong adsorption capacity for mineralizing bacteria. Mater. Lett. 2024, 363, 136313. [Google Scholar] [CrossRef]
- Yang, X.; Dai, X.; Jian, T.; Tian, W. Enhanced adsorption and reduction of Pb(II) from aqueous solution by sulfide-modified nanoscale zerovalent iron: Characterization, kinetics and mechanisms. Inorg. Chem. Commun. 2024, 170, 113496. [Google Scholar] [CrossRef]
- Lv, D.; Zhou, X.; Zhou, J.; Liu, Y.; Li, Y.; Yang, K.; Lou, Z.; Baig, S.A.; Wu, D.; Xu, X. Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium(II) removal from aqueous solutions. Appl. Surf. Sci. 2018, 442, 114–123. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Peng, T.; Ding, W.; Yin, H. Effect of Microwave Irradiation on Lead Adsorption Properties of Vermiculite with Different Particle Sizes. Materials 2024, 17, 4152. [Google Scholar] [CrossRef]
- Gao, R.; Hu, P.; Dai, Y.; Zhang, Y.; Liu, L.; Yang, W. Removal of cadmium(II) from aqueous solutions by a novel sulfide-modified nanoscale zero-valent iron supported on kaolinite: Treatment efficiency, kinetics and mechanisms. Appl. Surf. Sci. 2022, 602, 154353. [Google Scholar] [CrossRef]
