Review: The Application of MXene in Thermal Energy Storage Materials for Efficient Solar Energy Utilization
Abstract
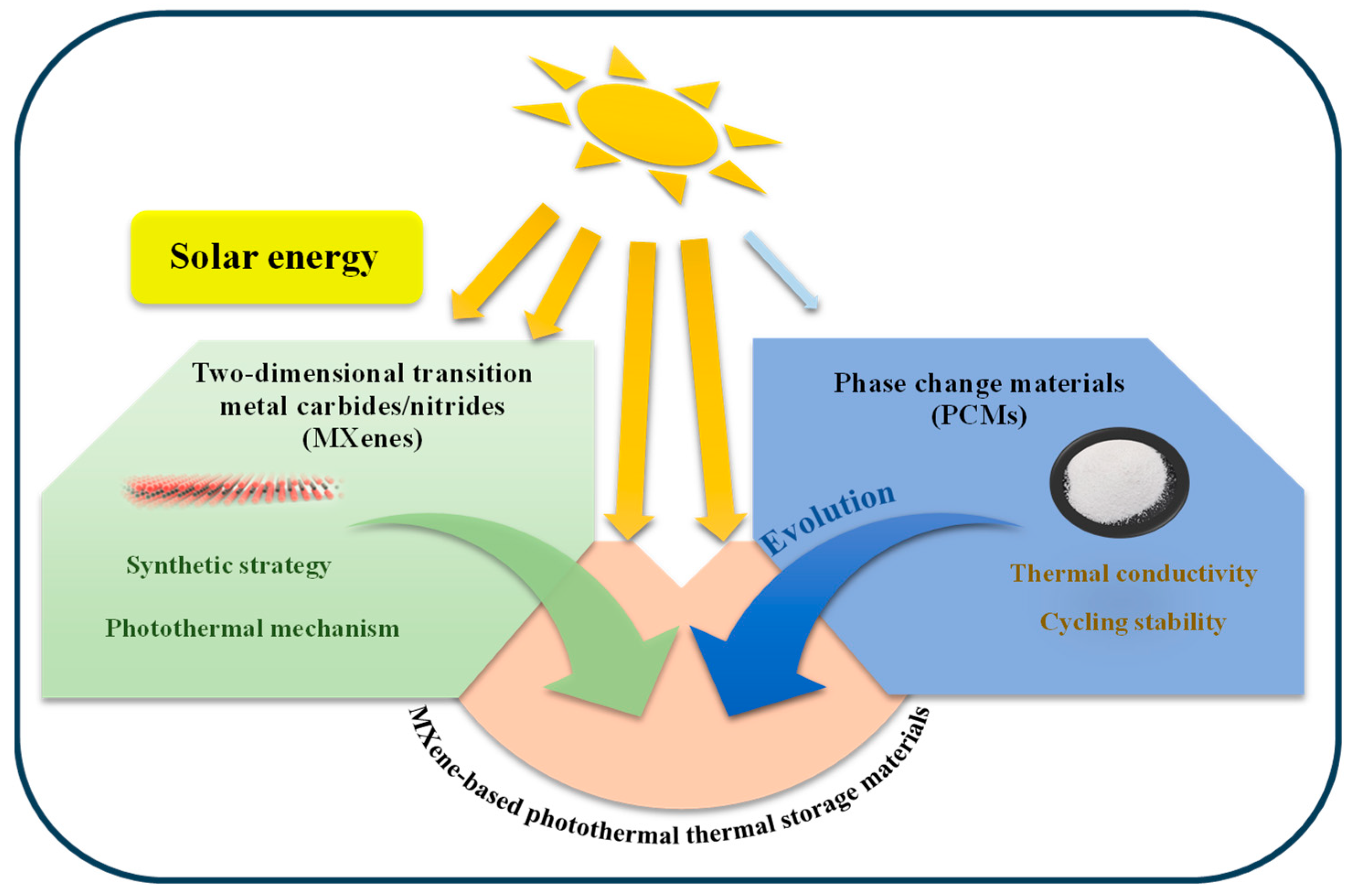
1. Introduction
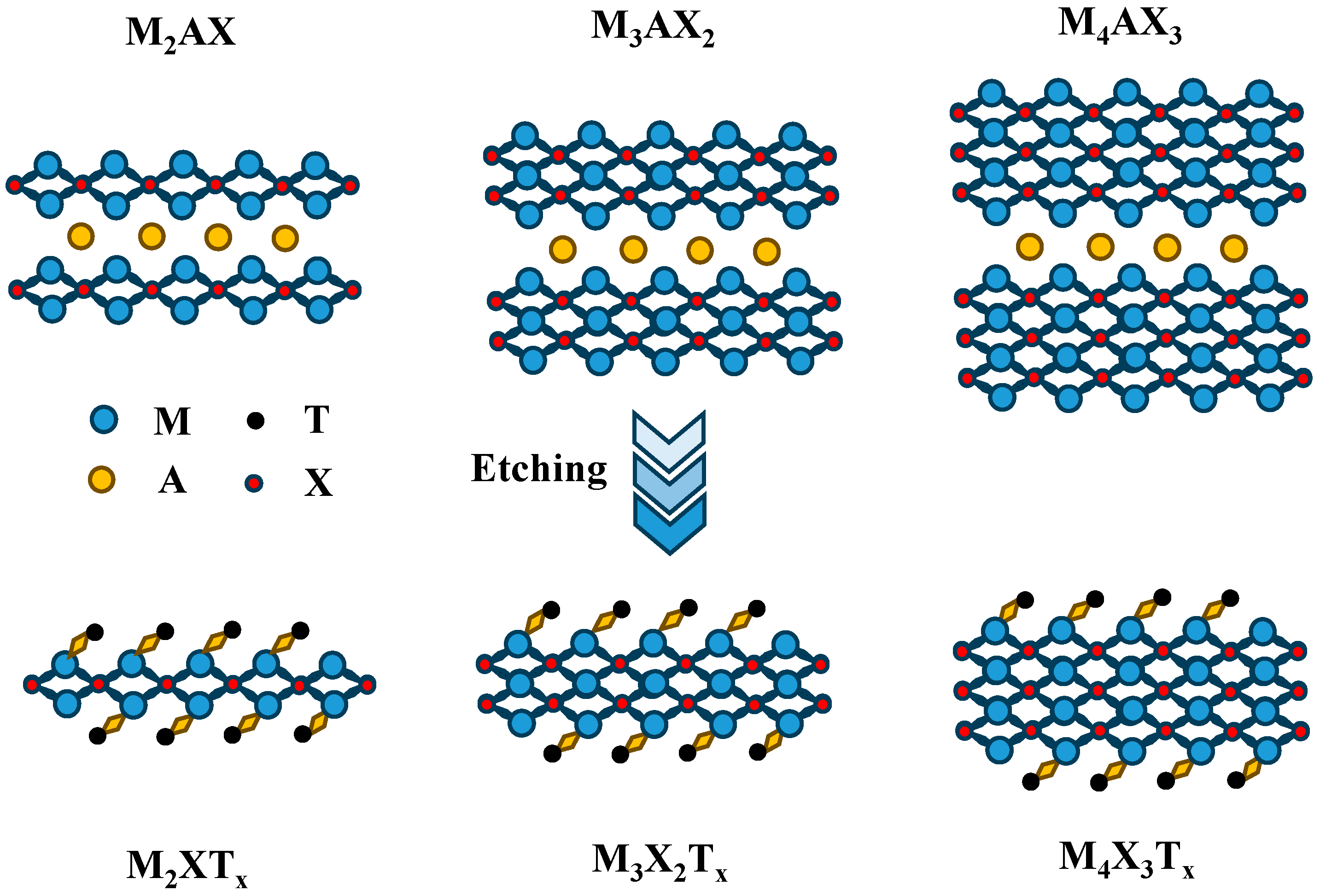
2. Preparation of MXene Materials
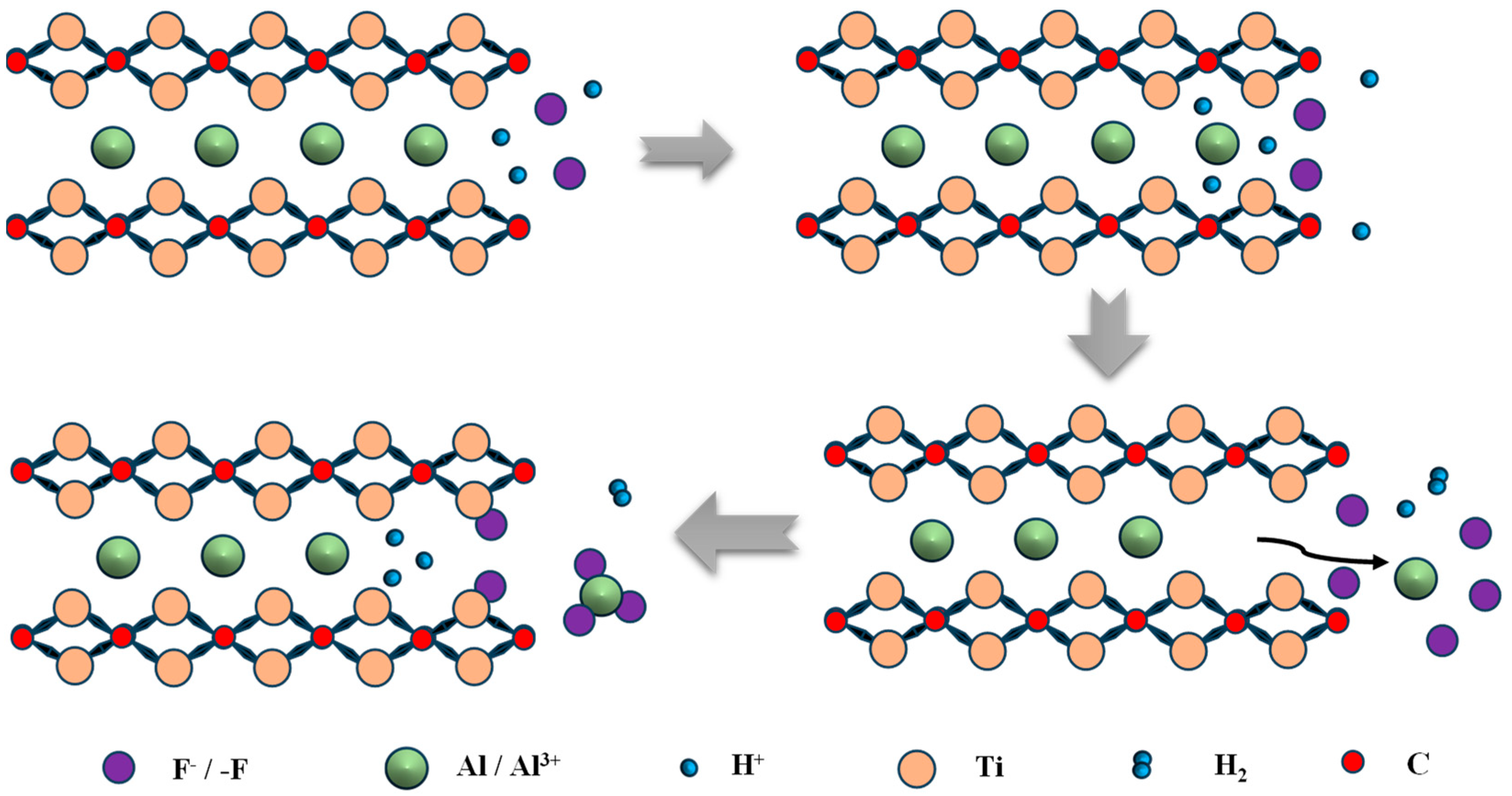
2.1. Fluorine-Containing Compound Etching
2.2. Fluorine-Free Preparation
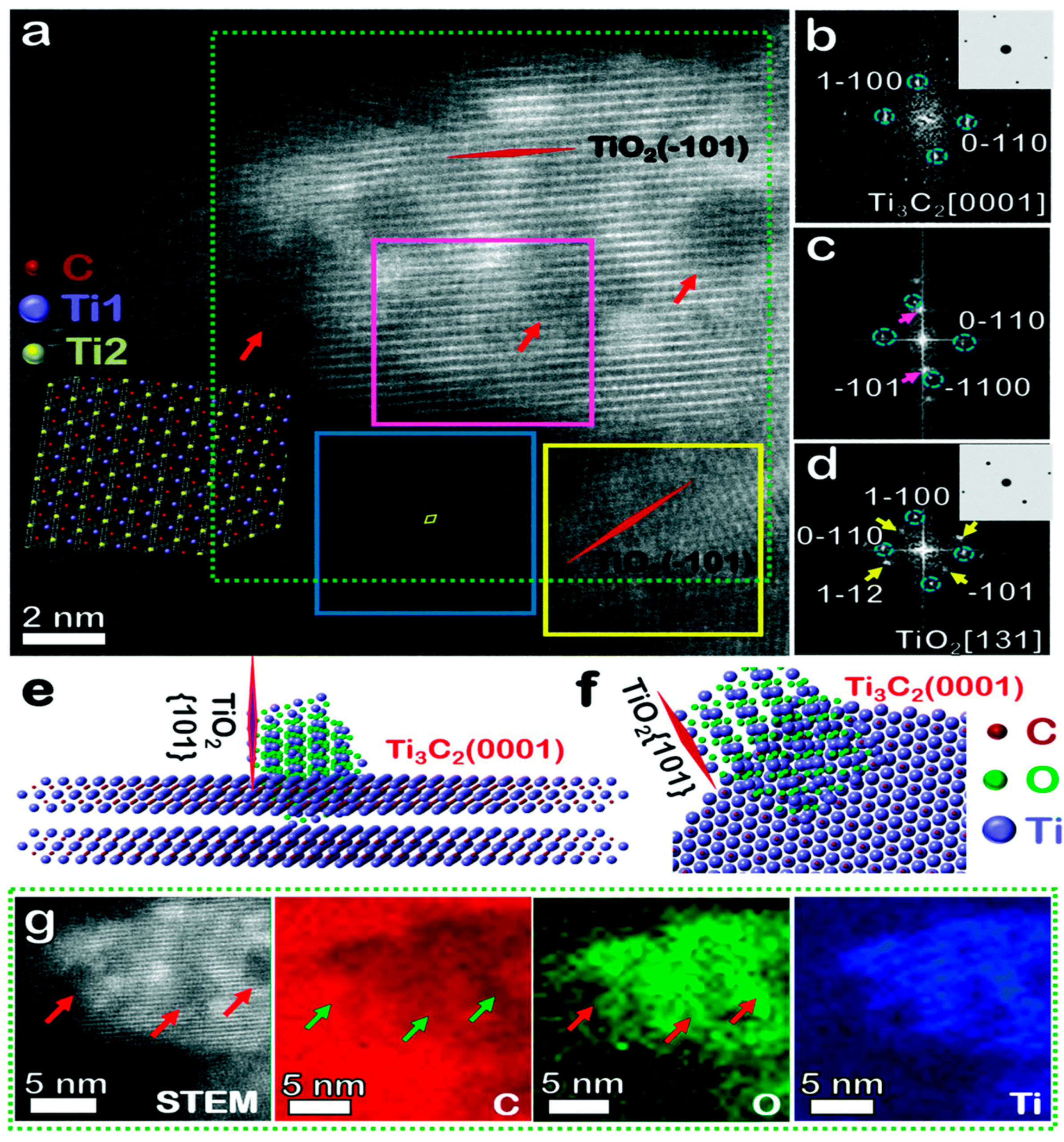
2.3. Degradation Issues of MXene
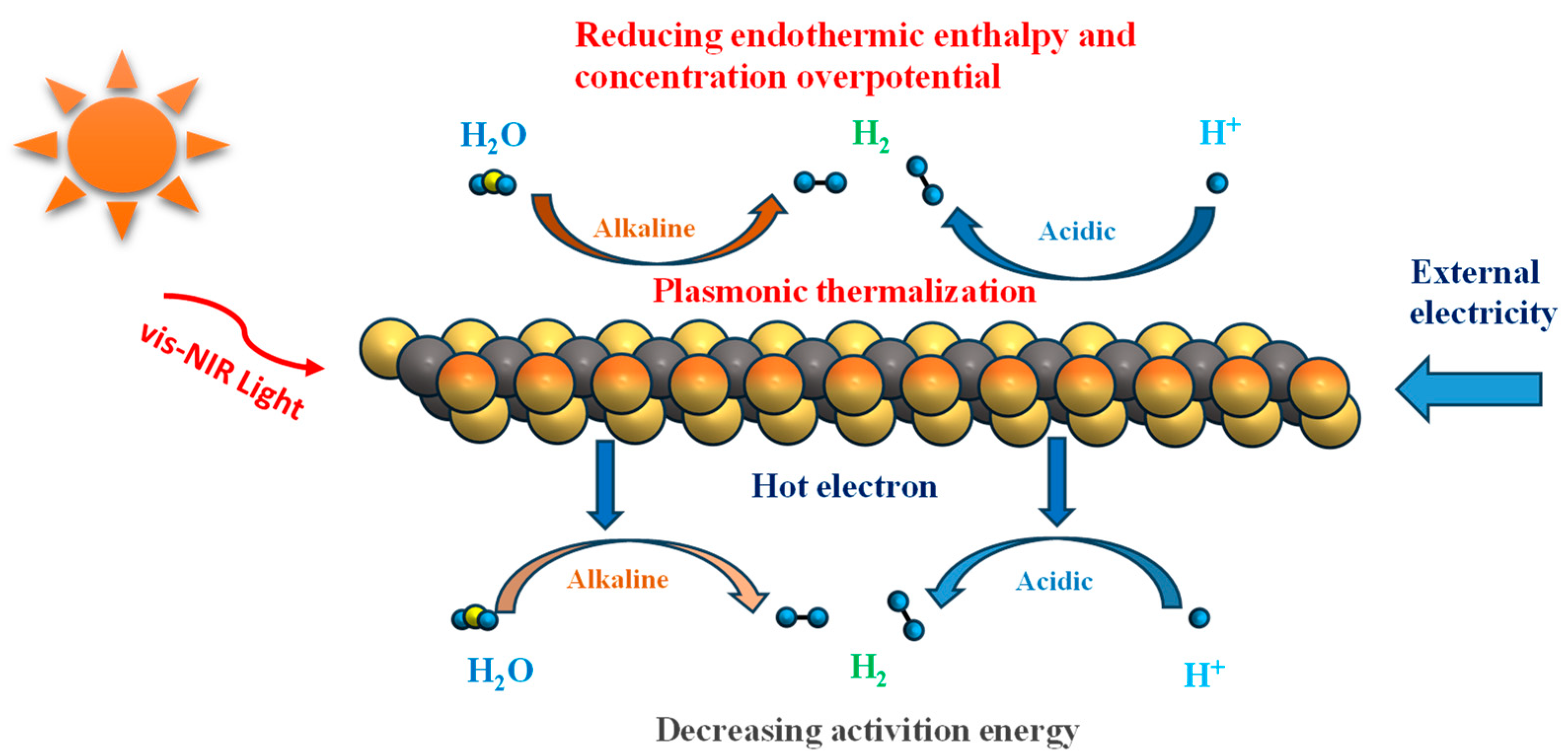
3. MXene Photothermal Conversion Mechanism
4. MXene-Based Composite Heat Storage Materials
4.1. Improvement of Heat Storage Capacity
4.2. Expansion of the Photothermal Capacity of PCMs
5. Discussion and Industrial Implications
- Quantum mechanical simulations of MXene/PCM interfacial bonding;
- Mesoscale finite element analysis of heat transfer pathways;
- Macroscopic system-level thermal performance modeling.
6. Conclusions and Prospects for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MXene | Two-dimensional transition metal carbides/nitrides |
| PCMs | Phase-change materials |
| IR | Infrared |
| UV | Ultraviolet |
| TES | Thermal energy storage |
| LSPR | Localized surface plasmon resonance |
| HF | Hydrofluoric acid |
| F- | Fluoride ions |
| OH- | Hydroxide ions |
| DMSO | Dimethyl sulfoxide |
| CVD | Chemical vapor deposition |
| STEM | Scanning transmission electron microscopy |
| EELS | Electron energy loss spectroscopy |
| NMP | N-methyl-2-pyrrolidone |
| DFT | Density functional theory simulations |
| SAT | Sodium acetate trihydrate |
| PEG | Polyethylene glycol |
| SAL | Stearyl alcohol |
| PW | Paraffin wax |
| C20 | N-eicosane |
| SSD | Na2SO4·10H2O |
| C18 | N-octadecane |
| DM | D-mannitol |
| MA | Myristic acid |
References
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Lin, J.; Su, J.; Redshaw, C.; Feng, X.; Min, Y. Novel Pyrene-Based Aggregation-Induced Emission Luminogen (AIEgen) Composite Phase Change Fibers with Satisfactory Fluorescence Anti-Counterfeiting, Temperature Sensing, and High-Temperature Warning Functions for Solar-Thermal Energy Storage. Adv. Compos. Hybrid Mater. 2023, 6, 126. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghose, J.; Datta, P.; Kumari, P.; Paul, S. Strategies for Phase Change Material Application in Latent Heat Thermal Energy Storage Enhancement: Status and Prospect. J. Energy Storage 2022, 53, 105179. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Wei, M.; Yang, Z.; Li, T.; Chen, C. Review: Photothermal Effect of Two-Dimensional Flexible Materials. J. Mater. Sci. 2025, 60, 1797–1825. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for Improved Photovoltaic Devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Rousta, M.; Kia, A.; Kasaeian, A. Application of Nanomaterials as Photo-Thermal Agents in Solar Desalination: A Review of Experimental Approaches. Desalination 2025, 598, 118379. [Google Scholar] [CrossRef]
- Bian, J.; Liao, L.; Lv, G. Preparation Strategy of Photo-Thermal Composite Phase Change Materials: A Review. J. Energy Storage 2024, 102, 114155. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Hang, T.; Wu, L.; Yang, X. Roles of MXenes in Photocatalysis. Sol. RRL 2023, 8, 2300961. [Google Scholar] [CrossRef]
- Ma, H.; Xue, M. Recent Advances in the Photothermal Applications of Two-Dimensional Nanomaterials: Photothermal Therapy and Beyond. J. Mater. Chem. A 2021, 9, 17569–17591. [Google Scholar] [CrossRef]
- Zhang, D.; Shah, D.; Boltasseva, A.; Gogotsi, Y. MXenes for Photonics. ACS Photonics 2022, 9, 1108–1116. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Lu, H.; Zhan, P.; Du, W.; Wan, M.; Wang, Z. Ultra-broadband Tunable Resonant Light Trapping in a Two-dimensional Randomly Microstructured Plasmonic-photonic Absorber. Sci. Rep. 2017, 7, 43803. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as Emerging Nanofillers for High-Performance Polymer Composites: A Review. Compos. Part B Eng. 2021, 217, 108867. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene Synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Khaledialidusti, R.; Khazaei, M.; Khazaei, S.; Ohno, K. High-throughput Computational Discovery of Ternary-layered MAX Phases and Prediction of Their Exfoliation for Formation of 2D MXenes. Nanoscale 2021, 13, 7294–7307. [Google Scholar] [CrossRef]
