Cold Consolidation of Waste Glass by Alkali Activation and Curing by Traditional and Microwave Heating
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preparation
2.2. Samples Characterization
2.3. Leaching Tests
2.4. Microbial and Cytotoxicity Assays
3. Results and Discussion
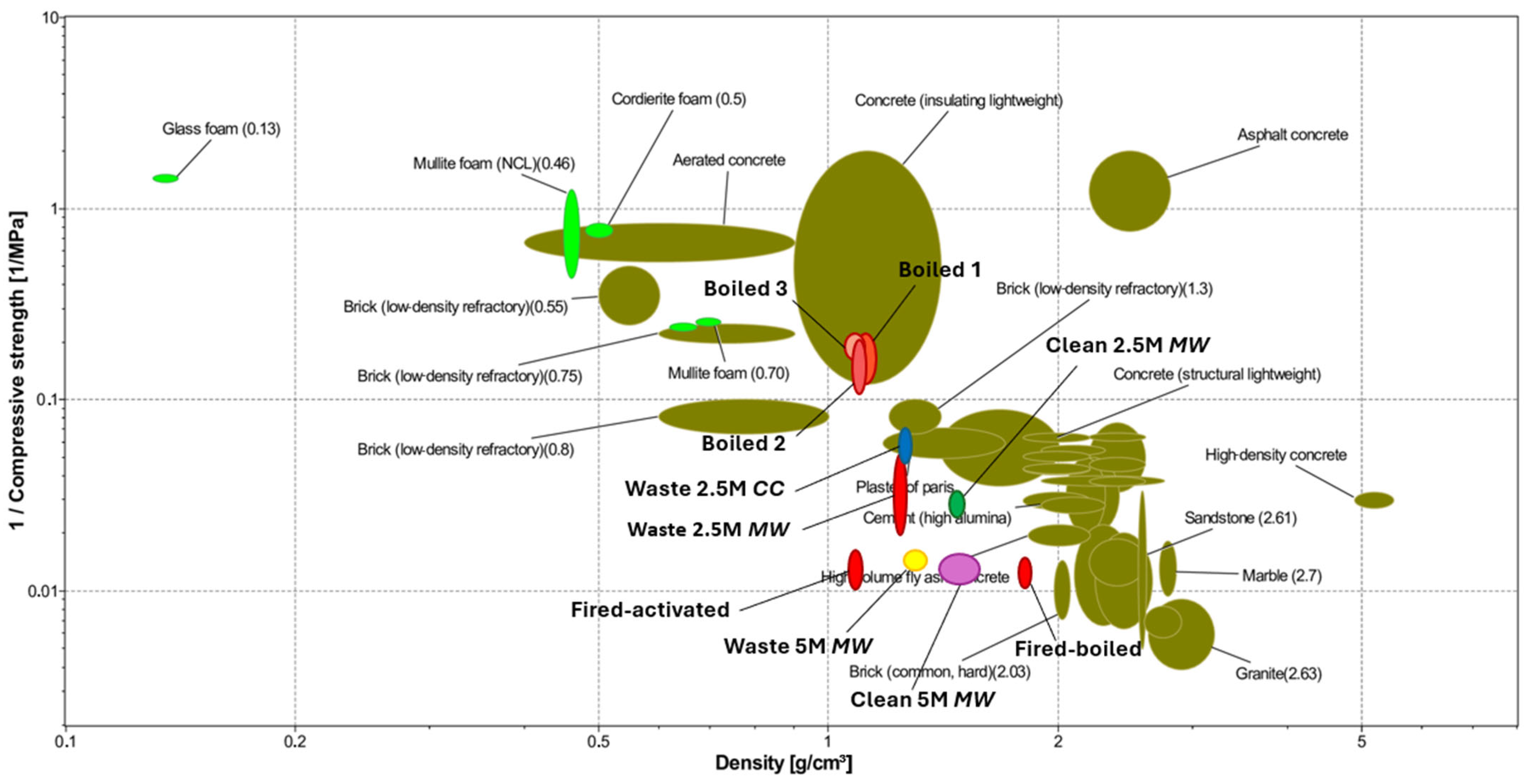
3.1. Mechanical Characterization and Mass Loss
