Recycled Clay Brick Powder as a Dual-Function Additive: Mitigating the Alkali–Silica Reaction (ASR) and Enhancing Strength in Eco-Friendly Mortar with Hybrid Waste Glass and Clay Brick Aggregates
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Sample Preparation
2.3. Testing
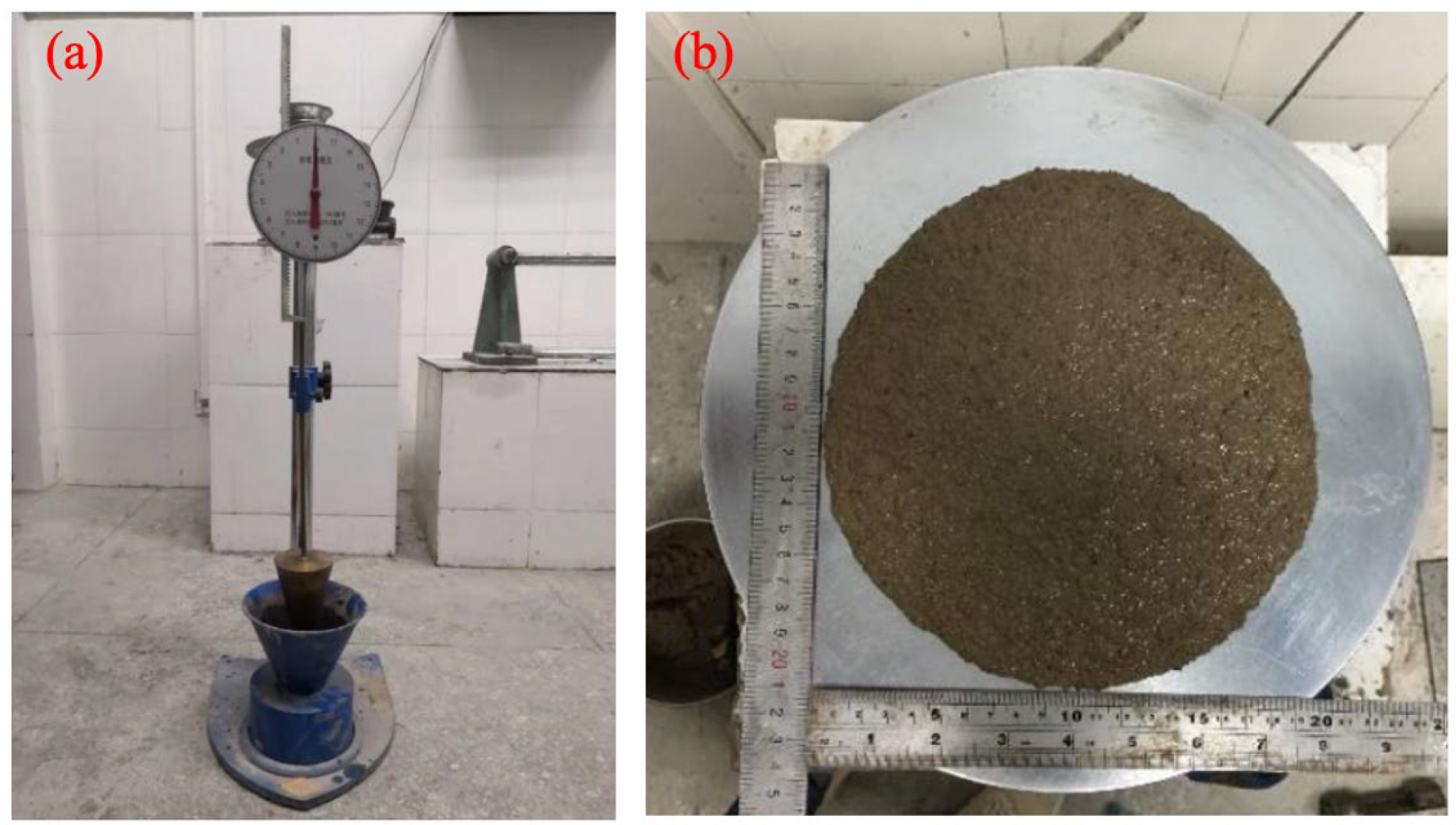
2.3.1. Consistency and Fluidity
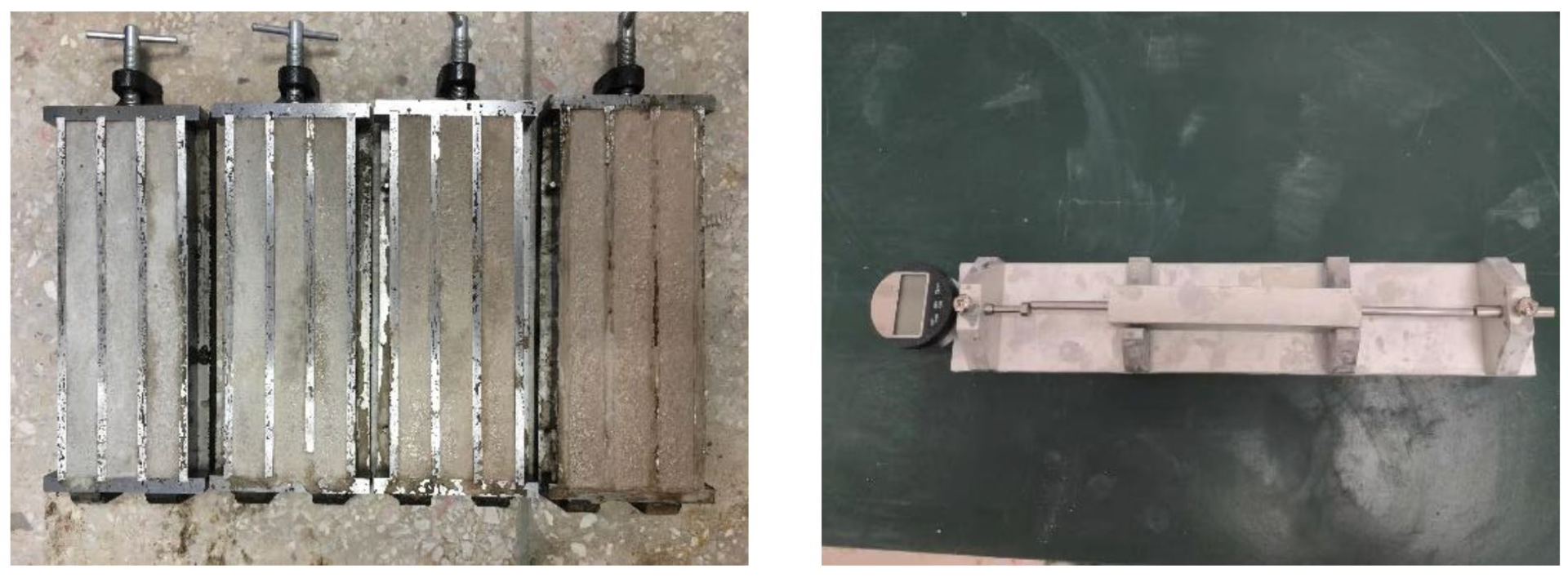
