Improving Energy Storage Properties of Barium Zirconate Titanate Ceramics via Defect Dipole Engineering
Highlights
- Defect dipole engineering was adopted and pinched hysteresis loop was achieved.
- Recoverable energy storage increased by 59% in BZT after doping MnO2.
- XPS and EPR confirmed the generation of a large amount of oxygen vacancies.
- SEM and TEM characterizations revealed changes in domains and defects.
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid-State Synthesis
2.2. Characterization
3. Results and Discussion
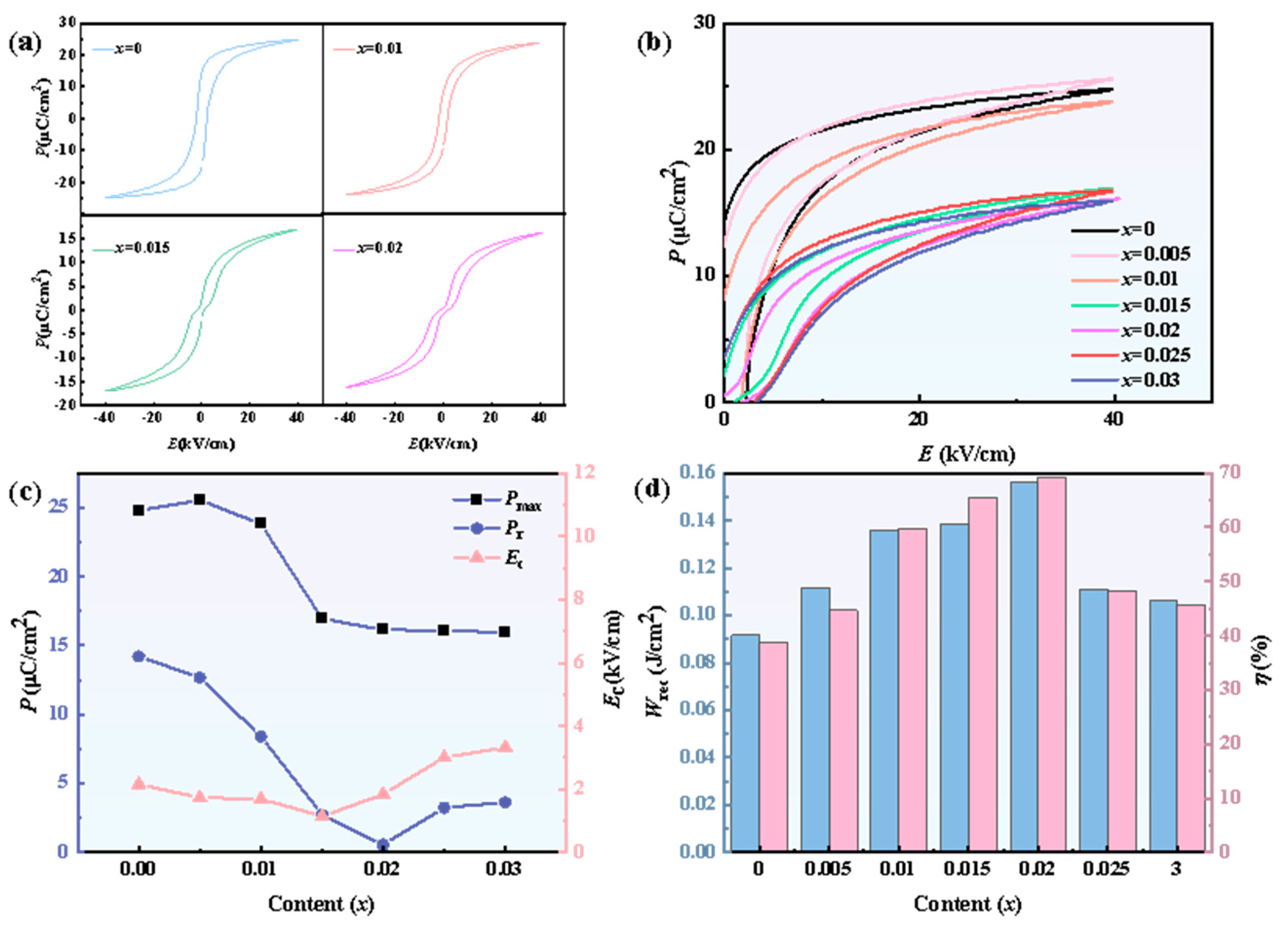
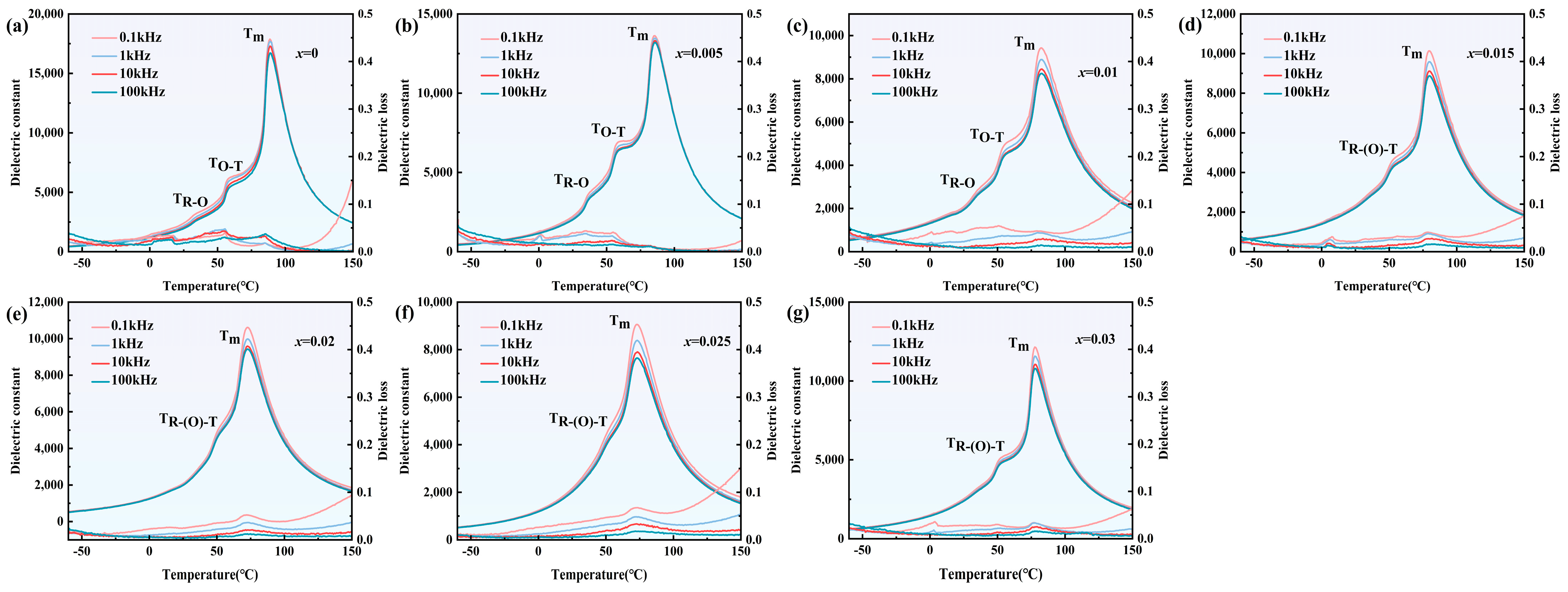
3.1. Ferroelectric and Dielectric Properties
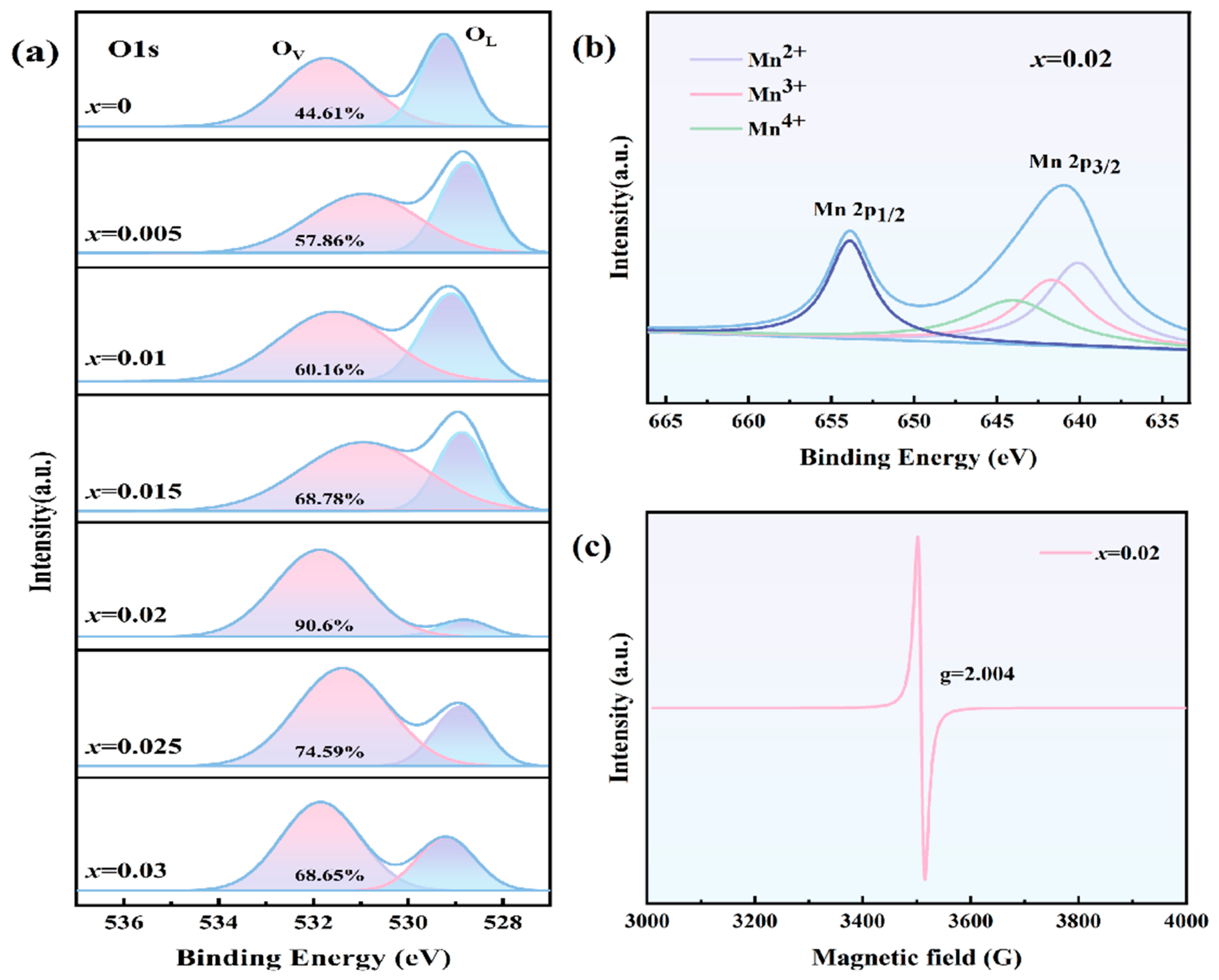
3.2. Defect Characterization
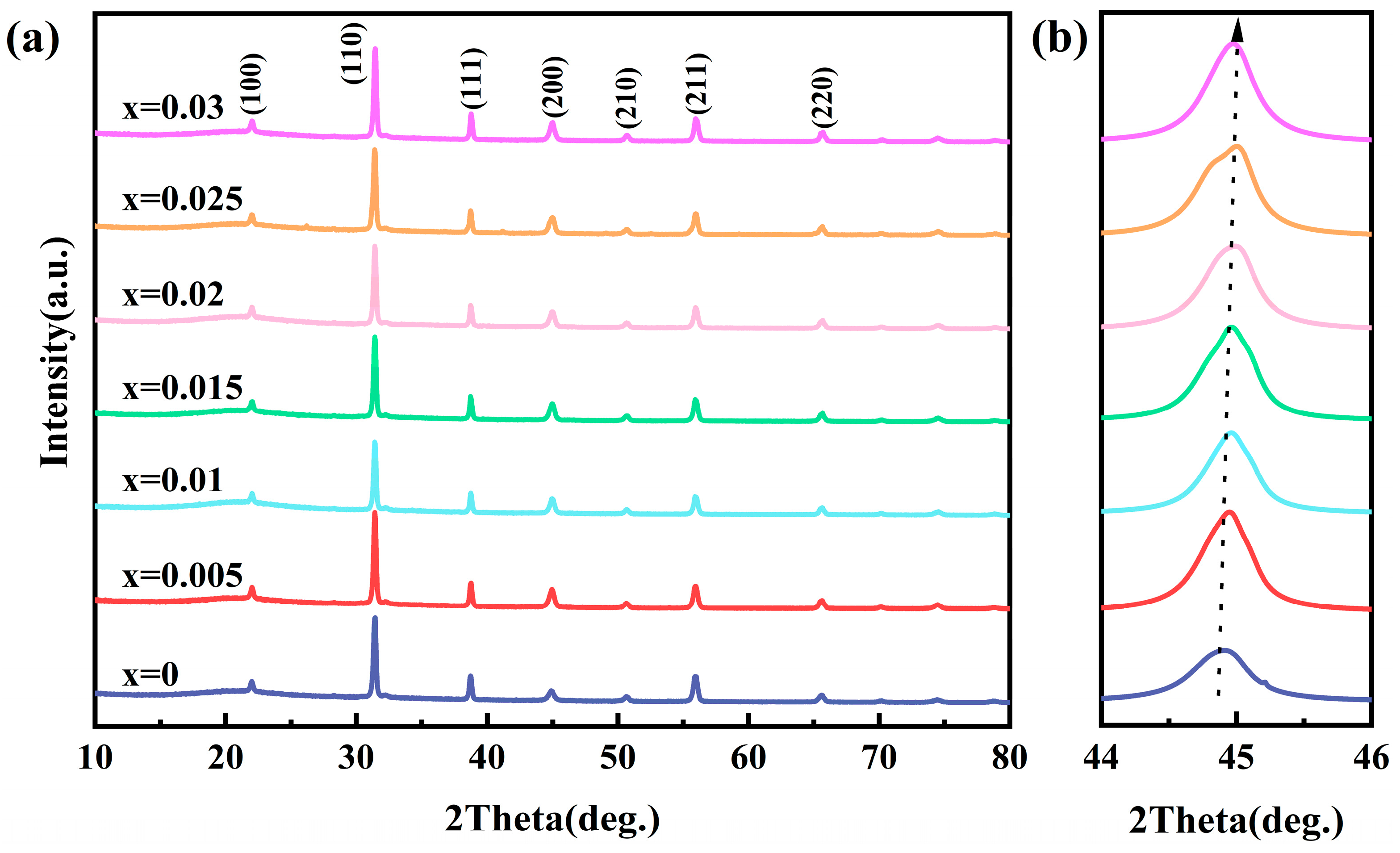
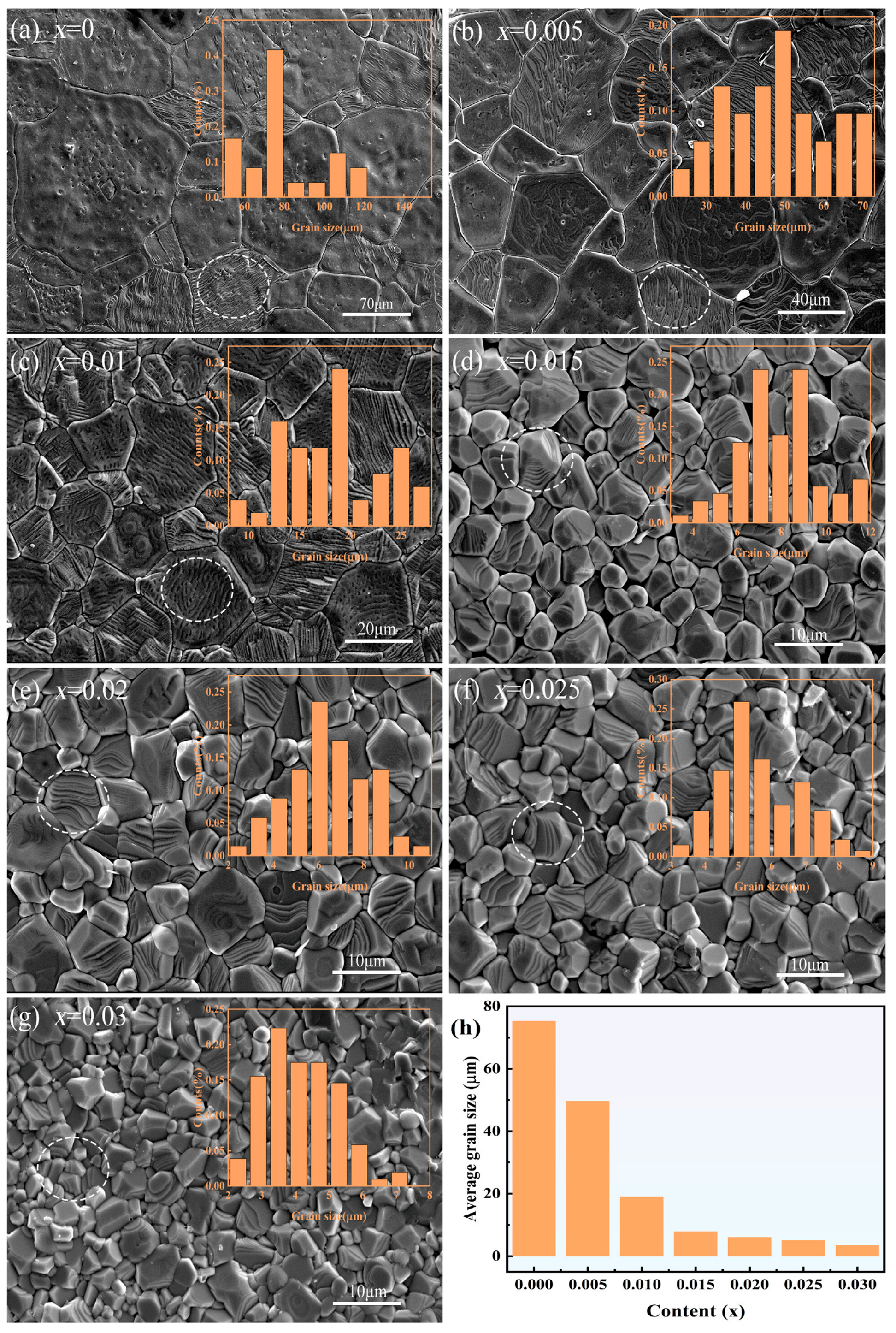
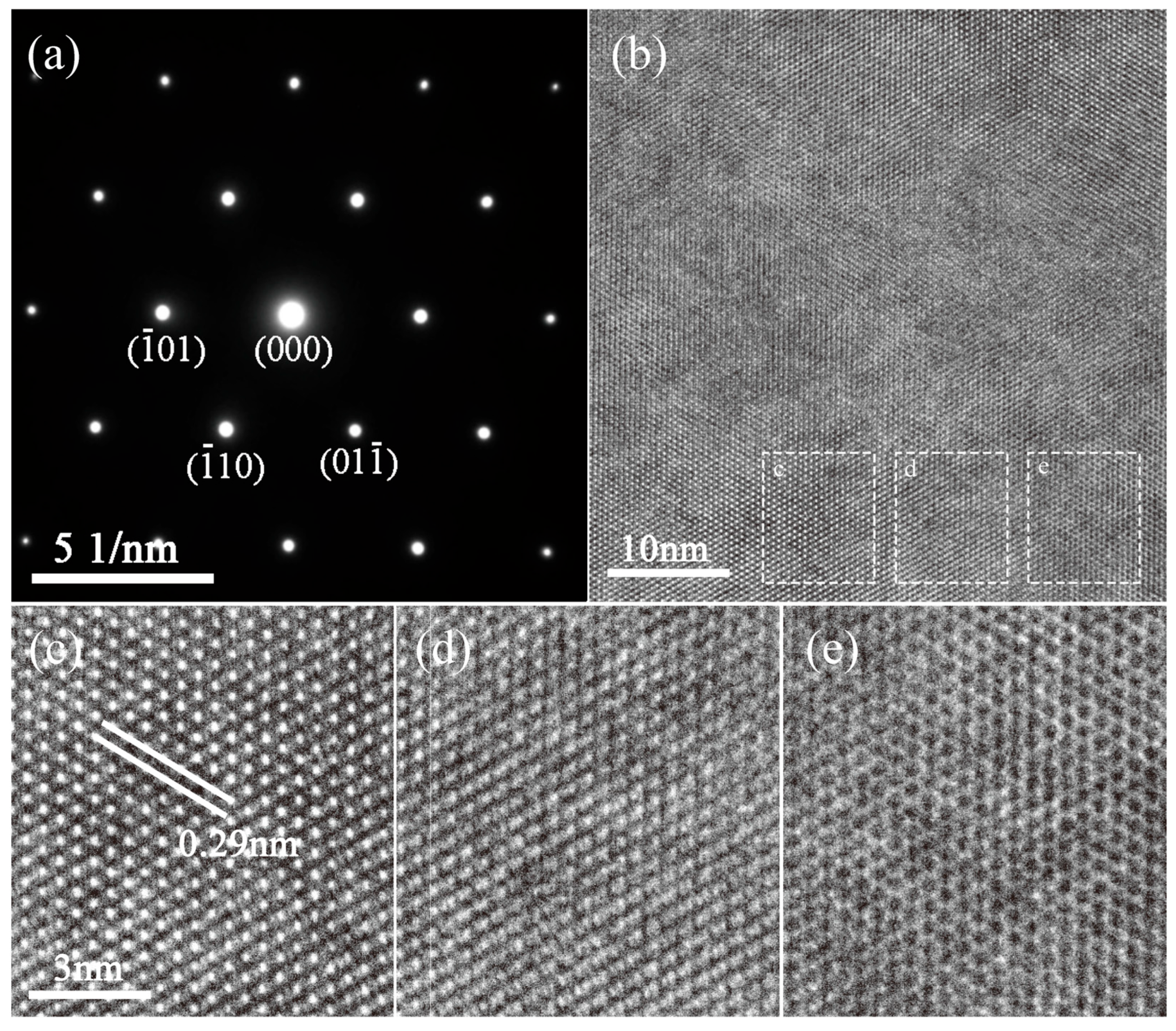
3.3. Microstructure Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Lu, Z.; Li, Y.; Li, L.; Ji, H.; Feteira, A.; Zhou, D.; Wang, D.; Zhang, S.; Reaney, I.M. Electroceramics for high-energy density capacitors: Current status and future perspectives. Chem. Rev. 2021, 121, 6124–6172. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Hou, Y.; Zhu, M.; Zhang, M.; Yan, H. Shift of morphotropic phase boundary in high-performance fine-grained PZN–PZT ceramics. J. Eur. Ceram. Soc. 2014, 34, 2275–2283. [Google Scholar] [CrossRef]