- Vilela, P.B.; Dalalibera, A.; Duminelli, E.C.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar] [CrossRef]
- Kera, N.H.; Bhaumik, M.; Pillay, K.; Ray, S.S.; Maity, A. Selective removal of toxic Cr(VI) from aqueous solution by adsorption combined with reduction at a magnetic nanocomposite surface. J. Colloid Interface Sci. 2017, 503, 214–228. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Tian, D.; Lin, R.; Wu, W.; Liu, C.; Xia, J.; Lu, M. Effective removal of Cr(VI) from aqueous solutions by covalent organic framework modified with the hyper-crosslinked aniline polymer: Adsorption performance and mechanism. J. Mol. Struct. 2025, 1339, 142410. [Google Scholar] [CrossRef]
- Tan, L.; Xu, J.; Xue, X.; Lou, Z.; Zhu, J.; Baig, S.A.; Xu, X. Multifunctional nanocomposite Fe3O4@SiO2-mPD/SP for selective removal of Pb(II) and Cr(VI) from aqueous solutions. RSC Adv. 2014, 4, 45920. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, F.; Cheng, S.; Zong, L.; Zhu, C.; Ling, C.; Li, A. Recyclable Nanocomposite of Flowerlike MoS2@Hybrid Acid-Doped PANI Immobilized on Porous PAN Nanofibers for the Efficient Removal of Cr(VI). ACS Sustain. Chem. Eng. 2018, 6, 447–456. [Google Scholar] [CrossRef]