2.3.2. Mechanical Properties and Water Absorption
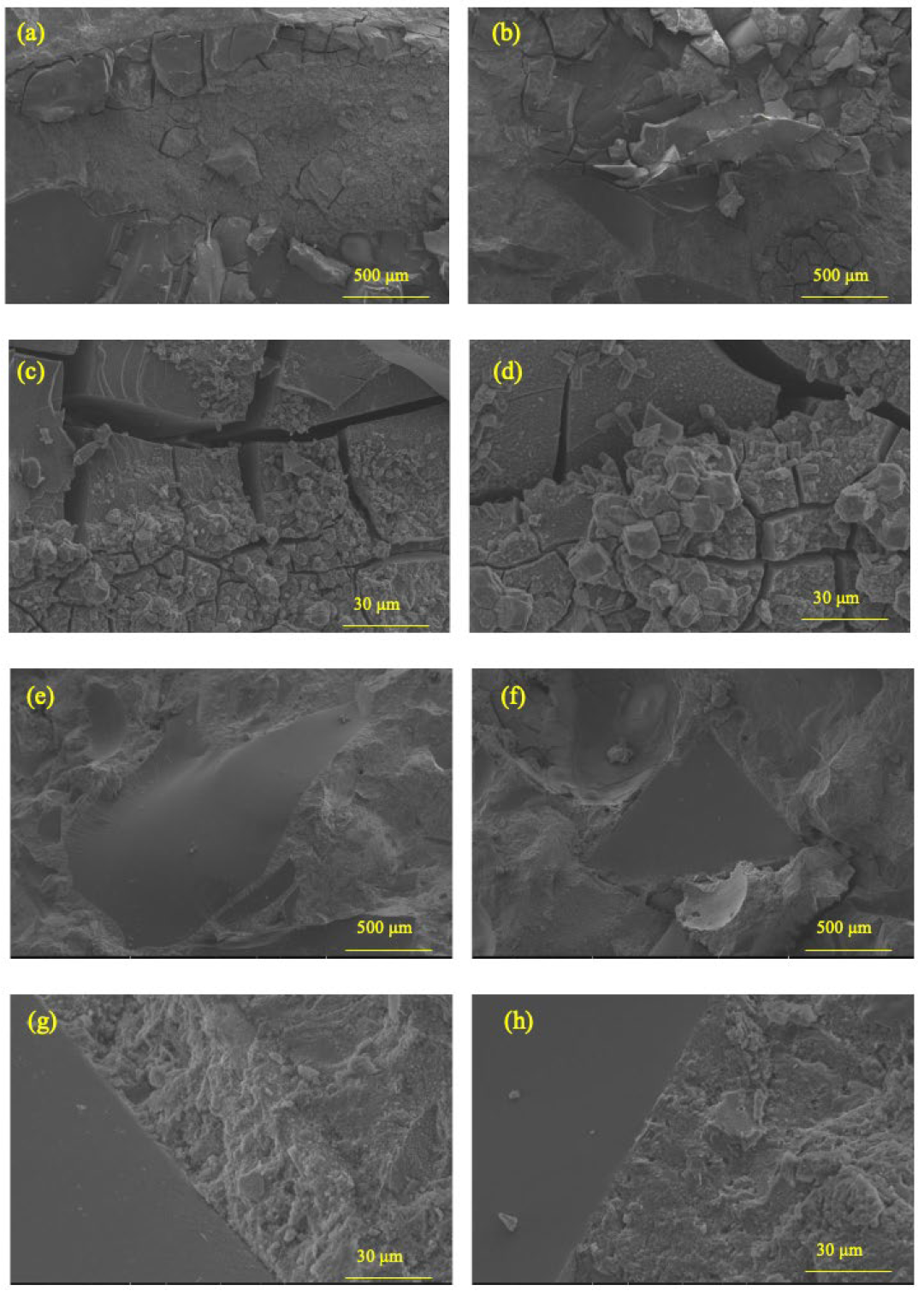
2.3.3. Micrographs via ESEM
2.3.4. Activity Index of Recycled Clay Brick Powder
2.3.5. Rapid Alkali–Silica Reaction (ASR)
3. Results and Discussion
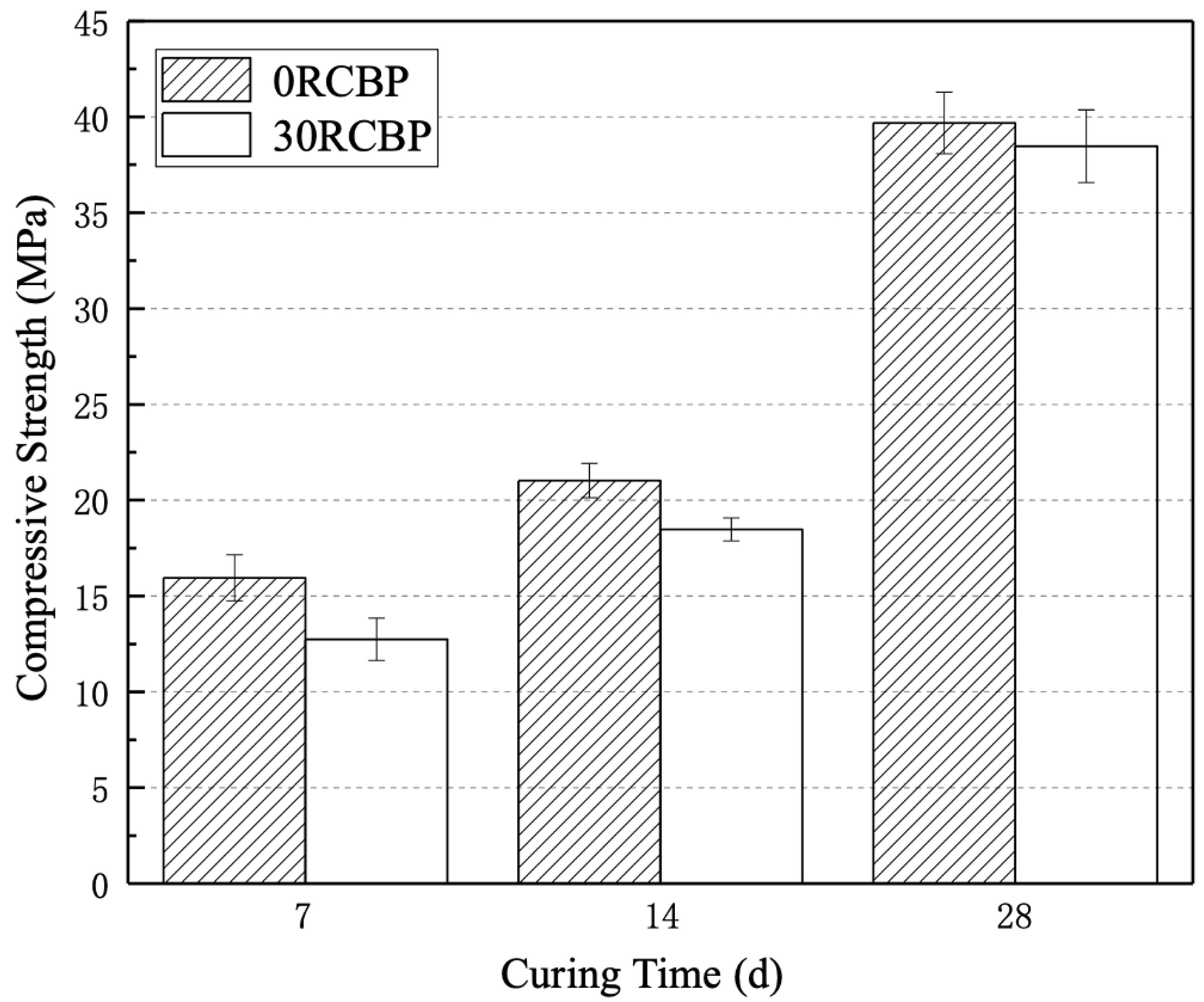
3.1. Activity of Recycled Clay Brick Powder
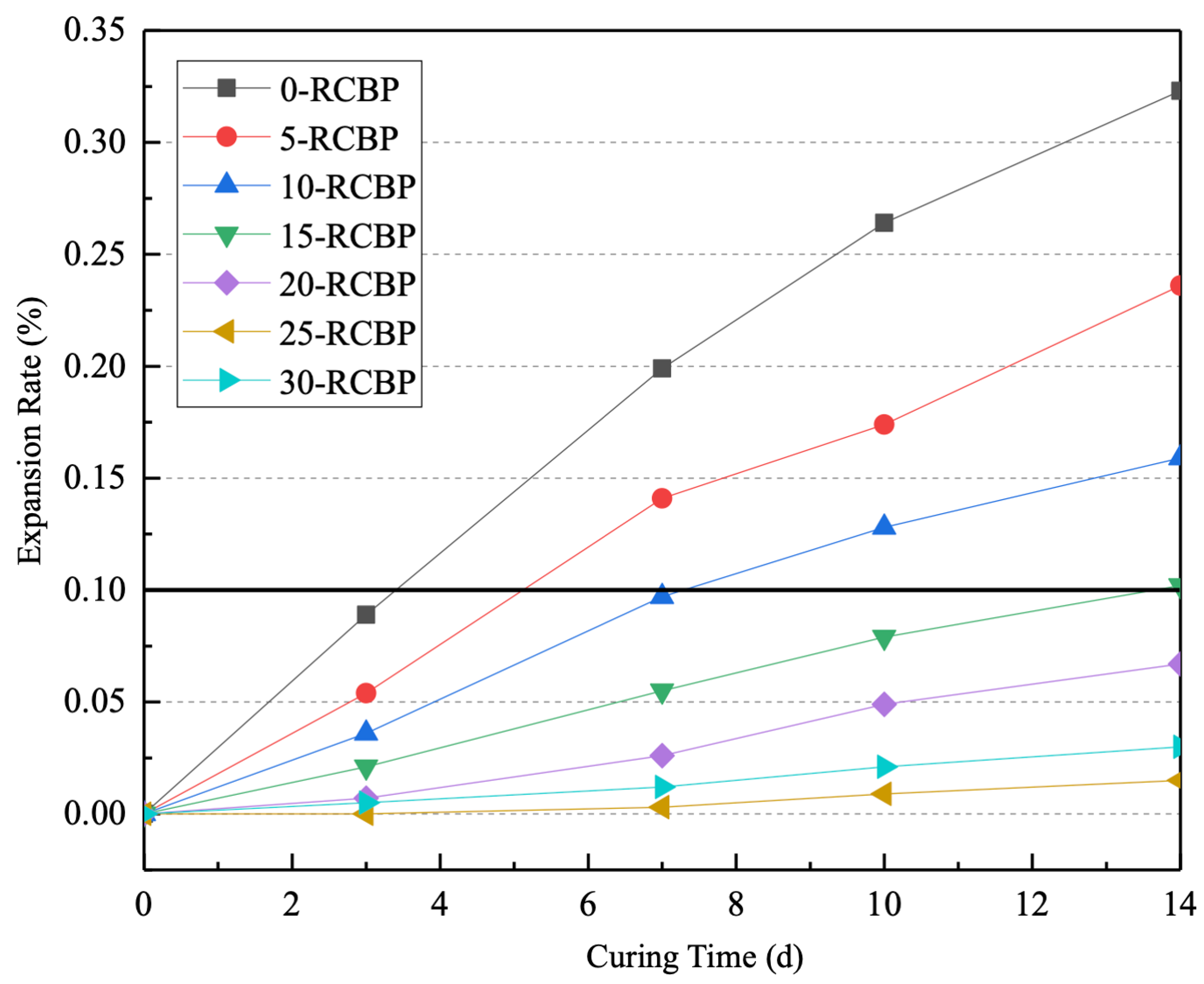
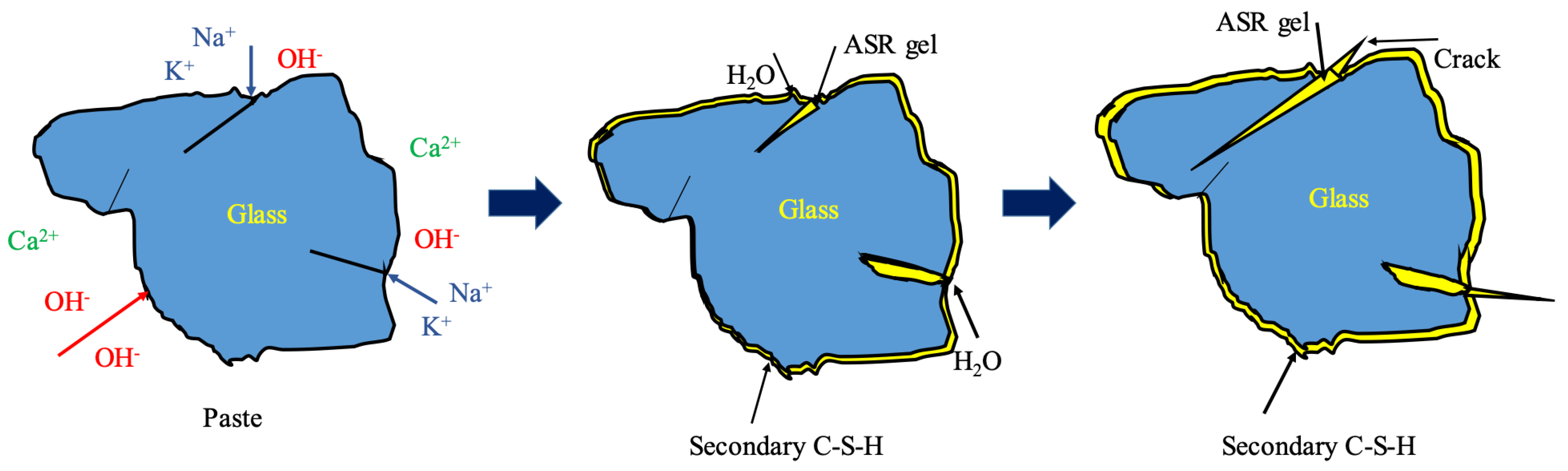
3.2. Rapid Alkali–Silica Reaction