- Johnson, D.; Qiao, Z.; Uwadiunor, E.; Djire, A. Holdups in Nitride MXene’s Development and Limitations in Advancing the Field of MXene. Small 2022, 18, 2106129. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Y.; Yan, D.; Huang, J.; Peng, S. Synthesis of MXene and Its Application for Zinc-ion Storage. Sustain. Mater. 2022, 2, 293–318. [Google Scholar] [CrossRef]
- Olshtrem, A.; Chertopalov, S.; Guselnikova, O.; Valiev, R.R.; Cieslar, M.; Miliutina, E.; Elashnikov, R.; Fitl, P.; Postnikov, P.; Lancok, J.; et al. Plasmon-assisted MXene Grafting: Tuning of Surface Termination and Stability Enhancement. 2D Mater. 2021, 8, 045037. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372, 1165. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zha, X.-H.; Luo, K.; Huang, Q.; He, J.; Liu, Y.; Deng, W.; Du, S. Structures and Mechanical and Electronic Properties of the Ti2CO2 MXene Incorporated with Neighboring Elements (Sc, V, B and N). J. Electron. Mater. 2017, 46, 2460–2466. [Google Scholar] [CrossRef]
- Mohanadas, D.; Rohani, R.; Sulaiman, Y.; Bakar, S.A.; Mahmoudi, E.; Zhang, L.-C. Heavy Metal Detection in Water Using MXene and Its Composites: A Review. Mater. Today Sustain. 2023, 22, 100411. [Google Scholar] [CrossRef]
- Paramasivam, G.; Yadavali, S.P.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Recent Advances in the Medical Applications of Two-Dimensional MXene Nanosheets. Nanomedicine 2024, 19, 2633–2654. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Al-Gheethi, A.A.; Morsin, M.; Tee, K.S. Recent Progress and New Perspective of MXene-based Membranes for Water Purification: A Review. Ceram. Int. 2022, 48, 16477–16491. [Google Scholar] [CrossRef]
- Yu, K.; Ren, J.; Liao, W.; Hu, B.; Bai, C.; Li, Z.; Zhang, X.; Chhattal, M.; Li, N.; Qiang, L. Maintaining the 2D Structure of MXene via Self-Assembled Monolayers for Efficient Lubrication in High Humidity. Small 2024, 20, 2402143. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.; Wang, W.; Han, Y.; Zhu, Z. High-intensity Ultrasonic Exfoliation-Assisted Rapid Preparation of MXene for Gas Sensing. Chem. Eng. J. 2024, 489, 151140. [Google Scholar] [CrossRef]
- He, Y.; Su, Q.; Liu, D.; Xia, L.; Huang, X.; Lan, D.; Liu, Y.; Huang, Y.; Zhong, B. Surface Engineering Strategy for MXene to Tailor Electromagnetic Wave Absorption Performance. Chem. Eng. J. 2024, 491, 152041. [Google Scholar] [CrossRef]
- Qin, M.; Ai, J.; Kuklin, A.V.; Zhang, R.; Hou, J.; Zhao, Y.; Zhang, H.; Zhang, J.; Ågren, H.; Gao, L.; et al. Surface Modification of Ti3CN MXene and Their Enhanced Performance in Photodetection. Small 2025, 21, 2412637. [Google Scholar] [CrossRef]
- Zhang, C.; Anasori, B.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Bai, L.; Yin, H.; Wu, L.; Zhang, X. First-principal Study of the Nbn+1CnT2 Systems as Electrode Materials for Supercapacitors. Comput. Mater. Sci. 2018, 143, 225–231. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Liu, Y.; Tekell, M.C.; Fan, D. Three Dimensional Nanosuperstructures Made of Two-Dimensional Materials by Design: Synthesis, Properties, and Applications. Nano Today 2019, 29, 100799. [Google Scholar] [CrossRef]
- Mohtasim, M.S.; Das, B.K. MXene Based Composite Phase Change Materials for Thermal Energy Storage Applications: Featuring Bio-Mimic Approaches. Renew. Sustain. Energy Rev. 2025, 207, 114952. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, X.; Jia, D.; Zhou, Y.; van der Zwaag, S. On the formation mechanisms and properties of MAX phases: A review. J. Eur. Ceram. Soc. 2021, 41, 3851–3878. [Google Scholar] [CrossRef]