- Rajput, S.; Singh, L.P.; Pittman, C.U.; Mohan, D. Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP). J. Colloid Interface Sci. 2017, 492, 176–190. [Google Scholar] [CrossRef]
- He, C.; Yang, Z.; Ding, J.; Chen, Y.; Tong, X.; Li, Y. Effective removal of Cr(VI) from aqueous solution by 3-aminopropyltriethoxysilane-functionalized graphene oxide. Colloid Surface A 2017, 520, 448–458. [Google Scholar] [CrossRef]
- Zhou, G.; Li, W.; He, C.; Liu, X.; Ding, R.; Wang, Y.; Mu, Y. Enhanced hydrodeiodination of iodinated contrast medium by sulfide-modified nano-sized zerovalent iron: Kinetics, mechanisms and application prospects. Chem. Eng. J. 2020, 401, 126050. [Google Scholar] [CrossRef]
- Blanes, P.S.; Bordoni, M.E.; González, J.C.; García, S.I.; Atria, A.M.; Sala, L.F.; Bellú, S.E. Application of soy hull biomass in removal of Cr(VI) from contaminated waters. Kinetic, thermodynamic and continuous sorption studies. J. Environ. Chem. Eng. 2016, 4, 516–526. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Wu, C. Adsorption of Cr(VI) polluted water by Fe3O4@SiO2-APTMS nanocomposites prepared in the presence of ultrasonic irradiation for sustainable water resources utilization. Ultrason. Sonochem. 2023, 96, 106439. [Google Scholar] [CrossRef]