3.3. Consistency and Fluidity
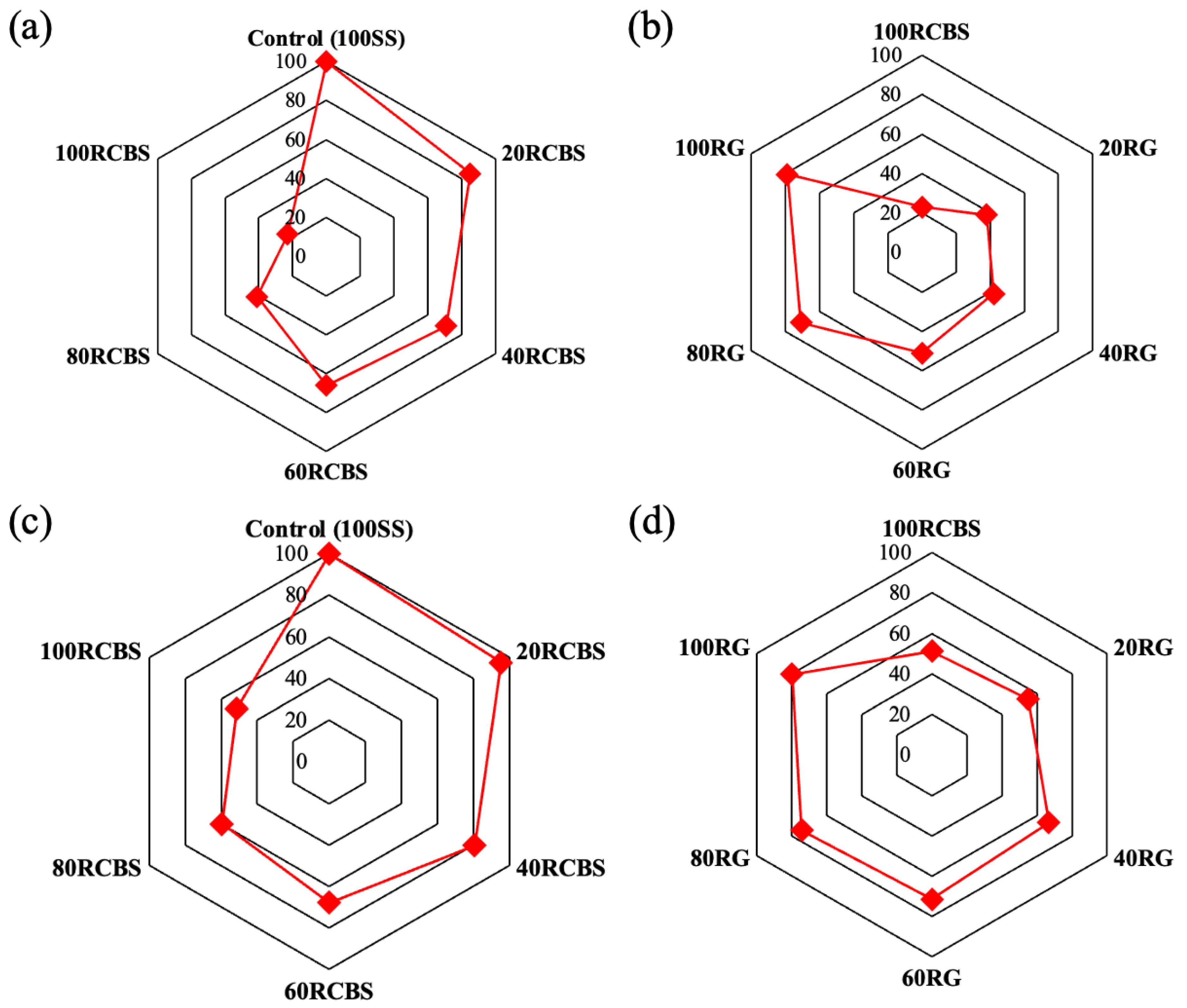
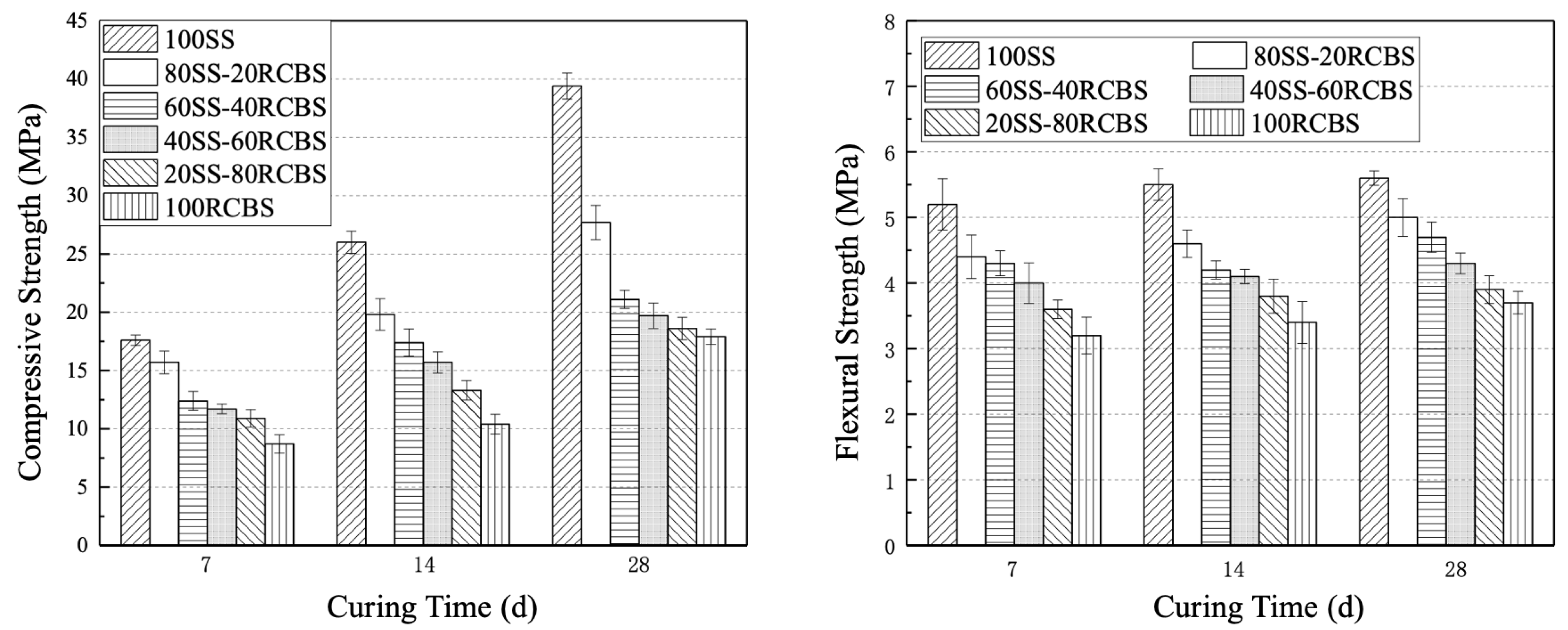
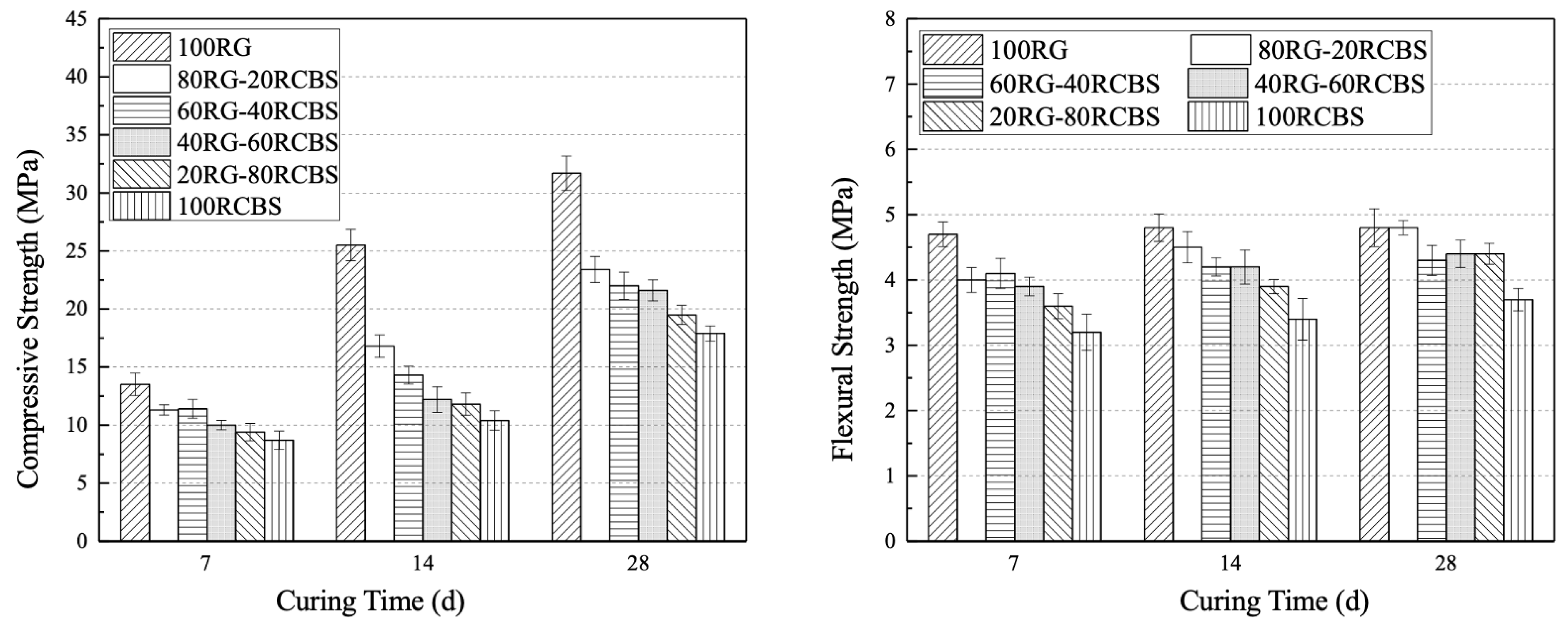
3.4. Mechanical Strength
4. Conclusions
- Firstly, as the curing time increases, the activity index of mortar specimens with a 30% substitution ratio of recycled clay brick powder demonstrates a gradual upward trend, reaching 96.95% at 28 days, which is indicative of significant pozzolanic activity. Secondly, a substitution ratio of recycled clay brick powder of no less than 20% effectively mitigates the alkali–silica reaction induced by recycled glass sand, with the optimal substitution ratio being 25%.
- Furthermore, the consistency and fluidity of mortar exhibit a decreasing trend with an increasing substitution ratio of clay brick sand. Notably, SS-RCBS mortar specimens exhibit higher consistency and fluidity than RG-RCBS mortar specimens at the same substitution ratio. However, both the compressive and flexural strengths of SS-RCBS and RG-RCBS mortars decline with increasing substitution ratio of clay brick sand. Additionally, the growth rate of compressive strength significantly surpasses that of flexural strength for all mortar specimens as the curing time extends.
