Mechanical and Pore Properties of Foam Concrete Under Salt Erosion Environment
Abstract
1. Introduction
2. Methodology
2.1. Test Materials
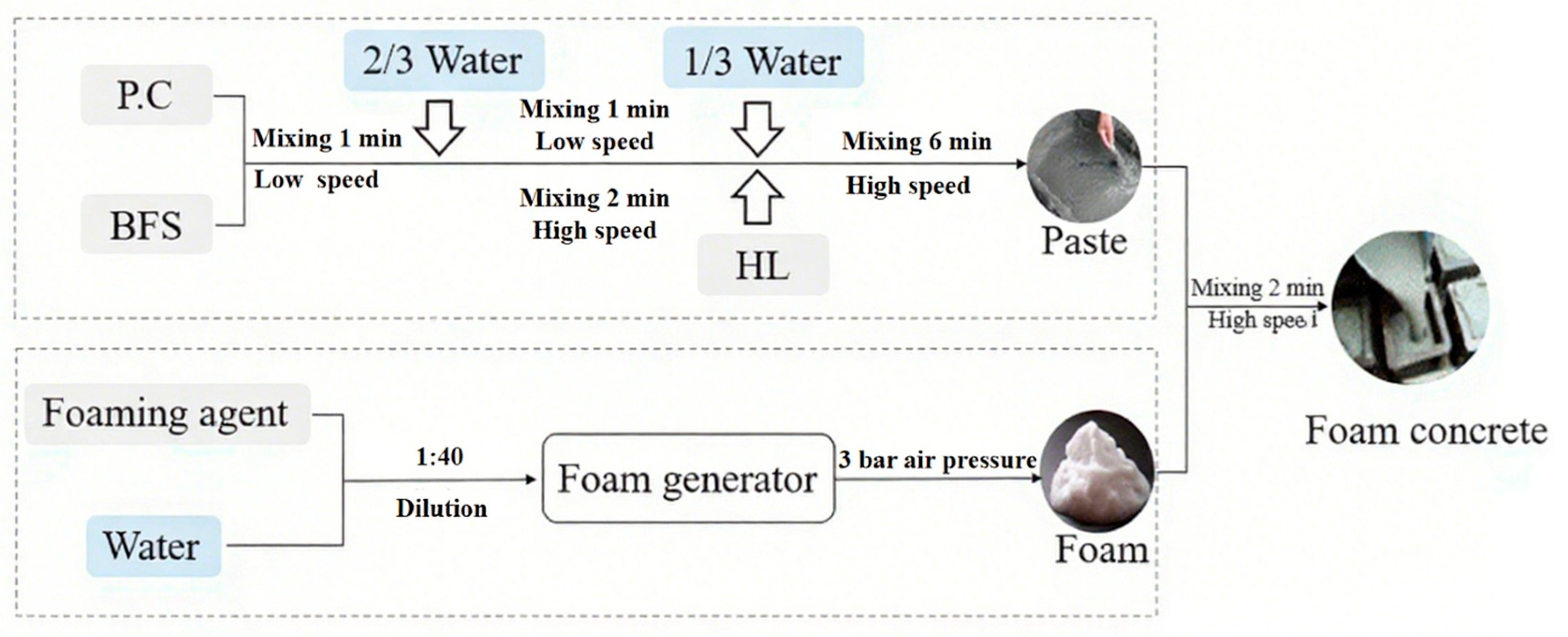
2.2. Sample Preparation
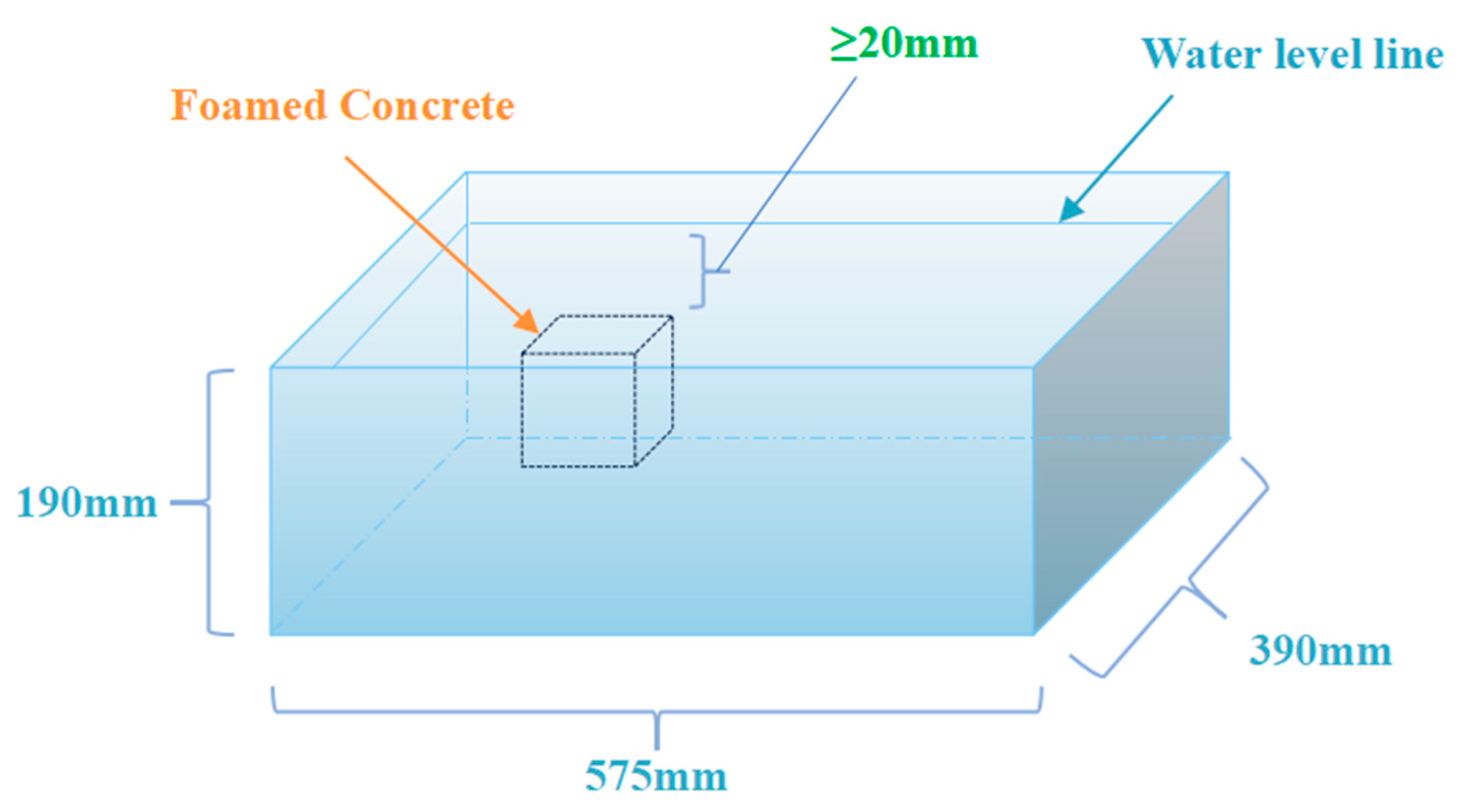
2.3. Salt Erosion Test Procedure
2.4. Test Methods
2.4.1. Compressive Strength Test
2.4.2. Mercury Intrusion Porosimetry (MIP) Analysis
2.4.3. Thermogravimetric Analysis (TGA)
3. Results and Analysis
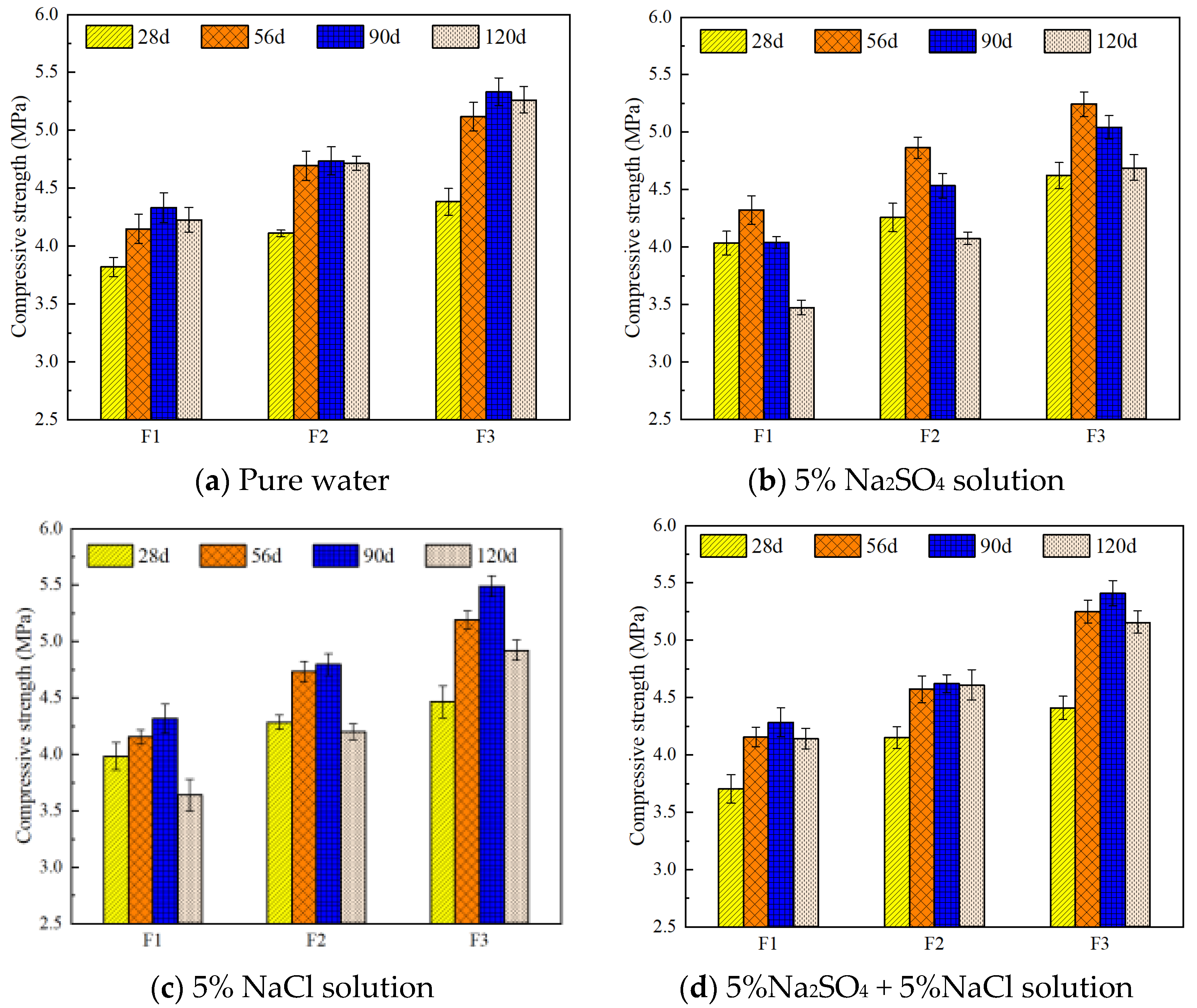
3.1. Effect of Different Salt Environments on the Compressive Strength of FC
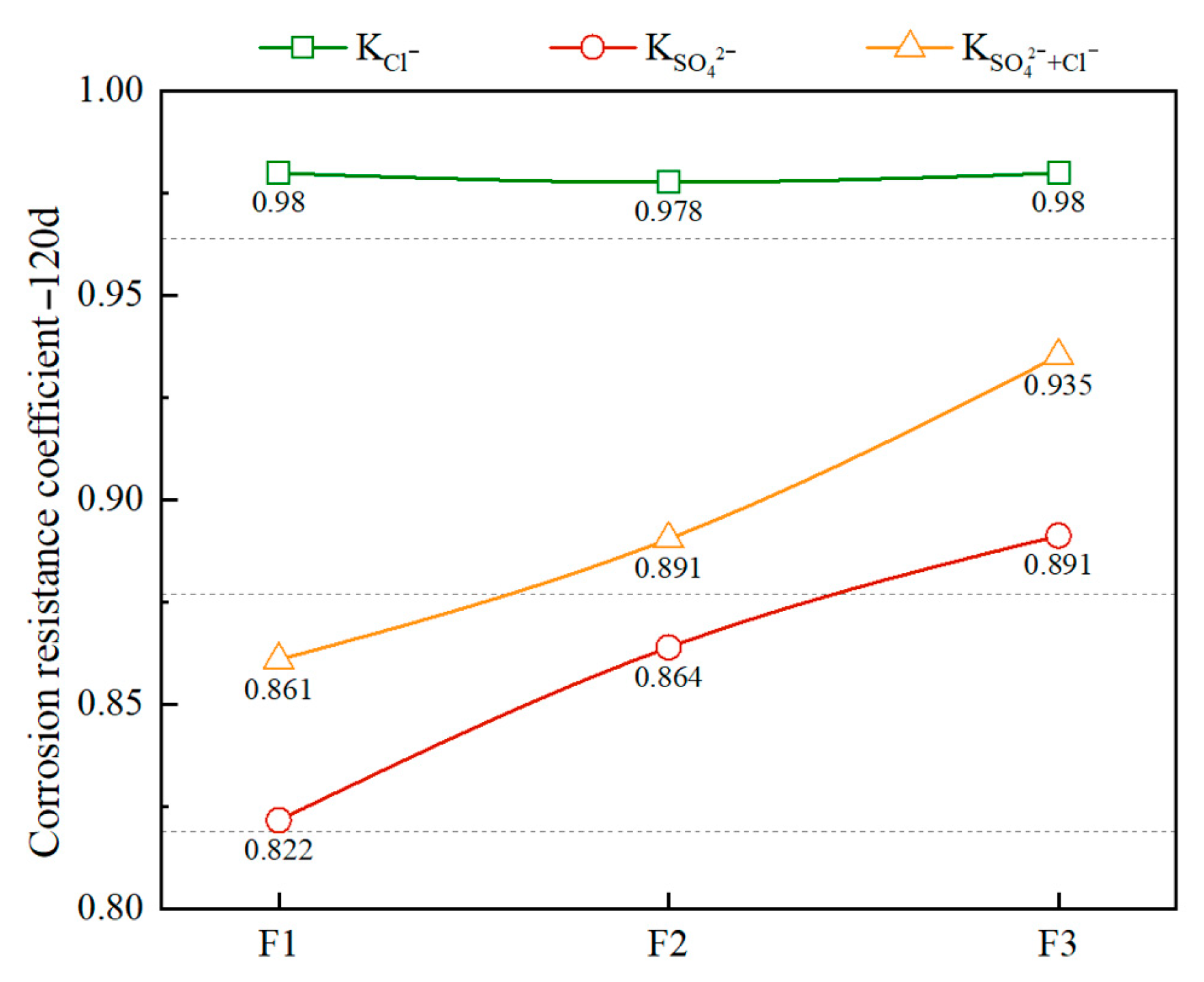
3.2. Effect of Different Densities on the Salt Corrosion Resistance of FC
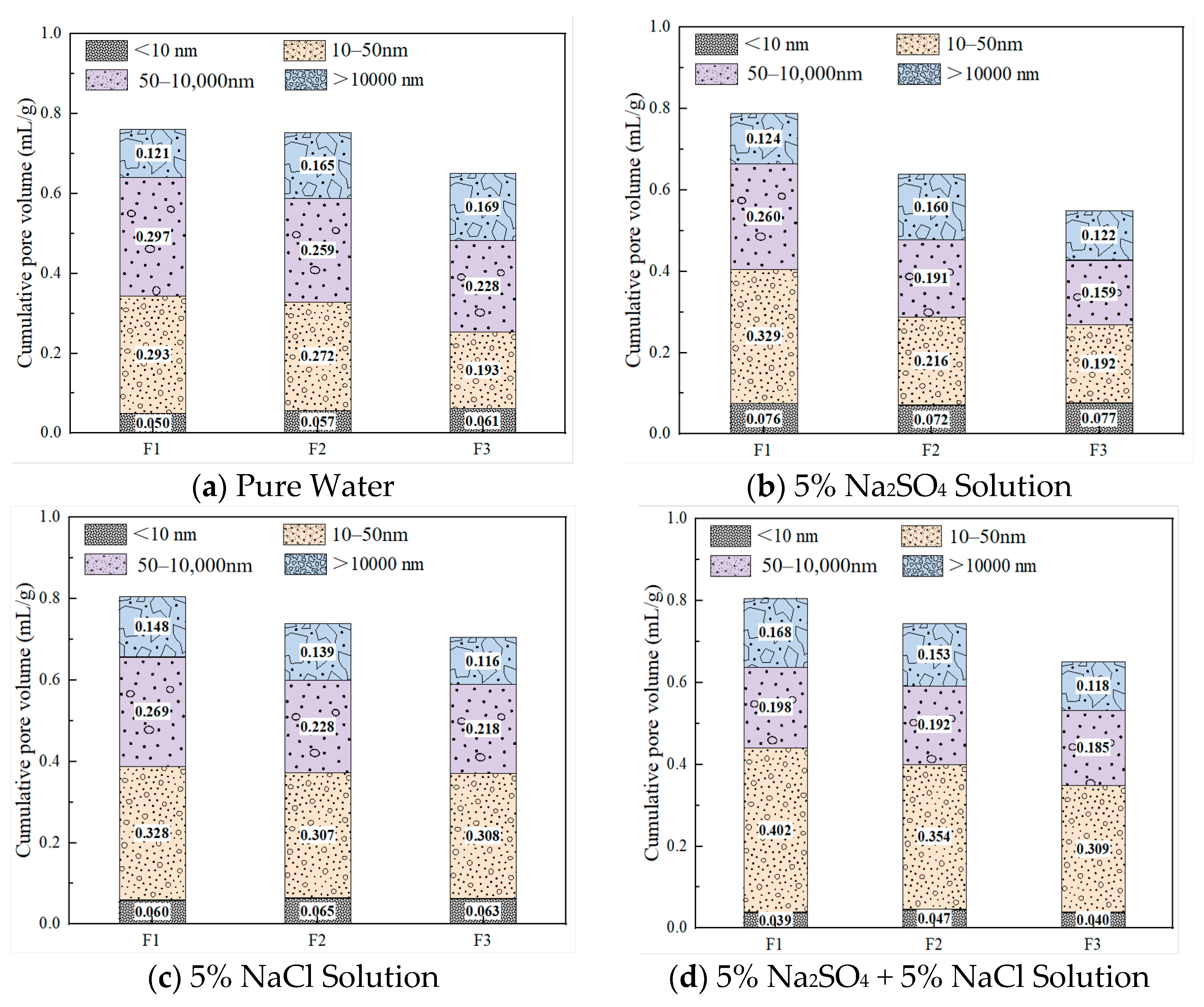
3.3. Effect of Different Salt Corrosion Environments on the Pore Structure Characteristics of FC
3.3.1. Influence of Different Salt Environments on Pore Size Distribution Under Short-Term Salt Exposure (28 Days)
3.3.2. Evolution of Pore Structure Under Long-Term Salt Exposure (120 Days)
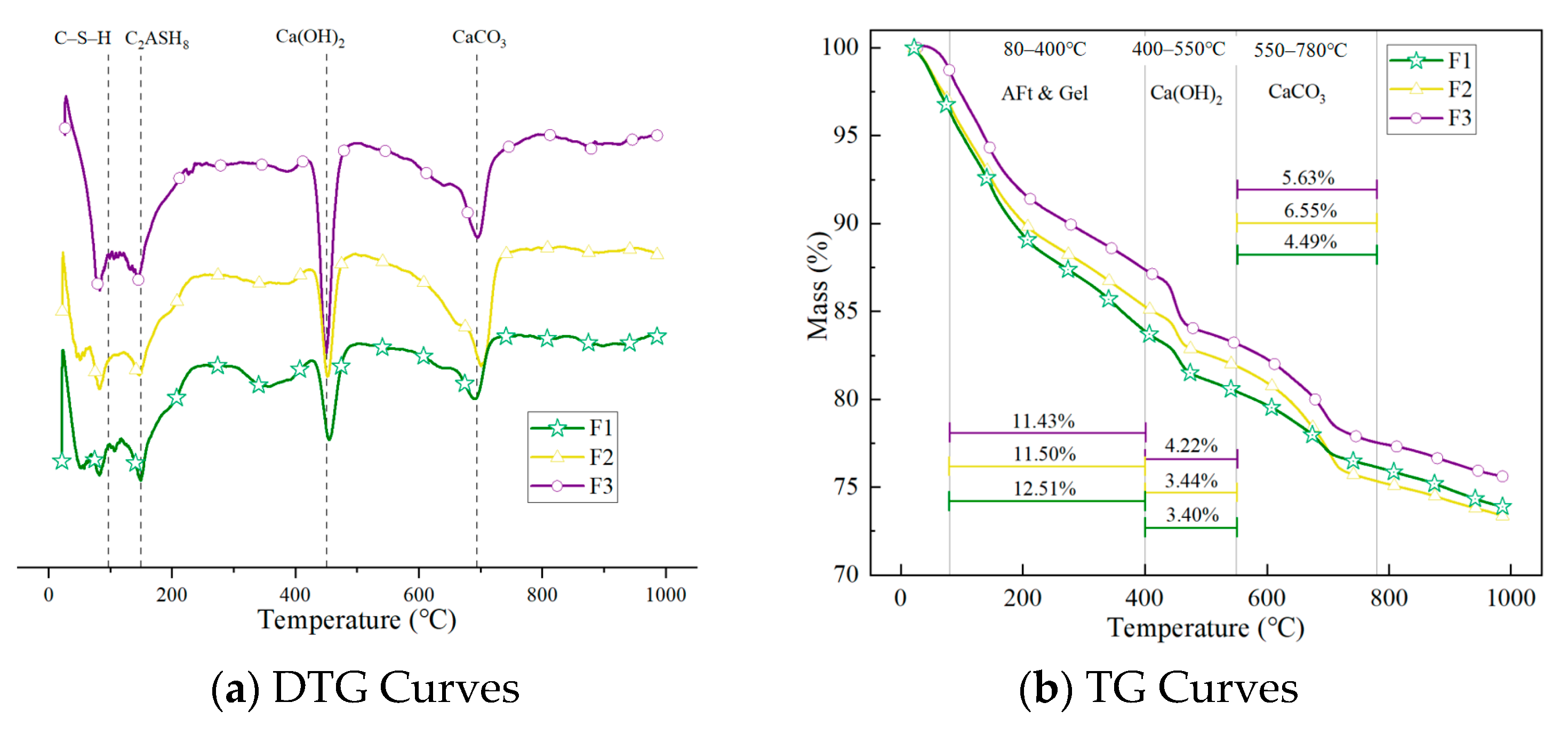
3.4. Influence of Different Salt Environments on the Phase Composition of FC
3.4.1. Hydration Process Evolution of Different Density Specimens Under Standard Curing (28 Days)
3.4.2. Hydration Process Evolution of Different Density Specimens Under Long-Term Curing (120 Days)
4. Conclusions
- (1)