- Qian, L.; Shang, X.; Zhang, B.; Zhang, W.; Su, A.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron. Chemosphere 2019, 215, 739–745. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, D.; Wu, C.; Xie, R. Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms. Materials 2022, 15, 3898. [Google Scholar] [CrossRef]
- Chen, A.; Huang, Y.; Liu, H. Fabrication of chitin microspheres supported sulfidated nano zerovalent iron and their performance in Cr (VI) removal. Chemosphere 2023, 338, 139609. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Wang, X.; Mu, Y.; Tian, W. Magnetic triiron tetraoxide/biochar-loaded nanoscale zero-valent iron for chromium(VI) removal from aqueous solution. J. Taiwan Inst. Chem. E 2024, 159, 105458. [Google Scholar] [CrossRef]
- Lv, X.; Qin, X.; Wang, K.; Peng, Y.; Wang, P.; Jiang, G. Nanoscale zero valent iron supported on MgAl-LDH-decorated reduced graphene oxide: Enhanced performance in Cr(VI) removal, mechanism and regeneration. J. Hazard. Mater. 2019, 373, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liang, B.; Wei, J.; Zhong, S.; Zhang, R.; Yin, Y.; Zhang, Y.; Hu, H.; Huang, Z. A cellulose-based adsorbent with pendant groups of quaternary ammonium and amino for enhanced capture of aqueous Cr(VI). Int. J. Biol. Macromol. 2020, 148, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, Z.; Zhao, X.; Jing, G. Enhanced Cr(VI) removal from simulated electroplating rinse wastewater by amino-functionalized vermiculite-supported nanoscale zero-valent iron. Chemosphere 2019, 218, 458–467. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef] [PubMed]

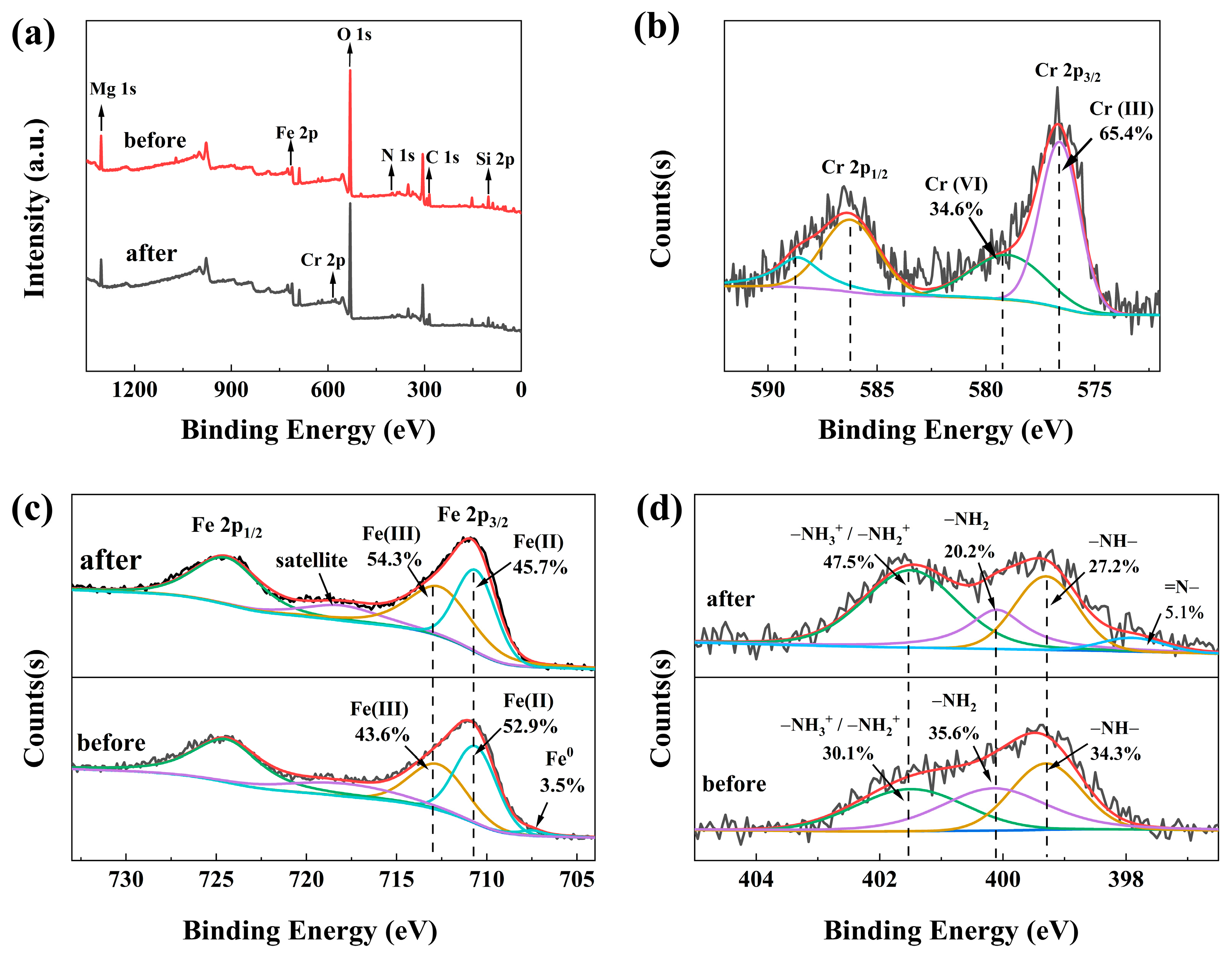
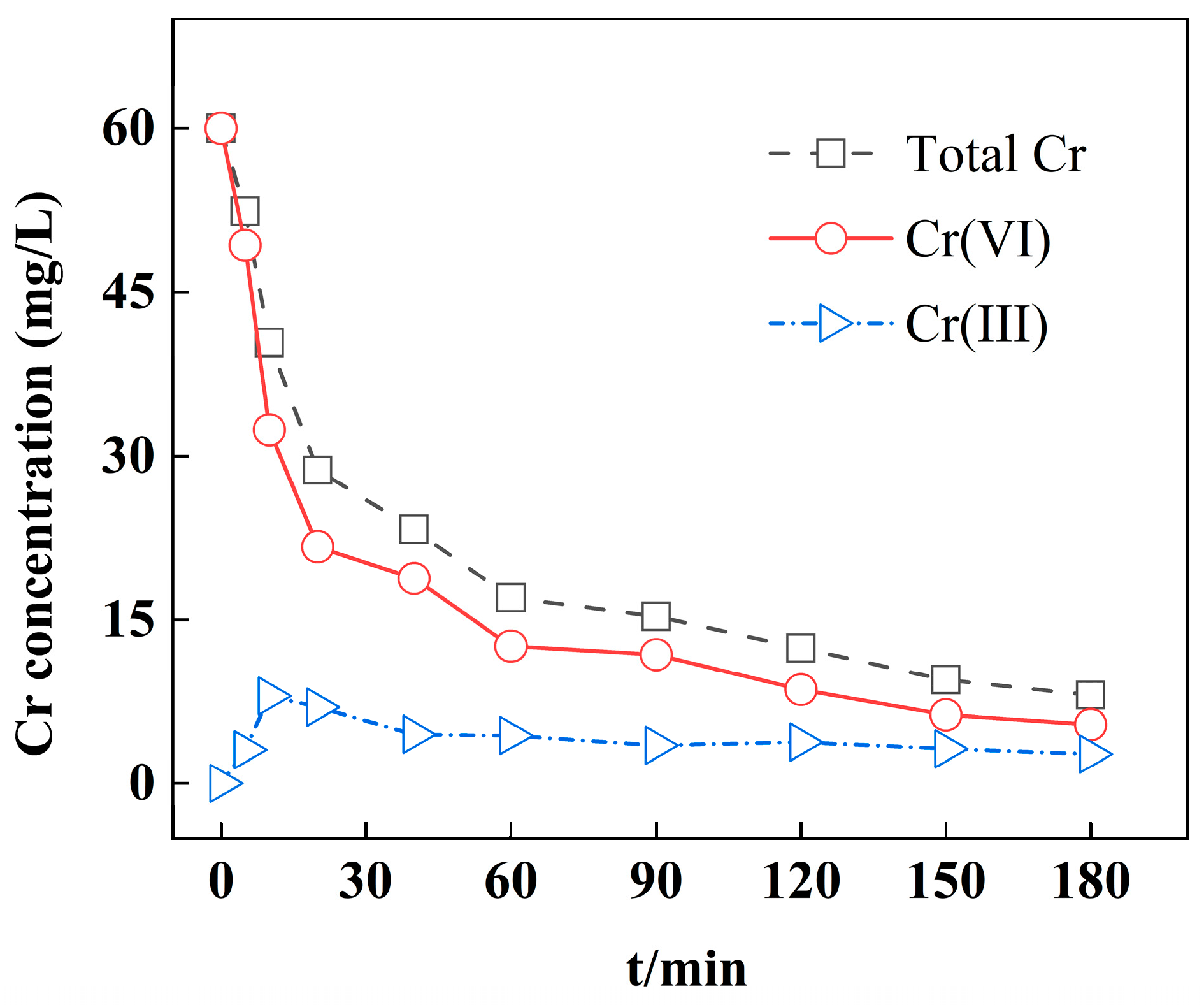
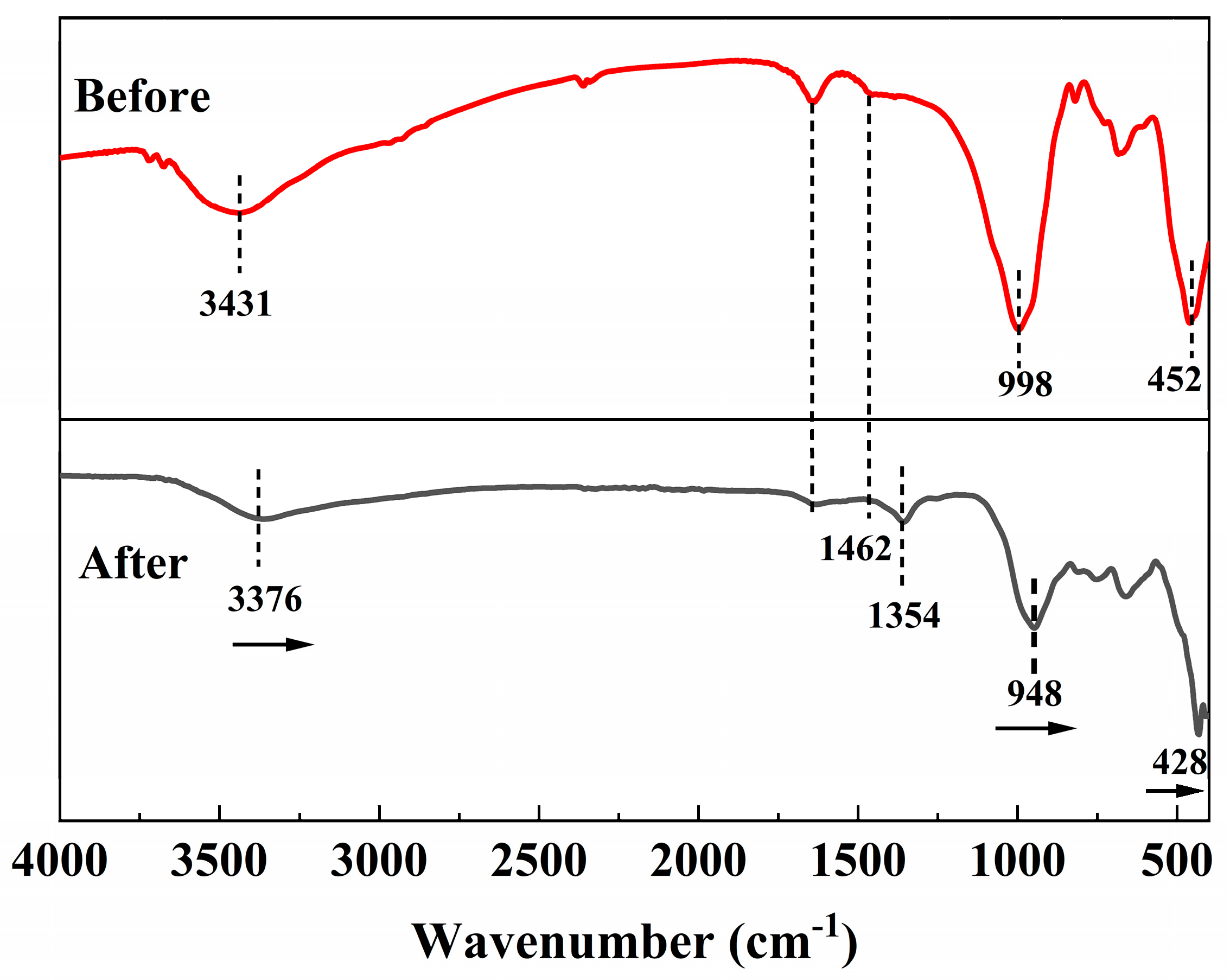
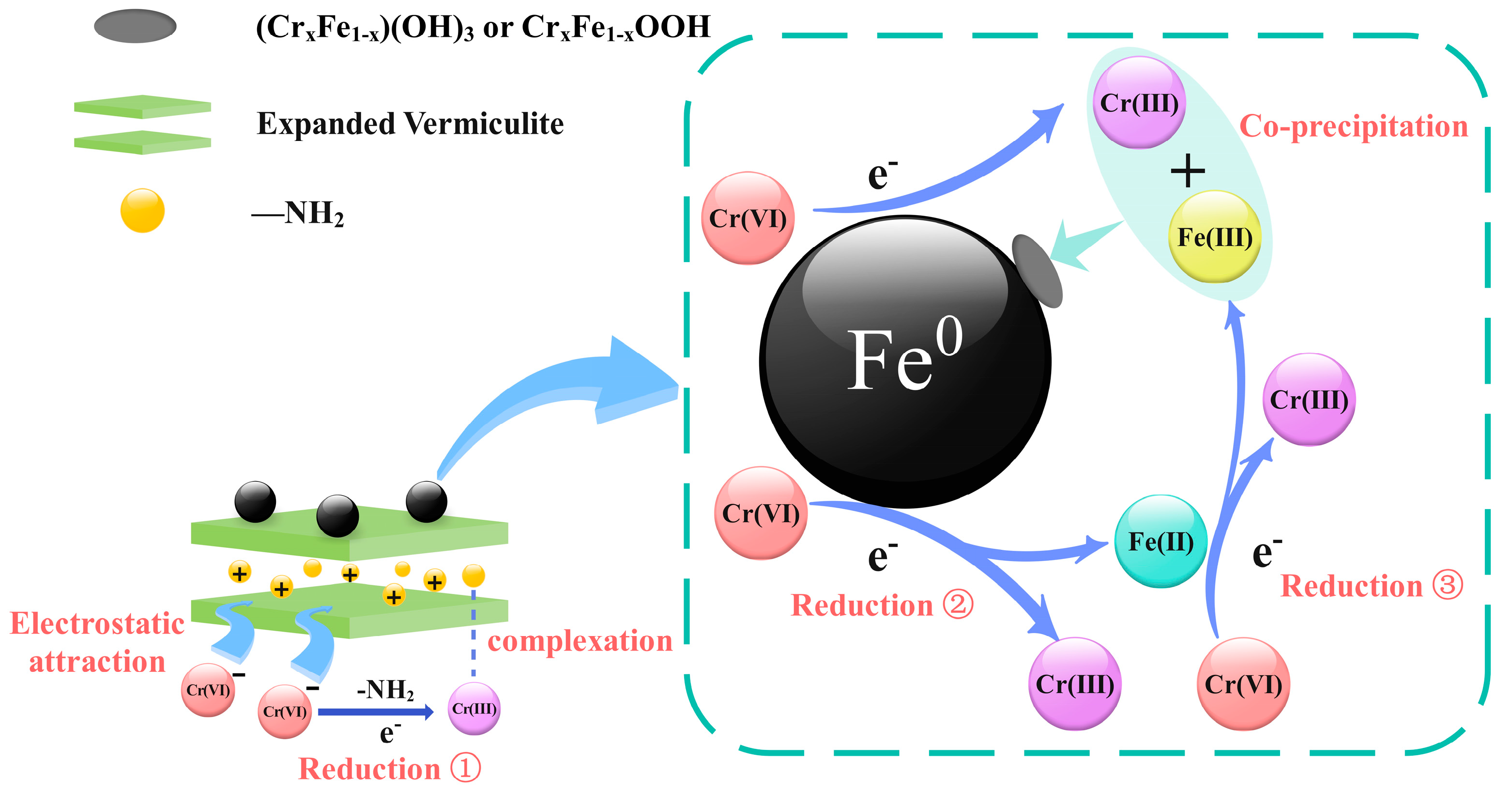
| C0 (mg/L) | qe,exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mg/g) | R2 | k2 (g/mg min) | qe,cal (mg/g) | R2 | ||
| 60 | 54.6 | 2.36 × 10−2 | 39.2 | 0.949 | 1.05 × 10−3 | 59.2 | 0.996 |
| 100 | 78.5 | 2.69 × 10−2 | 59.7 | 0.974 | 8.57 × 10−4 | 84.0 | 0.999 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | R2 | KF | 1/n | R2 |
| 0.131 | 116.2 | 0.958 | 50.2 | 0.156 | 0.918 |
| ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol K) | ||