- Kour, P.; Pradhan, S.K.; Kumar, P.; Sinha, S.K.; Kar, M. Enhanced ferroelectric and piezoelectric properties in La-modified PZT ceramics. Appl. Phys. A 2016, 122, 591. [Google Scholar] [CrossRef]
- Thong, H.-C.; Zhao, C.; Zhou, Z.; Wu, C.-F.; Liu, Y.-X.; Du, Z.-Z.; Li, J.-F.; Gong, W.; Wang, K. Technology transfer of lead-free (K,Na)NbO3-based piezoelectric ceramics. Mater. Today 2019, 29, 37–48. [Google Scholar] [CrossRef]
- Ge, P.-Z.; Liu, Z.-G.; Huang, X.-X.; Tang, X.-G.; Tang, Z.-H.; Li, S.-F.; Liu, Q.-X.; Jiang, Y.-P.; Guo, X.-B. Excellent energy storage properties realized in novel BaTiO3-based lead-free ceramics by regulating relaxation behavior. J. Mater. 2023, 9, 910–919. [Google Scholar] [CrossRef]
- Yan, F.; Qian, J.; Wang, S.; Zhai, J. Progress and outlook on lead-free ceramics for energy storage applications. Nano Energy 2024, 123, 109394. [Google Scholar] [CrossRef]
- Li, Y.; Tang, M.-Y.; Zhang, Z.-G.; Li, Q.; Li, J.-L.; Xu, Z.; Liu, G.; Li, F. BaTiO3-based ceramics with high energy storage density. Rare Met. 2023, 42, 1261–1273. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, H.; Hou, D.; Jia, Y.; Zhang, A.; Wang, W. Bismuth sodium titanate-barium titanate-barium zirconate titanate relaxor ferroelectric ceramics with high recoverable energy storage density. Ceram. Int. 2022, 48, 26894–26903. [Google Scholar] [CrossRef]