- This study shows that foamed concrete’s degradation under salt attack follows the sequence sulfate > composite salt > chloride, with sulfate ions causing the most severe deterioration. In mixed Na2SO4–NaCl solutions, chloride induces a modest passivation effect that retards sulfate corrosion. Moreover, increasing dry density markedly improves resistance: after 120 days in 5 wt % Na2SO4, strength losses for specimens at 500, 600, and 800 kg/m3 were 17.8%, 13.6%, and 10.9%, respectively. Under combined 5 wt % Na2SO4 + 5 wt % NaCl exposure, low-density samples remained unaffected until 120 days, while high-density samples retained over 95% of their initial strength, confirming density’s protective role.
- (2)
- A comparison between standard curing (28 days) and long-term curing (120 days) results shows that ettringite formed in the early stage temporarily fills pores and seals cracks, thereby reducing porosity. However, as the exposure period extends, the expansive nature of ettringite leads to intensified structural damage. This is especially evident in the composite salt environment, where excessive ettringite formation over time disrupts the original structure, increases pore volume, and induces cracking, ultimately resulting in strength degradation.
- (3)
- Ground granulated blast furnace slag (BFS) contributes to pore structure refinement in foamed concrete through both physical filling and chemical reactions. It acts as a fine aggregate to fill voids and enhance structural compactness. Moreover, the higher the dry density of the material, the more pronounced this refining effect becomes, significantly improving resistance to salt erosion.
- (4)
- High-density specimens, due to their adequate cementitious material and aggregate content, exhibit more complete hydration reactions, resulting in a higher volume fraction of solid products and promoting the formation of large amounts of C-S-H gel, thus optimizing pore size distribution. Furthermore, in saline environments, the formation of ettringite in high-density samples is limited, which helps reduce structural expansion and the risk of microcracking caused by salt erosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gwóźdź-Lasoń, M.; Brachaczek, W.; Kadela, M.; Kukiełka, A. Physical Properties of Foamed Concrete Based on Plaster Mortar with Polystyrene Granulate and Synthetic Foaming Agent. Materials 2025, 18, 2115. [Google Scholar] [CrossRef]
- Abdellatief, M.; Mortagi, M.; Hamouda, H.; Skrzypkowski, K.; Zagórski, K.; Zagórska, A. Influence of Silicate Modulus and Eggshell Powder on the Expansion, Mechanical Properties, and Thermal Conductivity of Lightweight Geopolymer Foam Concrete. Materials 2025, 18, 2088. [Google Scholar] [CrossRef]
