Preparation of Metallic Zr from ZrO2 via Carbothermal and Electrochemical Reduction in Molten Salts
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbothermal Reduction of ZrO2
2.2. Electrochemical Measurements and Electrodeposition
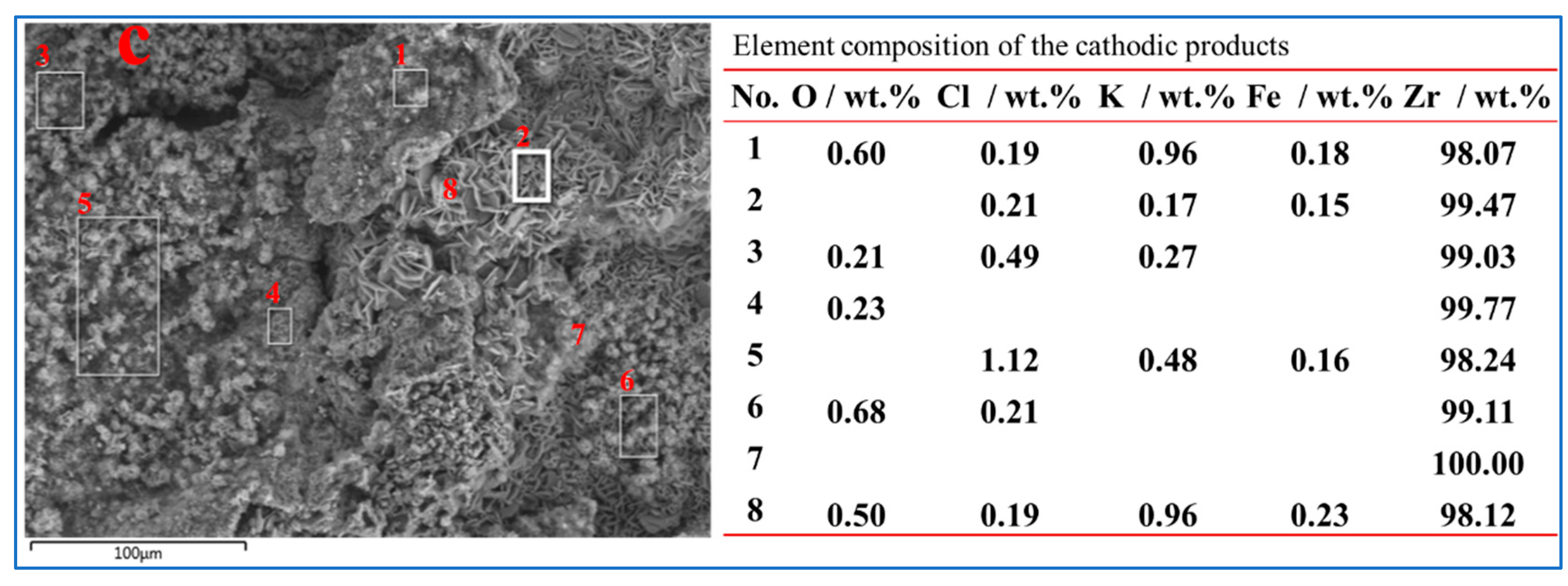
3. Results and Discussion
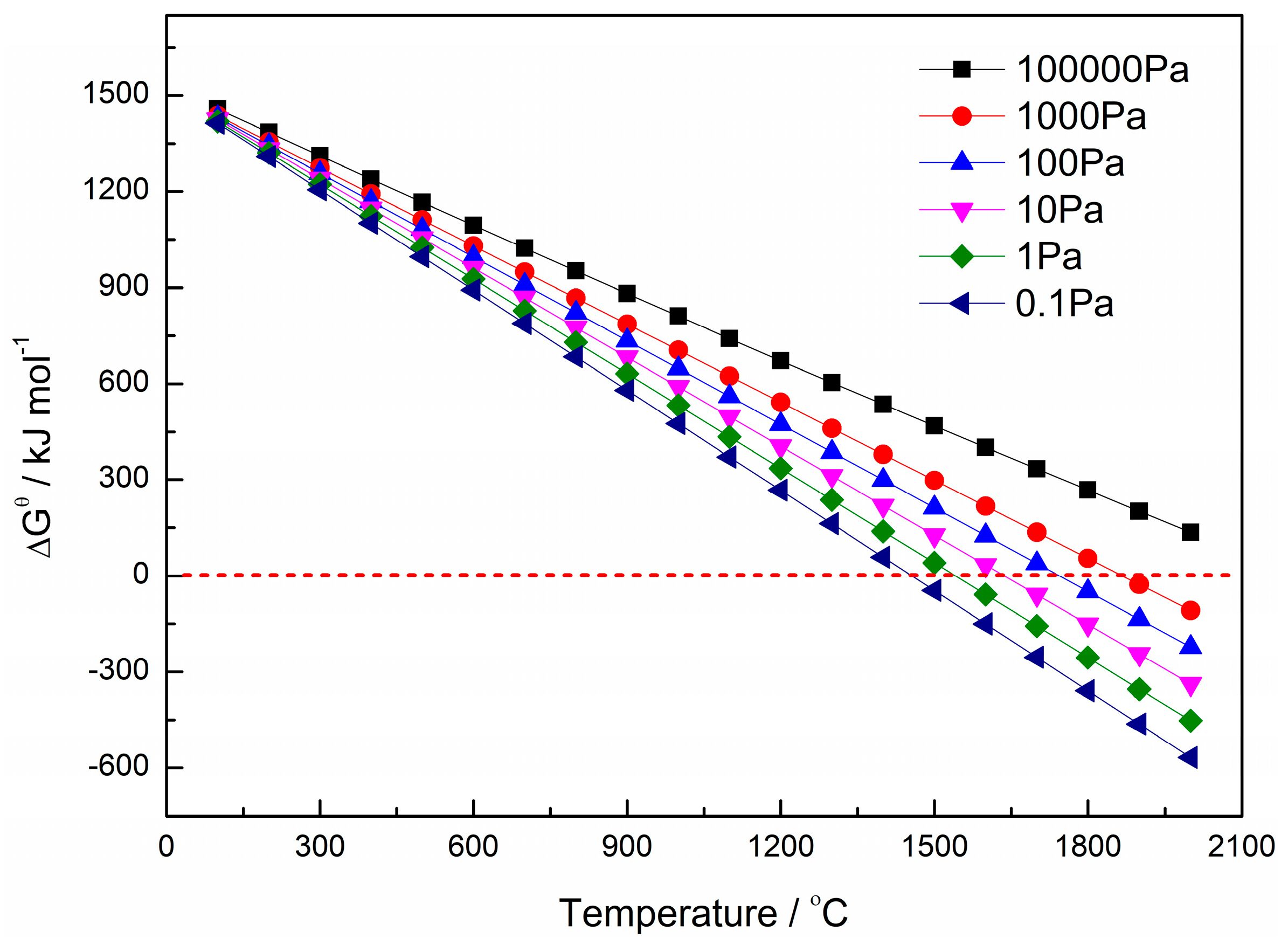
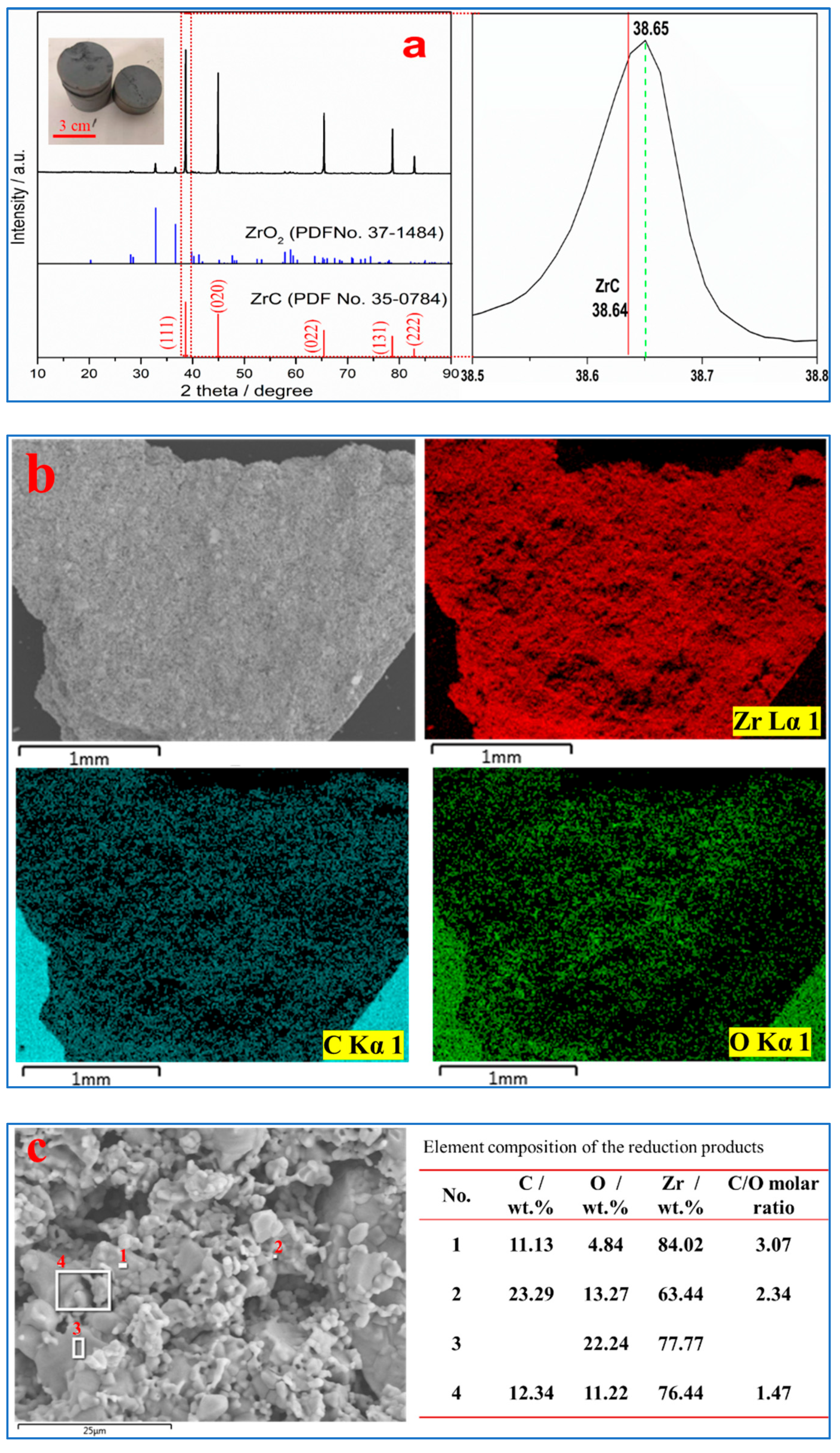
3.1. Carbothermal Reduction
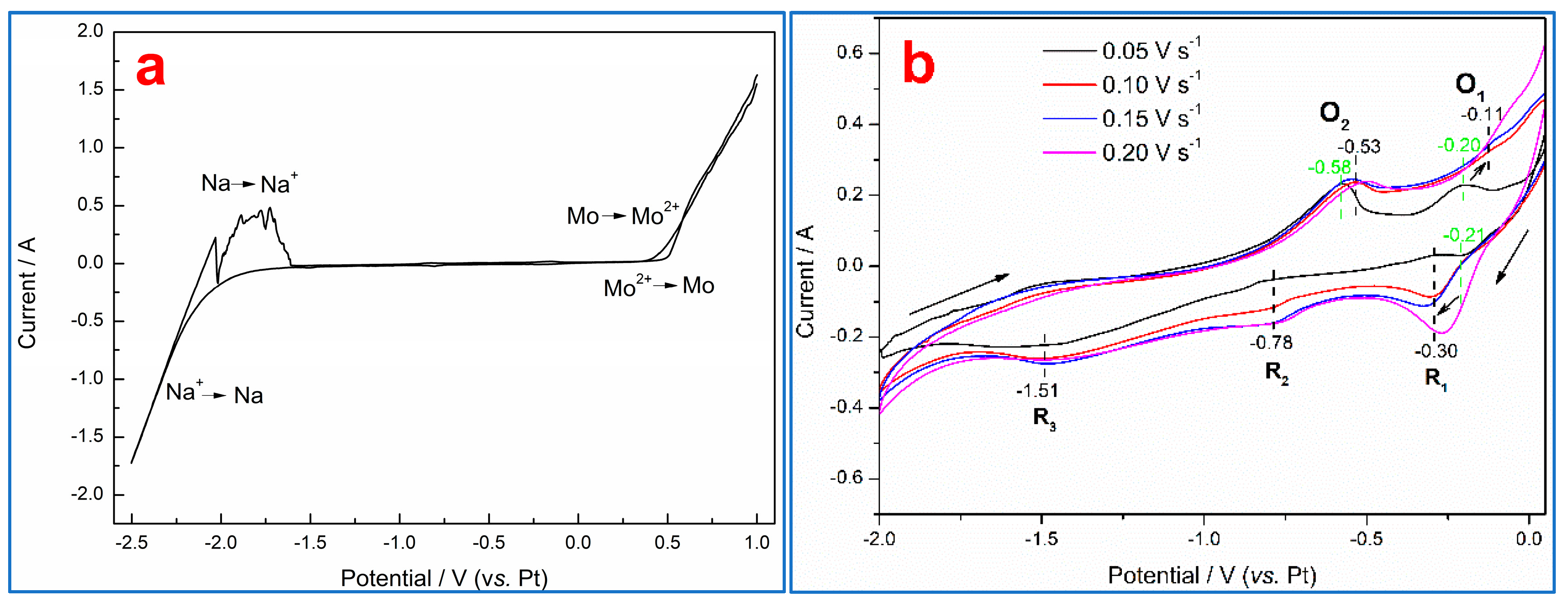
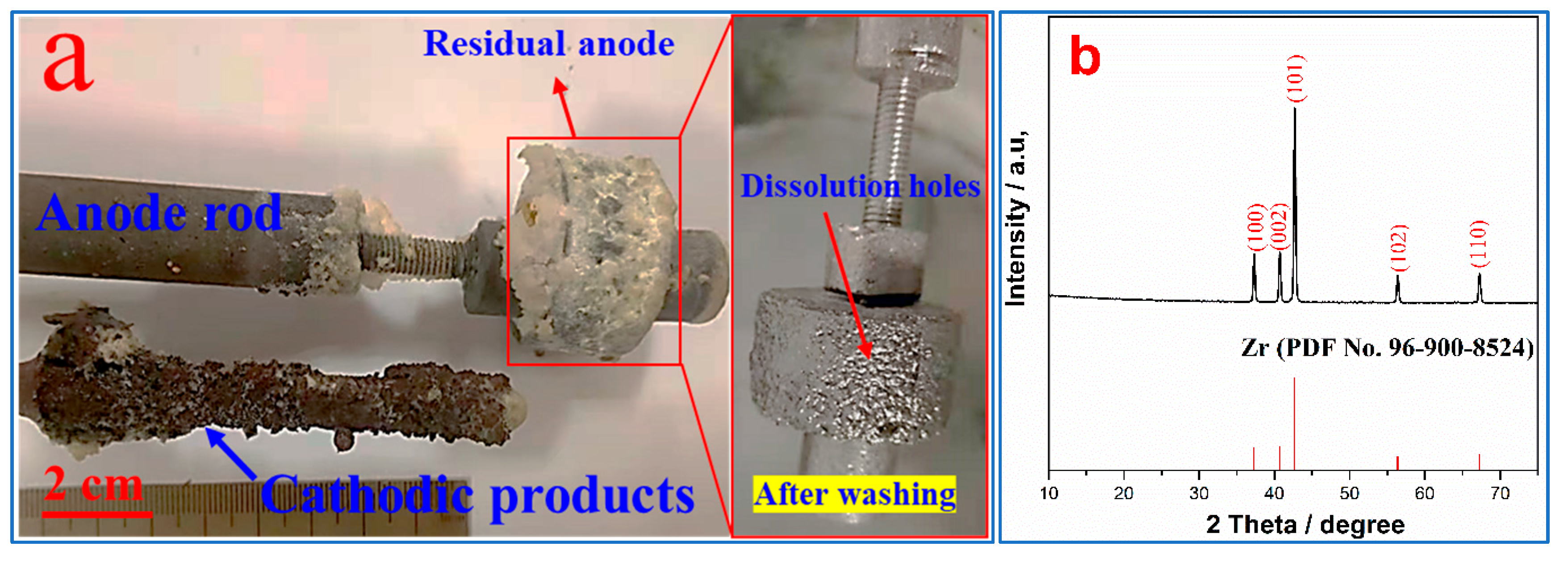
3.2. Electrochemical Behavior of Zirconium Oxycarbide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birch, R.M.; Douglas, J.O.; Britton, T.B. Characterization of local deformation around hydrides in Zircaloy-4 using conventional and high angular resolution electron backscatter diffraction. Mater. Charact. 2023, 202, 112988. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Zhao, X.; Wu, J.; Zhao, B.; Zhao, H.; Wu, J.; Zhang, Y.; Liu, C. Zirconium based neutron absorption material with outstanding corrosion resistance and mechanical properties. J. Nucl. Mater. 2022, 567, 153763. [Google Scholar] [CrossRef]
