Construction of Symmetric Flexible Electrochromic and Rechargeable Supercapacitors Based on a 1,3,6,8-Pyrenetetrasulfonic Acid Tetrasodium Salt-Loaded Polyaniline Nanostructured Film
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Au/Nylon 66 Porous Film
2.3. Electrodeposition of the Polymer Film
2.4. Fabrication of Flexible Supercapacitors
2.5. Characterization
2.6. Calculation Methods of the Electrochemical Date
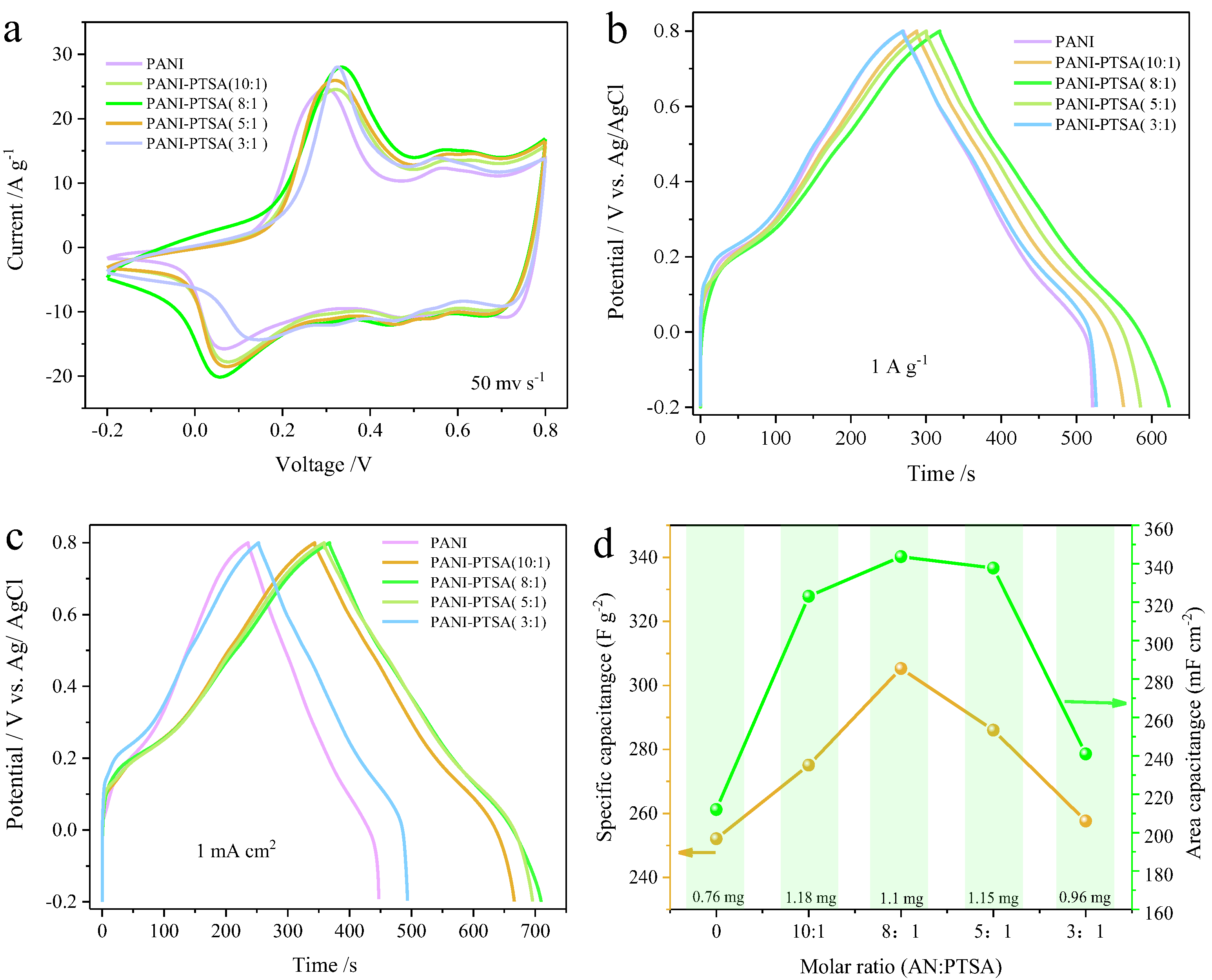
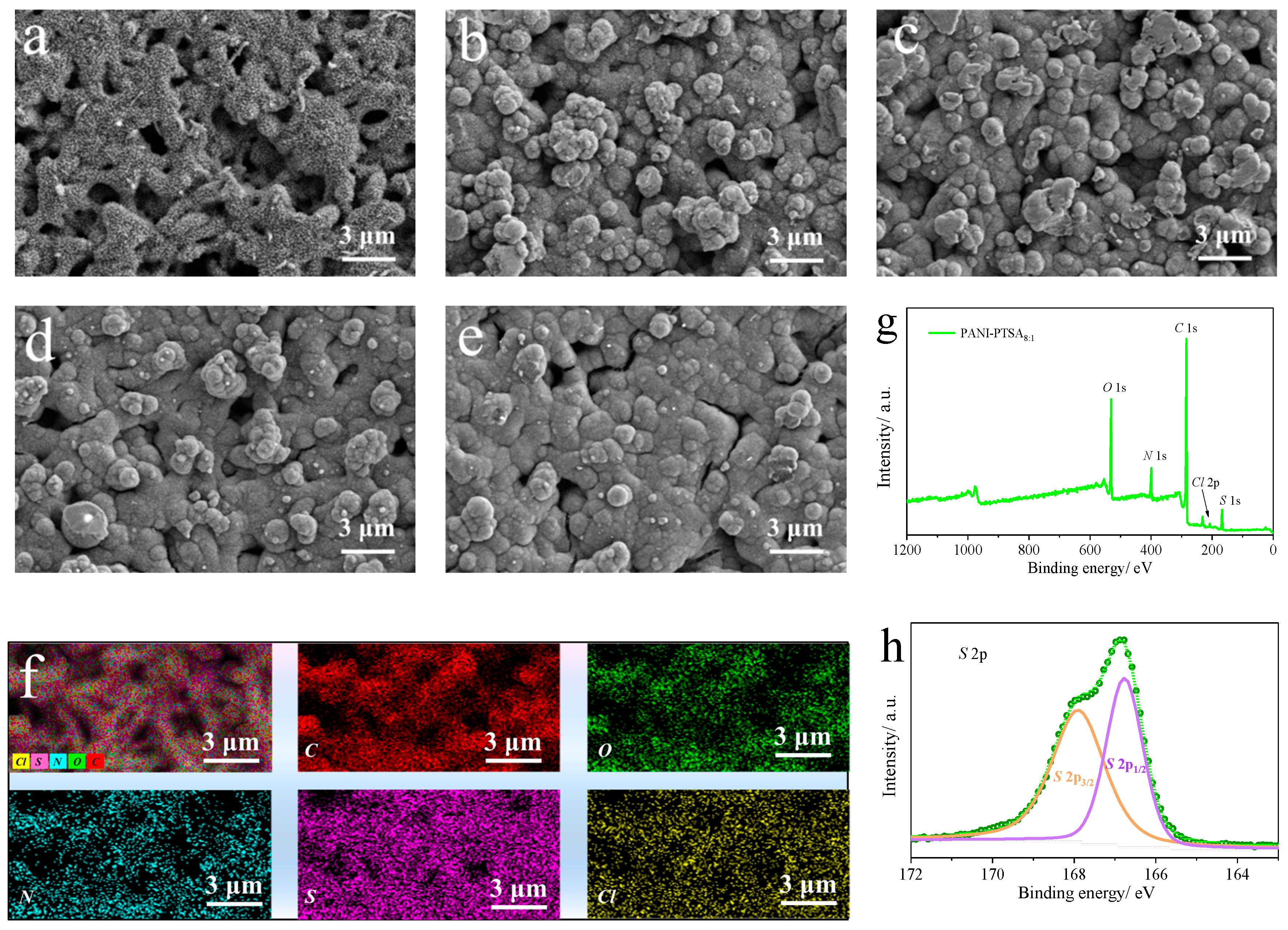
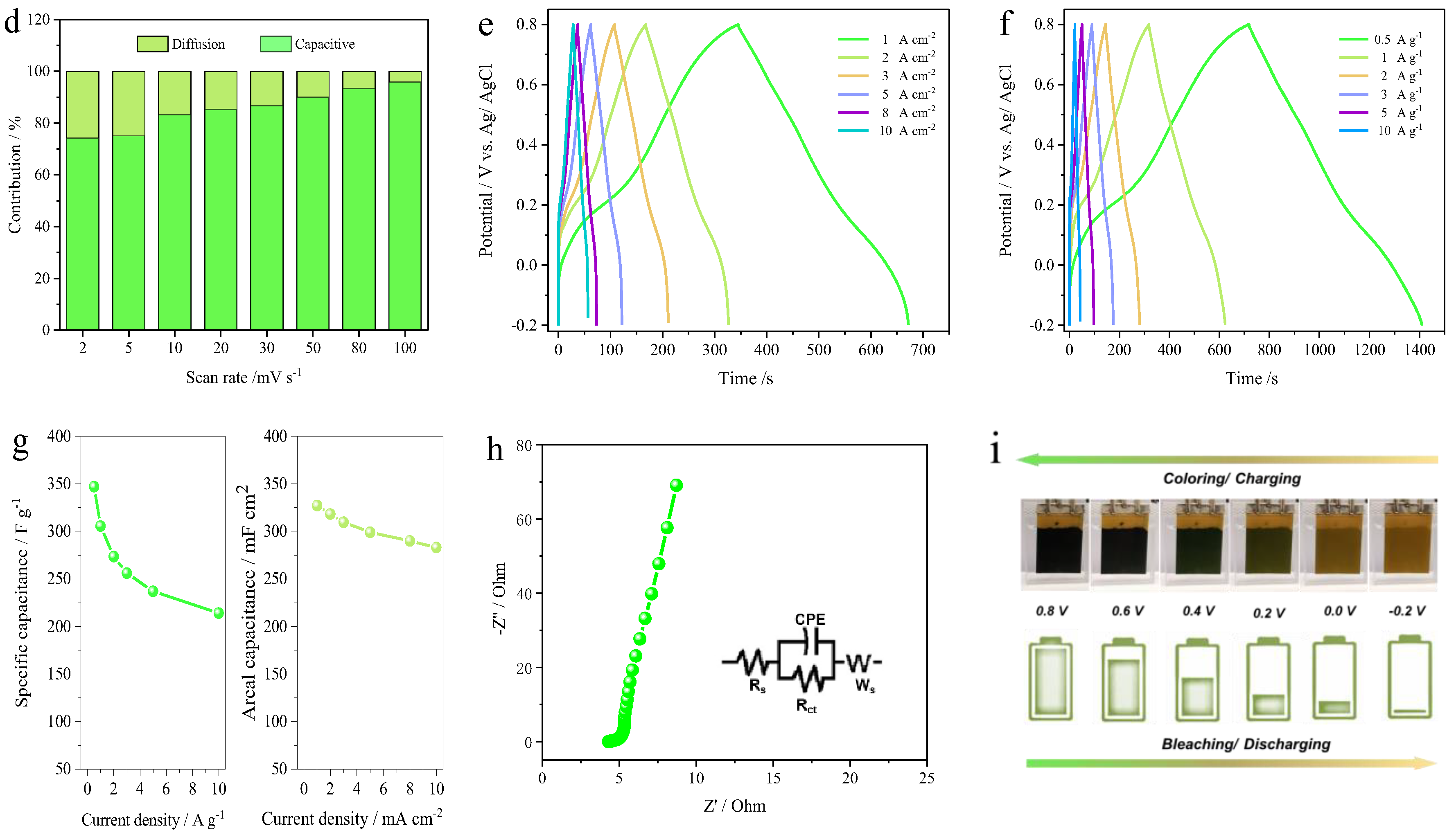
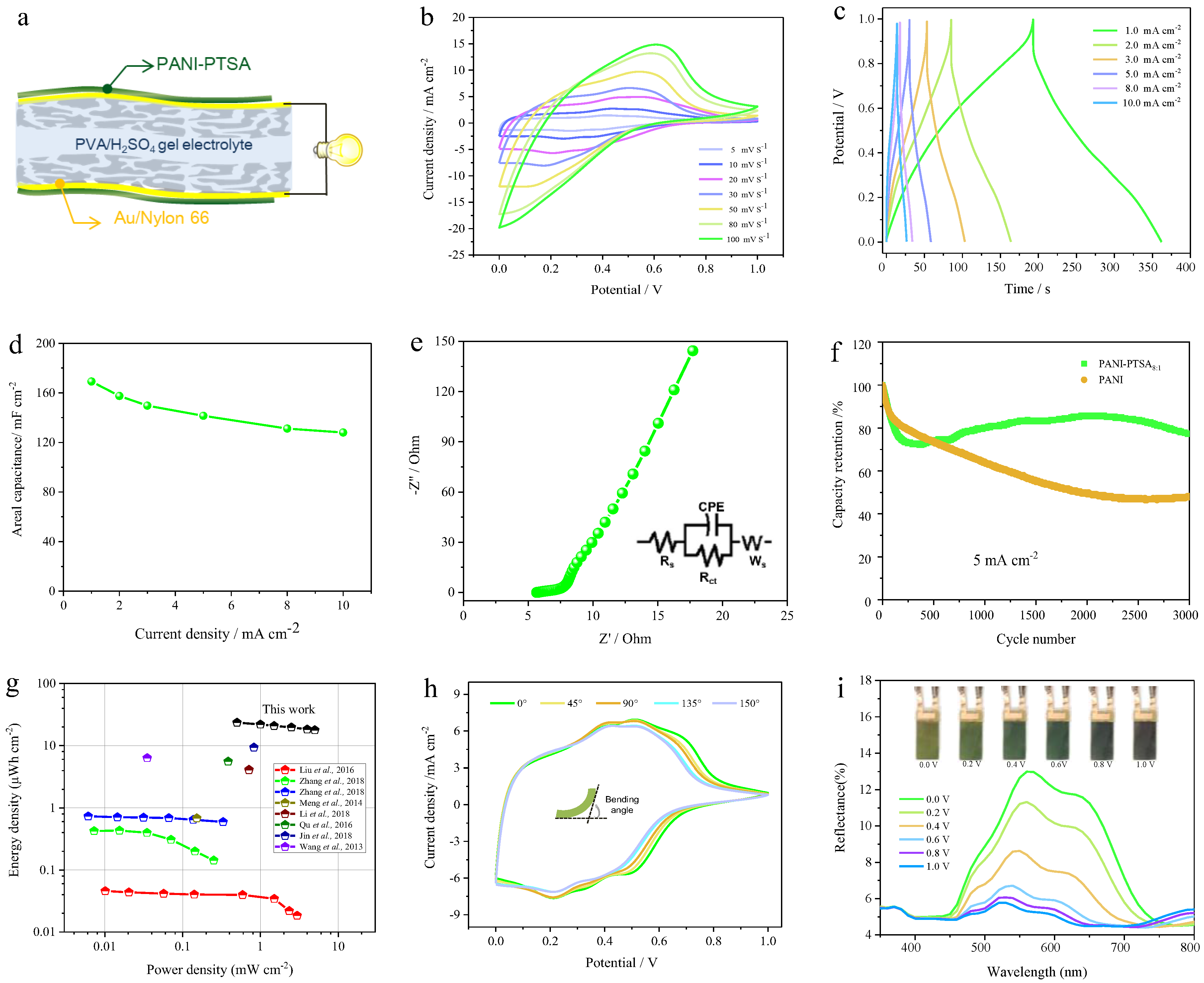
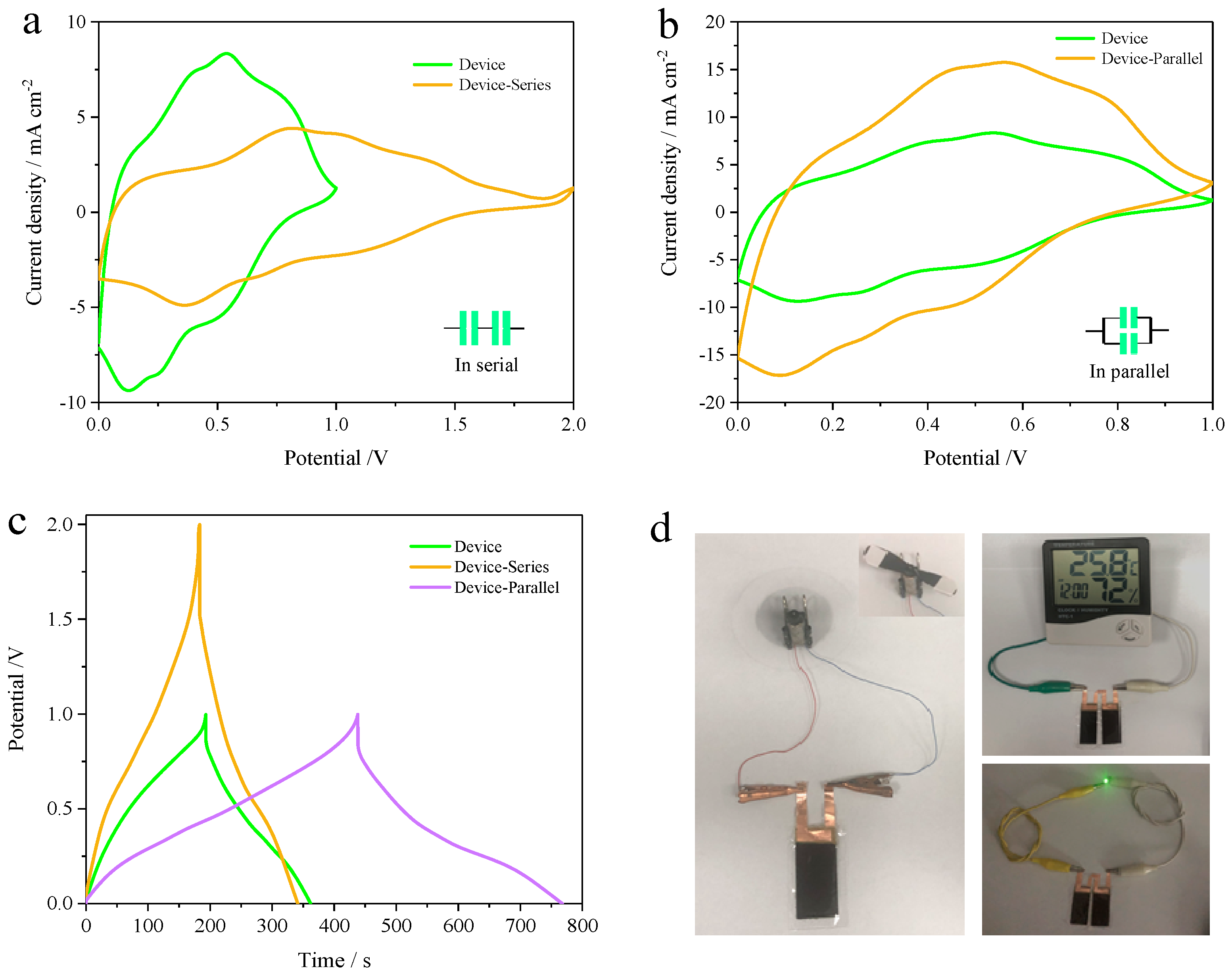
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Wang, H.; Xiang, Y.; Jiang, H.; Tang, L.; Luo, J.; Tian, Y. Quantum dots CdS-modified WO3 film for multi-color electrochromism. Electrochim. Acta 2023, 440, 141749. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Chen, H.; Jia, Y.; Ma, Y. Electropolymerized triphenylamine network films for high-performance transparent to black electrochromism and capacitance. Adv. Opt. Mater. 2022, 11, 2201572. [Google Scholar] [CrossRef]
- Gong, H.; Li, A.; Fu, G.; Zhang, M.; Zheng, Z.; Zhang, Q.; Zhou, K.; Liu, J.; Wang, H. Ultrathin flexible electrochromic devices enabled by highly transparent ion-conducting films. J. Mater. Chem. A 2023, 11, 8939–8949. [Google Scholar] [CrossRef]
- An, T.; Ling, Y.; Gong, S.; Zhu, B.; Zhao, Y.; Dong, D.; Yap, L.W.; Wang, Y.; Cheng, W. A wearable second skin-like multifunctional supercapacitor with vertical gold nanowires and electrochromic polyaniline. Adv. Mater. Technol. 2019, 4, 1800473. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Deng, J.; Zhang, Y.; Sun, X.; Chen, P.; Fang, X.; Zhang, Z.; Guan, G.; Peng, H. Electrochromic fiber-shaped supercapacitors. Adv. Mater. 2014, 26, 8126–8132. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Q.; Hao, Z.; Tang, Y.; Wang, H.; Liu, J.; Yan, H. Integrated electrochromic supercapacitors with visual energy levels boosted by coating onto carbon nanotube conductive networks. Sol. Energy Mater. Sol. Cells 2020, 206, 110330. [Google Scholar] [CrossRef]