- Yang, H.; Bao, W.; Lu, Z.; Li, L.; Ji, H.; Huang, Y.; Xu, F.; Wang, G.; Wang, D. High-energy storage performance in BaTiO3-based lead-free multilayer ceramic capacitors. J. Mater. Res. 2021, 36, 1285–1294. [Google Scholar] [CrossRef]
- Dai, Z.; Xie, J.; Liu, W.; Wang, X.; Zhang, L.; Zhou, Z.; Li, J.; Ren, X. Effective strategy to achieve excellent energy storage properties in lead-free BaTiO3-based bulk ceramics. ACS Appl. Mater. Interfaces 2020, 12, 30289–30296. [Google Scholar] [CrossRef]
- Tian, Y.; Xue, F.; Li, W.; Gan, Y.; Zheng, Y.; Du, G. Direct measurement of large electrocaloric effect in BZT-BST lead-free relaxor ferroelectrics near room temperature. Ceram. Int. 2025, 51, 12101–12108. [Google Scholar] [CrossRef]
- Jin, L.; Li, F.; Zhang, S. Decoding the Fingerprint of ferroelectric loops: Comprehension of the material properties and structures. J. Am. Ceram. Soc. 2014, 97, 1–27. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Li, Z.; Chen, W.; Xu, Q.; He, D.; Xi, D.; Zhang, Q.; Yuan, T.; Qu, Y.; et al. Solid-diffusion synthesis of single-atom catalysts directly from bulk metal for efficient CO2 reduction. Joule 2019, 3, 584–594. [Google Scholar] [CrossRef]
- Lin, J.; Qian, J.; Shi, Y.; Wang, S.; Lin, J.; Ge, G.; Hua, Y.; Shen, B.; Zhai, J. Enhanced piezoelectric response attained by defect dipoles in BiFeO3-based lead-free ceramics. ACS Appl. Mater. Interfaces 2024, 16, 67959–67969. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lu, Y.