Chiral Perovskite Single Crystals: Toward Promising Design and Application
Abstract
1. Introduction
2. Synthesis Strategies of Chiral Perovskites
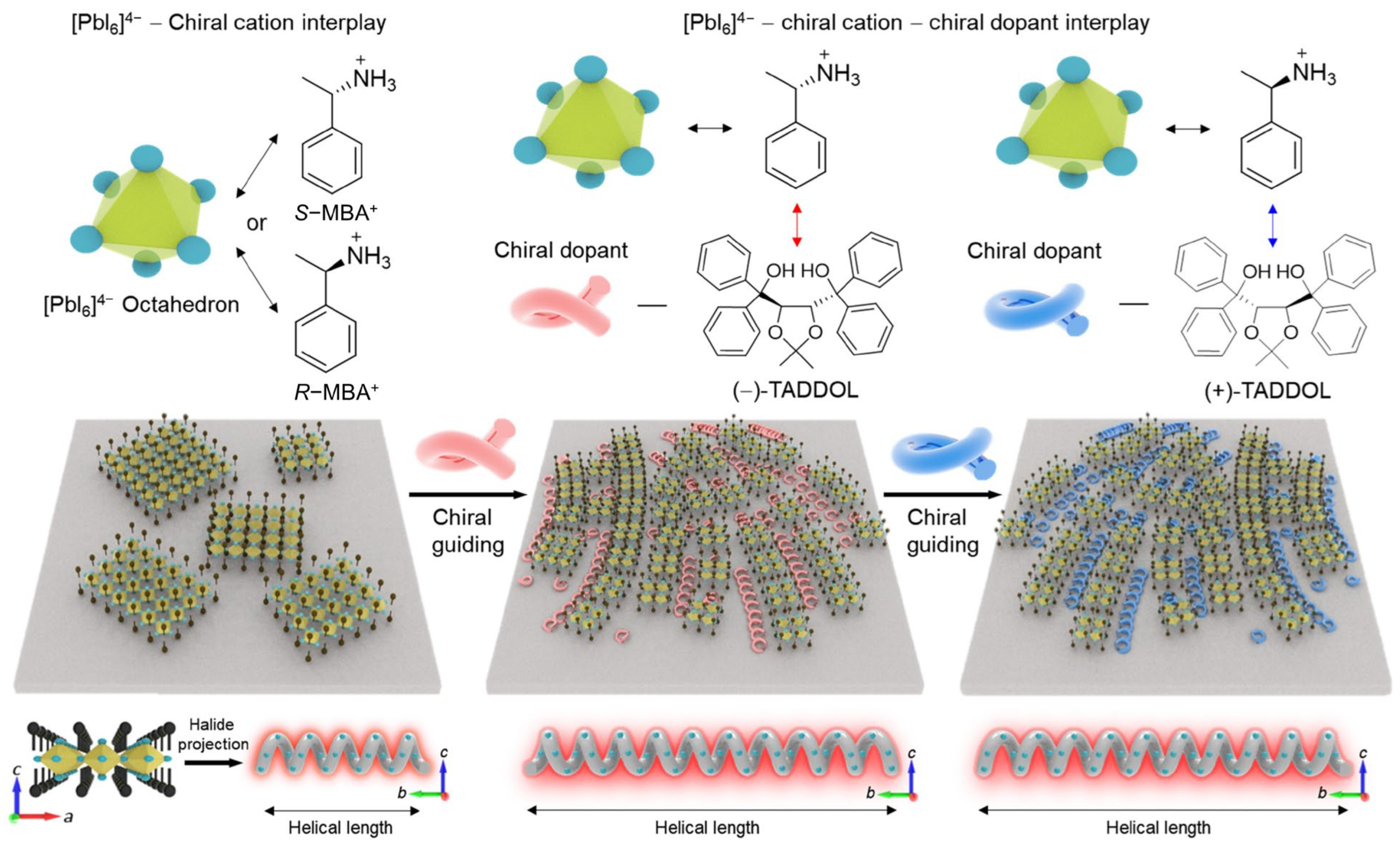
2.1. Chiral Perovskite Bulk SCs
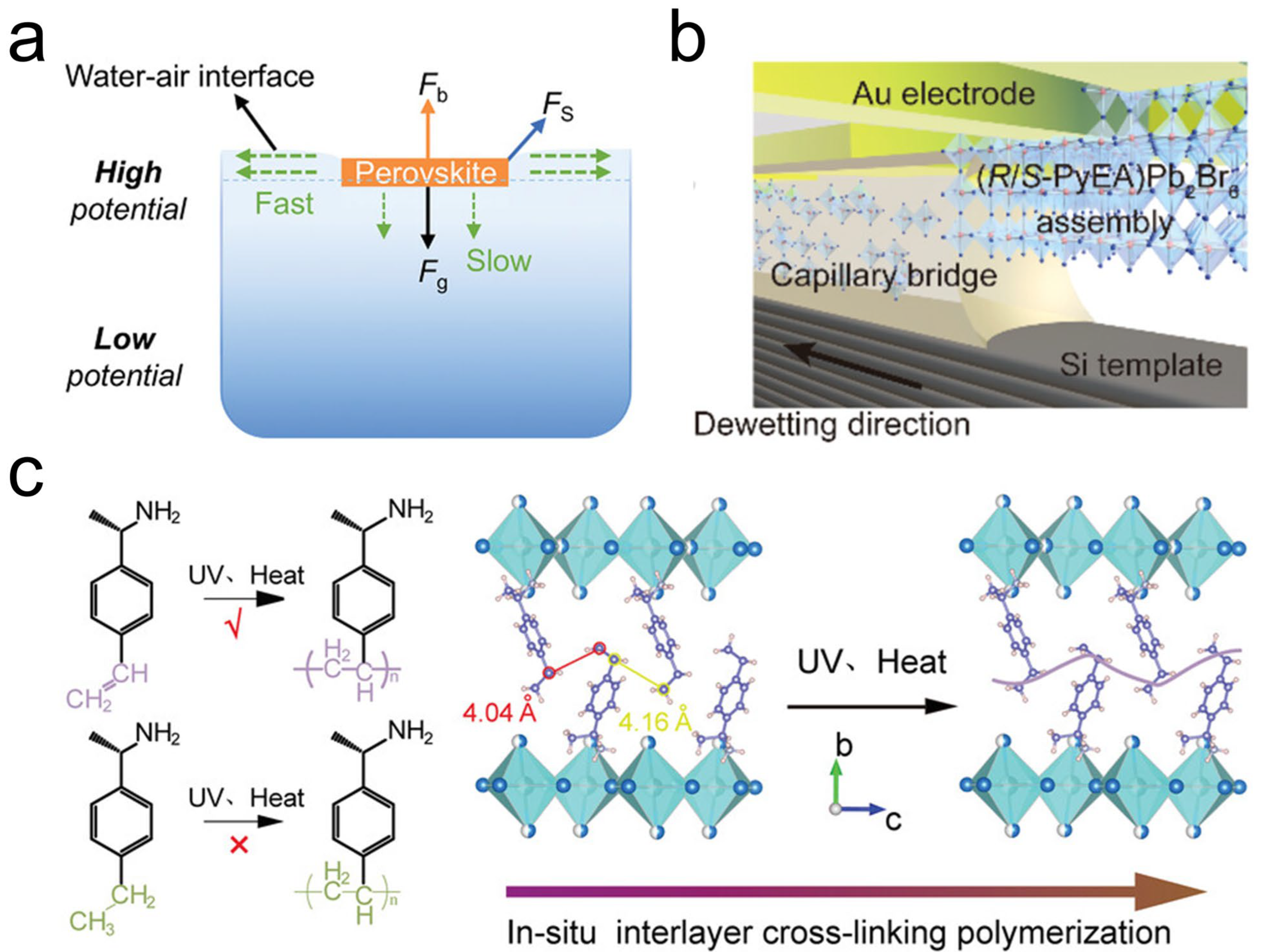
2.2. Chiral Perovskite SCTFs
2.3. Chiral Perovskites Nanocrystals
2.4. Chiral Perovskites Polycrystalline Thin Films
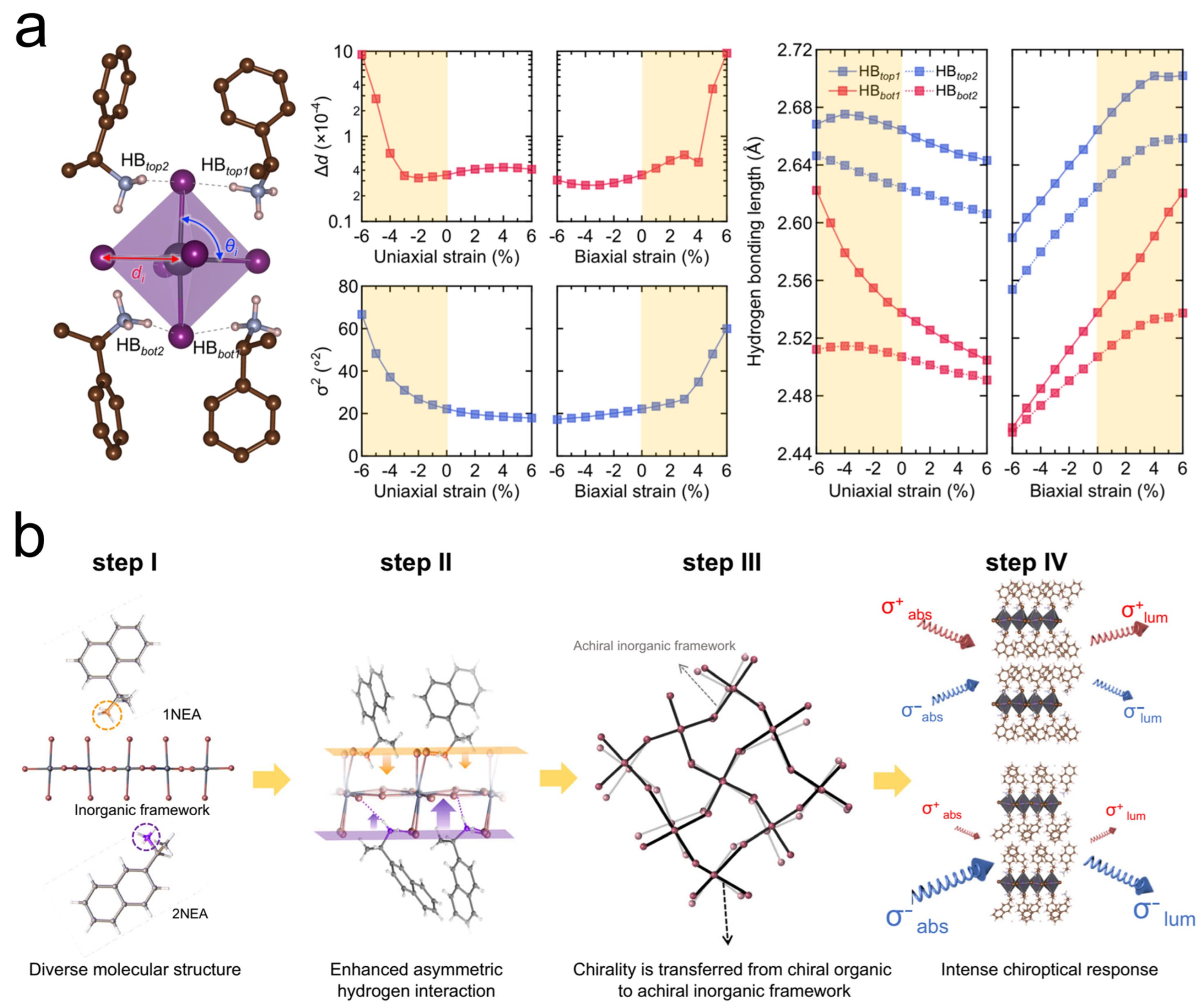
2.5. Defect Management Strategies in Chiral Perovskites
3. Chirality Induction Mechanisms
3.1. Asymmetric Hydrogen-Bonding Interaction
3.2. Interfacial Interaction
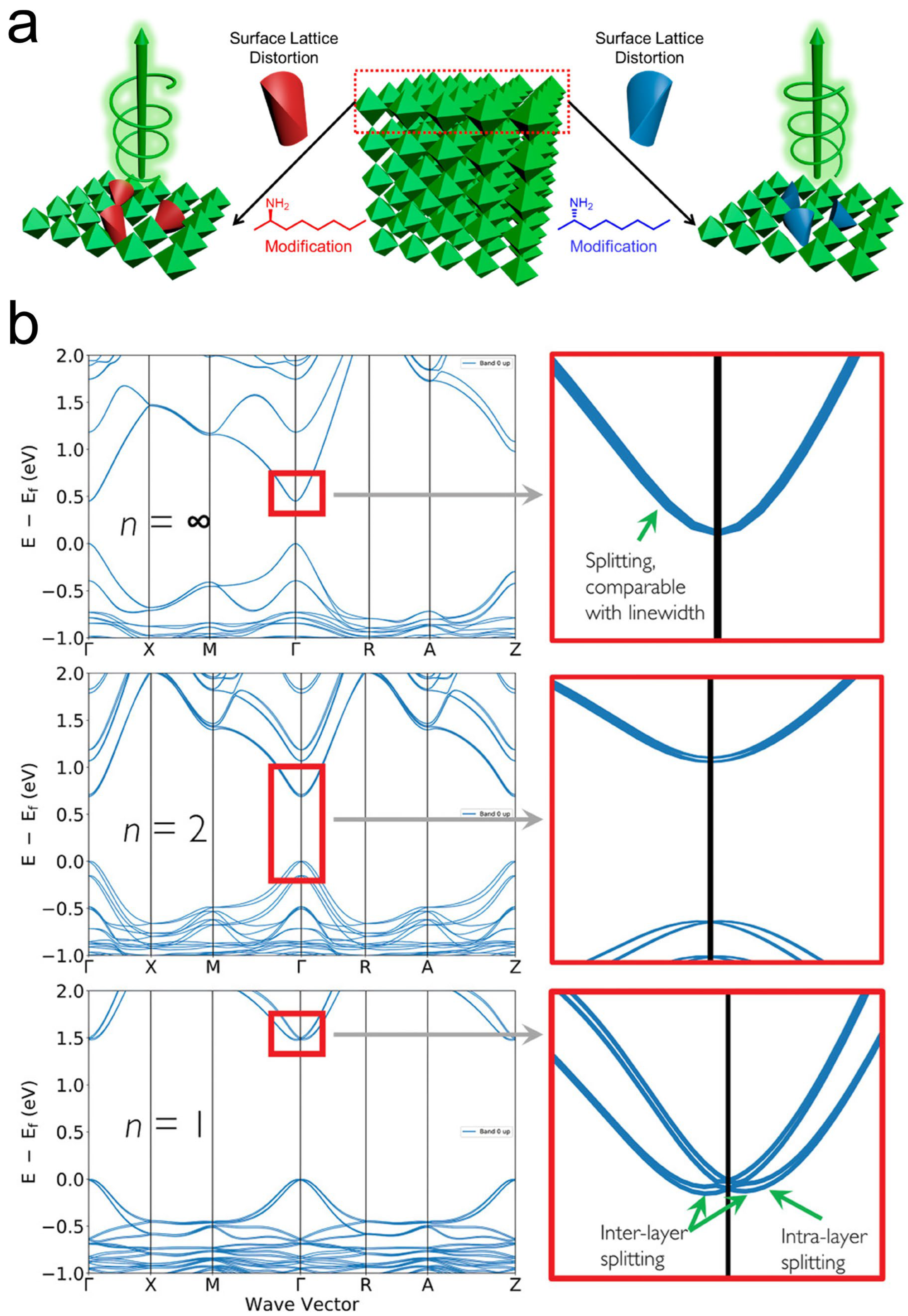
3.3. Rashba–Dresselhaus Splitting
4. Applications in Chiral Optics and Photonic Devices
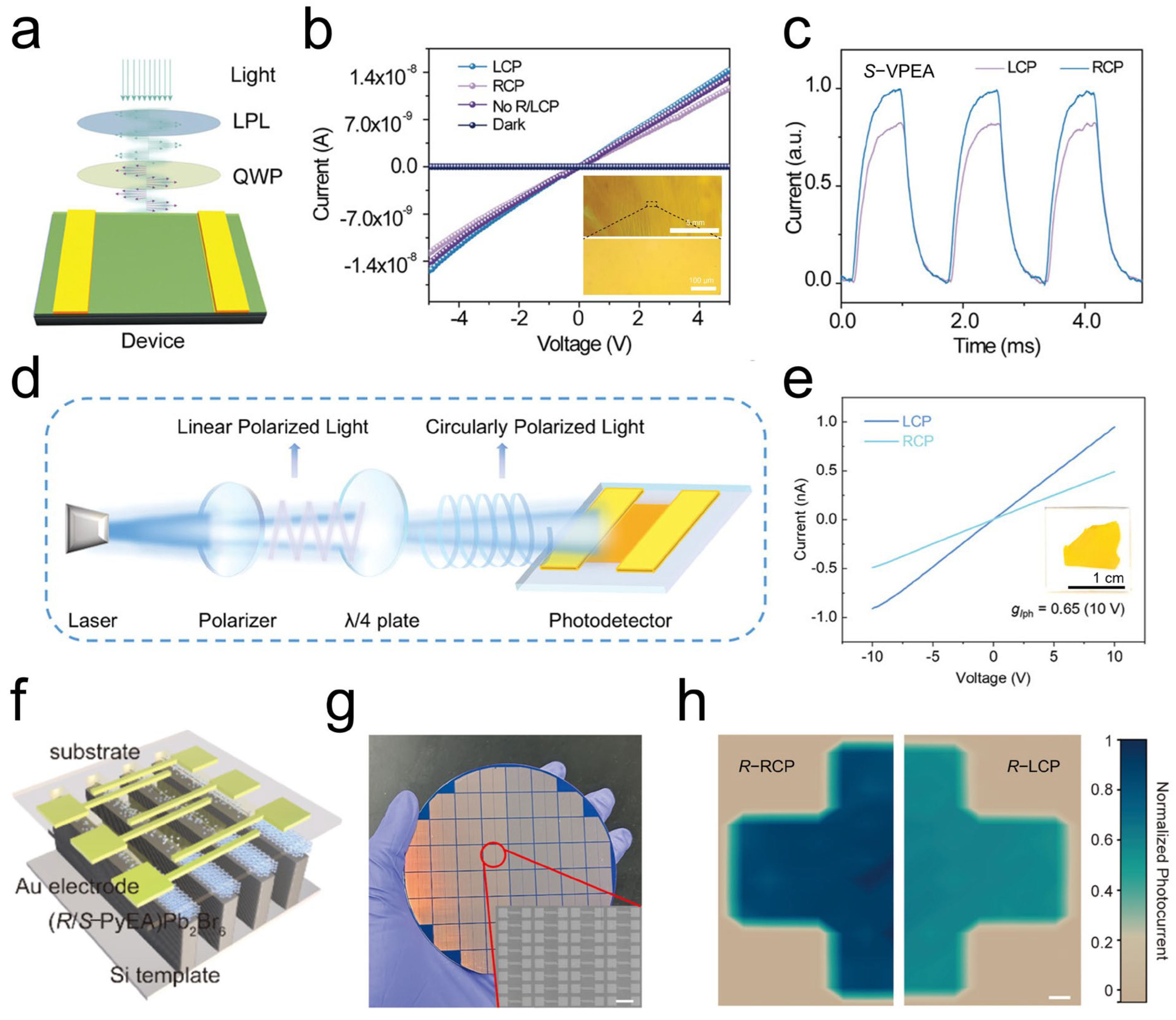
4.1. Chiral Perovskite SC-Based CPL Photodetector
4.2. NLO Response of Chiral Perovskite SCs
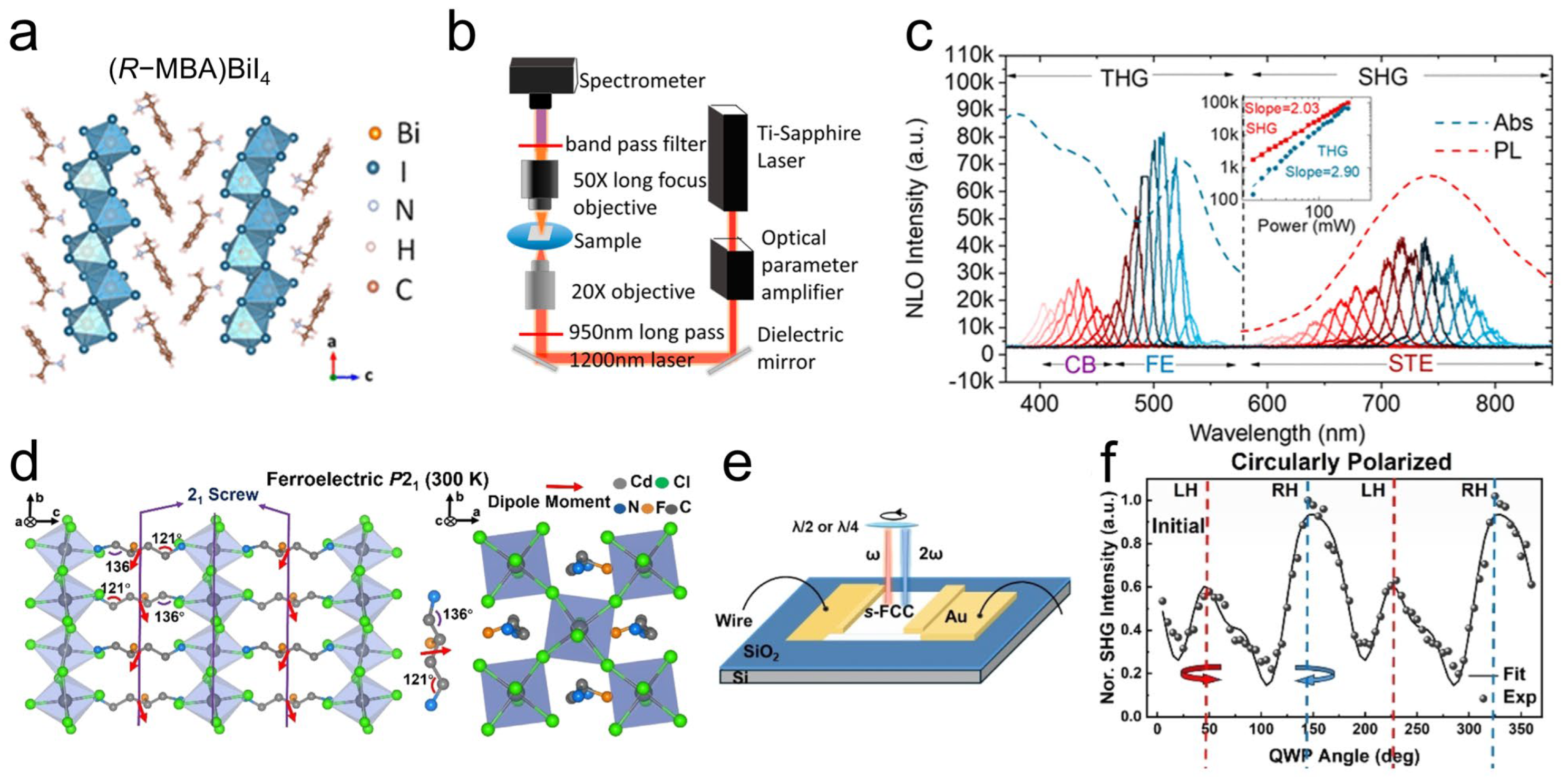
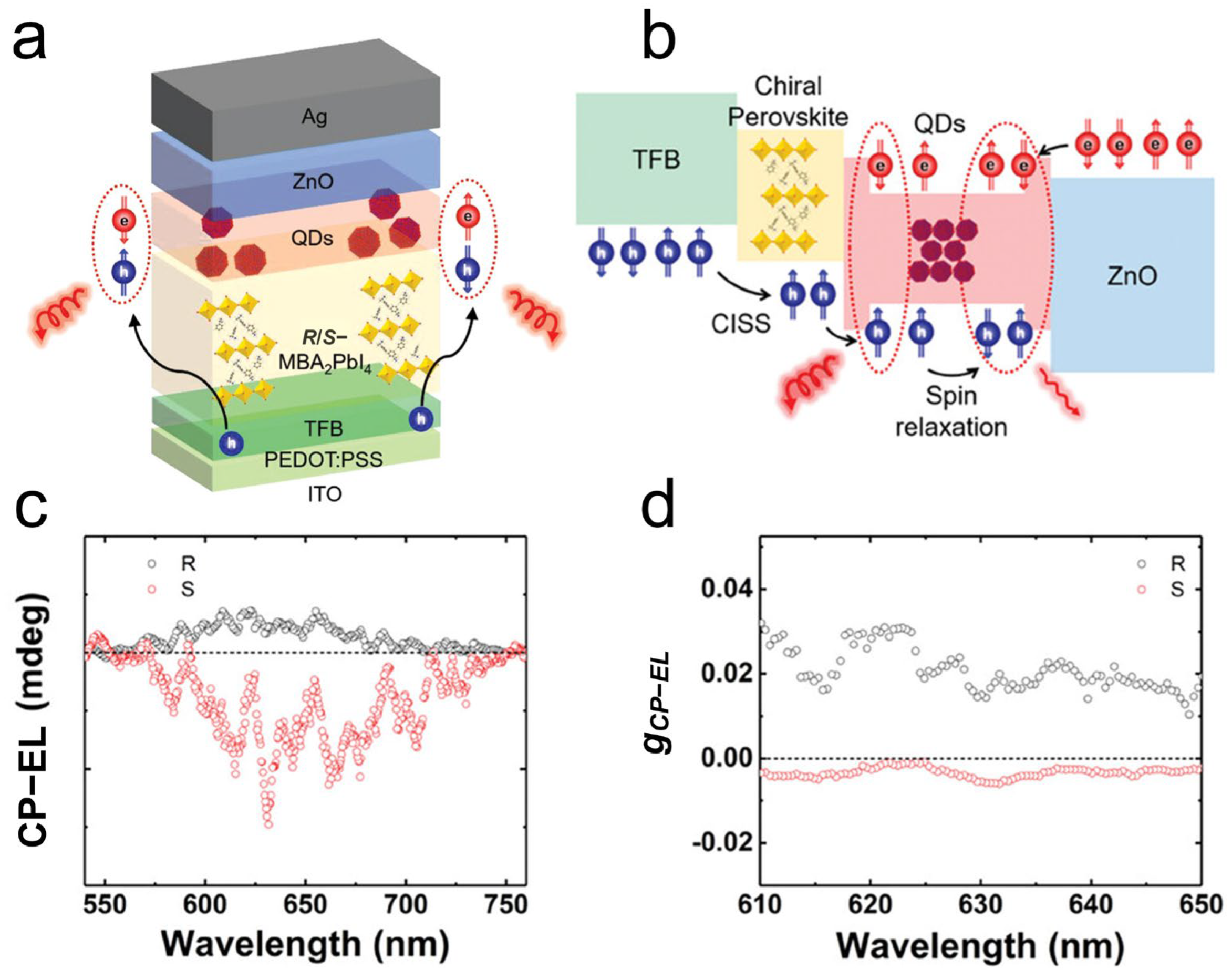
4.3. Other Emerging Chirality-Associated Optoelectronics
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, M.; Zhang, X.; Wang, F. Efficient Perovskite Nanograin Light-Emitting Diodes in Green-to-Blue Gamut with Co-Additive Engineering. Adv. Mater. 2024, 36, 2400565. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, M.; Xia, Z.; Cheng, Y.; Chu, Z.; Zhang, W.; Li, J.; Yin, Z.; You, J.; Zhang, X. Efficient Pure-Red Perovskite Light-Emitting Diodes with Strong Passivation via Ultrasmall-Sized Molecules. Sci. Adv. 2024, 10, eadn5683. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wan, H.; Teale, S.; Grater, L.; Zhao, F.; Zhang, Z.; Duan, H.-W.; Imran, M.; Wang, S.-D.; Hoogland, S.; et al. Long-Range Order Enabled Stability in Quantum Dot Light-Emitting Diodes. Nature 2024, 629, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Grede, A.J.; Cawthorn, R.; Zhao, L.; Murphy, J.P.; Roh, K.; Al Kurdi, K.; Barlow, S.; Marder, S.R.; Rand, B.P.; Giebink, N.C. Electrically Assisted Lasing in Metal Halide Perovskite Semiconductors. ACS Photonics 2024, 11, 1851–1856. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Z.; Ran, G.; Zhang, T.; Jiang, Z.; Liu, H.; Zhang, W.; Yan, Y.; Yao, J.; Dong, H.; et al. Spin-Polarized Lasing in Manganese Doped Perovskite Microcrystals. Nat. Commun. 2024, 15, 10880. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, D.; Vegso, K.; Baillard, G.; Nadazdy, P.; Mrkyvkova, N.; Siffalovic, P.; Chen, Y.; Coolen, L.; Pauporté, T.; et al. A Dual Strategy to Enhance the Photoelectric Performance of Perovskite-Based Photodetectors for Potential Applications in Optical Communications. Chem. Eng. J. 2024, 488, 151068. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Song, J.; He, Y.; Qu, W.; Li, W.; Guo, K.; Liu, L.; Yang, B.; Wei, H. Differential Perovskite Hemispherical Photodetector for Intelligent Imaging and Location Tracking. Nat. Commun. 2024, 15, 577. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, Y.; Pei, C.; Wang, Z.; Sun, Y.; Zhang, M.; Hao, Y.; Wei, X.; Zhao, Z.; Dong, K.; et al. Stress Release of Single Crystal Arrays Bridged by SAM Interface toward Highly Mechanically Durable Flexible Perovskite NIR Photodetector. Adv. Mater. 2025, 37, 2413721. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, Y.; Elmestekawy, K.A.; Caprioglio, P.; Li, Q.; Zhong, Q.; Ji, Y.; Huang, T.; Yan, H.; Yang, Y.; et al. Metastable Interphase Induced Pre-Strain Compensation Enables Efficient and Stable Perovskite Solar Cells. Energy Environ. Sci. 2025, 18, 246–255. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Fu, S.; Zheng, W.; Shen, W.; Jia, P.; Huang, L.; Zhou, S.; Zhou, J.; Wang, C.; et al. Boosting All-Perovskite Tandem Solar Cells by Revitalizing the Buried Tin-Lead Perovskite Interface. Adv. Mater. 2024, 36, 2401698. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Sun, Z.; Zhang, J.; Zhou, Z.; Shi, C.; Liu, S.; Ren, F.; Chen, R.; Cai, Y.; et al. Surface Chemical Polishing and Passivation Minimize Non-Radiative Recombination for All-Perovskite Tandem Solar Cells. Nat. Commun. 2024, 15, 7335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fu, S.; Zhou, S.; Huang, L.; Wang, C.; Guan, H.; Pu, D.; Cui, H.; Wang, C.; Wang, T.; et al. Mixed Tin-Lead Perovskites with Balanced Crystallization and Oxidation Barrier for All-Perovskite Tandem Solar Cells. Nat. Commun. 2024, 15, 2324. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.D.; Fan, C.C.; Zhang, W. Reversible Glass-Crystal Transition in a New Type of 2D Metal Halide Perovskites. Adv. Funct. Mater. 2024, 34, 2407143. [Google Scholar] [CrossRef]
- Schleusener, A.; Faraji, M.; Borreani, M.; Lauciello, S.; Pasquale, L.; Abkenar, S.K.; Divitini, G.; Krahne, R. Heterostructures via a Solution-Based Anion Exchange in Microcrystalline 2D Layered Metal-Halide Perovskites. Adv. Mater. 2024, 36, 2402924. [Google Scholar] [CrossRef]
- Jayanthi, K.; Spanopoulos, I.; Zibouche, N.; Voskanyan, A.A.; Vasileiadou, E.S.; Islam, M.S.; Navrotsky, A.; Kanatzidis, M.G. Entropy Stabilization Effects and Ion Migration in 3D “Hollow” Halide Perovskites. J. Am. Chem. Soc. 2022, 144, 8223–8230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Kepenekian, M.; Guo, P.; Ke, W.; Even, J.; Katan, C.; Stoumpos, C.C.; Schaller, R.D.; Kanatzidis, M.G. Three-Dimensional Lead Iodide Perovskitoid Hybrids with High X-Ray Photoresponse. J. Am. Chem. Soc. 2020, 142, 6625–6637. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Min Ok, K. Strategically Designed Metal-Free Deep-Ultraviolet Birefringent Crystals with Superior Optical Properties. Chem. Sci. 2024, 15, 15145–15151. [Google Scholar] [CrossRef]
- Ahn, J.; Ma, S.; Kim, J.Y.; Kyhm, J.; Yang, W.; Lim, J.A.; Kotov, N.A.; Moon, J. Chiral 2D Organic Inorganic Hybrid Perovskite with Circular Dichroism Tunable over Wide Wavelength Range. J. Am. Chem. Soc. 2020, 142, 4206–4212. [Google Scholar] [CrossRef]
- Billing, D.G.; Lemmerer, A. Bis[(S)-β-Phenethylammonium] Tribromoplumbate(II). Acta Crystallogr. 2003, 59, m381–m383. [Google Scholar] [CrossRef]
- Billing, D.G.; Lemmerer, A. Synthesis and Crystal Structures of Inorganic-Organic Hybrids Incorporating an Aromatic Amine with a Chiral Functional Group. CrystEngComm 2006, 8, 686–695. [Google Scholar] [CrossRef]
- Lemmerer, A.; Billings, D.G. Inorganic-Organic Hybrids Incorporating a Chiral Cyclic Ammonium Cation. S. Afr. J. Chem. 2013, 66, 263–272. [Google Scholar]
- Ahn, J.; Lee, E.; Tan, J.; Yang, W.; Kim, B.; Moon, J. A New Class of Chiral Semiconductors: Chiral-Organic-Molecule-Incorporating Organic-Inorganic Hybrid Perovskites. Mater. Horiz. 2017, 4, 851–856. [Google Scholar] [CrossRef]
- Ma, J.; Fang, C.; Chen, C.; Jin, L.; Wang, J.; Wang, S.; Tang, J.; Li, D. Chiral 2D Perovskites with a High Degree of Circularly Polarized Photoluminescence. ACS Nano 2019, 13, 3659–3665. [Google Scholar] [CrossRef]
- Coccia, C.; Morana, M.; Mahata, A.; Kaiser, W.; Moroni, M.; Albini, B.; Galinetto, P.; Folpini, G.; Milanese, C.; Porta, A.; et al. Ligand-Induced Chirality in ClMBA2SnI4 2D Perovskite. Angew. Chem. Int. Ed. 2024, 136, e202318557. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, D.G.; Yang, L.S.; Lin, T.C.; Liu, Y.H.; Chao, Y.-C.; Chou, P.-T.; Chiu, C.-W. Tuning the Circular Dichroism and Circular Polarized Luminescence Intensities of Chiral 2D Hybrid Organic-Inorganic Perovskites through Halogenation of the Organic Ions. Angew. Chem. Int. Ed. 2021, 60, 21434–21440. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, L.; Ma, J.; Li, J.; Li, W.; Liu, Z.; Li, H.; Chen, R.; Li, D. Thermally Assisted Rashba Splitting and Circular Photogalvanic Effect in Aqueously Synthesized 2D Dion-Jacobson Perovskite Crystals. Nano Lett. 2021, 21, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Wei, Q.; Yang, Y.; Zhou, W.; Jiang, X.; Wang, H.; Ma, M.; Yu, D.; Yang, Y.; Ning, Z. Symmetry-Broken 2D Lead-Tin Mixed Chiral Perovskite for High Asymmetry Factor Circularly Polarized Light Detection. Nano Lett. 2023, 23, 1938–1945. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Xiao, C.; Pan, X.; Chen, X.; Brunecky, R.; Berry, J.J.; Zhu, K.; Beard, M.C.; Vardeny, Z.V. Spin-Dependent Charge Transport through 2D Chiral Hybrid Lead-Iodide Perovskites. Sci. Adv. 2019, 5, eaay0571. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xiao, C.; Song, R.; Li, T.; Maughan, A.E.; Levin, A.; Brunecky, R.; Berry, J.J.; Mitzi, D.B.; Blum, V.; et al. Highly Distorted Chiral Two-Dimensional Tin Iodide Perovskites for Spin Polarized Charge Transport. J. Am. Chem. Soc. 2020, 142, 13030–13040. [Google Scholar] [CrossRef]
- Long, G.; Jiang, C.; Sabatini, R.; Yang, Z.; Wei, M.; Quan, L.N.; Liang, Q.; Rasmita, A.; Askerka, M.; Walters, G.; et al. Spin Control in Reduced-Dimensional Chiral Perovskites. Nat. Photonics 2018, 12, 528–533. [Google Scholar] [CrossRef]
- Wang, J.; Lu, H.; Pan, X.; Xu, J.; Liu, H.; Liu, X.; Khanal, D.R.; Toney, M.F.; Beard, M.C.; Vardeny, Z.V. Spin-Dependent Photovoltaic and Photogalvanic Responses of Optoelectronic Devices Based on Chiral Two-Dimensional Hybrid Organic-Inorganic Perovskites. ACS Nano 2021, 15, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.C.; Han, X.B.; Liang, B.D.; Shi, C.; Miao, L.P.; Chai, C.-Y.; Liu, C.-D.; Ye, Q.; Zhang, W. Chiral Rashba Ferroelectrics for Circularly Polarized Light Detection. Adv. Mater. 2022, 34, 2204119. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Florio, F.; Chen, Z.; Phelan, W.A.; Siegler, M.A.; Zhou, Z.; Guo, Y.; Hawks, R.; Jiang, J.; Feng, J.; et al. A Chiral Switchable Photovoltaic Ferroelectric 1D Perovskite. Sci. Adv. 2020, 6, eaay4213. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Huang, X.Q.; Huang, C.R.; Zhang, H.; Wang, F.; Wang, Z.X. Unprecedented 2D Homochiral Hybrid Lead-Iodide Perovskite Thermochromic Ferroelectrics with Ferroelastic Switching. Angew. Chem. Int. Ed. 2021, 60, 10730–10735. [Google Scholar] [CrossRef]
- He, T.; Li, J.; Li, X.; Ren, C.; Luo, Y.; Zhao, F.; Chen, R.; Lin, X.; Zhang, J. Spectroscopic Studies of Chiral Perovskite Nanocrystals. Appl. Phys. Lett. 2017, 111, 151102. [Google Scholar] [CrossRef]
- Chen, C.; Gao, L.; Gao, W.; Ge, C.; Du, X.; Li, Z.; Yang, Y.; Niu, G.; Tang, J. Circularly Polarized Light Detection Using Chiral Hybrid Perovskite. Nat. Commun. 2019, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Chen, W.N.; Ding, Y.T.; Wang, J.; Rao, Y.; Liao, W.-Q.; Tang, Y.-Y.; Li, P.-F.; Wang, Z.-X.; Xiong, R.-G. The First 2D Homochiral Lead Iodide Perovskite Ferroelectrics: [R- and S-1-(4-Chlorophenyl)Ethylammonium]2PbI4. Adv. Mater. 2019, 31, 1808088. [Google Scholar] [CrossRef]
- Yuan, C.; Li, X.; Semin, S.; Feng, Y.; Rasing, T.; Xu, J. Chiral Lead Halide Perovskite Nanowires for Second-Order Nonlinear Optics. Nano Lett. 2018, 18, 5411–5417. [Google Scholar] [CrossRef]
- Dang, Y.; Liu, X.; Sun, Y.; Song, J.; Hu, W.; Tao, X. Bulk Chiral Halide Perovskite Single Crystals for Active Circular Dichroism and Circularly Polarized Luminescence. J. Phys. Chem. Lett. 2020, 11, 1689–1696. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Li, X.; Feng, Y.; Zhang, H.; Xu, J. Chiral Perovskites: Promising Materials toward Next-generation Optoelectronics. Nano Micro Small 2019, 15, 1902237. [Google Scholar] [CrossRef]