- Zhong, Y.; Chai, Z.; Liang, Z.; Sun, P.; Xie, W.; Zhao, C.; Mai, W. Electrochromic asymmetric supercapacitor windows enable direct determination of energy status by the naked eye. ACS Appl. Mater. Interfaces 2017, 9, 34085–34092. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.-H.; Kim, K.-H.; Ahn, H.-J. P-doped carbon quantum dot graft-functionalized amorphous WO3 for stable and flexible electrochromic energy-storage devices. Chem. Eng. J. 2022, 445, 136826. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Zhang, Y.; Wei, Z. Integrated energy storage and electrochromic function in one flexible device: An energy storage smart window. Energy Environ. Sci. 2012, 5, 8384. [Google Scholar] [CrossRef]
- Kandpal, S.; Ghosh, T.; Rani, C.; Chaudhary, A.; Park, J.; Lee, P.S.; Kumar, R. Multifunctional electrochromic devices for energy applications. ACS Energy Lett. 2023, 8, 1870–1886. [Google Scholar] [CrossRef]
- Lim, B.H.; Kim, J.M.; Nguyen, V.-T.; Kim, H.; Park, C.W.; Lee, J.K.; Lee, C.-H.; Yoo, J.; Min, B.K.; Kim, S.K. Functionalized methyl cellulose/LiClO4 composite as an environmentally friendly quasi-solid polymer electrolyte for solid-state electrochromic devices and cellulose-based supercapacitors. Mater. Today Energy 2023, 33, 101263. [Google Scholar] [CrossRef]
- Lv, X.; Xu, H.; Yang, Y.; Ouyang, M.; Xia, M.; Liu, C.; Wright, D.S.; Zhang, C. Flexible laterally-configured electrochromic supercapacitor with feasible patterned display. Chem. Eng. J. 2023, 458, 141453. [Google Scholar] [CrossRef]