- Nashim, A.; Parida, K. A Glimpse on the Plethora of Applications of Prodigious Material MXene. Sustain. Mater. Technol. 2022, 32, e00439. [Google Scholar] [CrossRef]
- Magnuson, M.; Mattesini, M. Chemical Bonding and Electronic-Structure in MAX Phases as Viewed by X-ray Spectroscopy and Density Functional Theory. Thin Solid Film. 2017, 621, 108–130. [Google Scholar] [CrossRef]
- Zhang, L.; Song, W.; Liu, H.; Ding, H.; Yan, Y.; Chen, R. Influencing Factors on Synthesis and Properties of MXene: A Review. Processes 2022, 10, 1744. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Shen, M.; Zhao, Y.; Zhang, X.; Liu, S.; Liu, X.; Hou, L.; Yuan, C. In-Situ Construction of Functional Multi-Dimensional MXene-based Composites Directly from MAX Phases through Gas-Solid Reactions. Angew. Chem. Int. Ed. 2024, 63, e202412898. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide ‘Clay’ with High Volumetric Capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Zou, X.; Liu, H.; Xu, H.; Wu, X.; Han, X.; Kang, J.; Reddy, K.M. A Simple Approach to Synthesis Cr2CTx MXene for Efficient Hydrogen Evolution Reaction. Mater. Today Energy 2021, 20, 100668. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by Fluoride Salts Etching and Methane Adsorptive Properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical Etching of Ti2AlC to Ti2CTx (MXene) in Low-Concentration Hydrochloric Acid Solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, H.; Deng, Y.; Yang, T.; Huang, J.; Zhu, Y. Preparation of Fluorine-free MXene Ti3C2Tx and Its Electrical Properties. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2023, 38, 304–307. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, Z.; Chen, Y.; Liang, D.; Cheng, L.; Yang, X.; Liu, Z.; Ma, R.; Sasaki, T.; Geng, F. Organic-Base-Driven Intercalation and Delamination for the Production of Functionalized Titanium Carbide Nanosheets with Superior Photothermal Therapeutic Performance. Angew. Chem. Int. Ed. 2016, 55, 14569–14574. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T = OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal Strategy for HF-Free Facile and Rapid Synthesis of Two-dimensional MXenes as Multifunctional Energy Materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective Etching of Silicon from Ti3SiC2 (MAX) To Obtain 2D Titanium Carbide (MXene). Angew. Chem. Int. Ed. 2018, 57, 5444–5448. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A General Lewis Acidic Etching Route for Preparing MXenes with Enhanced Electrochemical Performance in Non-Aqueous Electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Orbay, M.; Luo, S.; Duluard, S.; Shao, H.; Harmel, J.; Rozier, P.; Taberna, P.-L.; Simon, P. Exfoliation and Delamination of Ti3C2Tx MXene Prepared via Molten Salt Etching Route. ACS Nano 2021, 16, 111–118. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area High-quality 2D Ultrathin Mo2C Superconducting Crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, G.; Fang, L.; Wang, Z.; Wu, F.; Liu, G.; Wang, Q.; Nian, H. Surface-Modification Strategy to Produce Highly Anticorrosive Ti3C2Tx MXene-Based Polymer Composite Coatings: A Mini-Review. Materials 2025, 18, 653. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liao, S.Y.; Huang, H.P.; Wang, Q.F.; Shi, Y.Y.; Zhu, P.L.; Hu, Y.G.; Sun, R.; Wan, Y.J. Enhancing the Chemical Stability of MXene Through Synergy of Hydrogen Bond and Coordination Bond in Aqueous Solution. Small Methods 2023, 7, 2201694. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Lao, J.; Yu, R.; Sang, X.; Luo, J.; Li, Y.; Wu, J. Ambient Oxidation of Ti3C2 MXene Initialized by Atomic Defects. Nanoscale 2019, 11, 23330–23337. [Google Scholar] [CrossRef]