- Perks, C.; Mudd, G. Titanium, zirconium resources and production: A state of the art literature review. Ore Geol. Rev. 2019, 107, 629–646. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, F.; Liu, J.; Zhang, J.; Zhang, H.; Zhang, S. Highly Efficient and Low-Temperature Preparation of Plate-Like ZrB2-SiC Powders by a Molten-Salt and Microwave-Modified Boro/Carbothermal Reduction Method. Materials 2018, 11, 1811. [Google Scholar] [CrossRef]
- Park, K.; Nersisyan, H.H.; Lee, H.; Hong, S.-I.; Cho, N.-C.; Lee, J.-H. Low-temperature synthesis of zirconium metal using ZrCl4–2Mg reactive mixtures. Int. J. Refract. Met. H 2012, 33, 33–37. [Google Scholar] [CrossRef]
- Li, S.; Che, Y.; Shu, Y.; He, J.; Song, J.; Yang, B. Review—Preparation of Zirconium Metal by Electrolysis. J. Electrochem. Soc. 2021, 168, 062508. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Ting, A.; Xiao, C. A review on the extraction and recovery of critical metals using molten salt electrolysis. J. Environ. Chem. Eng. 2023, 11, 109746. [Google Scholar] [CrossRef]
- Xi, X.; Feng, M.; Zhang, L.; Nie, Z.-R. Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. Int. J. Miner. Metall. Mater. 2020, 27, 1599–1617. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Min, D.; Wen, T.; Shi, Z.; Yang, S. Mechanism for electrochemical reduction of Zr(IV) in molten NaCl–KCl. Int. J. Hydrogen Energy 2022, 47, 6544–6551. [Google Scholar] [CrossRef]