- Long, G.; Sabatini, R.; Saidaminov, M.I.; Lakhwani, G.; Rasmita, A.; Liu, X.; Sargent, E.H.; Gao, W. Chiral-Perovskite Optoelectronics. Nat. Rev. Mater. 2020, 5, 423–439. [Google Scholar] [CrossRef]
- Wei, Q.; Ning, Z. Chiral Perovskite Spin-Optoelectronics and Spintronics: Toward Judicious Design and Application. ACS Mater. Lett. 2021, 3, 1266–1275. [Google Scholar] [CrossRef]
- Dang, Y.; Liu, X.; Cao, B.; Tao, X. Chiral Halide Perovskite Crystals for Optoelectronic Applications. Matter 2021, 4, 794–820. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; Li, D. Recent Progress of Chiral Perovskites: Materials, Synthesis, and Properties. Adv. Mater. 2021, 33, 2008785. [Google Scholar] [CrossRef]
- Ma, S.; Ahn, J.; Moon, J. Chiral Perovskites for Next-generation Photonics: From Chirality Transfer to Chiroptical Activity. Adv. Mater. 2021, 33, 2005760. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Mattoni, A.; Pica, G.; Fortino, M.; Schifino, G.; Grancini, G. Rationalizing the Design and Implementation of Chiral Hybrid Perovskites. Chem 2022, 8, 1231–1253. [Google Scholar] [CrossRef]
- Wang, J.; Fang, C.; Ma, J.; Wang, S.; Jin, L.; Li, W.; Li, D. Aqueous Synthesis of Low-Dimensional Lead Halide Perovskites for Room-Temperature Circularly Polarized Light Emission and Detection. ACS Nano 2019, 13, 9473–9481. [Google Scholar] [CrossRef]
- Tao, K.; Zhang, B.; Li, Q.; Yan, Q. Centimeter-sized Piezoelectric Single Crystal of Chiral Bismuth-based Hybrid Halide with Superior Electrostrictive Coefficient. Small 2023, 19, 2207663. [Google Scholar] [CrossRef]
- He, Q.; Feng, J.; Du, L.; Shen, Y.; Chen, H.; Yan, C.; Kang, S.; Wang, H.; Yin, Y.; Wan, L.; et al. Highly Emissive Chiral Indium-Based Perovskite Single Crystals for Efficient Optoelectronic Applications. Adv. Opt. Mater. 2024, 12, 2400682. [Google Scholar] [CrossRef]
- Chen, G.; Liu, X.; An, J.; Wang, S.; Zhao, X.; Gu, Z.; Yuan, C.; Xu, X.; Bao, J.; Hu, H.-S.; et al. Nucleation-Mediated Growth of Chiral 3D Organic-Inorganic Perovskite Single Crystals. Nat. Chem. 2023, 15, 1581–1590. [Google Scholar] [CrossRef]
- Wang, L.; Hao, W.; Peng, B.; Ren, J.; Li, H. Nucleation-Controlled Crystallization of Chiral 2D Perovskite Single Crystal Thin Films for High-Sensitivity Circularly Polarized Light Detection. Adv. Mater. 2025, 2414199. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, H.; Ma, J.; Zhao, Y.; Lu, H.; Zhang, Y.; Gull, S.; Qiao, T.; Qin, W.; Chen, Y.; et al. Wafer-Scale Patterning Integration of Chiral 3D Perovskite Single Crystals toward High-Performance Full-Stokes Polarimeter. J. Am. Chem. Soc. 2024, 146, 18771–18780. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, X.; Gu, Z.; Yuan, M.; Ma, J.; Li, T.; Jiang, L.; Wu, Y.; Song, Y. Interlayer Polymerization of 2D Chiral Perovskite Single-Crystal Films toward High-Performance Flexible Circularly Polarized Light Detection. Adv. Funct. Mater. 2023, 33, 2306199. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Zhou, M.; Zhao, T.; Qin, X.; Liu, X.; Liu, M.; Duan, P. Two-Photon Absorption-Based Upconverted Circularly Polarized Luminescence Generated in Chiral Perovskite Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 3290–3295. [Google Scholar] [CrossRef]
- Shi, Y.; Duan, P.; Huo, S.; Li, Y.; Liu, M. Endowing Perovskite Nanocrystals with Circularly Polarized Luminescence. Adv. Mater. 2018, 30, 1705011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, Y.; Feng, J.; Zhao, J.; Chen, G.; Gao, H.; Zhao, Y.; Jiang, L.; Wu, Y. Chiral 2D-Perovskite Nanowires for Stokes Photodetectors. J. Am. Chem. Soc. 2021, 143, 8437–8445. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, X.; Ren, A.; Zhou, Z.; Qiao, C.; Guan, Y.; Fan, Y.; Hu, F.; Zhao, Y.S. Chiral Hybrid Perovskite Single-Crystal Nanowire Arrays for High-Performance Circularly Polarized Light Detection. Adv. Sci. 2021, 8, 2102065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, M.; Feng, J.; Zhao, J.; Guo, Y.; Fu, Y.; Gao, H.; Yang, J.; Jiang, L.; Wu, Y. Lead-Free Chiral 2D Double Perovskite Microwire Arrays for Circularly Polarized Light Detection. Adv. Opt. Mater. 2022, 10, 2102227. [Google Scholar] [CrossRef]
