Resistance of Cereal-Husk-Reinforced PVC Terrace Profiles to Agaricomycetes Fungi
Abstract
1. Introduction
2. Materials and Methods
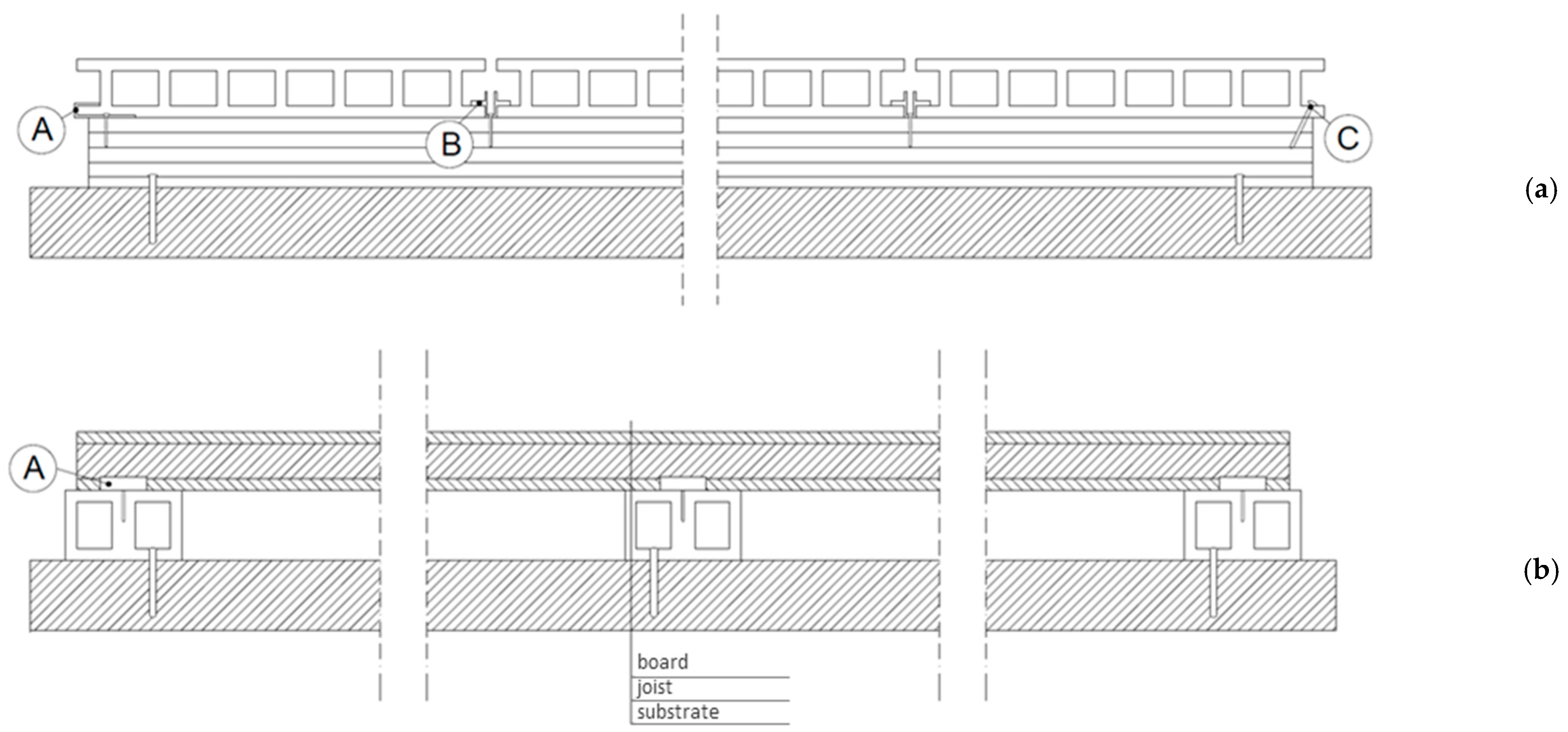
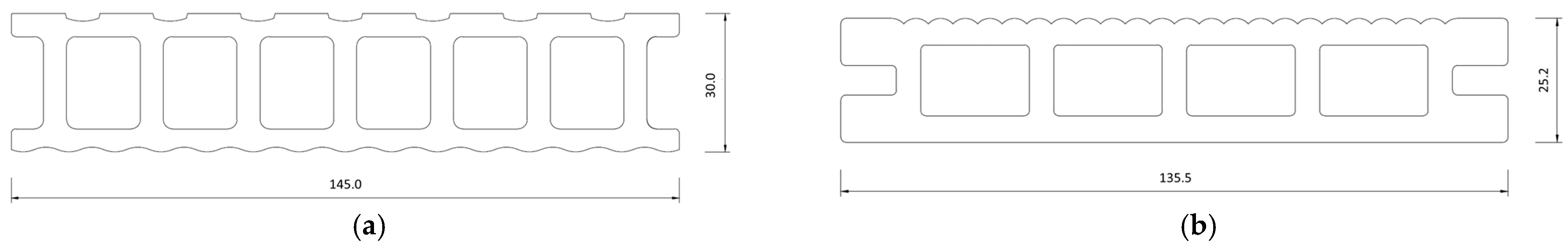
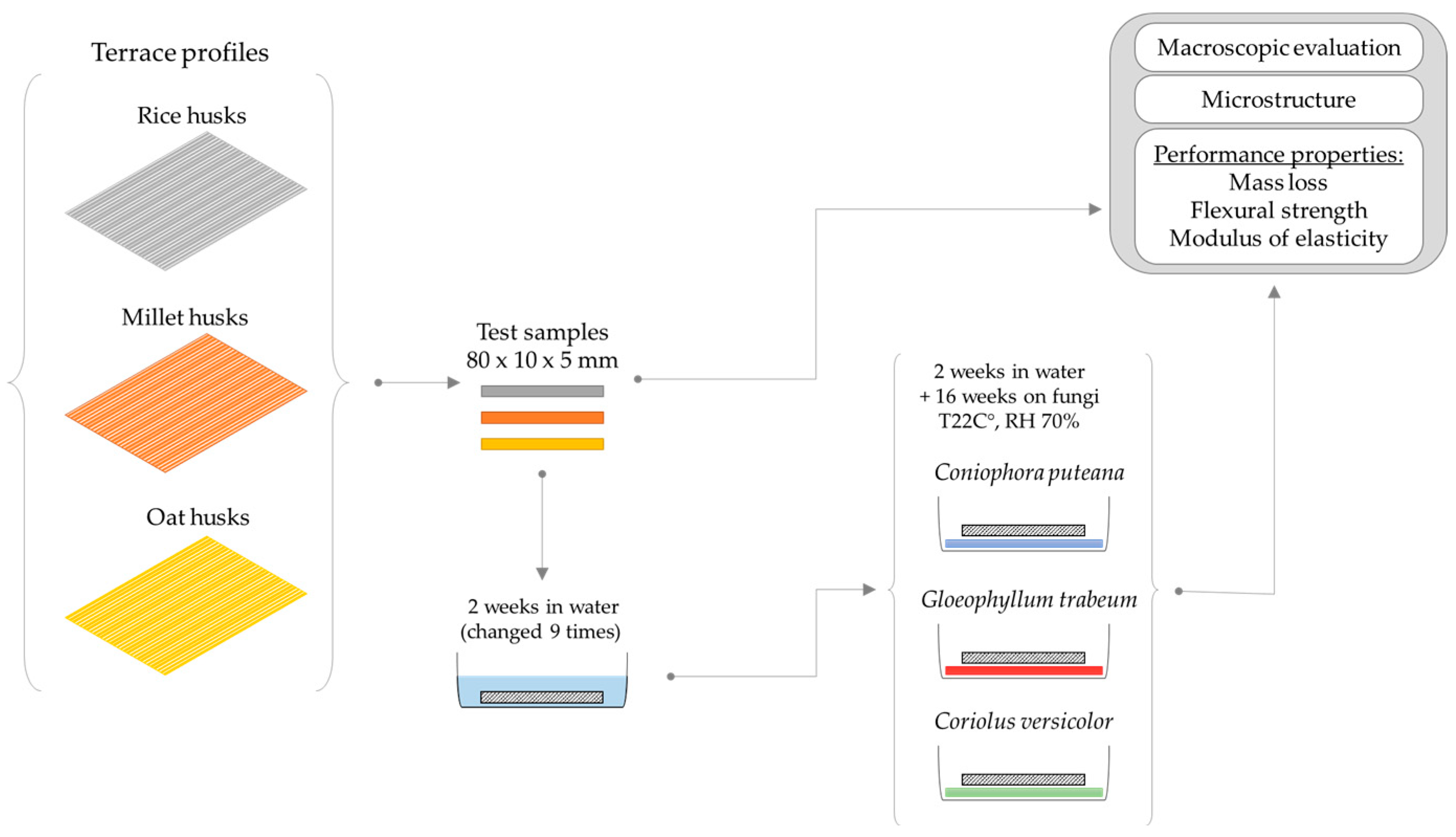
2.1. Test Specimens
2.2. Fungal Exposure
2.3. Macroscopic Analysis
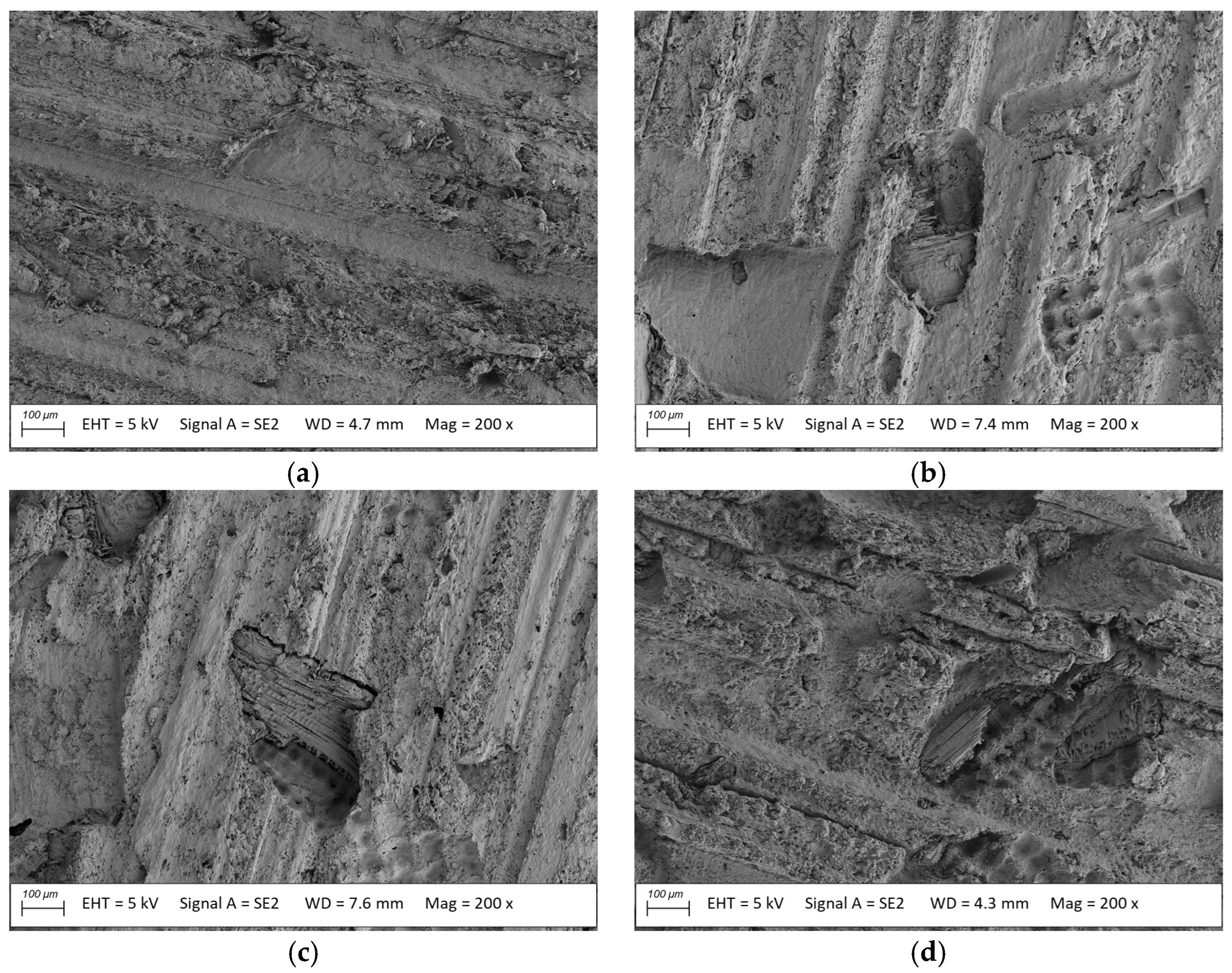
2.4. SEM Analysis
2.5. Performance
3. Results and Discussion
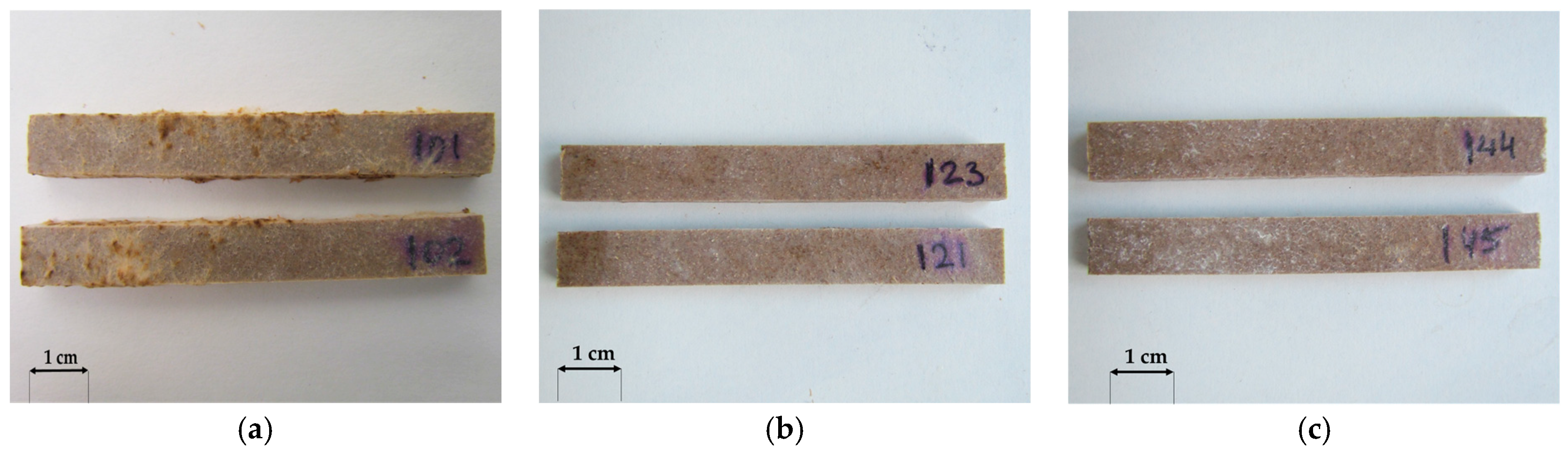
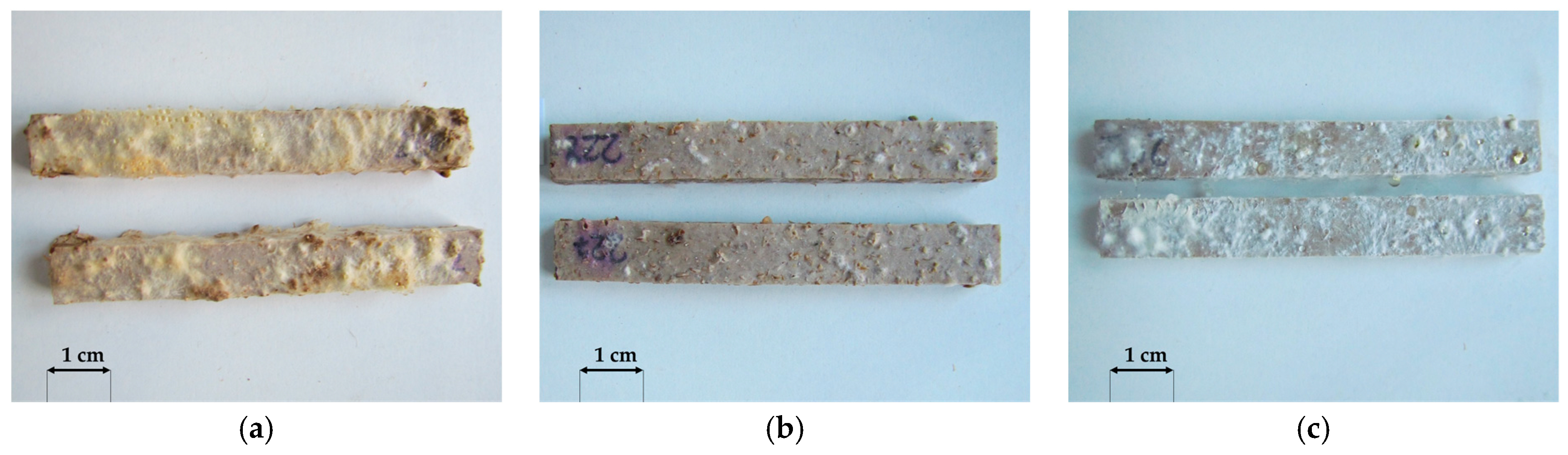
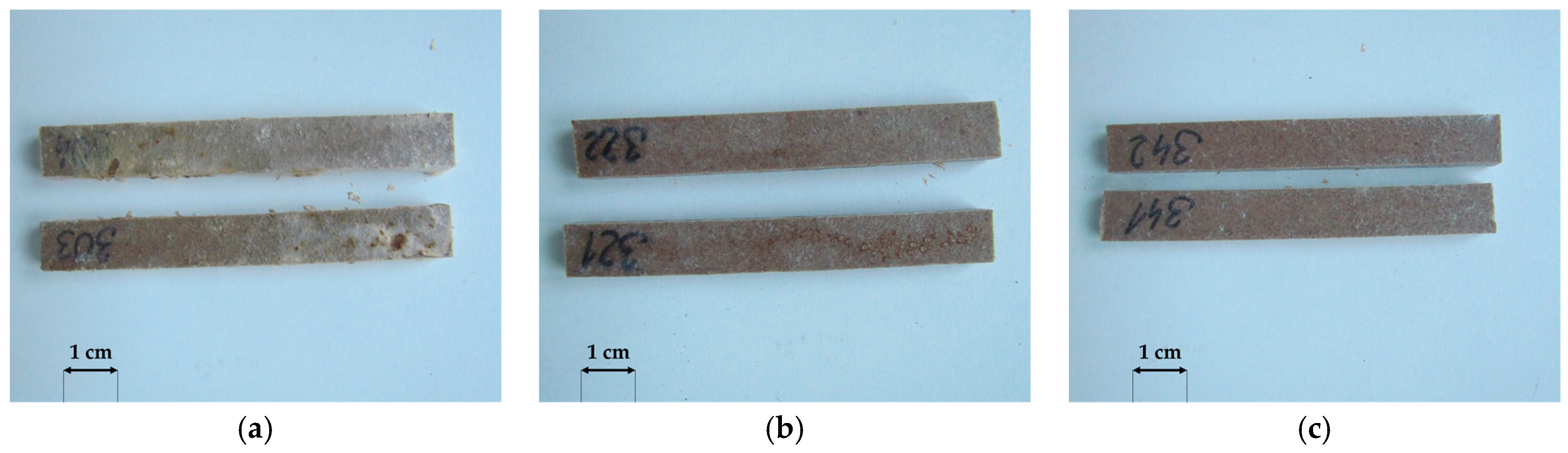
3.1. Macroscopic Analysis
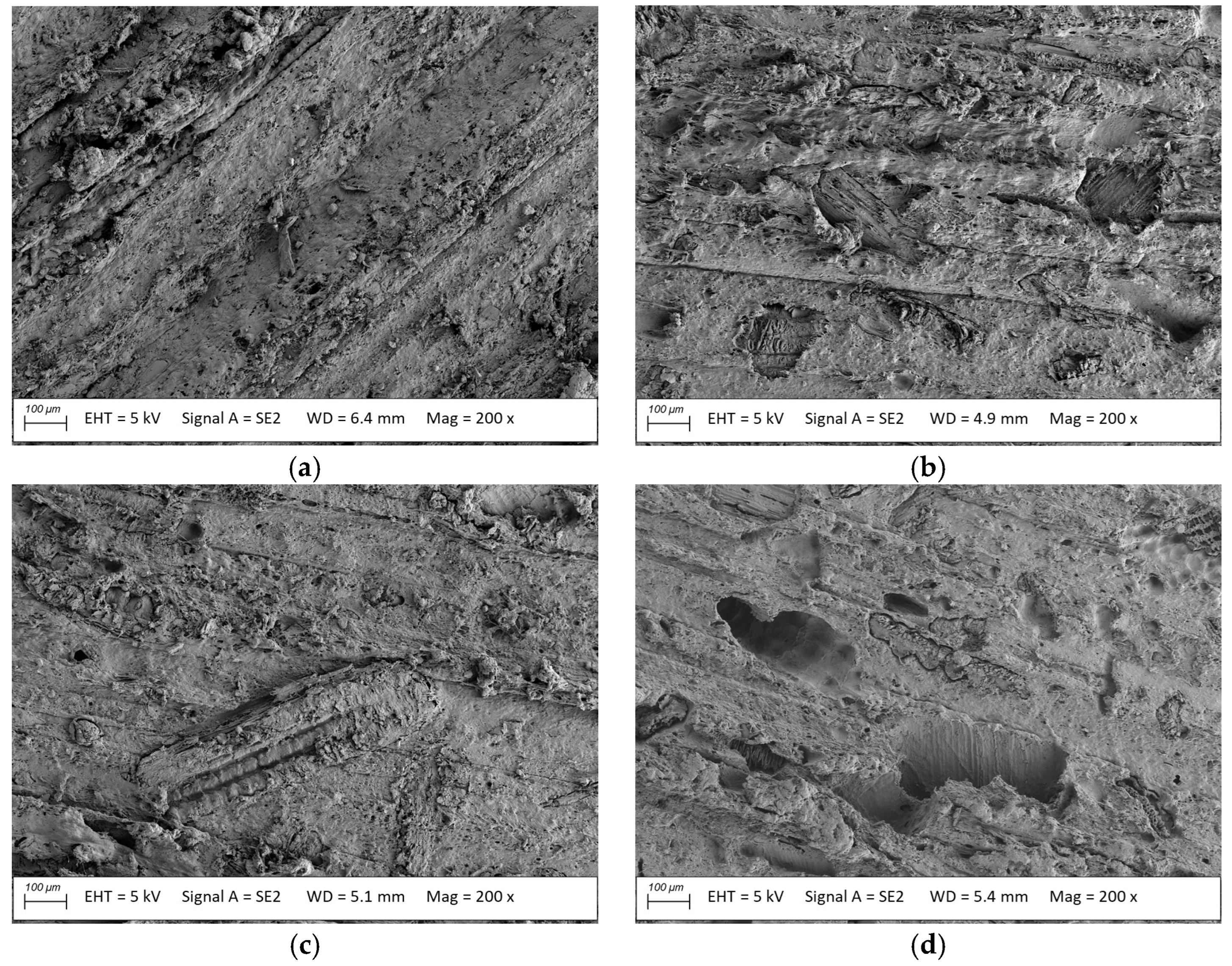
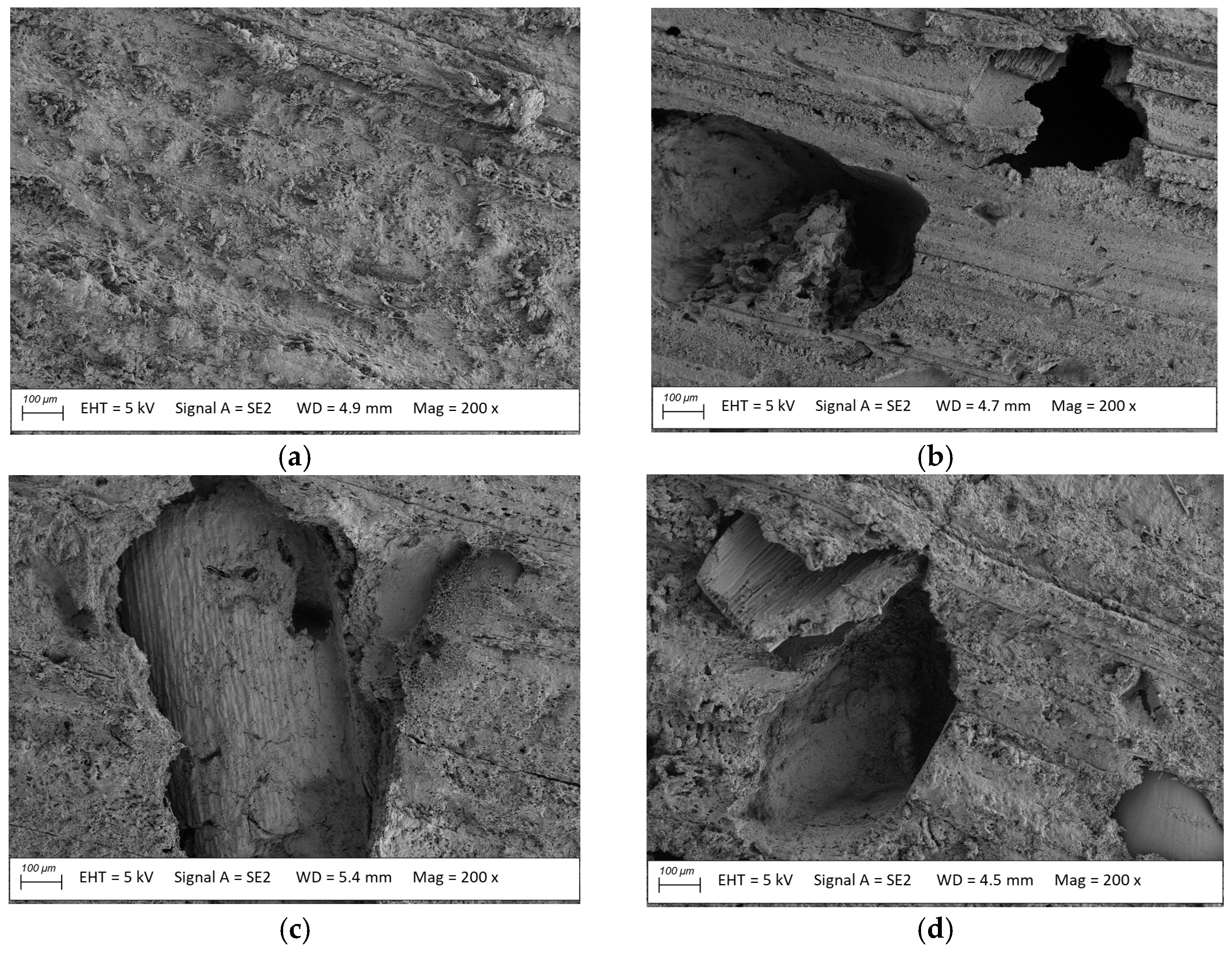
3.2. Microstructure Analysis
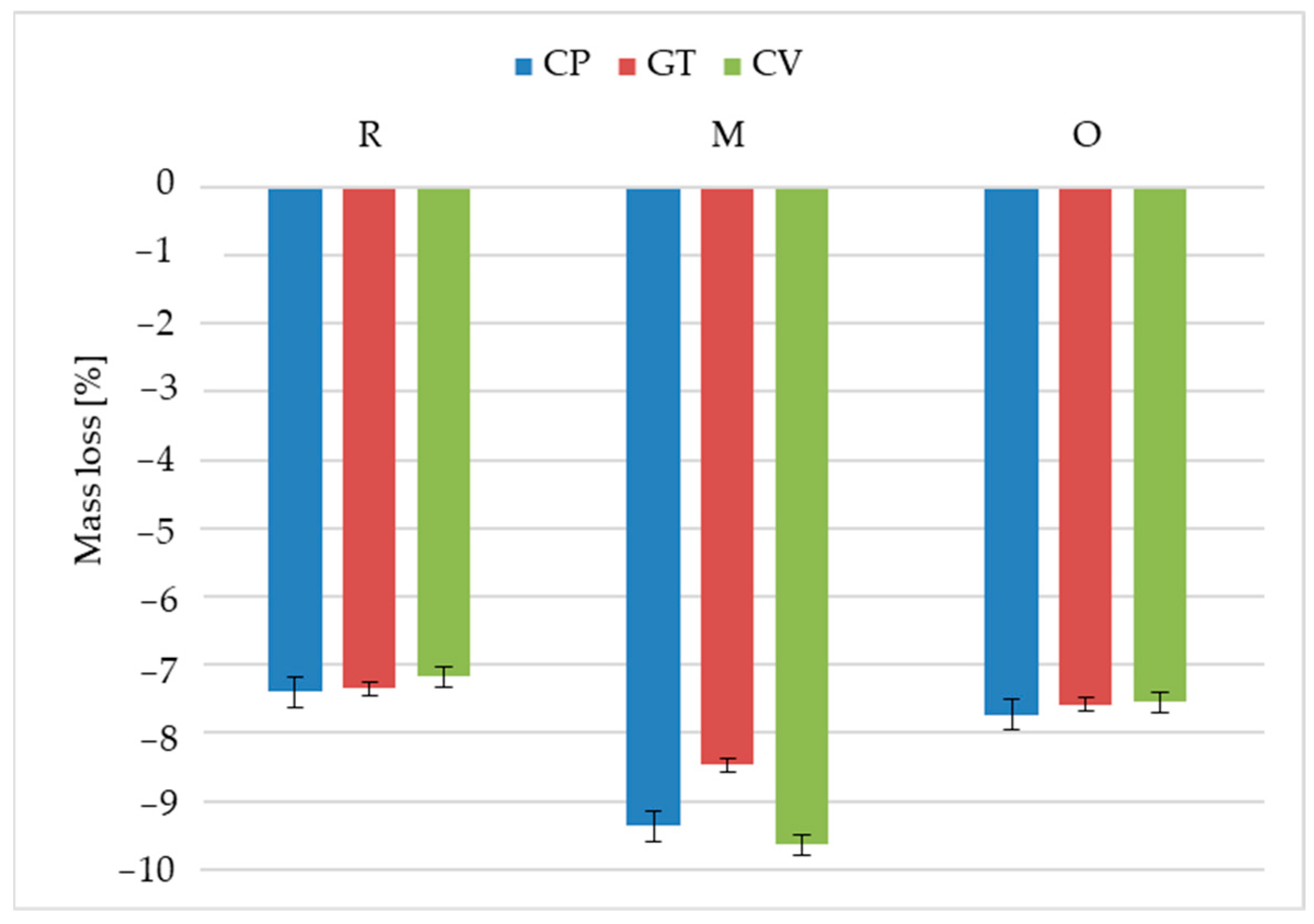
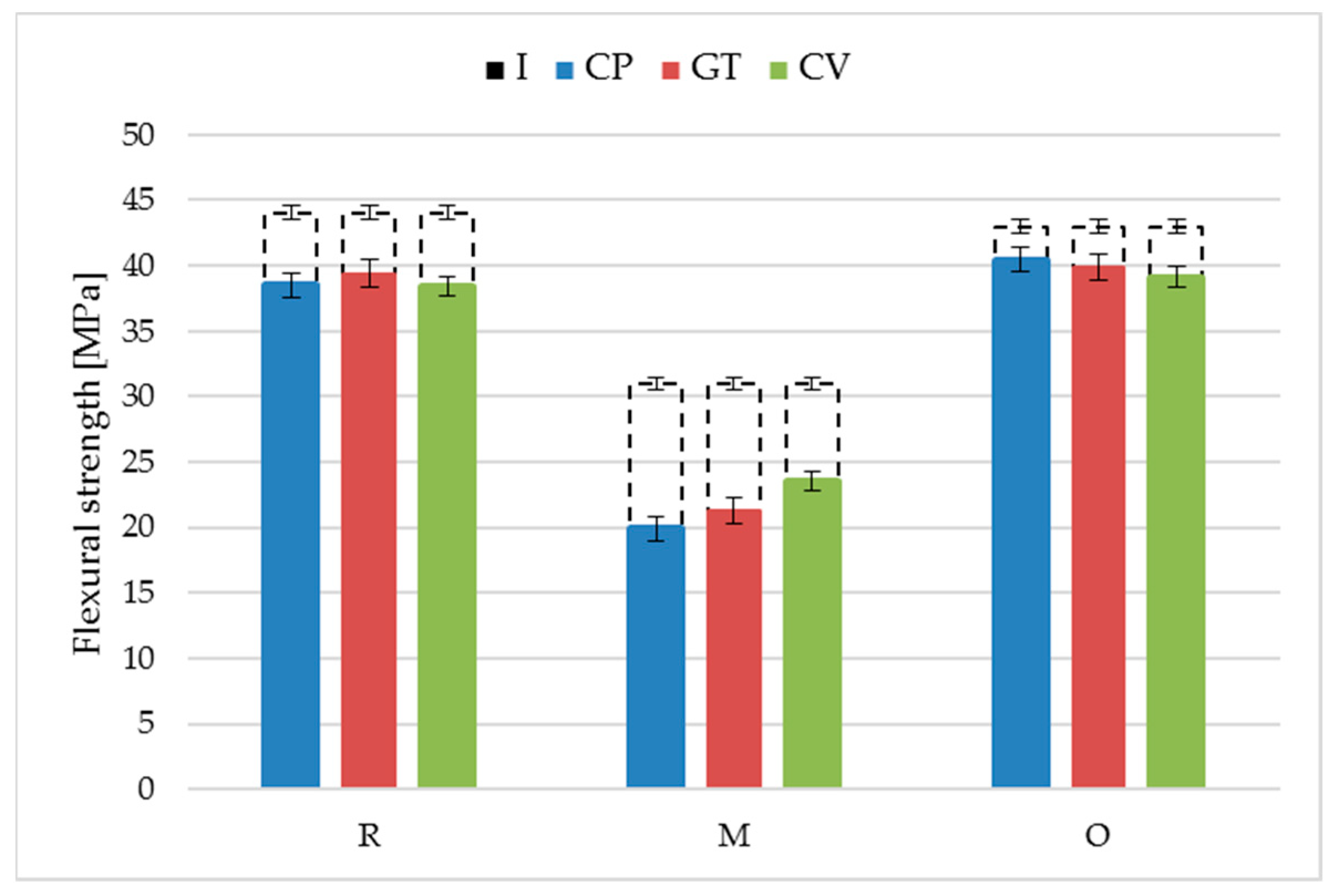
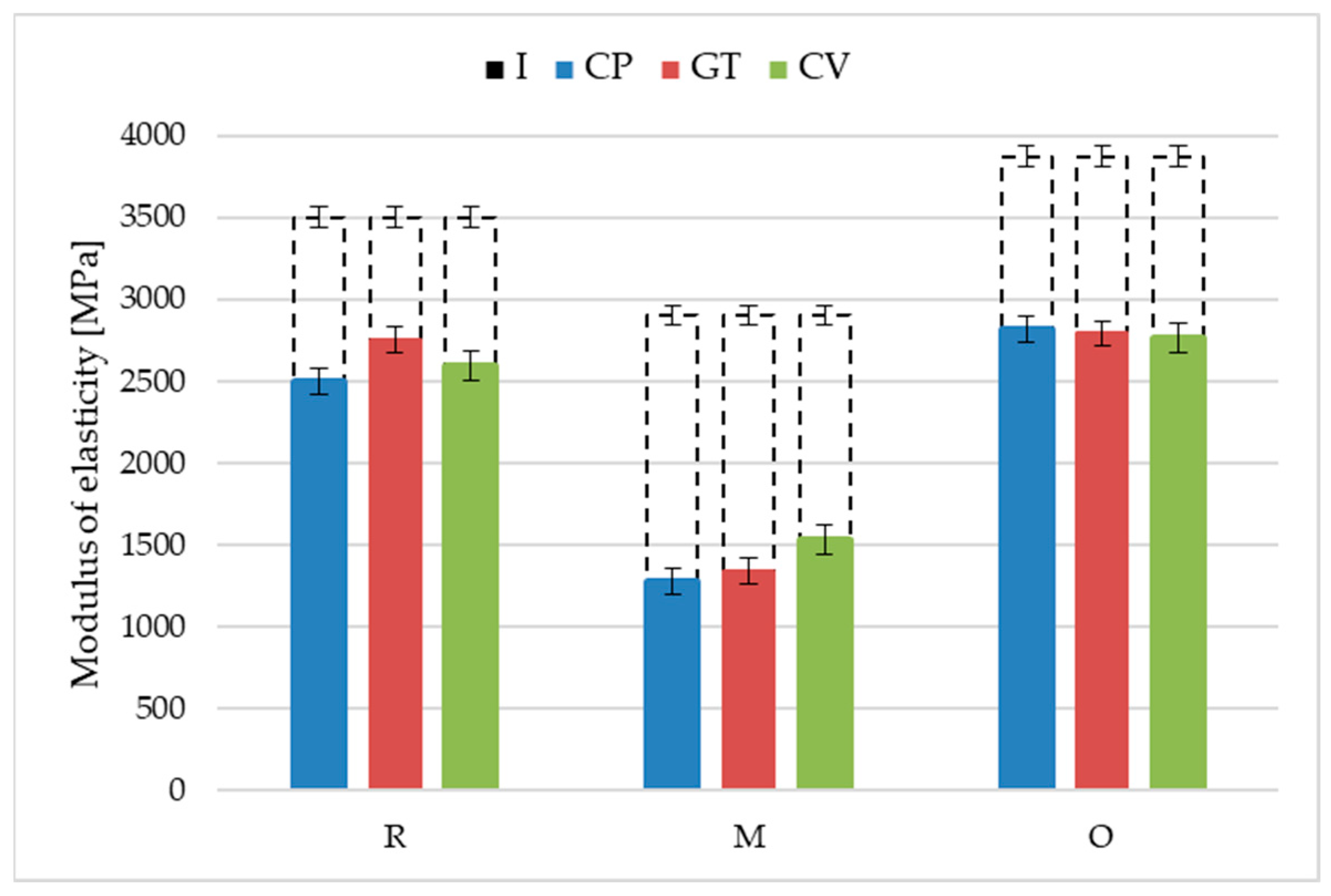
3.3. Performance
4. Conclusions
- The resistance of oat-husk-reinforced profiles to wood-decomposing fungi was similar to that of the reference rice-husk-reinforced profiles. A similar growth rate of Coniophora puteana, Gloeophyllum trabeum and Coriolus versicolor was found for both solutions. The changes in the surface morphology were also identical. The mass loss and decreases in flexural strength and modulus of elasticity after exposure to the fungi were similar.
- The millet-husk-reinforced profiles were susceptible to Agaricomycetes fungi. The mycelia were more developed, especially in the case of Coniophora puteana and Coriolus versicolor. Surface degradation was observed, including numerous voids left after the fillers, contributing to weight, flexural strength, and modulus of elasticity changes.
- Considering the biodegradation resistance of the oat-husk-reinforced profiles, they may be suitable for use in terraces. This may be a reason for further considering the use of residual materials from the hulling of oat grains in the production of polymer composites, which is an environmental benefit. The susceptibility of millet-husk-reinforced profiles to microbiological factors seems too high to be appropriate for such applications.