- Li, S.; Che, Y.; Song, J.; Shu, Y.; He, J.; Xu, B.; Yang, B. Preparation of zirconium metal through electrolysis of zirconium oxycarbonitride anode. Sep. Purif. Technol. 2021, 274, 118803. [Google Scholar] [CrossRef]
- Li, N.; Peng, Y.; Chen, Z.; Xiong, W.; Sun, J.; Chen, Y.; Zhang, P.; Liu, M.; Li, S. Preparation of Mg-Zr alloys through direct electro-deoxidation of MgO-ZrO2 in CaCl2-NaCl molten salt. Electrochim. Acta 2021, 372, 137816. [Google Scholar] [CrossRef]
- Abdelkader, A.; Daher, A.; Abdelkareem, R.A.; El-Kashif, E. Preparation of Zirconium Metal by the Electrochemical Reduction of Zirconium Oxide. Metall. Mater. Trans. B 2007, 38, 35–44. [Google Scholar] [CrossRef]
- Shang, X.; Li, S.; Che, Y.; Shu, Y.; He, J.; Song, J. Novel extraction of Zr based on an in-situ preparation of ZrCxOy. Sep. Purif. Technol. 2021, 275, 118096. [Google Scholar] [CrossRef]
- Liu, H.; Man, Z.; Liu, J.; Wang, X.-G.; Zhang, G.-J. Solid solution and densification behavior of zirconium oxycarbide (ZrCxOy) ceramics via doping ZrO2 and Zr in ZrC. J. Alloys Compd. 2017, 729, 492–497. [Google Scholar] [CrossRef]
- Li, S.; Che, Y.; Song, J.; Shu, Y.; Xu, B.; He, J.; Yang, B. Electrolytic Preparation of Zirconium Metal from a Consumable Zirconium Oxycarbide Anode. Metall. Mater. Trans. B 2021, 52B, 3276–3287. [Google Scholar] [CrossRef]