- Kim, Y.H.; Zhai, Y.; Gaulding, E.A.; Habisreutinger, S.N.; Moot, T.; Rosales, B.A.; Lu, H.; Hazarika, A.; Brunecky, R.; Wheeler, L.M.; et al. Strategies to Achieve High Circularly Polarized Luminescence from Colloidal Organic-Inorganic Hybrid Perovskite Nanocrystals. ACS Nano 2020, 14, 8816–8825. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Kim, Y.J.; Kim, J.; Ahn, J.; Song, I.; Kwak, M.; Kim, J.; Park, J.; Yoo, D.; et al. Giant Chiral Amplification of Chiral 2D Perovskites via Dynamic Crystal Reconstruction. Sci. Adv. 2024, 10, eado5942. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Fang, Y.; Zhou, Y.; Ni, Z.; Xiao, X.; Chen, S.; Huang, J. Ligand Assisted Growth of Perovskite Single Crystals with Low Defect Density. Nat. Commun. 2021, 12, 1686. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Qiu, Y.; Zhao, Y.; Zhao, Y.; Li, H.; Wei, X.; Chen, G.; Feng, J.; Gao, H.; Zhao, J.; et al. Chlorine-Substituted Chiral 2D Perovskite Microwires with Enhanced Lattice Rigidity for Sensitive Circularly Polarized Light Detection. Sci. China Chem. 2023, 66, 3602–3610. [Google Scholar] [CrossRef]
- Son, J.; Jang, G.; Ma, S.; Lee, H.; Lee, C.U.; Yang, S.; Lee, J.; Moon, S.; Jeong, W.; Park, J.H.; et al. Boosted Chirality Transfer in 2D Perovskites through Structural Isomer-Driven Halogen–Halogen Interaction. Adv. Funct. Mater. 2025, 35, 2413041. [Google Scholar] [CrossRef]
- Tsumatori, H.; Harada, T.; Yuasa, J.; Hasegawa, Y.; Kawai, T. Circularly Polarized Light from Chiral Lanthanide(III) Complexes in Single Crystals. Appl. Phys. Express 2011, 4, 11601. [Google Scholar] [CrossRef]
- Riehl, J.P.; Richardson, F.S. Circularly Polarized Luminescence Spectroscopy. Chem. Rev. 1986, 86, 1–16. [Google Scholar] [CrossRef]
- Guan, Q.; Ye, H.; Zhu, T.; Zhang, X.; You, S.; Wu, J.; Zheng, Y.; Liu, X.; Luo, J. Formamidine Engineering the Lattice Distortion of Chiral Halide Perovskites for Efficient Blue Circularly Polarized Emission. Adv. Opt. Mater. 2023, 11, 2202726. [Google Scholar] [CrossRef]
- Yu, M.; Qin, T.; Gao, G.; Zu, K.; Zhang, D.; Chen, N.; Wang, D.; Hua, Y.; Zhang, H.; Zhao, Y.-B.; et al. Multiple Defects Renovation and Phase Reconstruction of Reduced-Dimensional Perovskites via in Situ Chlorination for Efficient Deep-Blue (454 Nm) Light-Emitting Diodes. Light Sci. Appl. 2025, 14, 102. [Google Scholar] [CrossRef]
- Yang, S.; Jang, G.; Lee, C.U.; Son, J.; Lee, J.; Jeong, W.; Roe, D.G.; Cho, J.H.; Moon, J. Highly Circularly Polarized Light-Sensitive Chiral One-Dimensional Perovskites Enabled by Antisolvent Engineering. Adv. Funct. Mater. 2024, 34, 2310917. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, Y.; Gong, T.; Wang, Y.; Jiang, P.; Wei, W.; Yang, Y.; Chai, J.; Wu, Z.; Wang, X.; et al. Spatial and Energetic Mapping of Traps in FeFET during Endurance Process by Advanced Trap Characterization Platform. IEEE Electron. Device Lett. 2024, 45, 2371–2374. [Google Scholar] [CrossRef]
- Gao, W.; Guo, Z.; Zhao, H.; Xu, Y.; Gu, X.; Guan, W.; Li, W.; Lewandowski, J.J. Improving Intergranular Cracking and Stress Corrosion Cracking Resistance of Highly Sensitized AA5083 Al-Mg Alloy via Reversion Heat Treatment. J. Alloys Compd. 2024, 1002, 175538. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Y.; Chang, X.; Zhou, M.; Yu, X.; Zhao, X.; Wang, T.; Cai, Z.; Zhu, X.; Wang, H.; et al. Self-Induced Bi-Interfacial Modification via Fluoropyridinic Acid for High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 2025, 15, 2404335. [Google Scholar] [CrossRef]