- Various fungal species had different effects on cereal-husk-reinforced profiles. Coniophora puteana and Coriolus versicolor affected the performance of the composites. Further studies are planned to determine the susceptibility of cereal-husk-reinforced profiles to other microorganism groups and environmental factors. This project was undertaken with the main goal of evaluating the changes in the surface properties of terrace profiles under abiotic factors and the effect of these changes on the susceptibility to fungal and algal growth. The influence of abiotic factors was simulated by artificial methods, as well as several years of observations under natural conditions. The new experimental data will be analysed in terms of the service life of terrace profiles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef] [PubMed]
- Buchner, J.; Irle, M.; Belloncle, C.; Michaud, F.; Macchioni, N. Fungal and bacterial colonies growing on weathered wood surfaces. Wood Mater. Sci. Eng. 2019, 14, 33–41. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Maraveas, C. Production of Sustainable Construction Materials Using Agro-Wastes. Materials 2020, 13, 262. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Sudoł, E.; Kozikowska, E.; Szewczak, E. Artificial Weathering Resistance Test Methods for Building Performance Assessment of Profiles Made of Natural Fibre-Reinforced Polymer Composites. Materials 2022, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Sudoł, E. Outdoor floors made of composite boards. In Technical Conditions for the Execution and Acceptance of Construction Works. Part B. Finishing Works; Research Building Institute: Warsaw, Poland, 2021; Volume 17. (In Polish) [Google Scholar]
- Irbe, I.; Zommere, Ž.; Grinins, J. Water-Related Properties and Biological Durability of Wood-Based Composites. Drv. Ind. 2024, 75, 99–106. [Google Scholar] [CrossRef]
- Carlsson, L.A.; Du, E. Water Uptake in Polymer Composites with Voids. In Durability of Composites in a Marine Environment 2. Solid Mechanics and Its Applications; Davies, P., Rajapakse, Y., Eds.; Springer: Cham, Switzerland, 2018; Volume 245. [Google Scholar] [CrossRef]
- Ibach, R.; Gnatowski, M.; Sun, G.; Glaeser, J.; Leung, M.; Haight, J. Laboratory and environmental decay of wood–plastic composite boards: Flexural properties. Wood Mater. Sci. Eng. 2017, 13, 81–96. [Google Scholar] [CrossRef]