- Lastly, the 28-day water absorption rates of both SS-RCBS and RG-RCBS mortars show an increasing trend with the substitution ratio of clay brick sand. These findings collectively underscore the intricate interplay between the substitution ratio of clay brick sand and the performance characteristics of mortar specimens, providing valuable insights into the optimization of mortar formulations for enhanced durability and functionality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Hu, T.; Liu, J. Decoupling economic growth from construction waste generation: Comparative analysis between the EU and China. J. Environ. Manag. 2024, 353, 120144. [Google Scholar] [CrossRef] [PubMed]
- Hernández García, L.C.; Monteiro, S.N.; Lopera, H.A.C. Recycling Clay Waste from Excavation, Demolition, and Construction: Trends and Challenges. Sustainability 2024, 16, 6265. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Lin, W.; Zheng, J.; Yan, G.; Chen, X.-F. Enhanced Photocatalytic Activity in Photocatalytic Concrete: Synthesis, Characterization, and Comprehensive Performance Assessment of Nano-TiO2-Modified Recycled Aggregates. Catalysts 2024, 14, 711. [Google Scholar] [CrossRef]
- Chen, X.-F.; Chen, W.-Z.; Zhang, X.-C.; Lin, W.-C.; Zheng, J.-S.; Yan, G.-H. Nano-TiO2-Enhanced Surface Functionalization of Recycled Concrete Aggregates for Improved Degradation Efficiency of Low-Concentration Sulfur Dioxide. Catalysts 2024, 14, 709. [Google Scholar] [CrossRef]
- Chen, X.-F.; Kou, S.-C.; Sun Poon, C. Rheological behaviour, mechanical performance, and NOx removal of photocatalytic mortar with combined clay brick sands-based and recycled glass-based nano-TiO2 composite photocatalysts Constr. Build. Mater. 2020, 240, 117698. [Google Scholar] [CrossRef]
- Chen, X.-F.; Zhang, X.-C.; Yan, G.-H. Multiscale Investigation of Modified Recycled Aggregate Concrete on Sulfate Attack Resistance. Materials 2025, 18, 1450. [Google Scholar] [CrossRef]
- Qasim, O.A.; Hilal, N.; Al Biajawi, M.I.; Sor, N.H.; Tawfik, T.A. Studying the usability of recycled aggregate to produce new concrete. J. Eng. Appl. Sci. 2024, 71, 129. [Google Scholar] [CrossRef]
- Wang, A.; Lyu, B.; Zhu, Y.; Liu, K.; Guo, L.; Sun, D. A gentle acid-wash and pre-coating treatment of coral aggregate to manufacture high-strength geopolymer concrete. Constr. Build. Mater. 2021, 274, 121780. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Bedon, C.; Barluenga, G. Study on the Compressive Behaviour of Sustainable Cement-Based Composites under One-Hour of Direct Flame Exposure. Sustainability 2020, 12, 10548. [Google Scholar] [CrossRef]
- Wang, W.; Noguchi, T.; Tomoyose, A.; Zhang, Y.; Maruyama, I. Influence of volcanic glass powder on alkali-silica reaction expansion in alkali-activated slag mortars Cem. Concr. Compos. 2024, 152, 105665. [Google Scholar] [CrossRef]
- Shafaie, V.; Ghodousian, O.; Ghodousian, A.; Gorji, M.; Mehdikhani, H.; Movahedi Rad, M. Shear Bond Strength in Stone-Clad Façades: Effect of Polypropylene Fibers, Curing, and Mechanical Anchorage. Polymers 2024, 16, 2975. [Google Scholar] [CrossRef] [PubMed]
- Banik, D.; He, R.; Lu, N.; Feng, Y. Mitigation mechanisms of alkali silica reaction through the incorporation of colloidal nanoSiO2 in accelerated mortar bar testing Constr. Build. Mater. 2024, 422, 135834. [Google Scholar] [CrossRef]
- Lu, C.-G.; Jiao, C.-J.; Zhang, X.-C.; Lin, W.-C.; Chen, X.-F. Fly Ash-Supported Photocatalysts: Synthesis, Applications, and Advances in Modification Technology. Crystals 2025, 15, 223. [Google Scholar] [CrossRef]
- Chen, X.-F.; Quan, C.-Q.; Jiao, C.-J. Experimental Study of Chloride Resistance of Polypropylene Fiber Reinforced Concrete with Fly Ash and Modeling. Materials 2021, 14, 4417. [Google Scholar] [CrossRef]
- Wang, D.; Shen, X.; Wang, Z.; Zhang, X.; Chen, X.-F. Effect of Quicklime Substitution for Cement on the Physical and Mechanical Properties of Autoclaved Fly Ash Aggregates via Hydrothermal Synthesis. Materials 2025, 18, 707. [Google Scholar] [CrossRef]
- de Oliveira, V.M.; de Souza, F.; da Cruz, R.T.; Py, L.G.; Kirchheim, A.P.; Bragança, S.R. Valorization of non-beneficiated clays as supplementary cementitious materials in the production of cement-based mortar J. Build. Eng. 2021, 42, 102474. [Google Scholar] [CrossRef]
- Meddah, M.S.; Al Owaisi, M.; Abedi, M.; Hago, A.W. Mortar and concrete with lime-rich calcined clay pozzolana: A sustainable approach to enhancing performances and reducing carbon footprint Constr. Build. Mater. 2023, 393, 132098. [Google Scholar] [CrossRef]