- Cao, W.; Nie, J.; Cao, Y.; Gao, C.; Wang, M.; Wang, W.; Lu, X.; Ma, X.; Zhong, P. A Review of how to Improve Ti3C2Tx MXene Stability. Chem. Eng. J. 2024, 496, 154097. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Maleski, K.; Mochalin, V.N.; Gogotsi, Y. Dispersions of Two-Dimensional Titanium Carbide MXene in Organic Solvents. Chem. Mater. 2017, 29, 1632–1640. [Google Scholar] [CrossRef]
- Aftab, S.; Abbas, A.; Iqbal, M.Z.; Hussain, S.; Kabir, F.; Hegazy, H.H.; Xu, F.; Kim, J.H.; Goud, B.S. Two-dimensional MXene Incorporating for Electron and Hole Transport in High-Performance Perovskite Solar Cells. Mater. Today Energy 2023, 36, 101366. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for Extreme Light Concentration and Manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Wang, Z.; Sun, F.; Liu, Y.; Wu, K.; Meng, X.; Qiu, J. Boosting the Electrocatalysis of MXenes by Plasmon-Induced Thermalization and Hot-Electron Injection. Angew. Chem. Int. Ed. 2021, 60, 9416–9420. [Google Scholar] [CrossRef]
- Maleski, K.; Shuck, C.E.; Fafarman, A.T.; Gogotsi, Y. The Broad Chromatic Range of Two-Dimensional Transition Metal Carbides. Adv. Opt. Mater. 2021, 9, 2001563. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal Conductivity Enhancement on Phase Change Materials for Thermal Energy Storage: A Review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Zhang, B.; Chu, T.; Bai, Q.; Zhu, C.; Francisco, J.S.; Xuan, F. Unconventional Photoconversion from In-Plane 2D Heterostructures of 2D Transition Metal Carbides/Semiconductors. Sol. RRL 2022, 6, 2200620. [Google Scholar] [CrossRef]
- Ali, H.M.; Rehman T-u Arıcı, M.; Said, Z.; Duraković, B.; Mohammed, H.I.; Kumar, R.; Rathod, M.K.; Buyukdagli, O.; Teggar, M. Advances in Thermal Energy Storage: Fundamentals and Applications. Prog. Energy Combust. Sci. 2024, 100, 101109. [Google Scholar] [CrossRef]
- Man, X.; Lu, H.; Xu, Q.; Wang, C.; Ling, Z. Review on the Thermal Property Enhancement of Inorganic Salt Hydrate Phase Change Materials. J. Energy Storage 2023, 72, 108699. [Google Scholar] [CrossRef]
- Lu, J.; Deng, Y.; Luo, D.; Wu, F.; Dai, X. Preparation and Performance Enhancement of MXene/Na2HPO4·12H2O@SiO2 Phase Change Microcapsule. J. Energy Storage 2024, 91, 112079. [Google Scholar] [CrossRef]
- Woods, J.; Mahvi, A.; Goyal, A.; Kozubal, E.; Odukomaiya, A.; Jackson, R. Rate Capability and Ragone plots for Phase Change Thermal Energy Storage. Nat. Energy 2021, 6, 295–302. [Google Scholar] [CrossRef]
- Wang, S.; Du, R.; Li, T. Progress and Perspective on Thermal Conductivity Enhancement of Phase Change Materials. Sci. Bull. 2024, 69, 3176–3179. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Chen, X.; Gao, H.; Zhang, X.; Tang, Z.; Li, A.; Wang, G. Different Dimensional Nanoadditives for Thermal Conductivity Enhancement of Phase Change Materials: Fundamentals and Applications. Nano Energy 2021, 85, 105948. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.; Meng, L.; Pan, W. Preparation and Photothermal Properties of Accordion-like MXene/Sodium Acetate Trihydrate Composite Phase Change Materials. Sol. RRL 2024, 8, 2300972. [Google Scholar] [CrossRef]
- Panda, D.; Sahu, A.K.; Gangawane, K.M. Eutectic Phase Change Composites with MXene Nanoparticles for Enhanced Photothermal Absorption and Conversion Capacity. Sol. Energy Mater. Sol. Cells 2024, 272, 112911. [Google Scholar] [CrossRef]
- Sheng, X.; Dong, D.; Lu, X.; Zhang, L.; Chen, Y. MXene-wrapped Bio-Based Pomelo Peel Foam/Polyethylene Glycol Composite Phase Change Material with Enhanced Light-to-Thermal Conversion Efficiency, Thermal Energy Storage Capability and Thermal Conductivity. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106067. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of Phase-Change Materials and the Techniques used to Mitigate the Phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Shamseddine, I.; Pennec, F.; Biwole, P.; Fardoun, F. Supercooling of Phase Change Materials: A Review. Renew. Sustain. Energy Rev. 2022, 158, 112172. [Google Scholar] [CrossRef]