- Meyer, L.; Brischke, C. Fungal decay at different moisture levels of selected European-grown wood species. Int. Biodeterior. Biodegrad. 2015, 103, 23–29. [Google Scholar] [CrossRef]
- Wiejak, A.; Francke, B. Testing and Assessing Method for the Resistance of Wood-Plastic Composites to the Action of Destroying Fungi. Materials 2021, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Brischke, C.; Koddenberg, T.; Buschalsky, A.; Militz, H.; Krause, A. Resistance of Injection Molded Wood-Polypropylene Composites against Basidiomycetes According to EN 15534-1: New Insights on the Test Procedure, Structural Alterations, and Impact of Wood Source. Fibers 2019, 7, 92. [Google Scholar] [CrossRef]
- Catto, A.; Montagna, L.; Almeida, S.; Silveira, R.; Santana, R. Wood plastic composites weathering: Effects of compatibilization on biodegradation in soil and fungal decay. Int. Biodeterior. Biodegrad. 2016, 109, 11–22. [Google Scholar] [CrossRef]
- Altuntas, E.; Yilmaz, E.; Salan, T.; Alma, M.H. Biodegradation properties of wood-plastic composites containing high content of lignocellulosic filler and zinc borate exposed to two different brown-rot fungi. BioResources 2017, 12, 7161–7177. [Google Scholar] [CrossRef]
- Floudas, D.; Held, B.W.; Riley, R.; Nagy, L.G.; Koehler, G.; Ransdell, A.S.; Younus, H.; Chow, J.; Chiniquy, J.; Lipzen, A.; et al. Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genet. Biol. 2015, 76, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Sudoł, E.; Wasiak, M. Slip resistance of wood-polymer composite decking profiles. Ann. WULS-SGGW For. Wood Technol. 2018, 104, 31–35. [Google Scholar]
- Stark, N.M.; Matuana, L.M.; Clemons, C.M. Effect of processing method on surface and weathering characteristics of wood-flour/HDPE composites. J. Appl. Polym. Sci. 2004, 93, 1021–1030. [Google Scholar] [CrossRef]
- Stark, N.M.; Matuana, L.M. Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym. Degrad. Stab. 2004, 86, 1–9. [Google Scholar] [CrossRef]
- Stark, N.M. Effect of weathering cycle and manufacturing method on performance of wood flour and high-density polyethylene composites. J. Appl. Polym. Sci. 2006, 100, 3131–3140. [Google Scholar] [CrossRef]