- Rehman, M.U.L.; MacLeod, A.J.N.; Gates, W.P. Phase development and mechanical strength of limestone calcined clay cement utilising Australian bentonite and plasterboard waste Constr. Build. Mater. 2024, 445, 137937. [Google Scholar] [CrossRef]
- Pinheiro, V.D.; Alexandre, J.; Xavier, G.d.C.; Marvila, M.T.; Monteiro, S.N.; de Azevedo, A.R.G. Methods for Evaluating Pozzolanic Reactivity in Calcined Clays: A Review. Materials 2023, 16, 4778. [Google Scholar] [CrossRef]
- Overmann, S.; Vollpracht, A.; Matschei, T. Reactivity of Calcined Clays as SCM—A Review. Materials 2024, 17, 312. [Google Scholar] [CrossRef]
- Li, S.; Chen, G.; Zhao, Y.; Xu, Z.; Luo, X.; Liu, C.; Gao, J. Investigation on the reactivity of recycled brick powder Cem. Concr. Compos. 2023, 139, 105042. [Google Scholar] [CrossRef]
- Chen, X.-F.; Kou, S.-C.; Xing, F. Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder. Materials 2019, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, C.; Shi, Q.; Tan, X.; Meng, W. Recycling waste glass aggregate in concrete: Mitigation of alkali-silica reaction (ASR) by carbonation curing J. Clean. Prod. 2022, 370, 133545. [Google Scholar] [CrossRef]
- Wang, T.; San Nicolas, R.; Ngoc Nguyen, T.; Kashani, A.; Ngo, T. Experimental and numerical study of long-term alkali-silica reaction (ASR) expansion in mortar with recycled glass Cem. Concr. Compos. 2023, 139, 105043. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Saha, A.K.; Sarker, P.K. Evaluation of the ASR of waste glass fine aggregate in alkali activated concrete by concrete prism tests. Constr. Build. Mater. 2021, 266, 121121. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kassem, E.; Ibrahim, A. ASR mitigation using binary and ternary blends with waste glass powder Constr. Build. Mater. 2021, 280, 122425. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, K.H.; Du, H.; Ling, T.-C. Effects of CO2 curing treatment on alkali-silica reaction of mortars containing glass aggregate. Constr. Build. Mater. 2022, 323, 126637. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Turning sandstone clay into supplementary cementitious material: Activation and pozzolanic reactivity evaluation. Compos. B Eng. 2021, 223, 109137. [Google Scholar] [CrossRef]
- Al-Shmaisani, S.; Kalina, R.D.; Ferron, R.D.; Juenger, M.C.G. Critical assessment of rapid methods to qualify supplementary cementitious materials for use in concrete. Cem. Concr. Res. 2022, 153, 106709. [Google Scholar] [CrossRef]
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Navarrete, I.; Valdes, J.; Lopez, M.; Vargas, F. Replacement of pozzolanic blended cement by supplementary cementitious materials: Mechanical and environmental approach. Constr. Build. Mater. 2023, 394, 132263. [Google Scholar] [CrossRef]
- Yamamoto, T. Formulation optimization and proposal of Assessed Pozzolanic-activity Index (API) method for rapid evaluation of pozzolanic activity of fly ash. Constr. Build. Mater. 2024, 432, 136394. [Google Scholar] [CrossRef]
- Chen, X.-F.; Jiao, C.-J. Experimental Investigation and Modeling of the Sulfur Dioxide Abatement of Photocatalytic Mortar Containing Construction Wastes Pre-Treated by Nano TiO2. Catalysts 2022, 12, 708. [Google Scholar] [CrossRef]
- Wang, W.; Noguchi, T.; Maruyama, I. Mechanism understanding of alkali-silica reaction in alkali-activated materials system Cem. Concr. Res. 2022, 156, 106768. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, H.; Hu, X.; Yang, H. Experiment study on the mechanical properties and alkali silica reaction (ASR) of mortar blended rice husk ash (RHA). Case Stud. Constr. Mater. 2023, 18, e02028. [Google Scholar] [CrossRef]
- Joo, H.E.; Takahashi, Y. Analytical and experimental studies on alkali-silica reaction mechanism: Aggregate cracking and chemical composition change of gel. Cem. Concr. Compos. 2023, 139, 105003. [Google Scholar] [CrossRef]
- Sinngu, F.; Ekolu, S.O.; Naghizadeh, A.; Quainoo, H.A. Evaluation of metakaolin pozzolan for cement in South Africa. Dev. Built Environ. 2023, 14, 100154. [Google Scholar] [CrossRef]
- Hay, R.; Ostertag, C.P. New insights into the role of fly ash in mitigating alkali-silica reaction (ASR) in concrete. Cem. Concr. Res. 2021, 144, 106440. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kolawole, J.T.; Almakrab, A. Alkali-silica reaction (ASR) in concrete structures: Mechanisms, effects and evaluation test methods adopted in the United States, Case Studies in Construction. Materials 2021, 15, e00563. [Google Scholar] [CrossRef]