- Xu, W.; Su, J.; Lin, J.; Huang, J.; Weng, M.; Min, Y. Enhancing the Light-Thermal Absorption and Conversion Capacity of Diatom-Based Biomass/Polyethylene Glycol Composites Phase Change Material by Introducing MXene. J. Energy Storage 2023, 72, 108253. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Jin, L.; Deng, S.; Dong, Y.; Lin, S. Dopamine-Decorated Ti3C2Tx MXene/Cellulose Nanofiber Aerogels Supported Form-Stable Phase Change Composites with Superior Solar–Thermal Conversion Efficiency and Extremely High Thermal Storage Density. ACS Appl. Mater. Interfaces 2022, 14, 15225–15234. [Google Scholar] [CrossRef]
- Weng, M.; Lin, J.; Yang, Y.; Su, J.; Huang, J.; Lu, X.; Sheng, X. MXene-based Phase Change Materials for Multi-Source Driven Energy Storage, Conversion and Applications. Sol. Energy Mater. Sol. Cells 2024, 272, 112915. [Google Scholar] [CrossRef]
- Jiao, K.; Lu, L.; Wen, T.; Wang, Q. Endowing Photothermal Materials with Latent Heat Storage: A State-of-art Review on Photothermal PCMs. Chem. Eng. J. 2024, 500, 156498. [Google Scholar] [CrossRef]
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Jatoi, A.S.; Koduru, J.R.; Dehghani, M.H. MXene-based Phase Change Materials for Solar Thermal Energy Storage. Energy Convers. Manag. 2022, 273, 116432. [Google Scholar] [CrossRef]
- Bark, H.; Thangavel, G.; Liu, R.J.; Chua, D.H.C.; Lee, P.S. Effective Surface Modification of 2D MXene toward Thermal Energy Conversion and Management. Small Methods 2023, 7, 2300077. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, L.; Jin, X.; Wang, W.; Zhang, S.; Tang, B. MXene Ti3C2Tx for Phase Change Composite with Superior Photothermal Storage Capability. J. Mater. Chem. A 2019, 7, 14319–14327. [Google Scholar] [CrossRef]
- Chen, J.; Mo, Z.; Chen, Y.; Mo, P.; Hu, Z.; Chen, X.; Yu, J.; Hao, Z.; Zeng, X.; Sun, R.; et al. Highly Stable MXene-Based Phase Change Composites with Enhanced Thermal Conductivity and Photothermal Storage Capability. ACS Appl. Energy Mater. 2022, 5, 11669–11683. [Google Scholar] [CrossRef]
- Lin, P.; Xie, J.; He, Y.; Lu, X.; Li, W.; Fang, J.; Yan, S.; Zhang, L.; Sheng, X.; Chen, Y. MXene Aerogel-based Phase Change Materials Toward Solar Energy Conversion. Sol. Energy Mater. Sol. Cells 2020, 206, 110229. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Saidur, R.; Han, T.K.; Mishra, Y.N. MXene-based Eutectic Salt Hydrate Phase Change Material for Efficient Thermal Features, Corrosion Resistance & Photo-Thermal Energy Conversion. Mater. Today Sustain. 2024, 25, 100634. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Zhao, T.; Shah, M.Z.; Khan, Y.; Hayat, A.; Dang, C.; Zheng, M.; Yun, S. Nanoengineering of MgSO4 Nanohybrid on MXene Substrate for Efficient Thermochemical Heat Storage Material. Appl. Energy 2023, 332, 120549. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Flame-retardant and Form-stable Phase Change Composites Based on MXene with High Thermostability and Thermal Conductivity for Thermal Energy Storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Y.; Liu, Y.; Wu, F.; Wang, W.; Wang, H.; Sun, S.; Lu, J. Paraffin/Polyvinyl Alcohol/MXene Flexible Phase Change Composite Films for Thermal Management Applications. Chem. Eng. J. 2023, 453, 139727. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Y.; Luo, D.; Wu, F.; Dai, X. Preparation and Performance enhancement of N-Eicosane/Polyvinyl Alcohol/MXene Flexible Phase Change Composites with Sandwich Structure. Compos. Struct. 2024, 331, 117930. [Google Scholar] [CrossRef]
- Gong, S.; Ding, Y.; Li, X.; Liu, S.; Wu, H.; Lu, X.; Qu, J. Novel Flexible Polyurethane/MXene Composites with Sensitive Solar Thermal Energy Storage Behavior. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106505. [Google Scholar] [CrossRef]