- Yin, Q.; Jia, H.; Mohamed, A.; Ji, Q.; Hong, L. Highly flexible and mechanically strong polyaniline nanostructure @ aramid nanofiber films for free-standing supercapacitor electrodes. Nanoscale 2020, 12, 5507–5520. [Google Scholar] [CrossRef]
- Shah, S.S.; Alfasane, M.A.; Bakare, I.A.; Aziz, M.A.; Yamani, Z.H. Polyaniline and heteroatoms-enriched carbon derived from Pithophora polymorpha composite for high performance supercapacitor. J. Energy Storage 2020, 30, 101562. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, J.-M.; Kim, S.J.; Kim, K.H.; Lee, S.H.; Bae, N.H.; Lee, K.G.; Choi, B.G. Large-area and 3d polyaniline nanoweb film for flexible supercapacitors with high rate capability and long cycle life. ACS Appl. Energy Mater. 2020, 3, 7746–7755. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Q.; Yang, Y.; Chen, J.; Yan, B.; Gu, Y.; Fu, R.; Chen, S. PANI doped with carbon quantum dots for the asymmetric high-performance solid-state electrochromic supercapacitor. J. Power Sources 2025, 633, 236407. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Zhang, F.; Zhang, Y.; Liu, C.; Liu, B. One stone, two birds: Polyaniline film with the difunctional characteristic of fast-response multi-color electrochromic and excellent super-capacitive behaviors. Appl. Surf. Sci. 2025, 707, 163642. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Haider, I.; Zheng, Y.; Xue, M.; Ge, J.; Wang, B.; Zhuiykov, S.; Cui, Y. High-performance electrochromic energy storage devices based on WO3 nanoflower via in situ intercalation with polyaniline. Adv. Mater. Technol. 2025, 2500260. [Google Scholar] [CrossRef]
- Shen, Y.; Qin, Z.; Li, T.; Zeng, F.; Chen, Y.; Liu, N. Boosting the supercapacitor performance of polyaniline nanofibers through sulfonic acid assisted oligomer assembly during seeding polymerization process. Electrochim. Acta 2020, 356, 136841. [Google Scholar] [CrossRef]