- Ban, J.; Fan, D.; Li, K.; Yao, J.; Lu, J.-X.; Wang, Z.; Poon, C.-S. Mechanisms on the inhibition of alkali-silica reaction in supersulfated cement. Cem. Concr. Compos. 2024, 145, 105320. [Google Scholar] [CrossRef]
- Afshinnia, K.; Poursaee, A. The potential of ground clay brick to mitigate Alkali–Silica Reaction in mortar prepared with highly reactive aggregate. Constr. Build. Mater. 2015, 95, 164–170. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Effect of particle size on alkali–silica reaction in recycled glass mortars. Constr. Build. Mater. 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D.A. Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Luo, D.; Sinha, A.; Adhikari, M.; Wei, J. Mitigating alkali-silica reaction through metakaolin-based internal conditioning: New insights into property evolution and mitigation mechanism Cem. Concr. Res. 2022, 159, 106888. [Google Scholar] [CrossRef]
- Urhan, S. Alkali silica and pozzolanic reactions in concrete. Part 1: Interpretation of published results and an hypothesis concerning the mechanism. Cem. Concr. Res. 1987, 17, 141–152. [Google Scholar] [CrossRef]
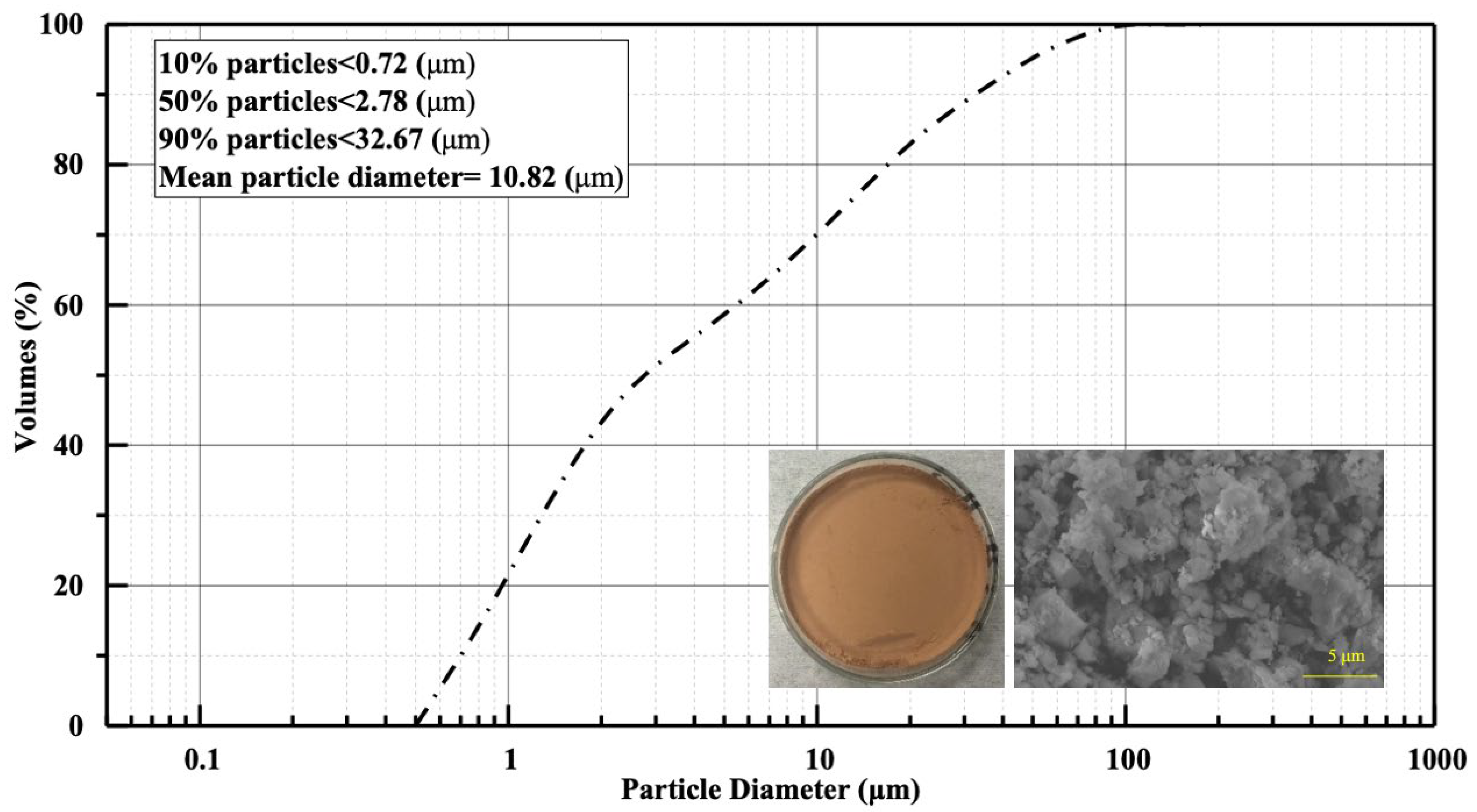





| Notation (%) | Mass |
|---|---|
| Calcium oxide (CaO) | 66.43 |
| Silica (SiO2) | 18.4 |
| Ferric oxide (Fe2O3) | 4.03 |
| Alumina (Al2O3) | 3.73 |
| Sulphuric anhydride (SO3) | 2.65 |
| Magnesium oxide (MgO) | 2.11 |
| Sodium oxide (Na2O) | 1.36 |
| Potassium oxide (K2O) | 0.43 |
| LOI | 0.86 |
| Density (kg/m3) | 3160 |
| Notation | SiO2 (%) | Fe2O3 (%) | Al2O3 (%) | CaO (%) | MgO (%) | K2O (%) | Na2O (%) | SO3 (%) | BD (kg/m3) | WA (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| RCBS | 66.52 | 5.45 | 14.2 | 6.06 | 2.35 | 2.09 | 0.64 | 0.75 | 1360 | 17.1 |
| RG | 69.74 | 0.33 | 2.47 | 11.8 | 1.03 | 0.39 | 10.95 | - | 1480 | 0 |
| Notation | Water | Binder | Functionalized Aggregates | Total Alkali Content (Na2Oeq) (%) | |||
|---|---|---|---|---|---|---|---|
| Cement | RCBP | RCBS | SS | RGS | |||
| 100SS | 0.5 | 0.75 | 0.25 | 0.0 | 2.5 | 0.0 | 0.126 |
| 80SS-20RCBS | 0.5 | 2.0 | 0.0 | 0.252 | |||
| 60SS-40RCBS | 1.0 | 1.5 | 0.0 | 0.504 | |||
| 40SS-60RCBS | 1.5 | 1.0 | 0.0 | 0.756 | |||
| 20SS-80RCBS | 2.0 | 0.5 | 0.0 | 1.008 | |||
| 100RCBS | 2.5 | 0.0 | 0.0 | 1.260 | |||
| 20RG-80RCBS | 2.0 | 0.0 | 0.5 | 2.408 | |||
| 40RG-60RCBS | 1.5 | 0.0 | 1.0 | 3.557 | |||
| 60RG-40RCBS | 1.0 | 0.0 | 1.5 | 4.706 | |||
| 80RG-20RCBS | 0.5 | 0.0 | 2.0 | 5.855 | |||
| 100RG | 0.0 | 0.0 | 2.5 | 7.004 | |||
| Notation | Cement, g | RCBP, g | Standard Sand, g | Water, mL |
|---|---|---|---|---|
| 0RCBP | 450 | - | 1350 | 225 |
| 30RCBP | 315 | 135 | 1350 | 225 |
| Notation | RCBP (g) | Cement (g) | RG (g) | Water (g) |
|---|---|---|---|---|
| 0-RCBP | - | 440 | 990 | 206.8 |
| 5-RCBP | 22 | 418 | 990 | 206.8 |
| 10-RCBP | 44 | 396 | 990 | 206.8 |
| 15-RCBP | 66 | 374 | 990 | 206.8 |
| 20-RCBP | 88 | 352 | 990 | 206.8 |
| 25-RCBP | 110 | 330 | 990 | 206.8 |
| 30-RCBP | 132 | 308 | 990 | 206.8 |
| Size | 150–300 μm | 300–600 μm | 600 μm–1.18 mm | 1.18–2.36 mm | 2.36–4.75 mm |
| Mass/g | 148.5 | 247.5 | 247.5 | 247.5 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-F.; Zhang, X.-C.; Peng, Y. Recycled Clay Brick Powder as a Dual-Function Additive: Mitigating the Alkali–Silica Reaction (ASR) and Enhancing Strength in Eco-Friendly Mortar with Hybrid Waste Glass and Clay Brick Aggregates. Materials 2025, 18, 2838. https://doi.org/10.3390/ma18122838
Chen X-F, Zhang X-C, Peng Y. Recycled Clay Brick Powder as a Dual-Function Additive: Mitigating the Alkali–Silica Reaction (ASR) and Enhancing Strength in Eco-Friendly Mortar with Hybrid Waste Glass and Clay Brick Aggregates. Materials. 2025; 18(12):2838. https://doi.org/10.3390/ma18122838
Chicago/Turabian StyleChen, Xue-Fei, Xiu-Cheng Zhang, and Ying Peng. 2025. "Recycled Clay Brick Powder as a Dual-Function Additive: Mitigating the Alkali–Silica Reaction (ASR) and Enhancing Strength in Eco-Friendly Mortar with Hybrid Waste Glass and Clay Brick Aggregates" Materials 18, no. 12: 2838. https://doi.org/10.3390/ma18122838
APA StyleChen, X.-F., Zhang, X.-C., & Peng, Y. (2025). Recycled Clay Brick Powder as a Dual-Function Additive: Mitigating the Alkali–Silica Reaction (ASR) and Enhancing Strength in Eco-Friendly Mortar with Hybrid Waste Glass and Clay Brick Aggregates. Materials, 18(12), 2838. https://doi.org/10.3390/ma18122838