- Väisänen, T.; Das, O.; Tomppo, L. A review on new bio-based constituents for natural fiber-polymer composites. J. Clean. Prod. 2017, 149, 582–596. [Google Scholar] [CrossRef]
- Vercher, J.; Fombuena, V.; Diaz, A.; Soriano, M. Influence of fibre and matrix characteristics on properties and durability of wood–plastic composites in outdoor applications. J. Thermoplast. Compos. Mater. 2020, 33, 477–500. [Google Scholar] [CrossRef]
- Pratheep, V.; Priyanka, E.; Hare Prasad, P. Characterization and Analysis of Natural Fibre-Rice Husk with Wood Plastic Composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 561, 012066. [Google Scholar]
- Sudoł, E.; Kozikowska, E.; Choińska, E. The Utility of Recycled Rice Husk-Reinforced PVC Composite Profiles for Façade Cladding. Materials 2022, 15, 3418. [Google Scholar] [CrossRef] [PubMed]
- Azman, M.A.; Asyraf, M.R.M.; Khalina, A.; Petrů, M.; Ruzaidi, C.M.; Sapuan, S.M.; Wan Nik, W.B.; Ishak, M.R.; Ilyas, R.A.; Suriani, M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers 2021, 13, 1917. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Lau, K.; Hung, P.; Zhu, M.; Hui, D. Properties of natural fibre composites for structural engineering applications. Compos. Part B Eng. 2008, 136, 222–233. [Google Scholar] [CrossRef]
- Miller, S.; Srubar, W., III.; Billington, S.; Lepech, M. Integrating durability-based service-life predictions with environmental impact assessments of natural fiber–reinforced composite materials. Resour. Conserv. Recycl. 2008, 99, 72–83. [Google Scholar] [CrossRef]
- Sethi, S.; Ray, B.C. Environmental effects on fibre reinforced polymeric composites: Evolving reasons and remarks on interfacial strength and stability. Adv. Colloid Interface Sci. 2015, 217, 43–67. [Google Scholar] [CrossRef]
- Czarnecki, L.; Van Gemert, D. Scientific basis and rules of thumb in civil engineering: Conflict or harmony? Bull. Pol. Acad. Sci. Tech. Sci. 2016, 64, 665–673. [Google Scholar] [CrossRef][Green Version]
- Jin, D.; Meyer, T.K.; Chen, S.; Boateng, K.A.; Pearce, J.M.; You, Z. Evaluation of lab performance of stamp sand and acrylonitrile styrene acrylate waste composites without asphalt as road surface materials. Constr. Build. Mater. 2022, 338, 127569. [Google Scholar] [CrossRef]