-q.; Han, Q.; Hu, T.-Y.; Duan, T.; Liu, Y.; Cheng, S.-D.; Dai, Y.; Liu, M.; Ma, C. Enhanced energy storage performance of 0.85BaTiO3–0.15Bi(Mg0.5Hf0.5)O3 films via synergistic effect of defect dipole and oxygen vacancy engineering. Acta Mater. 2025, 283, 120522. [Google Scholar] [CrossRef]
- Kim, J.; Saremi, S.; Acharya, M.; Velarde, G.; Parsonnet, E.; Donahue, P.; Qualls, A.; Garcia, D.; Martin, L.W. Ultrahigh capacitive energy density in ion-bombarded relaxor ferroelectric films. Science 2020, 369, 81–84. [Google Scholar] [CrossRef]
- Wang, H.Q.; Dai, Y.J.; Zhang, X.W. Microstructure and hardening mechanism of K0.5Na0.5NbO3 Lead-Free Ceramics with CuO Doping Sintered in Different Atmospheres. J. Am. Ceram. Soc. 2012, 95, 1182–1184. [Google Scholar] [CrossRef]
- Zhang, L.X.; Ren, X. In situ observation of reversible domain switching in aged Mn-doped BaTiO3 single crystals. Phys. Rev. B 2005, 71, 174108. [Google Scholar] [CrossRef]
- Ren, X.; Otsuka, K. Universal symmetry property of point defects in crystals. Phys. Rev. Lett. 2000, 85, 1016–1019. [Google Scholar] [CrossRef]
- Liu, S.; Cohen, R.E. Multiscale simulations of defect dipole–enhanced electromechanical coupling at dilute defect concentrations. Appl. Phys. Lett. 2017, 111, 082903. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, A.; Zhang, G.; Zheng, Y.; Zheng, A.; Luo, G.; Tu, R.; Sun, Y.; Zhang, J.; Shen, Q. Optimization of energy storage properties in lead-free barium titanate-based ceramics via B-site defect dipole engineering. ACS Sustain. Chem. Eng. 2022, 10, 2930–2937. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, J.; Chi, Q.; Li, W.; Yu, Y.; Fei, W. Defects and aliovalent doping engineering in electroceramics. Chem. Rev. 2020, 120, 1710–1787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Dai, Y.; Huang, F. The formation and effect of defect dipoles in lead-free piezoelectric ceramics: A review. Sustain. Mater. Technol. 2019, 20, e00092. [Google Scholar] [CrossRef]
- Peng, J.; Shan, D.; Liu, Y.; Pan, K.; Lei, C.; He, N.; Zhang, Z.; Yang, Q.J. A thermodynamic potential for barium zirconate titanate solid solutions. Npj Comput. Mater. 2018, 4, 66. [Google Scholar] [CrossRef]
- Maiti, T.; Guo, R.; Bhalla, A.S. Structure-property phase diagram of BaZrxTi1−xO3 System. J. Am. Ceram. Soc. 2008, 91, 1769–1780. [Google Scholar] [CrossRef]
- Sundarakannan, B.; Kakimoto, K.; Ohsato, H.J.J.o.A.P. Frequency and temperature dependent dielectric and conductivity behavior of KNbO3 ceramics. J. Appl. Phys. 2003, 94, 5182–5187. [Google Scholar] [CrossRef]
- Prieto, C.; Arizmendi, L.; Gonzalo, J.A.; Jaque, F.; Agulló-López, F. Point-defect contribution to the low-frequency dielectric response of LiTaO3 Reanalysis. Phys. Rev. B 1986, 34, 7396–7399. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of oxygen 1s X-ray photoelectron spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Shi, Z.; Tong, S.; Wei, J.; Guo, Y.; Zhang, Y.; Wang, L.; Zhang, J. Regulating Multiscale Defects to enhance the thermoelectric performance of Ca0.87Ag0.1Dy0.03MnO3 ceramics. ACS Appl. Mater. Interfaces 2022, 14, 32166–32175. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Yan, Y.; Li, Z.; Li, F.; Yang, S. Piezoelectric ceramic hardening through defect distribution optimization in multicomponent systems. Adv. Funct. Mater. 2024, 35, 2413130. [Google Scholar] [CrossRef]
- Sun, Z.; Bai, Y.; Jing, H.; Hu, T.; Du, K.; Guo, Q.; Gao, P.; Tian, Y.; Ma, C.; Liu, M.; et al. A polarization double-enhancement strategy to achieve super low energy consumption with ultra-high energy storage capacity in BCZT-based relaxor ferroelectrics. Mater. Horiz. 2024, 11, 3330–3344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Du, P.; Li, W.; Yuan, G.; Liu, Z.; Luo, L. Reversible and color controllable emissions in Er3+/Pr3+-codoped K0.5Na0.5NbO3 ceramics with splendid photochromic properties for anti-counterfeiting applications. J. Eur. Ceram. Soc. 2021, 41, 1904–1916. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Luo, H.; Zhang, Y.; Yao, Y.; Liu, G.; Qi, H.; Chen, J. Outstanding electromechanical performance in piezoelectric ceramics via synergistic effects of defect engineering and domain miniaturization. J. Mater. Sci. Technol. 