- Jiao, S.; Zhu, H. Novel metallurgical process for titanium production. J. Mater. Res. 2006, 21, 2172–2175. [Google Scholar] [CrossRef]
- Katea, S.N.; Riekehr, L.; Westin, G. Synthesis of nano-phase ZrC by carbothermal reduction using a ZrO2–carbon nano-composite. J. Eur. Ceram. Soc. 2021, 41, 62–67. [Google Scholar] [CrossRef]
- Wang, J.; Jia, C.; Li, C.; Peng, X.-L.; Zhang, L.-H.; Liu, J.-Y. Thermodynamic Properties for Carbon Dioxide. ACS Omega 2019, 21, 19193–19198. [Google Scholar] [CrossRef]
- Hamm, D.; Ogle, K.; Olsson, C.-O.A.; Weber, S.; Landolt, D. Passivation of Fe–Cr alloys studied with ICP-AES and EQCM. Corros. Sci. 2002, 44, 1443–1456. [Google Scholar] [CrossRef]
- Mehdi, N.; Pouyan, S. A simple method for determining Hf in Zr and Zr alloys by ICP-AES. Nucl. Sci. Tech. 2009, 20, 340–343. [Google Scholar] [CrossRef]
- Long, W.; Gao, F.; Wang, D.; Zou, B.; Wang, X.; Wang, Y.; Zhan, F.; Wei, Y. Forming thermodynamics, structure, and electrical conductivity of TiCxOy compounds fabricated through the carbothermal reduction process. J. Alloys Compd. 2022, 892, 162201. [Google Scholar] [CrossRef]
- Pham, D.; Gai, F.; Rochester, J.; Dycus, J.H.; LeBeau, J.M.; Manga, V.R.; Corral, E.L. Thermodynamic assessment within the Zr–B–C–O quaternary system. J. Am. Ceram. Soc. 2023, 106, 3127–3140. [Google Scholar] [CrossRef]