- Decky, M.; Hodasova, K.; Papanova, Z.; Remisova, E. Sustainable Adaptive Cycle Pavements Using Composite Foam Concrete at High Altitudes in Central Europe. Sustainability 2022, 14, 9034. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Y.; Ma, Q.; Li, X. Performance and mechanism analysis of natural fiber-reinforced foamed concrete. Case. Stud. Constr. Mater. 2024, 21, e03476. [Google Scholar] [CrossRef]
- Niu, W.; Guo, B.; Li, K.; Ren, Z.; Zheng, Y.; Liu, J.; Lin, H.; Men, X. Cementitous material based stabilization of soft soils by stabilizer: Feasibility and durabiliy assessment. Constr. Build. Mater. 2024, 425, 136046. [Google Scholar] [CrossRef]
- Bayraktar, O.Y.; Yarar, G.; Benli, A.; Kaplan, G.; Gencel, O.; Sutcu, M.; Kozlowski, M.; Kadela, M. Basalt fiber reinforced foam concrete with marble waste and calcium aluminate cement. Struct. Concr. 2023, 24, 1152–1178. [Google Scholar] [CrossRef]
- Mydin, M.; Nawi, M.; Omar, R.; Dulaimi, A.; Najm, H.M.; Mahmood, S.; Sabri, M. Mechanical, durability and thermal properties of foamed concrete reinforced with synthetic twisted bundle macro-fibers. Front. Mater. 2023, 10, 1158675. [Google Scholar]
- Chen, L.L.; Chen, X.D.; Wang, L.; Ning, Y.J.; Ji, T. Compressive strength, pore structure, and hydration products of slag foam concrete under sulfate and chloride environment. Constr. Build. Mater. 2023, 394, 132141. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.G.; Ye, G.B.; Zhang, S.Y.; Shen, H.H.; Chen, Y.G. Strength Degradation of Foamed Lightweight Soil Due to Chemical Erosion and Wet-Dry Cycle and Its Empirical Model. Materials 2023, 16, 6505. [Google Scholar] [CrossRef]