- Qi, X.; Zhu, T.; Hu, W.; Jiang, W.; Yang, J.; Lin, Q.; Wang, Y. Multifunctional Polyacrylamide/Hydrated Salt/Mxene Phase Change Hydrogels with High Thermal Energy Storage, Photothermal Conversion Capability and Strain Sensitivity for Personal Healthcare. Compos. Sci. Technol. 2023, 234, 109947. [Google Scholar] [CrossRef]
- Mo, S.; Xiao, B.; Mo, B.; Chen, J.; Jia, L.; Wang, Z.; Chen, Y. Improving the Thermal and Photothermal Performances of MXene-Doped Microencapsulated Molten Salts for Medium-Temperature Solar Thermal Energy Storage. Energy Fuels 2023, 37, 7490–7500. [Google Scholar] [CrossRef]
- Zhao, K.; Guo, Z.; Wang, J.; Xie, H. Enhancing Solar Photothermal Conversion and Energy Storage with Titanium Carbide (Ti3C2) MXene Nanosheets in Phase-Change Microcapsules. J. Colloid Interface Sci. 2023, 650, 1591–1604. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, S. MXene/d-Mannitol Aerogel Phase Change Material Composites for Medium-temperature Energy Storage and Solar-Thermal Conversion. J. Energy Storage 2023, 67, 107498. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Deng, Y.; Zheng, J.; Wu, F.; Dai, X.; Deng, C. ZIF-67@MXene structure Synergistically Improve Heat Storage and Photothermal Conversion of Phase Change Material. J. Energy Storage 2023, 67, 107641. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Zhang, L.; Zhao, W.; Cao, Y.; Liu, X. Dual-Mode MXene-Based Phase-Change Composite Towards Enhanced Photothermal Utilization and Excellent Infrared Stealth. Small 2024, 20, 2405694. [Google Scholar] [CrossRef]
- Usman, A.; Qin, M.; Xiong, F.; Aftab, W.; Shen, Z.; Bashir, A.; Han, H.; Han, S.; Zou, R. MXene-Integrated Solid-Solid Phase Change Composites for Accelerating Solar-Thermal Energy Storage and Electric Conversion. Small Methods 2024, 8, 2301458. [Google Scholar] [CrossRef]
- Liu, X.; Lin, F.; Zhang, X.; Liu, M.; Sun, Z.; Zhang, L.; Min, X.; Mi, R.; Huang, Z. Paraffin/Ti3C2Tx Mxene@Gelatin Aerogels Composite Phase-Change Materials with High Solar-Thermal Conversion Efficiency and Enhanced Thermal Conductivity for Thermal Energy Storage. Energy Fuels 2021, 35, 2805–2814. [Google Scholar] [CrossRef]
- Chen, N.; Yang, W.; Zhang, C. Perspectives on Preparation of Two-dimensional MXenes. Sci. Technol. Adv. Mater. 2021, 22, 917–930. [Google Scholar] [CrossRef]
- Shuck, C.E.; Gogotsi, Y. Taking MXenes from the Lab to Commercial Products. Chem. Eng. J. 2020, 401, 125786. [Google Scholar] [CrossRef]
- Vasyukova, I.A.; Zakharova, O.V.; Kuznetsov, D.V.; Gusev, A.A. Synthesis, Toxicity Assessment, Environmental and Biomedical Applications of MXenes: A Review. Nanomaterials 2022, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
| Materials | Advantages | Disadvantages | References | Title 1 | Title 2 | Title 3 |
|---|---|---|---|---|---|---|
| Noble metals (e.g., Au, Ag, Pt). | High-efficiency solar thermal conversion can be realized by LSPR. | The material has a narrow absorption band, which is expensive and oxidizes easily. | [6,12] | entry 1 | data | data |
| Carbon-based material. | Wide spectral absorption. | Low launch rate. | [6] | entry 2 | data | data |
| Semiconductor material. | Low cost, easy to synthesize, and not susceptible to photodegradation or bleaching. | Low concentration of free charge carriers and poor infrared absorption. | [6] | |||
| Two-dimensional transition metal nitrides/carbides. | The bandgap is easy to adjust, and the in-plane electron mobility is high, which theoretically provides the most excellent photothermal conversion efficiency. | Poor long-term stability and toxicity of the synthesis process make it difficult to scale up. | [6,13,14] |
| PCMs | Preparation Process | Changes in Thermal Parameters | Photothermal Capability | Research Gap | References |
|---|---|---|---|---|---|
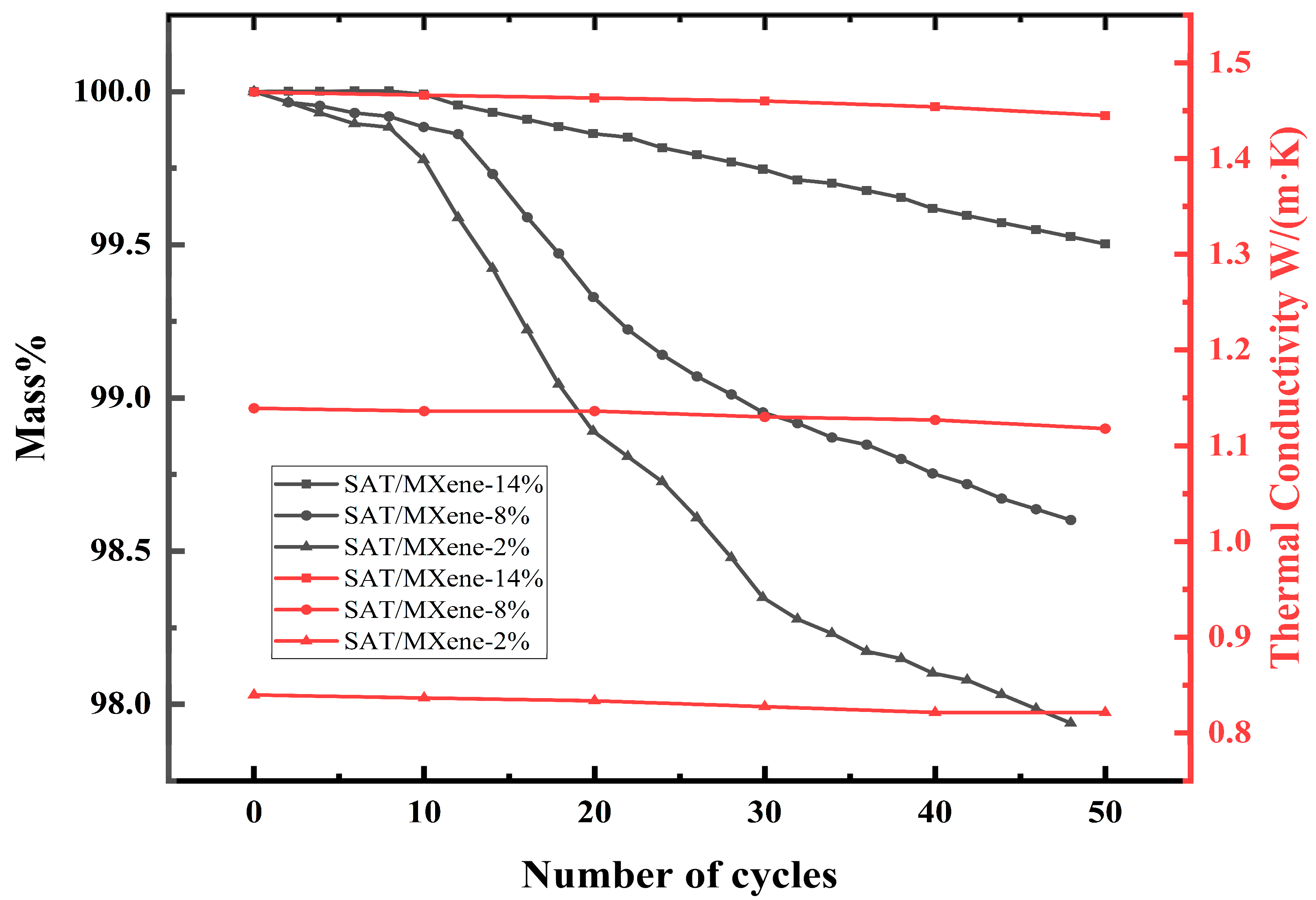
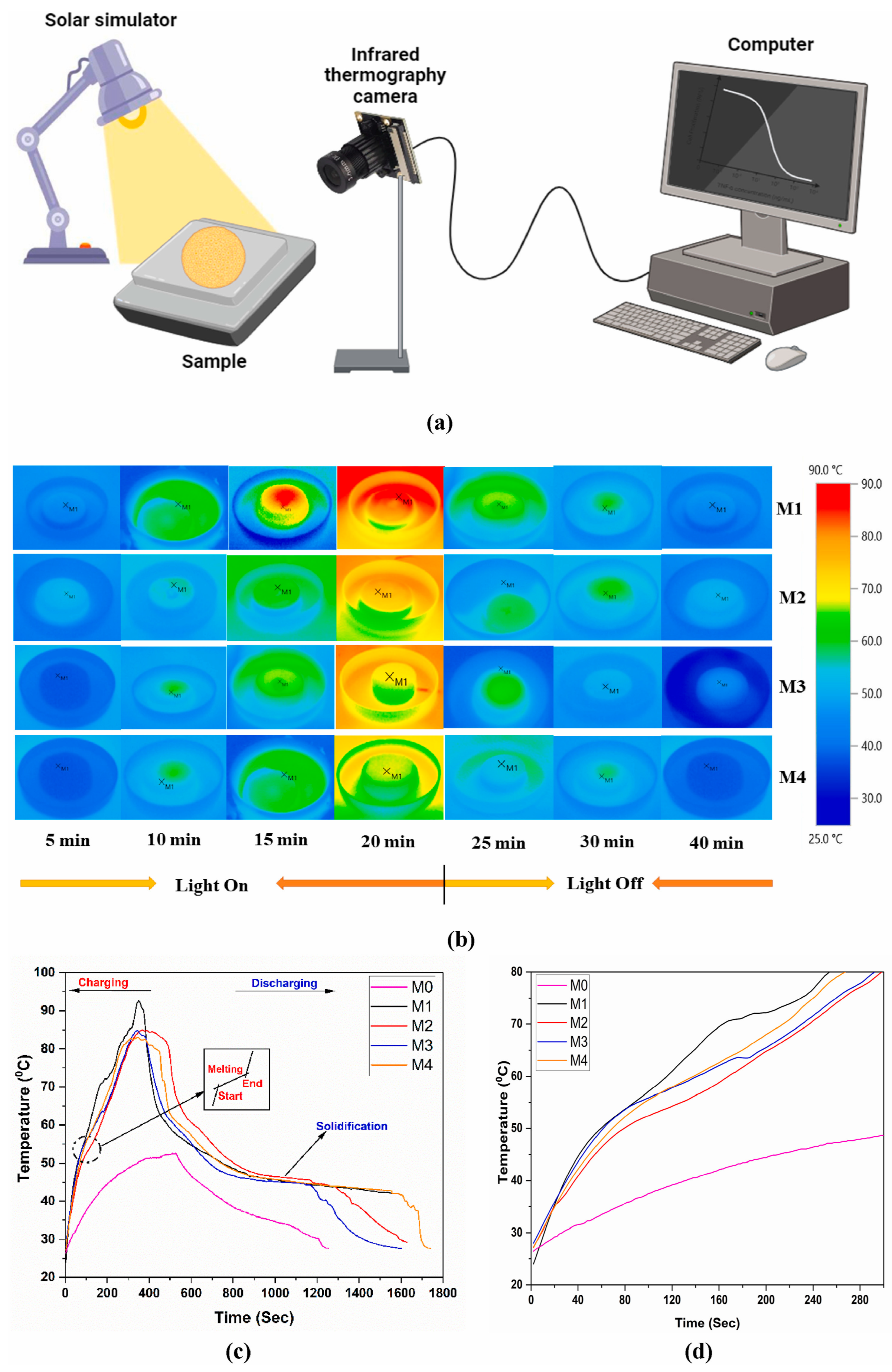