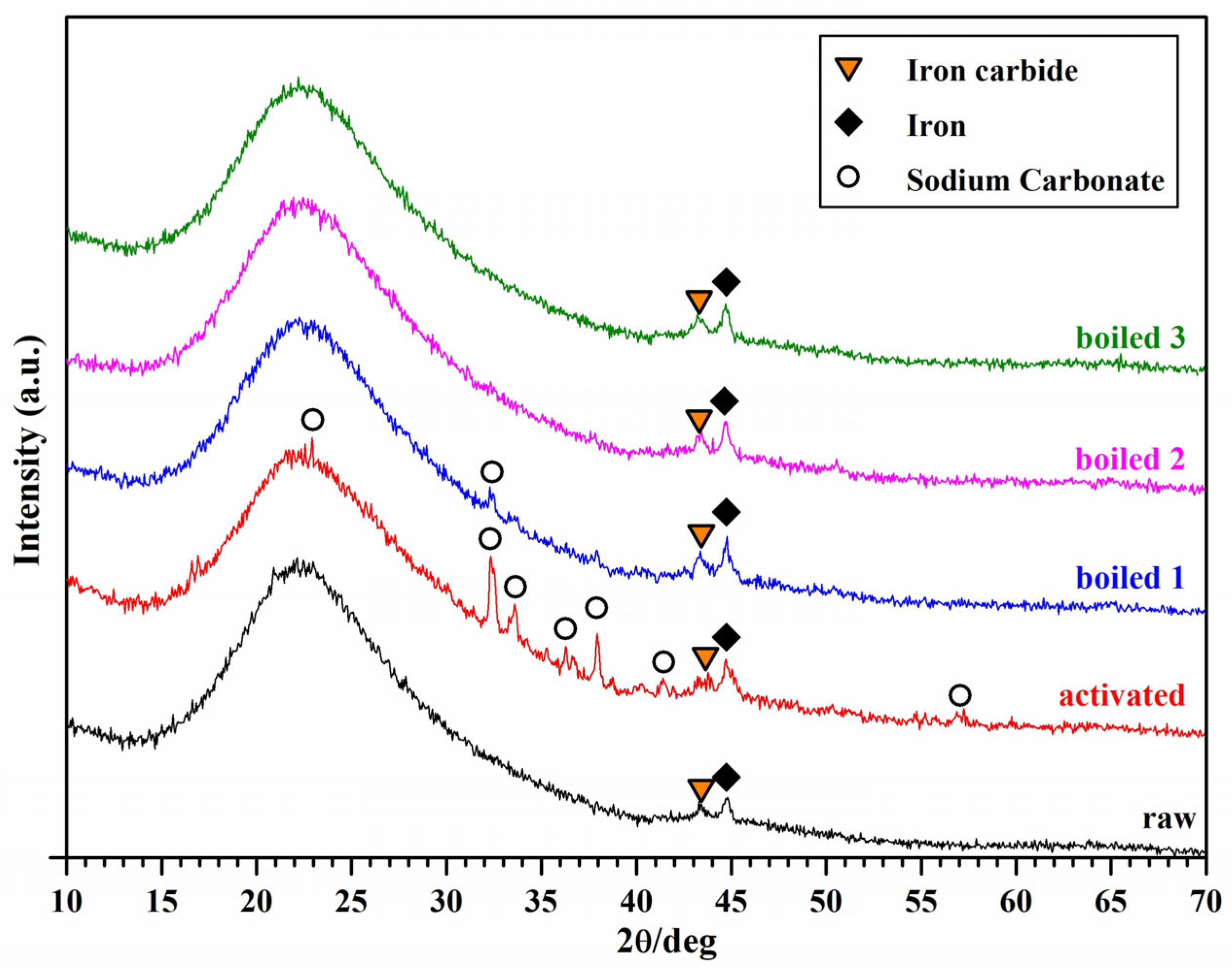
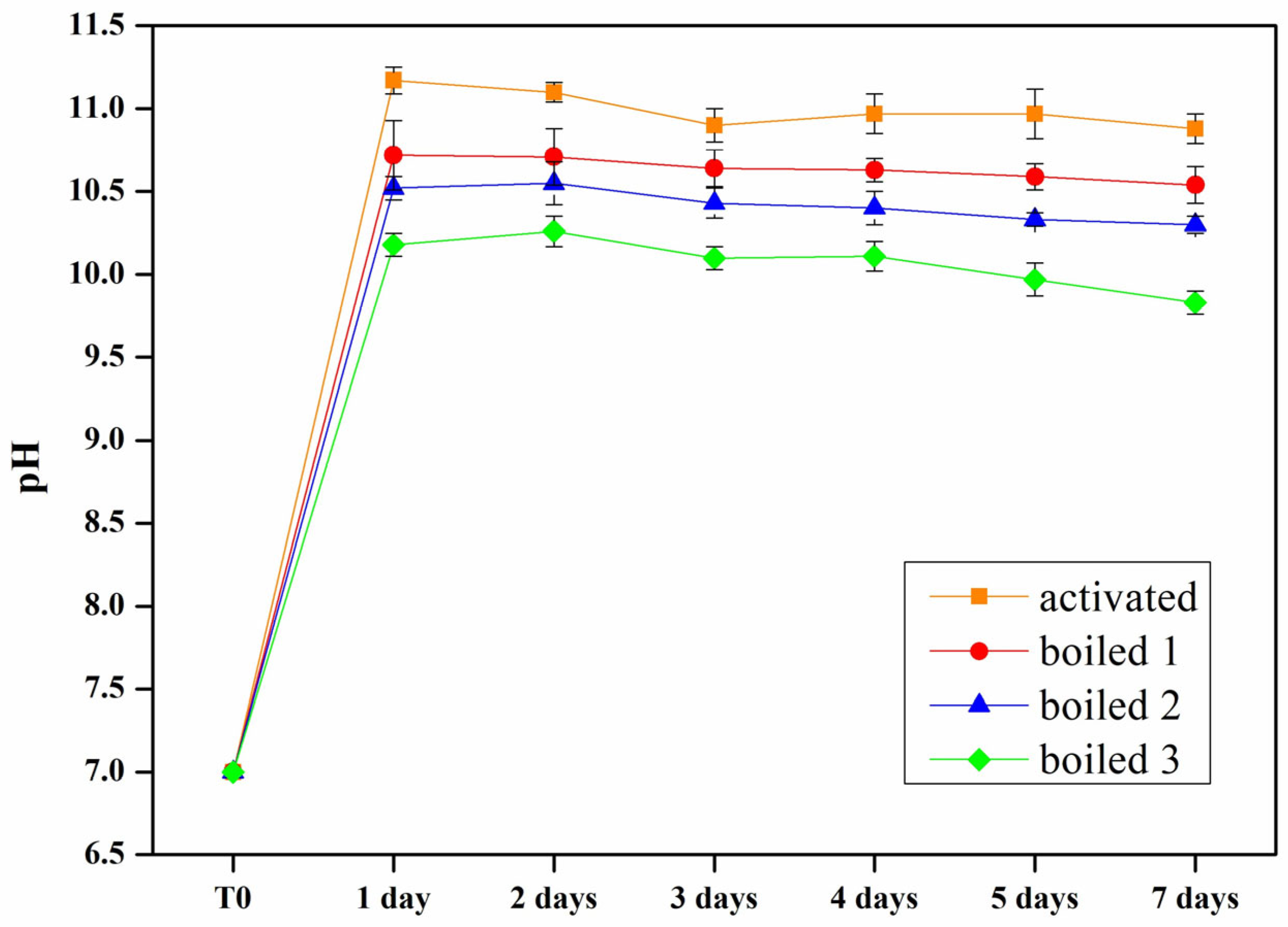
3.2. Material Stabilization
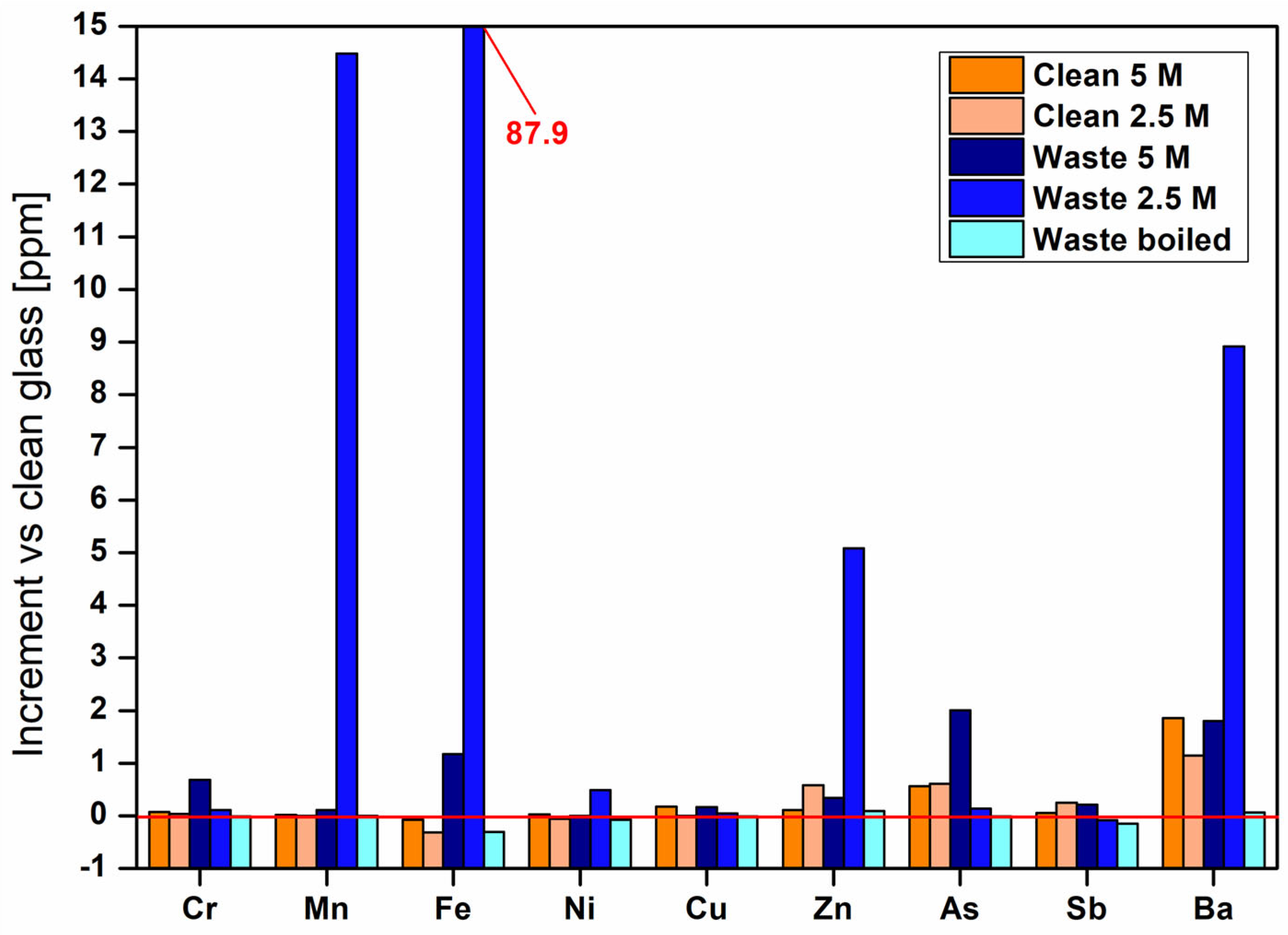
3.3. Leaching Tests
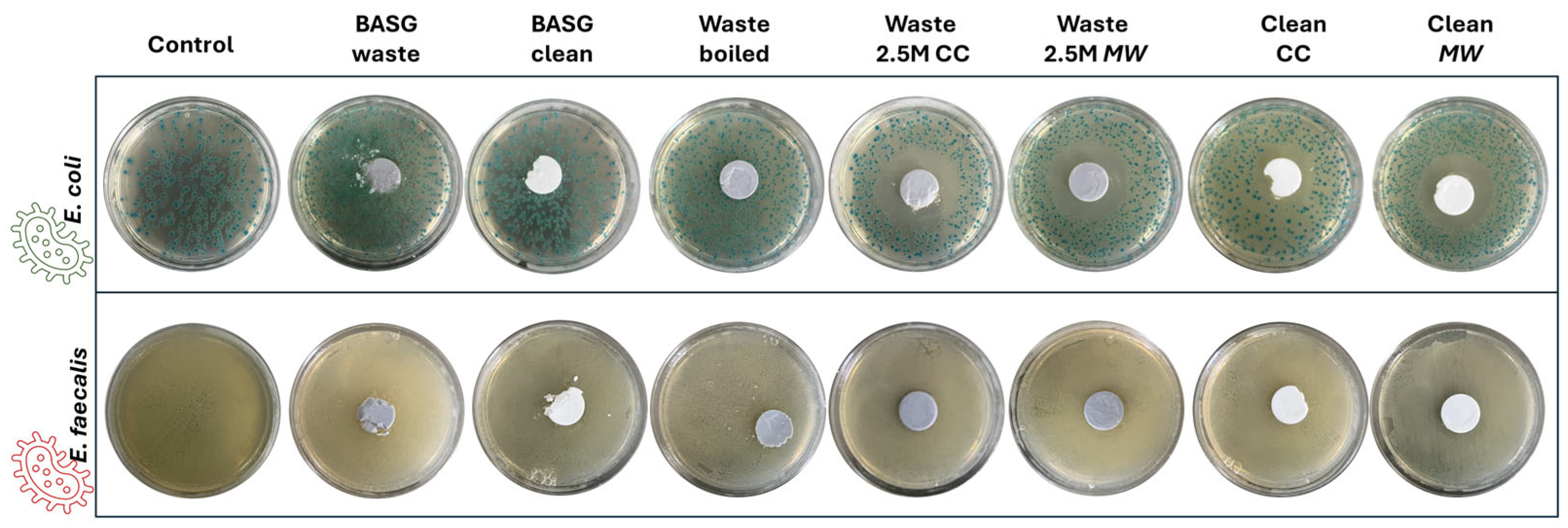
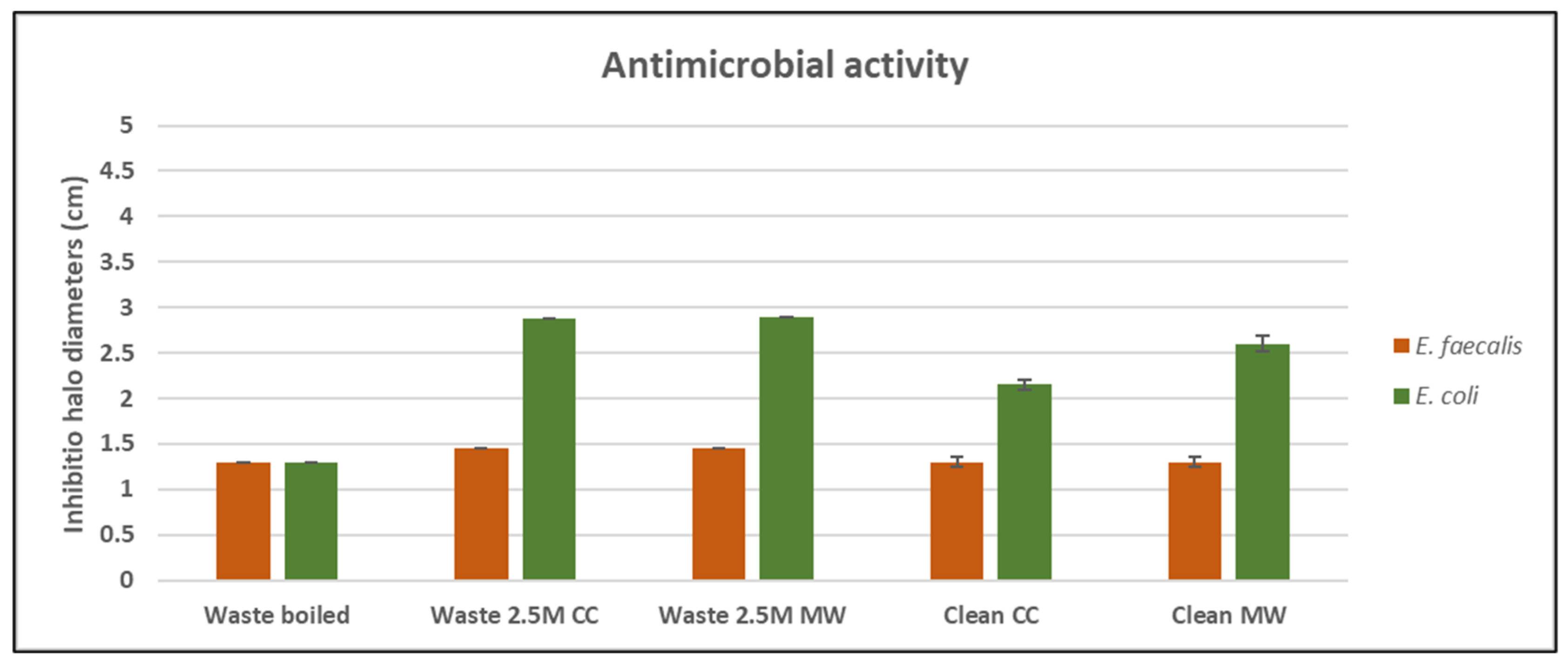
3.4. Antimicrobial Properties and Cytotoxicity Test
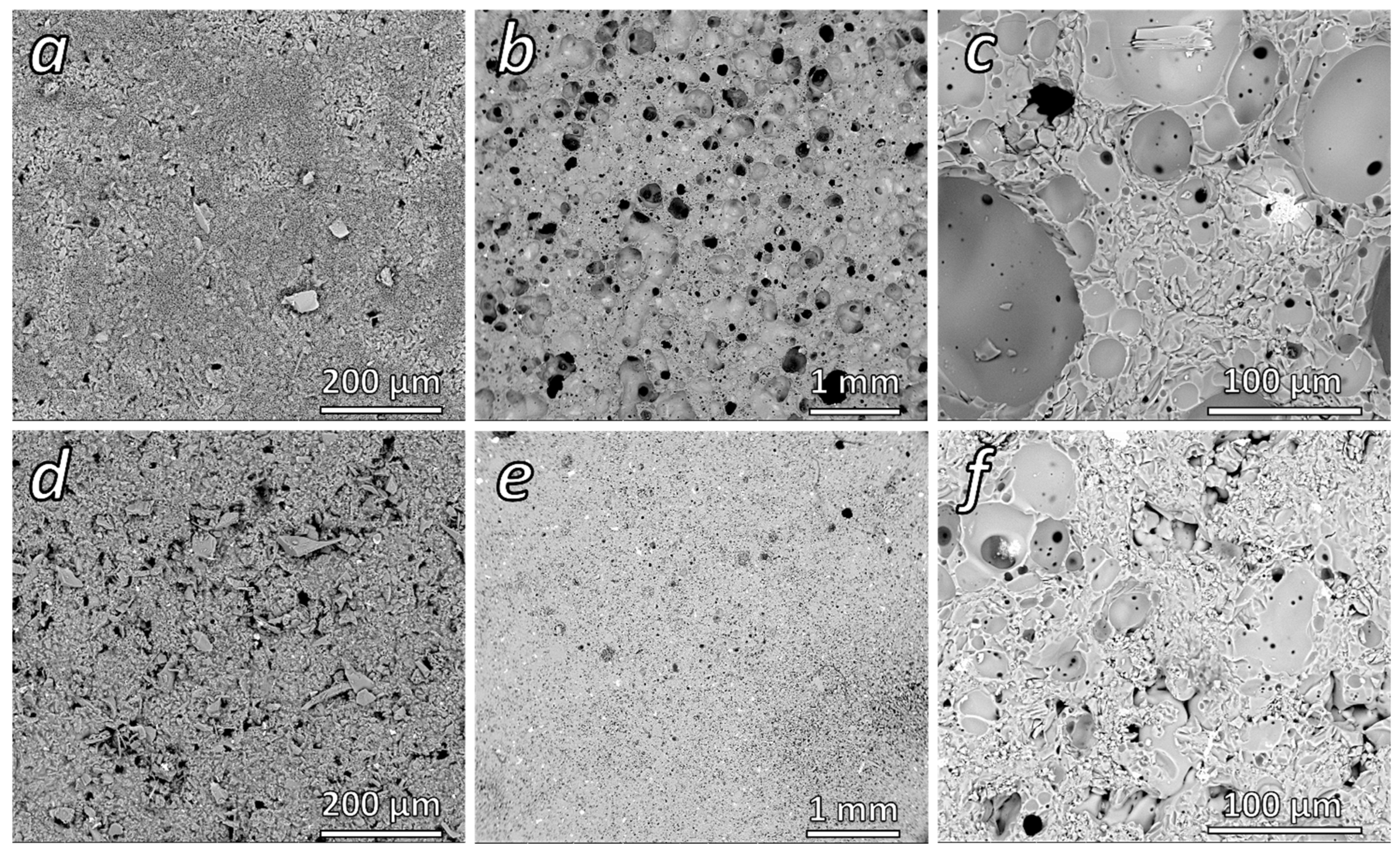