- EN 15534-1:2017; Composites Made from Cellulose-Based Materials and Thermoplastics (Usually Called Wood-Polymer Composites (WPC) or Natural Fibre Composites (NFC)). Part 1: Test Methods for Characterisation of Compounds and Products. European Committee for Standardization (CEN): Brussels, Belgium, 2017.
- ISO 178:2019; Plastics. Determination of Flexural Properties. International Organization for Standardization ISO: Geneva, Switzerland, 2019.
- Sudoł, E.; Szewczak, E.; Goron, M. Precision of fungal resistance test method for cereal husk-reinforced composite construction profiles considering mycelium removal techniques. Materials 2025, 18, 411. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers- A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Bader, T.; Hofstetter, K.; Alfredsen, G.; Bollmus, S. Microstructure and stiffness of Scots pine (Pinus sylvestris L) sapwood degraded by Gloeophyllum trabeum and Trametes versicolor—Part I: Changes in chemical composition, density and equilibrium moisture content. Holzforschung 2012, 66, 191–198. [Google Scholar] [CrossRef]
- Piekarczuk, A.; Szewczak, E.; Kozikowska, E.; Gołębiowski, Ł. 3D Scanning of wood–plastic composite decking after cyclic thermal action. Materials 2025, 18, 97. [Google Scholar] [CrossRef]
- Xu, K.; Feng, J.; Zhong, T.; Zheng, Z.; Chen, T. Effects of volatile chemical components of wood species on mould growth susceptibility and termite attack resistance of wood plastic composites. Int. Biodeterior. Biodegrad. 2015, 100, 106–115. [Google Scholar] [CrossRef]
- Nadali, E.; Tajvidi, M.; Naghdi, R. Effects of fungal biodegradation on structure–property relationships of medium density fibre board and hybrid polypropylene composite made from sugar-cane residue. Int. Wood Prod. J. 2021, 12, 152–163. [Google Scholar] [CrossRef]
- Bao, M.; Li, N.; Bao, Y.; Li, J.; Zhong, H.; Chen, Y.; Yu, Y. Outdoor Wood Mats-Based Engineering Composite: Influence of Process Parameters on Decay Resistance against Wood-Degrading Fungi Trametes versicolor and Gloeophyllum trabeum. Polymers 2021, 13, 3173. [Google Scholar] [CrossRef]