- Wang, D.K.; Zhang, G.Z.; Ding, Q.J.; Yan, P.; Li, Y. Microstructural evolution and degradation mechanisms of tricalcium silicate and tricalcium aluminate composite pastes under sulfate exposure. Case Stud. Constr. Mat. 2024, 21, e04050. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Zhang, Z.; Shen, H.; Chen, Y. Strength degradation of foamed cement paste under sulfate attack. In GEO Shanghai 2024 International Conference, Volume 5, Proceedings of the 5th GeoShanghai International Conference, Shanghai, China, 26–29 May 2024; Qiu, T., Wang, C., Ziotopoulou, K., Deng, L., Wang, X., Eds.; Institute of Physics Publishing: Bristol, UK, 2024; Volume 1334. [Google Scholar]
- Zhang, Z.; Zhang, Y.G.; Ye, G.B.; Shen, H.H.; Chen, Y.G. Dynamic Strength Degradation Prediction Research of Foamed Lightweight Soil Under Chemical Erosion and Wet-Dry Cycle. In Engineering Geology for a Habitable Earth, Volume 3, Iaeg Xiv Congress 2023, Proceedings of the 14th Congress of the International-Association-for-Engineering-Geology-and-the-Environment (IAEG), Chengdu, China, 21–27 September 2023; Wang, S., Huang, R., Azzam, R., Marinos, V.P., Eds.; Springer: Singapore, 2024; pp. 731–741. [Google Scholar]