- Mooss, V.A.; Vijayakumar, V.; Kurungot, S.; Athawale, A.A. Interconnected polyaniline nanostructures: Enhanced interface for better supercapacitance retention. Polymer 2021, 212, 123169. [Google Scholar] [CrossRef]
- Peng, C.; Yang, H.; Chen, S.; Wang, L. Supercapacitors based on three-dimensional porous carbon/covalent-organic framework/polyaniline array composites. J. Energy Storage 2020, 32, 101786. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, M.; Li, H. Preparation of 1, 3, 6, 8-pyrenesulfonic acid tetrasodium salt dye-doped silica nanoparticles and their application in water-based anti-counterfeit ink. Materials 2020, 13, 4074. [Google Scholar] [CrossRef]
- Arvas, M.B.; Yazar, S.; Sahin, Y. An ultra-high power density supercapacitor: Cu(II) phthalocyanine tetrasulfonic acid tetrasodium salt doped polyaniline. J. Alloys Compd. 2022, 919, 165689. [Google Scholar] [CrossRef]
- Sheng, L.; Fang, D.; Wang, X.; Tang, J.; Han, Q.; Zhou, J.; Tang, W. Boosting PEDOT energy storage with redox dopant and electrolyte additive. Chem. Eng. J. 2020, 401, 126123. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Han, J.; Wang, T.; Leng, Y.; Wang, Y.; Li, T.; Han, Y. Preparation of polyaniline onto dl-tartaric acid assembled mxene surface as an electrode material for supercapacitors. ACS Appl. Energy Mater. 2020, 3, 9326–9336. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Zhong, Q.; Qin, Y.; Xu, A.; Li, W.; Shi, J. In situ synthesis of trifluoroacetic acid-doped polyaniline/reduced graphene oxide composites for high-performance all-solid-state supercapacitors. ACS Appl. Energy Mater. 2020, 3, 8774–8785. [Google Scholar] [CrossRef]
- Chu, X.; Huang, H.; Zhang, H.; Zhang, H.; Gu, B.; Su, H.; Liu, F.; Han, Y.; Wang, Z.; Chen, N.; et al. Electrochemically building three-dimensional supramolecular polymer hydrogel for flexible solid-state micro-supercapacitors. Electrochim. Acta 2019, 301, 136–144. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, K.; Li, S.; Ji, S.; Ye, C. Flexible, in-plane, and all-solid-state micro-supercapacitors based on printed interdigital Au/polyaniline network hybrid electrodes on a chip. J. Mater. Chem. A 2014, 2, 20916–20922. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Sheng, F.; Li, W.; Li, J.; Huang, D.; Guo, P.; Wang, Y.; Zhu, H. Graphene film with a controllable microstructure for efficient electrochemical energy storage. ACS Appl. Mater. Interfaces 2023, 15, 13086–13096. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, D.; Huang, D.; Lin, B.; Li, J.; Guo, P.; Wang, Y. Towards high rate and high areal capacity Zn ion hybrid supercapacitor: Fluffy graphene architecture anchored with ultrathin redox-active molecule. Appl. Surf. Sci. 2022, 585, 152695. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Zheng, R.; Pan, J.; Niu, J.; Zou, X.; Jia, C. A flexible, electrochromic, rechargeable Zn-ion battery based on actiniae-like self-doped polyaniline cathode. J. Mater. Chem. A 2020, 8, 12799–12809. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, Y.; Lin, Y.; Jia, C. Fully integrated pressure-controlled electrochromic E-skins. J. Mater. Chem. A 2021, 9, 9134–9144. [Google Scholar] [CrossRef]
- Salah, N.; Shehab, M.; Nady, J.E.; Ebrahim, S.; El-Maghraby, E.M.; Sakr, A.-H. Polyaniline/ZnS quantum dots nanocomposite as supercapacitor electrode. Electrochim. Acta 2023, 449, 142174. [Google Scholar] [CrossRef]
- Bi, X.; Li, M.; Zhou, G.; Liu, C.; Huang, R.; Shi, Y.; Xu, B.B.; Guo, Z.; Fan, W.; Algadi, H.; et al. High-performance flexible all-solid-state asymmetric supercapacitors based on binder-free MXene/cellulose nanofiber anode and carbon cloth/polyaniline cathode. Nano Res. 2023, 16, 7696–7709. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, J.; Wang, Y.; Hu, L.; Tang, S.; Wan, Z.; Jia, C.; Weng, X.; Deng, L. A light-weight, thin-thickness, flexible multifunctional electrochromic device integrated with variable optical, thermal management and energy storage. Electrochim. Acta 2022, 435, 141274. [Google Scholar] [CrossRef]