|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 30.1 | 118.9 |
| −4.78 | −5.83 | −7.16 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Mu, Y.; Zhang, L.; Sun, D.; Jian, T.; Tian, W. Polyethyleneimine Modified Expanded Vermiculite-Supported Nano Zero-Valent Iron for Cr(VI) Removal from Aqueous Solution. Materials 2025, 18, 2930. https://doi.org/10.3390/ma18132930
Yang X, Mu Y, Zhang L, Sun D, Jian T, Tian W. Polyethyleneimine Modified Expanded Vermiculite-Supported Nano Zero-Valent Iron for Cr(VI) Removal from Aqueous Solution. Materials. 2025; 18(13):2930. https://doi.org/10.3390/ma18132930
Chicago/Turabian StyleYang, Xinyu, Yan Mu, Lina Zhang, Dan Sun, Tiantian Jian, and Weiliang Tian. 2025. "Polyethyleneimine Modified Expanded Vermiculite-Supported Nano Zero-Valent Iron for Cr(VI) Removal from Aqueous Solution" Materials 18, no. 13: 2930. https://doi.org/10.3390/ma18132930
APA StyleYang, X., Mu, Y., Zhang, L., Sun, D., Jian, T., & Tian, W. (2025). Polyethyleneimine Modified Expanded Vermiculite-Supported Nano Zero-Valent Iron for Cr(VI) Removal from Aqueous Solution. Materials, 18(13), 2930. https://doi.org/10.3390/ma18132930