- Vedalakshmi, R.; Raj, A.S.; Srinivasan, S.; Babu, K.G. Effect of magnesium and sulphate ions on the sulphate resistance of blended cements in low- and medium-strength concretes. Adv. Cem. Res. 2005, 17, 47–55. [Google Scholar] [CrossRef]
- Huang, Q.; Xiong, G.Q.; Fang, Z.; Wang, S.J.; Wang, C.; Sun, H.Y.; Yuan, S.C.; Zhu, X.H. Long-term performance and microstructural characteristics of cement mortars containing nano-SiO2 exposed to sodium sulfate attack. Constr. Build. Mater. 2023, 364, 130011. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, G.; Li, Y. Research on the Influence of Recycled Fine Powder on Chloride Ion Erosion of Concrete in Different Chloride Salt Environments. Materials 2025, 18, 2018. [Google Scholar] [CrossRef]
- Yu, X.G.; Luo, S.S.; Gao, Y.N.; Wang, H.F.; Li, Y.X.; Wei, Y.R.; Wang, X.J. Pore Structure and Microstructure of Foam Concrete. Adv. Mater. Res. 2010, 177, 530–532. [Google Scholar] [CrossRef]
- Feng, W.; Jin, Y.; Zheng, D.; Fang, Y.; Dong, Z.; Cui, H. Study of triethanolamine on regulating early strength of fly ash-based chemically foamed geopolymer. Cem. Concr. Res 2022, 162, 107005. [Google Scholar] [CrossRef]
- Bochen, J. Study on the microstructure of thin-layer facade plasters of thermal insulating system during artificial weathering. Constr. Build. Mater. 2009, 23, 2559–2566. [Google Scholar] [CrossRef]