2023, 158, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Huang, P.; Chen, Z.; Bai, W.; Li, L.; Wen, F.; Zheng, P.; Wu, W.; Zheng, L.; et al. Reduction of oxygen vacancy concentration and large enhancement of electrical performances in Cu/Sb co-doped Bi4Ti3O12 high temperature piezoelectric ceramics. J. Appl. Phys. 2020, 127, 044102. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Chikada, S.; Kubota, T.; Honda, A.; Higai, S.I.; Motoyoshi, Y.; Wada, N.; Shiratsuyu, K. Interactions between Mn dopant and oxygen vacancy for insulation performance of BaTiO3. J. Appl. Phys. 2016, 120, 1221. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Zhao, Y.; Zhang, H.G.; Zhang, N. Aging-induced hysteresis loop evolution of Ba(Ti0.99Mn0.01)O3 ceramics during ferroelectric-ferroelectric transition cycle. J. Alloys Compd. 2017, 696, 814–819. [Google Scholar] [CrossRef]
- Yin, Y.; Yu, J.-R.; Tang, Y.-C.; Song, A.-Z.; Liu, H.; Yang, D.; Li, J.-F.; Zhao, L.; Zhang, B.-P. Enhanced energy storage properties and antiferroelectric stability of Mn-doped NaNbO3-CaHfO3 lead-free ceramics: Regulating phase structure and tolerance factor. J. Mater. 2022, 8, 611–617. [Google Scholar] [CrossRef]
- Shang, F.; Wei, J.; Xu, J.; Zhang, H.; Xia, Y.; Zhu, G.; Jiang, K.; Chen, G.; Ye, Z.; Xu, H. Boosting energy storage performance of glass ceramics via modulating defect formation during crystallization. Adv. Sci. 2023, 11, 2307011. [Google Scholar] [CrossRef]