| MgSO4·7H2O | The MXene colloidal solution was mixed with MgSO4·7H2O according to 1:1 and stirred and sonicated for 30 min. | During the 20 hydration/dehydration cycles, the heat release fluctuated less, and the thermal conductivity was 3.25 times higher than the original. | The average temperature of composite PCM was 15 °C higher than that of pure substance for the same light duration and intensity. | There is water vapor sorption and desorption in the storage heat cycle of hydrated salts, and the degradation of MXene under this condition needs to be considered. | [88] |
| Stearyl alcohol (SAL) | The feed ratio of SAL/MXene = 19:1 was placed in a vacuum chamber at 100 °C for 12 h at 0.08 MPa for melt adsorption. | The composite PCM did not leak throughout the heat charging process. The thermal conductivity improved from 0.353 W/(m·K) to 0.486 W/(m·K). | The average temperature of the composite PCM was 10 °C higher than that of the control at the same light duration and intensity. | The increase in MXene gravity can enhance thermal parameters, but there is a lack of discussion on the critical value of positive gains; for example, in combination with cost and the actual usage environment. | [89] |
| Paraffin Wax (PW) | PW and MXene were added to 20 mL of aqueous solution in different mass ratios and stirred for 3 h at 90 °C. | The thermal conductivity of composite PCM increases with increasing MXene loading up to 0.62 W/(m·K). | The temperature of the composite PCM with the highest loading was 38.25 °C higher than that of the lowest when the light duration and intensity were the same. | The paper points out that the latent heat and weight losses of the material are more severe after several phase change cycles and need to be further optimized. | [90] |
| n-eicosane (C20) | C20 and MXene were mixed in different mass ratios, stirred at 90 °C for 2 h, and uniformly coated on the upper and lower surfaces of the porous phase change layer. | After 200 phase change cycles, the latent heat of the composite PCM was reduced by only 2.58%. | Simulating sunlight irradiation, the maximum content of composite PCM reaches 89.07 °C, which is 22.82 °C higher than the minimum average temperature. | There is an ambivalence in the simultaneous improvement of thermal properties and mechanical strength of composites, and the choice of additive ratios should be discussed. | [91] |
| PEG | Different volumes of MXene aqueous dispersions were mixed with PEG and sonicated separately, and the precipitates were heated and melted at 80 °C. | The enthalpy remained almost constant after 200 thermal cycles. | Solar thermal conversion efficiency up to 90.45%. | Lack of a comparison with other composites in the same field. | [92] |
| Na2SO4·10H2O (SSD) | A certain mass of modified material and PCM were dissolved in MXene solution, irradiated with UV light at 50 °C for 2 h, and placed in an environment with 80% humidity for 24 h. The modified material and PCM were dissolved in MXene solution and irradiated with UV light at 50 °C for 2 h. | The phase change temperature and latent heat value remained almost unchanged after 50 cycles, with no obvious signs of leakage. | In the case of light irradiation, the composite PCM is able to absorb enough heat to complete the phase transition process, while the pure substance only increases in temperature and does not undergo a phase transition. | There is a lack of shape stability testing of the material after it has been subjected to multiple phase change cycles. | [93] |
| NaNO3 | The aqueous phase was prepared by mixing and stirring 10 g of PCM, active agent with 5 mL of MXene aqueous dispersion, which was mixed with the oil phase and stirred thoroughly to form composite microcapsules. | The thermal conductivity of composite PCM was improved by 156.2%, the subcooling was reduced by 49.6%, and the thermal reliability of the composite PCM was 94% after 50 cycles. | Photothermal conversion efficiency increased by 169.4%. | The paper points out that the composites have a large degree of subcooling and need to be improved by the addition of nucleating agents to improve the deficiencies. | [94] |