- Xu, H.; Wu, N.; Ji, B.; Cai, J.; Yao, W.; Wang, Z.; Zhang, Y.; Zhang, X.; Guo, S.; Zhou, X.; et al. Lattice Water Deprotonation Enables Potassium-Ion Chemistries. Angew. Chem. Int. Ed. 2025, e202503904. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, S.; Chen, L. Can Pb-Free Halide Perovskites Be Realized by Incorporating the Neutral or Anionic Molecule? Adv. Theor. Simul. 2025, 2401546. [Google Scholar] [CrossRef]
- Ma, S.; Jung, Y.K.; Ahn, J.; Kyhm, J.; Tan, J.; Lee, H.; Jang, G.; Lee, C.U.; Walsh, A.; Moon, J. Elucidating the Origin of Chiroptical Activity in Chiral 2D Perovskites through Nano-Confined Growth. Nat. Commun. 2022, 13, 3259. [Google Scholar] [CrossRef]
- Son, J.; Ma, S.; Jung, Y.K.; Tan, J.; Jang, G.; Lee, H.; Lee, C.U.; Lee, J.; Moon, S.; Jeong, W.; et al. Unraveling Chirality Transfer Mechanism by Structural Isomer-Derived Hydrogen Bonding Interaction in 2D Chiral Perovskite. Nat. Commun. 2023, 14, 3124. [Google Scholar] [CrossRef]
- Pham, M.T.; Amerling, E.; Ngo, T.A.; Luong, H.M.; Hansen, K.; Pham, H.T.; Vu, T.N.; Tran, H.; Whittaker-Brooks, L.; Nguyen, T.D. Strong Rashba-Dresselhaus Effect in Nonchiral 2D Ruddlesden-Popper Perovskites. Adv. Opt. Mater. 2022, 10, 2101232. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Cui, M.; Huang, Y.; Xu, H.; Qin, C.; Yang, J.; Dai, H.; Yuan, M. A Chiral Reduced-Dimension Perovskite for an Efficient Flexible Circularly Polarized Light Photodetector. Angew. Chem. Int. Ed. 2020, 132, 6504–6512. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Li, L.; Ji, C.; Yao, Y.; Luo, J. Great Amplification of Circular Polarization Sensitivity via Heterostructure Engineering of a Chiral Two-Dimensional Hybrid Perovskite Crystal with a Three-Dimensional MAPbI3 Crystal. ACS Cent. Sci. 2021, 7, 1261–1268. [Google Scholar] [CrossRef]
- Zhu, T.; Weng, W.; Ji, C.; Zhang, X.; Ye, H.; Yao, Y.; Li, X.; Li, J.; Lin, W.; Luo, J. Chain-to-Layer Dimensionality Engineering of Chiral Hybrid Perovskites to Realize Passive Highly Circular-Polarization-Sensitive Photodetection. J. Am. Chem. Soc. 2022, 144, 18062–18068. [Google Scholar] [CrossRef]
- Ma, J.; Fang, C.; Liang, L.; Wang, H.; Li, D. Full-Stokes Polarimeter Based on Chiral Perovskites with Chirality and Large Optical Anisotropy. Small 2021, 17, 2103855. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, W.; Li, L.; Wu, H.; Yao, Y.; Wang, Z.; Liu, X.; Lin, W.; Luo, J. Heterogeneous Integration of Chiral Lead-Chloride Perovskite Crystals with Si Wafer for Boosted Circularly Polarized Light Detection in Solar-Blind Ultraviolet Region. Small 2021, 17, 2102884. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, K.; Zhang, X.; Wang, S.; Wu, S.; Huang, W. Butylammonium-Based Chiral 2D Perovskite Single Crystals for Efficient UV Circularly Polarized Light Differentiation and High-Performance X-Ray Detection. ACS Appl. Mater. Interfaces 2025, 17, 17127–17134. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Li, L.; Yao, Y.; Ye, H.; Shang, X.; Chen, X.; Luo, J. Realization of Vis-NIR Dual-Modal Circularly Polarized Light Detection in Chiral Perovskite Bulk Crystals. J. Am. Chem. Soc. 2021, 143, 14077–14082. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Li, B.; Wang, J.Y.; Xu, L.J.; Chen, Z.N. Chiral 3D Perovskite Formation Induced by Chiral Templates. Nano Lett. 2024, 24, 9569–9574. [Google Scholar] [CrossRef]
- Yao, L.; Zeng, Z.; Cai, C.; Xu, P.; Gu, H.; Gao, L.; Han, J.; Zhang, X.; Wang, X.; Wang, X.; et al. Strong Second- and Third-Harmonic Generation in 1D Chiral Hybrid Bismuth Halides. J. Am. Chem. Soc. 2021, 143, 16095–16104. [Google Scholar] [CrossRef]