- Böke, N.; Birch, G.D.; Nyale, S.M.; Petrik, L.F. New synthesis method for the production of coal fly ash-based foamed geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Chen, X.; Shi, D.; Zhang, J.; Cheng, X. Experimental Investigation of the Residual Physical and Mechanical Properties of Foamed Concrete Exposed to High Temperatures. J. Mater. Civ. Eng. 2021, 33, 04021162. [Google Scholar] [CrossRef]
- Gencel, O.; Benli, A.; Bayraktar, O.Y.; Kaplan, G.; Sutcu, M.; Elabade, W.A.T. Effect of waste marble powder and rice husk ash on the microstructural, physico-mechanical and transport properties of foam concretes exposed to high temperatures and freeze–thaw cycles. Constr. Build. Mater. 2021, 291, 123374. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, M.; Wang, J.; Tian, Y.; Wang, X. Optimization of coal-based solid waste ceramsite foam concrete mix proportions and performance study. Constr. Build. Mater. 2024, 416, 135226. [Google Scholar] [CrossRef]
- Tang, K.; An, H.; Liu, C.; Li, Y.; Jia, L.; Tang, Y.; Wang, Q.; Jiang, Y.; Song, Z. Safety and environmental protection application of high performance solid waste unburned ceramsite and its lightweight high strength concrete. Sustain. Chem. Pharm. 2024, 40, 101611. [Google Scholar] [CrossRef]
- GB 175-2023; Common Portland Cement. National Standard of the People’s Republic of China: Beijing, China, 2023.