- Efaw, C.M.; Vandegrift, J.L.; Reynolds, M.; McMurdie, S.; Jaques, B.J.; Hu, H.; Xiong, H.; Hurley, M.F. Characterization of zirconium oxides part I: Raman mapping and spectral feature analysis. Nucl. Mater. Energy 2019, 21, 100707. [Google Scholar] [CrossRef]
- Li, S.; Lv, Z.; He, J.; Song, J. Anodic dissolution of consumable ZrCxOy and cathodic deposition of zirconium in NaCl-KCl based melts. J. Mol. Liq. 2025, 420, 126831. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.; Xia, D.; Meng, J.; Zhao, Z. A fundamental study on indium electrochemistry in molten NaCl-KCl eutectic: Toward new technology development for indium secondary resources recycling. Ionics 2023, 29, 4379–4387. [Google Scholar] [CrossRef]
- Wang, Z.; Tada, E.; Nishikata, A. In Situ Analysis of Scan Rate Effects on Pt Dissolution Under Potential Cycling Using a Channel Flow Double Electrode. Electrocatalysis 2015, 6, 179–184. [Google Scholar] [CrossRef]
- B551/B551M-12; Standard Specification for Zirconium and Zirconium Alloy Strip, Sheet, and Plate. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
| Weight Loss Rate/% | Electrical Resistivity/Ω cm | |
|---|---|---|
| Before reduction | / | 0.0440 |
| After reduction | 26.91 | 0.0169 |
| Batch Number | Zr/wt.% | Fe + Cr/wt.% | H/wt.% | O/wt.% | C/wt.% | N/wt.% |
|---|---|---|---|---|---|---|