- Bao, M.; Zhang, W.; He, L.; Bao, Y.; Wu, Z.; Yu, W.; Chen, Y.; Li, N. Changes in chemical composition, crystallinity, and microstructure of wood fiber mat-reinforced composite caused by white-rot fungus Trametes versicolor and brown-rot fungus Gloeophyllum trabeum. Wood Mater. Sci. Eng. 2023, 18, 1715–1723. [Google Scholar] [CrossRef]
- Schirp, A.; Wolcott, M.P. Influence of fungal decay and moisture absorption on mechanical properties of extruded wood-plastic composites. Wood Fiber Sci. 2005, 37, 643–652. [Google Scholar]
- Segerholm, B.; Ibach, R.; Wålinder, M. Moisture sorption in artificially aged wood-plastic composites. BioResources 2012, 7, 1283–1293. [Google Scholar] [CrossRef]
- Machado, J.; Santos, S.; Pinho, F.; Luís, F.; Alves, A.; Simões, R.; Rodrigues, J. Impact of high moisture conditions on the serviceability performance of wood plastic composite decks. Mater. Des. 2016, 103, 122–131. [Google Scholar] [CrossRef]
- Witomski, P.; Olek, W.; Bonarski, J.T. Changes in strength of Scots pine wood (Pinus silvestris L.) decayed by brown rot (Coniophora puteana) and white rot (Trametes versicolor). Constr. Build. Mater. 2016, 102, 162–166. [Google Scholar] [CrossRef]
- Bader, T.; Hofstetter, K.; Alfredsen, G.; Bollmus, S. Changes in microstructure and stiffness of Scots pine (Pinus sylvestris L) sapwood degraded by Gloeophyllum trabeum and Trametes versicolor—Part II: Anisotropic stiffness properties. Holzforschung 2012, 66, 199–206. [Google Scholar] [CrossRef]
- Bochkov, I.A.; Varkale, M.; Meri, R.M.; Zicans, J.; Franciszczak, P.; Bledzki, A.K. Polypropylene composites wear resistance properties due to spelt and oat grain husks short fiber preparation technology. In Proceedings of the International Scientific Conference “BALTTRIB 2019”, Kaunas, Lithuania, 14–16 November 2019; Volume 2019, pp. 1–6. [Google Scholar] [CrossRef]
- Grewal, R. Investigations on Biocomposites from Oat Hull and Biodegradable Polymers. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2016. [Google Scholar]
- Coffta, G.; Borysiak, S.; Doczekalska, B.; Garbrczyk, J. Resistance of polypropylene-wood composites to fungi. Polimery 2006, 51, 276–279. [Google Scholar] [CrossRef][Green Version]