- Chen, J.; He, Y.; Li, L. Real-time probing electrodeposition growth of polyaniline thin film via in-situ spectroscopic ellipsometry. Thin Solid Films 2022, 762, 139565. [Google Scholar] [CrossRef]
- Devendrachari, M.C.; Shimoga, G.; Lee, S.-H.; Heo, Y.H.; Kotresh, H.M.N.; Thotiyl, M.O.; Kim, S.-Y.; Choi, D.-S. Anthraquinone-2-sulfonic acid-loaded polyaniline nanostructures: Construction of symmetric supercapacitor electrodes thereof. J. Energy Storage 2022, 56, 106033. [Google Scholar] [CrossRef]
- Shi, H.Y.; Ye, Y.J.; Liu, K.; Song, Y.; Sun, X. A long-cycle-life self-doped polyaniline cathode for rechargeable aqueous zinc batteries. Angew. Chem. 2018, 57, 16359–16363. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhang, Y.; Hu, X.; Fu, X.; Dai, L.; Yu, D. Tactile UV- and Solar-Light Multi-Sensing Rechargeable Batteries with Smart Self-Conditioned Charge and Discharge. Angew. Chem. 2019, 58, 9248–9253. [Google Scholar] [CrossRef]
- Luo, S.; Xie, L.; Han, F.; Wei, W.; Huang, Y.; Zhang, H.; Zhu, M.; Schmidt, O.G.; Wang, L. Nanoscale parallel circuitry based on interpenetrating conductive assembly for flexible and high-power zinc ion battery. Adv. Funct. Mater. 2019, 29, 1901336. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, X.; Lin, G.; Peng, Y.; Chao, J.; Yi, L.; Huang, X.; Li, C.; Liao, W. Structure engineering in hexagonal tungsten trioxide/oriented titanium dioxide nanorods arrays with high performances for multi-color electrochromic energy storage device applications. Chem. Eng. J. 2021, 420, 129871. [Google Scholar] [CrossRef]