- Cheng, P.; Jia, X.; Chai, S.; Li, G.; Xin, M.; Guan, J.; Han, X.; Han, W.; Zeng, S.; Zheng, Y.; et al. Boosted Second Harmonic Generation of a Chiral Hybrid Lead Halide Resonant to Charge Transfer Exciton from Metal Halide Octahedra to Ligand. Angew. Chem. Int. Ed. 2024, 136, e202400644. [Google Scholar] [CrossRef]
- Dong, M.; Wang, B.; Yu, Z.; Zhao, J.; Li, X.; Fu, Y.; Guo, Y.; Zhao, Y.; Gao, H.; Jiang, L.; et al. Strong Anisotropic Second-Order Nonlinear Optical Responses in 0D Lead-Free Chiral Perovskite Single-Crystalline Microwire Arrays. Inorg. Chem. Front. 2023, 10, 3396–3405. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, S.; Zhao, Y.; Guo, Y.; Dong, M.; Fu, Y.; Zhao, J.; Yang, J.; Jiang, L.; Wu, Y. Chiral Lead-Free Double Perovskite Single-Crystalline Microwire Arrays for Anisotropic Second-Harmonic Generation. ACS Appl. Mater. Interfaces 2022, 14, 39451–39458. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Guo, Y.; Zhan, X.; Feng, J.; Geng, Y.; Yuan, M.; Fan, X.; Gao, H.; Jiang, L.; et al. Layered Metal-Halide Perovskite Single-Crystalline Microwire Arrays for Anisotropic Nonlinear Optics. Adv. Funct. Mater. 2021, 31, 2105855. [Google Scholar] [CrossRef]
- Tao, K.; Li, Q.; Yan, Q. 1D Tin(II)-Based Chiral Hybrid Perovskite Single Crystals with Extremely Distorted Inorganic Chains for Second Harmonic Generation. Adv. Opt. Mater. 2024, 12, 2400018. [Google Scholar] [CrossRef]
- Fu, D.; Xin, J.; He, Y.; Wu, S.; Zhang, X.; Zhang, X.-M.; Luo, J. Chirality-Dependent Second-Order Nonlinear Optical Effect in 1D Organic-Inorganic Hybrid Perovskite Bulk Single Crystal. Angew. Chem. Int. Ed. 2021, 60, 20021–20026. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, C.; Wu, S.; Wong, W.P.D.; Wu, Z.; Chang, K.; Wang, J.; Gao, H.; Loh, K.P. Dion-Jacobson Perovskites with a Ferroelectrically Switchable Chiral Nonlinear Optical Response. J. Am. Chem. Soc. 2025, 147, 811–820. [Google Scholar] [CrossRef]
- You, S.; Zhu, Z.-K.; Dai, S.; Wu, J.; Guan, Q.; Zhu, T.; Yu, P.; Chen, C.; Chen, Q.; Luo, J. Inch-Size Single Crystals of Lead-Free Chiral Perovskites with Bulk Photovoltaic Effect for Stable Self-Driven X-Ray Detection. Adv. Funct. Mater. 2023, 33, 2303523. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Yang, L.; Chai, Z.; Wang, Y.; Wang, S. Circularly Polarized Radioluminescence from Chiral Perovskite Scintillators for Improved X-Ray Imaging. Angew. Chem. Int. Ed. 2022, 134, e202208440. [Google Scholar] [CrossRef]
- Song, Z.; Yu, B.; Liu, G.; Meng, L.; Dang, Y. Chiral Hybrid Copper(I) Iodide Single Crystals Enable Highly Selective Ultraviolet-Pumped Circularly Polarized Luminescence Applications. J. Phys. Chem. Lett. 2022, 13, 2567–2575. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, H.; Tan, Y.; Hao, J.; Ye, T.; Tang, H.; Wang, Z.; Ma, J.; Sun, J.; Zhang, T.; et al. Spin Quantum Dot Light-Emitting Diodes Enabled by 2D Chiral Perovskite with Spin-Dependent Carrier Transport. Adv. Mater. 2024, 36, 2305604. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.M.; Gao, F.F.; Gong, Y.J.; Li, Z.G.; Wei, F.X.; Li, W.; Bu, X.H. Chiral Two-Dimensional Hybrid Organic-Inorganic Perovskites for Piezoelectric Ultrasound Detection. J. Am. Chem. Soc. 2023, 145, 22475–22482. [Google Scholar] [CrossRef]
- Geng, X.; He, Y.; Tang, L.; Chang, S.; Zhang, J.; Yang, W.; Yuan, M.; Wang, G.; Li, Q.; Ping, Y.; et al. Investigation of the Effect of Bonding Wires Degradation on Switching Stress Waves Released during Power Cycling in Discrete IGBT. IEEE J. Emerg. Sel. Top. Power Electron. 2025, 13, 2057–2069. [Google Scholar] [CrossRef]
- Duan, T.; You, S.; Chen, M.; Yu, W.; Li, Y.; Guo, P.; Berry, J.J.; Luther, J.M.; Zhu, K.; Zhou, Y. Chiral-Structured Heterointerfaces Enable Durable Perovskite Solar Cells. Science 2024, 384, 878–884. [Google Scholar] [CrossRef]