| No. 1 | 99.5 | 0.18 | 0.002 | 0.13 | 0.021 | 0.012 |
| No. 2 | 99.0 | 0.31 | 0.004 | 0.19 | 0.018 | 0.024 |
| No. 3 | 99.1 | 0.27 | 0.003 | 0.17 | 0.024 | 0.019 |
| Average | 99.2 ± 0.3 | 0.25 ± 0.07 | 0.003 ± 0.001 | 0.16 ± 0.03 | 0.021 ± 0.003 | 0.018 ± 0.06 |
| R60704 | ≥97.5 | 0.2 to 0.4 | ≤0.005 | ≤0.18 | ≤0.05 | ≤0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Chen, X.; Jia, Y.; Yang, M.; Ye, G.; Zhu, F. Preparation of Metallic Zr from ZrO2 via Carbothermal and Electrochemical Reduction in Molten Salts. Materials 2025, 18, 2634. https://doi.org/10.3390/ma18112634
Song W, Chen X, Jia Y, Yang M, Ye G, Zhu F. Preparation of Metallic Zr from ZrO2 via Carbothermal and Electrochemical Reduction in Molten Salts. Materials. 2025; 18(11):2634. https://doi.org/10.3390/ma18112634
Chicago/Turabian StyleSong, Wenchen, Xu Chen, Yanhong Jia, Mingshuai Yang, Guoan Ye, and Fuxing Zhu. 2025. "Preparation of Metallic Zr from ZrO2 via Carbothermal and Electrochemical Reduction in Molten Salts" Materials 18, no. 11: 2634. https://doi.org/10.3390/ma18112634
APA StyleSong, W., Chen, X., Jia, Y., Yang, M., Ye, G., & Zhu, F. (2025). Preparation of Metallic Zr from ZrO2 via Carbothermal and Electrochemical Reduction in Molten Salts. Materials, 18(11), 2634. https://doi.org/10.3390/ma18112634