- Wang, M.; He, Y.; Da Rocha, M.; Rougier, A.; Diao, X. Temperature dependence of the electrochromic properties of complementary NiO//WO3 based devices. Sol. Energy Mater. Sol. Cells 2021, 230, 111239. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; He, Z.; Yi, Y.; Wang, M.; Luo, Z.; Liu, Q.; Huang, J.; Zhong, X.; Du, K.; et al. All-solid-state electrochromic Li-ion hybrid supercapacitors for intelligent and wide-temperature energy storage. Chem. Eng. J. 2021, 414, 128892. [Google Scholar] [CrossRef]
- Liu, W.; Lu, C.; Li, H.; Tay, R.Y.; Sun, L.; Wang, X.; Chow, W.L.; Wang, X.; Tay, B.K.; Chen, Z.; et al. Paper-based all-solid-state flexible micro-supercapacitors with ultra-high rate and rapid frequency response capabilities. J. Mater. Chem. A 2016, 4, 3754–3764. [Google Scholar] [CrossRef]
- Zhang, C.J.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.-H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of flexible, coplanar micro-supercapacitors using mxene inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, K.; Guo, W.; Fang, J.; Wei, Z.; She, X. Thread-like supercapacitors based on one-step spun nanocomposite yarns. Small 2014, 10, 3187–3193. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, S.; Cheng, J.; Zhang, Y.; Zhou, J.; Yang, D.; Tong, D.G.; Wang, B. Interfacial engineered polyaniline/sulfur-doped TiO2 nanotube arrays for ultralong cycle lifetime fiber-shaped, solid-state supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 18390–18399. [Google Scholar] [CrossRef]
- Qu, G.; Cheng, J.; Li, X.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A fiber supercapacitor with high energy density based on hollow graphene/conducting polymer fiber electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, H.-T.; Liu, Y.-N.; Kang, X.-H.; Liu, P.; Zhang, J.-N.; Jin, L.-N.; Bian, S.-W.; Zhu, Q. High-performance yarn electrode materials enhanced by surface modifications of cotton fibers with graphene sheets and polyaniline nanowire arrays for all-solid-state supercapacitors. Electrochim. Acta 2018, 270, 205–214. [Google Scholar] [CrossRef]