- Zhang, F.; Tan, J.; Wang, P.; Huang, R.; Lin, H.-T.; Huang, X.; Yang, J.; Fu, Z.; Cao, X.; Zhang, L.; et al. Defect dipole engineering enhanced the dielectric performance and reliability of Mn-doped BaTiO3-based multilayer ceramic capacitor. Ceram. Int. 2024, 50, 38263–38273. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Qin, Z.; Gao, S.; Zheng, H.; Luo, J.; Liu, Y.; Lyu, Y. Improving Energy Storage Properties of Barium Zirconate Titanate Ceramics via Defect Dipole Engineering. Materials 2025, 18, 2809. https://doi.org/10.3390/ma18122809
Wang Z, Qin Z, Gao S, Zheng H, Luo J, Liu Y, Lyu Y. Improving Energy Storage Properties of Barium Zirconate Titanate Ceramics via Defect Dipole Engineering. Materials. 2025; 18(12):2809. https://doi.org/10.3390/ma18122809
Chicago/Turabian StyleWang, Zhiyi, Zhengchao Qin, Si Gao, Hongjuan Zheng, Jin Luo, Yunfei Liu, and Yinong Lyu. 2025. "Improving Energy Storage Properties of Barium Zirconate Titanate Ceramics via Defect Dipole Engineering" Materials 18, no. 12: 2809. https://doi.org/10.3390/ma18122809
APA StyleWang, Z., Qin, Z., Gao, S., Zheng, H., Luo, J., Liu, Y., & Lyu, Y. (2025). Improving Energy Storage Properties of Barium Zirconate Titanate Ceramics via Defect Dipole Engineering. Materials, 18(12), 2809. https://doi.org/10.3390/ma18122809