- GB/T 18046-2017; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. Standards Press of China: Beijing, China, 2017.
- JGJ/T 341-2014; Technical Specification for Application of Foamed Concrete. National Standard of the People’s Republic of China: Beijing, China, 2014.
- CJJ/T 177-2012; Technical Specification for Foamed Mixture Lightweight Soil Filling Engineering. China Architecture & Building Press: Beijing, China, 2012.
- JG/T 266-2011; Foamed Concrete. National Standard of the People’s Republic of China: Beijing, China, 2011.
- JC/T1011-2006; Sulfate Corrosion-Resistance Admixtures for Concrete. National Standard of the People’s Republic of China: Beijing, China, 2006.
- Dhouib, M.; Conciatori, D.; Sorelli, L. Optical Fiber Chloride Sensor for Health Monitoring of Structures in Cold Regions. In Cold Regions Engineering 2019; American Society of Civil Engineers: Reston, VA, USA, 2019; pp. 391–397. [Google Scholar]
- Zhang, C.; Chang, C.C.; Jamshidi, M. Concrete bridge surface damage detection using a single-stage detector. Comput.-Aided Civ. Infrastruct. Eng. 2019, 35, 389–409. [Google Scholar] [CrossRef]
- Cha, Y.J.; Choi, W.; Büyüköztürk, O. Deep Learning-Based Crack Damage Detection Using Convolutional Neural Networks. Comput.-Aided Civ. Infrastruct. Eng. 2017, 32, 361–378. [Google Scholar] [CrossRef]
- Tang, P.; Huber, D.; Akinci, B. Characterization of Laser Scanners and Algorithms for Detecting Flatness Defects on Concrete Surfaces. J. Comput. Civ. Eng. 2011, 25, 31–42. [Google Scholar] [CrossRef]




| Property | PC | BFS |
|---|---|---|
| CaO (%) | 63.255 | 45.22 |
| SiO2 (%) | 22.77 | 29.845 |
| Al2O3 (%) | 5.115 | 13.305 |
| Fe2O3 (%) | 4.125 | - |
| MgO (%) | 1.665 | 6.545 |
| SO3 (%) | 1.25 | 1.105 |
| Specific surface (m2/kg) | 350 | 436 |
| specific gravity (g/cm3) | 3.15 | 2.9 |
| Loss on ignition (%) | 1.985 | 0.46 |
| Mix ID | PC (kg/m3) | BFS (kg/m3) | HL (kg/m3) | w/b | Expected Density (kg/m3) |
|---|---|---|---|---|---|
| F1 | 467 | 180 | 20 | 0.5 | 500 |
| F2 | 560 | 216 | 24 | 0.5 | 600 |
| F3 | 747 | 288 | 32 | 0.5 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Liu, J.; Shi, Q.; Niu, W. Mechanical and Pore Properties of Foam Concrete Under Salt Erosion Environment. Materials 2025, 18, 2810. https://doi.org/10.3390/ma18122810
Huang W, Liu J, Shi Q, Niu W. Mechanical and Pore Properties of Foam Concrete Under Salt Erosion Environment. Materials. 2025; 18(12):2810. https://doi.org/10.3390/ma18122810
Chicago/Turabian StyleHuang, Weihong, Jiankun Liu, Qinyuan Shi, and Weiwei Niu. 2025. "Mechanical and Pore Properties of Foam Concrete Under Salt Erosion Environment" Materials 18, no. 12: 2810. https://doi.org/10.3390/ma18122810
APA StyleHuang, W., Liu, J., Shi, Q., & Niu, W. (2025). Mechanical and Pore Properties of Foam Concrete Under Salt Erosion Environment. Materials, 18(12), 2810. https://doi.org/10.3390/ma18122810