| n-Octadecane (C18) | Trace amount of MXene was poured into the prepared emulsion and stirred at 800 rpm for 30 min. It was heated to 85 °C and then stirred for another 5 h and then dehydrated for 24 h. A sample was extracted from the sample and then dried. | Composite PCM showed the highest encapsulation rate, and the encapsulation process hardly affected the latent heat of the microcapsules, and the thermal conductivity increased by 52.3% compared with pure C18. | The solar thermal conversion efficiency reaches 85.7%, which is 240% higher than the undoped sample. | Some of the characterization experiments tested incomplete and insufficiently comparable materials. | [95] |
| D-mannitol (DM) | Different masses of DM were added to the MXene colloidal solution and stirred under nitrogen atmosphere for 48 h at room temperature and dried for 60 h to obtain the composite aerogel. | During continuous heating, aerogels with higher MXene content are less likely to leak and have faster heating and cooling rates, indicating better thermal conductivity. | Solar thermal conversion efficiency of up to 88.1%. | Lack of cycling performance tests for photothermal conversion. | [96] |
| PEG | A certain amount of MXene was added to 10 mL of PEG anhydrous ethanol solution of different concentrations and stirred at 80 °C for 30 min. | After 100 phase change cycles, the latent heat loss of the composite PCM was only 1%, and the acceleration of the charging and discharging process proved that the thermal conductivity was improved. | The higher the content of MXene, the higher the surface temperature of the composite PCM for the same irradiation time. | According to experimental data, sunlight radiation alone cannot raise the surface temperature of phase change materials to the melting temperature. | [97] |
| Myristic acid (MA) | Laser-processed MXene aerogel was immersed in the melted MA at 80 °C and vacuumed for 2 h | Composite PCM achieved 94.4% encapsulation with no leakage during the phase transition. | The solar energy absorption rate reaches 96% and the photothermal conversion efficiency reaches 93.5%. | Lack of cycling performance tests for photothermal conversion. | [98] |
| PEG | The MXene dispersion was mixed with PEG at a molar ratio of 1:2 and reacted for 1 h at 70 °C. | Composite PCM remains dimensionally stable during heating and is leak-free with higher thermal conductivity. | The thermal energy storage efficiency of composite PCM was 94.5% in the full solar spectrum. | Lack of a comparison with other composites in the same field. | [99] |
| PW | The T@G compound was immersed in the PW melt and dried and degassed for 30 min. | The thermal conductivity of composite PCM is 0.919 W/(m·K), which is 3.48 times higher than that of PW. | The sample with the most MXene doping had the highest temperature for the same light time and intensity. | Lack of cycling performance tests for photothermal conversion. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Jin, Y.; Alam, F. Review: The Application of MXene in Thermal Energy Storage Materials for Efficient Solar Energy Utilization. Materials 2025, 18, 2839. https://doi.org/10.3390/ma18122839
Sun H, Jin Y, Alam F. Review: The Application of MXene in Thermal Energy Storage Materials for Efficient Solar Energy Utilization. Materials. 2025; 18(12):2839. https://doi.org/10.3390/ma18122839
Chicago/Turabian StyleSun, Han, Yingai Jin, and Firoz Alam. 2025. "Review: The Application of MXene in Thermal Energy Storage Materials for Efficient Solar Energy Utilization" Materials 18, no. 12: 2839. https://doi.org/10.3390/ma18122839
APA StyleSun, H., Jin, Y., & Alam, F. (2025). Review: The Application of MXene in Thermal Energy Storage Materials for Efficient Solar Energy Utilization. Materials, 18(12), 2839. https://doi.org/10.3390/ma18122839