- Wang, X.; Sumboja, A.; Foo, W.L.; Yan, C.Y.; Tsukagoshi, K.; Lee, P.S. Rational design of a high performance all solid state flexible micro-supercapacitor on paper. RSC Adv. 2013, 3, 15827–15833. [Google Scholar] [CrossRef]
- Chu, X.; Chen, G.; Xiao, X.; Wang, Z.; Yang, T.; Xu, Z.; Huang, H.; Wang, Y.; Yan, C.; Chen, N.; et al. Air-stable conductive polymer ink for printed wearable micro-supercapacitors. Small 2021, 17, 2100956. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Zhang, Z.; Yan, Y.; Xie, A.; Feng, T.; Jia, C. Construction of Symmetric Flexible Electrochromic and Rechargeable Supercapacitors Based on a 1,3,6,8-Pyrenetetrasulfonic Acid Tetrasodium Salt-Loaded Polyaniline Nanostructured Film. Materials 2025, 18, 2836. https://doi.org/10.3390/ma18122836
Wang Y, Wang Z, Zhang Z, Yan Y, Xie A, Feng T, Jia C. Construction of Symmetric Flexible Electrochromic and Rechargeable Supercapacitors Based on a 1,3,6,8-Pyrenetetrasulfonic Acid Tetrasodium Salt-Loaded Polyaniline Nanostructured Film. Materials. 2025; 18(12):2836. https://doi.org/10.3390/ma18122836
Chicago/Turabian StyleWang, Yi, Ze Wang, Zilong Zhang, Yujie Yan, An Xie, Tong Feng, and Chunyang Jia. 2025. "Construction of Symmetric Flexible Electrochromic and Rechargeable Supercapacitors Based on a 1,3,6,8-Pyrenetetrasulfonic Acid Tetrasodium Salt-Loaded Polyaniline Nanostructured Film" Materials 18, no. 12: 2836. https://doi.org/10.3390/ma18122836
APA StyleWang, Y., Wang, Z., Zhang, Z., Yan, Y., Xie, A., Feng, T., & Jia, C. (2025). Construction of Symmetric Flexible Electrochromic and Rechargeable Supercapacitors Based on a 1,3,6,8-Pyrenetetrasulfonic Acid Tetrasodium Salt-Loaded Polyaniline Nanostructured Film. Materials, 18(12), 2836. https://doi.org/10.3390/ma18122836