- Lucejko, J.; Mattonai, M.; Zborowska, M.; Tamburini, D.; Cofta, G.; Cantisani, E.; Kúdela, J.; Cartwright, C.; Colombini, M.; Ribechini, E.; et al. Deterioration effects of wet environments and brown rot fungus Coniophora puteana on pine wood in the archaeological site of Biskupin (Poland). Microchem. J. 2018, 138, 132–146. [Google Scholar] [CrossRef]
- Schmidt, O.; Sheng Wei, D.; Liese, W.; Wollenberg, E. Fungal degradation of bamboo samples. Holzforschung 2011, 65, 883–888. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Brunetti, M.; Macchioni, N.; Nocetti, M.; Palanti, S. Mechanical and physical properties of Cunninghamia lanceolata wood decayed by brown rot. Iforest Biogeosci. For. 2019, 12, 317–322. [Google Scholar] [CrossRef]
- Umezawa, K.; Niikura, M.; Kojima, Y.; Goodell, B.; Yoshida, M. Transcriptome analysis of the brown rot fungus Gloeophyllum trabeum during lignocellulose degradation. PLoS ONE 2020, 15, 0243984. [Google Scholar] [CrossRef]
- Benthien, J.; Thoemen, H.; Maikowski, S.; Lenz, M. Resistance of Flat-Pressed Wood-Plastic Composites to Fungal Decay: Effects of Wood Flour Content, Density, and Manufacturing Technology. Wood Fiber Sci. 2012, 44, 422–429. [Google Scholar]
- Hamzeh, Y.; Ashori, A.; Marvast, E.; Rashedi, K.; Olfat, A. A comparative study on the effects of Coriolus versicolor on properties of HDPE/wood flour/paper sludge composites. Compos. Part B Eng. 2012, 43, 2409–2414. [Google Scholar] [CrossRef]
| Profile | Plant Filler | Matrix | Mineral Filler |
|---|---|---|---|
| O | pulverised oat husks (30 phr) | PVC (100 phr) | CaCO3 (50 phr) |
| M | pulverised millet husks (30 phr) | ||
| R | rice husks (details no available) | no data available |
| Profile | Fungi | Min | Max | Medium | Median |
|---|---|---|---|---|---|
| R | Coniophora puteana | −7.49 | −7.23 | −7.40 | −7.44 |
| Gloeophyllum trabeum | −7.56 | −7.19 | −7.35 | −7.31 | |
| Coriolus versicolor | −7.42 | −6.98 | −7.17 | −7.13 | |
| M | Coniophora puteana | −10.07 | −8.43 | −9.37 | −9.47 |
| Gloeophyllum trabeum | −9.20 | −8.08 | −8.47 | −8.39 | |
| Coriolus versicolor | −10.42 | −8.75 | −9.64 | −9.74 | |