- Sheikh, T.; Maqbool, S.; Mandal, P.; Nag, A. Introducing Intermolecular Cation-π Interactions for Water-stable Low Dimensional Hybrid Lead Halide Perovskites. Angew. Chem. Int. Ed. 2021, 60, 18265–18271. [Google Scholar] [CrossRef]
- Guan, Q.; Zhu, T.; Zhu, Z.-K.; Ye, H.; You, S.; Xu, P.; Wu, J.; Niu, X.; Zhang, C.; Liu, X.; et al. Unprecedented Chiral Three-Dimensional Hybrid Organic-Inorganic Perovskitoids. Angew. Chem. Int. Ed. 2023, 62, e202307034. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.Y.; Fan, C.C.; Liang, B.D.; Liu, C.D.; Jin, M.L.; Chai, C.Y.; Zhang, W. Chirality Triggered Biferroicity in a 3D Rubidium Based Perovskite. Adv. Funct. Mater. 2024, 34, 2316747. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Liu, Y.H.; Peng, H.; Deng, B.B.; Cheng, T.T.; Hu, Y.T. Three-Dimensional Lead Bromide Hybrid Ferroelectric Realized by Lattice Expansion. J. Am. Chem. Soc. 2020, 142, 19698–19704. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, N.; Jiang, C.; Lv, M.; Wu, J.; Chen, Z. Perovskite Single Crystals with Self-Cleaning Surface for Efficient Photovoltaics. Angew. Chem. Int. Ed. 2024, 63, e202314089. [Google Scholar] [CrossRef]
- Gao, C.; Li, R.; Li, Y.; Wang, R.; Wang, M.; Gan, Z.; Bai, L.; Liu, Y.; Zhao, K.; Liu, S.F.; et al. Direct-Indirect Transition of Pressurized Two-Dimensional Halide Perovskite: Role of Benzene Ring Stack Ordering. J. Phys. Chem. Lett. 2019, 10, 5687–5693. [Google Scholar] [CrossRef]
- Chen, Q.; Ge, M.; Geng, C.; Zhang, J.; Gao, L.; Huang, Z.; Wang, S.; Feng, Y.; Yue, X.; Qaid, S.M.H.; et al. Manipulating Perovskite Structural Asymmetry for High-Performing Self-Powered Full-Stokes Polarimetry. Sci. Adv. 2025, 11, eads6123. [Google Scholar] [CrossRef]

| Materials | Synthesis Methods | Solvents | Temperature | Size of Crystals |
|---|---|---|---|---|
| (MPEA)1.5PbBr3.5(DMSO)0.5 SCs [38] | AVC | DMF and DMSO | Room temperature | Millimeter size |
| (R/S)-α-(PEA)2PbI4 SCs [47] | Aqueous synthesis | Deionized water | Room temperature | Millimeter size |
| (R/S-α-PEA)4Bi2I10 SCs [48] | Temperature-lowering | 55–57% HI | 70 to 35 °C | 1.1 cm |
| (R- and S-α-PEA)PbI3 SCs [36] | Temperature-lowering | 45% HI | 90 to 40 °C | 3 mm × 2 mm × 1 mm |
| (R-/S-/rac-MBA)3InCl6 SCs [49] | Slow evaporation | HCl | Room temperature | 1.5 mm × 0.6 mm × 0.4 mm |
| MAPbBr3 SCs [50] | Inverse temperature | DMF | 60–90 °C | 5–10 mm |
| [(R/S)-3APr]PbI4 SCTFs [51] | Nucleation-controlled | 55–57% HI | 90 °C | >1 cm |
| (R/S-PyEA)Pb2Br6 microwire arrays [52] | Capillary-bridge confined assembly | DMSO | 70 °C | 4 inch |
| R/S-VPEA and R/S-EPEA SCTFs [53] | In situ crosslinking polymerization | DMF/GBL | 70 °C | Exceeding 100 mm2 |
| CsPb(I/Br)3 NC [35] | Post-synthetic ligand exchange | n-hexane | 100 °C | ~100 nm |
| CsPbBr3 NCs [54] | Single-step ultrasonication | 1-octadecene | N/A | ~20 nm |
| CsPbX3 NCs (X = Cl, Br, and I) [55] | Supramolecular self-assembly | 1-octadecene | N/A | 10–15 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ren, J.; Li, H. Chiral Perovskite Single Crystals: Toward Promising Design and Application. Materials 2025, 18, 2635. https://doi.org/10.3390/ma18112635
Wang L, Ren J, Li H. Chiral Perovskite Single Crystals: Toward Promising Design and Application. Materials. 2025; 18(11):2635. https://doi.org/10.3390/ma18112635
Chicago/Turabian StyleWang, Lin, Jie Ren, and Hanying Li. 2025. "Chiral Perovskite Single Crystals: Toward Promising Design and Application" Materials 18, no. 11: 2635. https://doi.org/10.3390/ma18112635
APA StyleWang, L., Ren, J., & Li, H. (2025). Chiral Perovskite Single Crystals: Toward Promising Design and Application. Materials, 18(11), 2635. https://doi.org/10.3390/ma18112635