| O | Coniophora puteana | −7.98 | −7.55 | −7.73 | −7.65 |
| Gloeophyllum trabeum | −7.84 | −7.35 | −7.58 | −7.57 | |
| Coriolus versicolor | −7.80 | −7.44 | −7.55 | −7.48 |
| Profile | Fungi | Min | Max | Medium | Median |
|---|---|---|---|---|---|
| R | Coniophora puteana | 36.63 | 40.72 | 38.50 | 38.26 |
| Gloeophyllum trabeum | 37.03 | 40.82 | 39.41 | 39.53 | |
| Coriolus versicolor | 36.61 | 40.68 | 38.42 | 38.45 | |
| M | Coniophora puteana | 15.54 | 25.17 | 19.95 | 18.52 |
| Gloeophyllum trabeum | 19.34 | 22.95 | 21.25 | 21.46 | |
| Coriolus versicolor | 20.78 | 27.88 | 23.51 | 23.11 | |
| O | Coniophora puteana | 39.46 | 42.04 | 40.48 | 40.23 |
| Gloeophyllum trabeum | 36.63 | 42.63 | 39.83 | 39.96 | |
| Coriolus versicolor | 36.42 | 40.40 | 39.15 | 39.79 |
| Profile | Fungi | Min | Max | Medium | Median |
|---|---|---|---|---|---|
| R | Coniophora puteana | 2288 | 2884 | 2495 | 2424 |
| Gloeophyllum trabeum | 2434 | 2910 | 2749 | 2784 | |
| Coriolus versicolor | 2276 | 2786 | 2594 | 2605 | |
| M | Coniophora puteana | 1085 | 1515 | 1274 | 1217 |
| Gloeophyllum trabeum | 1119 | 1522 | 1337 | 1352 | |
| Coriolus versicolor | 1204 | 1695 | 1529 | 1619 | |
| O | Coniophora puteana | 2611 | 2923 | 2819 | 2846 |
| Gloeophyllum trabeum | 2521 | 3031 | 2791 | 2811 | |
| Coriolus versicolor | 2473 | 3089 | 2766 | 2747 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goron, M.; Sudoł, E.; Kozikowska, E. Resistance of Cereal-Husk-Reinforced PVC Terrace Profiles to Agaricomycetes Fungi. Materials 2025, 18, 2860. https://doi.org/10.3390/ma18122860
Goron M, Sudoł E, Kozikowska E. Resistance of Cereal-Husk-Reinforced PVC Terrace Profiles to Agaricomycetes Fungi. Materials. 2025; 18(12):2860. https://doi.org/10.3390/ma18122860
Chicago/Turabian StyleGoron, Mariia, Ewa Sudoł, and Ewelina Kozikowska. 2025. "Resistance of Cereal-Husk-Reinforced PVC Terrace Profiles to Agaricomycetes Fungi" Materials 18, no. 12: 2860. https://doi.org/10.3390/ma18122860
APA StyleGoron, M., Sudoł, E., & Kozikowska, E. (2025). Resistance of Cereal-Husk-Reinforced PVC Terrace Profiles to Agaricomycetes Fungi. Materials, 18(12), 2860. https://doi.org/10.3390/ma18122860