3.5. Effect of Heat Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussein, H.; Alyaa, A.; Bassam, T.; Fadzil, Y.; Blessen, T. Effect of recycled waste glass on the properties of high-performance concrete: A critical review. Case Stud. Constr. Mater. 2022, 17, e01149. [Google Scholar] [CrossRef]
- Chong, F.; Jianwei, L.; Gao, Y.; Abd Alwahed, D.; Wei, L.; Xudong, L.; Baobao, Z.; Haidong, W.; Meipeng, H.; Lifu, L.; et al. Recycling of waste glass as raw materials for the preparation of self-cleaning, light-weight and high-strength porous ceramics. J. Clean. Prod. 2021, 317, 128395. [Google Scholar] [CrossRef]
- Tameni, G.; Lago, D.; Kaňková, H.; Buňová, L.; Kraxner, J.; Galusek, D.; Dawson, D.M.; Ashbrook, S.E.; Bernardo, E. Alkaline attack of boro-alumino-silicate glass: New insights of the molecular mechanism of cold consolidation and new applications. Open Ceram. 2025, 21, 100726. [Google Scholar] [CrossRef]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Salmenperä, H.; Pitkänen, K.; Kautto, P.; Saikku, L. Critical factors for enhancing the circular economy in waste management. J. Clean. Prod. 2021, 280, 124339. [Google Scholar] [CrossRef]
- Reike, D.; Vermeulen, W.J.V.; Witjes, S. The circular economy: New or Refurbished as CE 3.0?—Exploring Controversies in the Conceptualization of the Circular Economy through a Focus on History and Resource Value Retention Options. Resour. Conserv. Recycl. 2018, 135, 246–264. [Google Scholar] [CrossRef]
- John, L.; Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Altimari, F.; Lancellotti, I.; Leonelli, C.; Andreola, F.; Elsayed, H.; Bernardo, E.; Barbieri, L. Green materials for construction industry from Italian volcanic quarry scraps. Mater. Lett. 2023, 333, 133615. [Google Scholar] [CrossRef]
- Lancellotti, I.; Cannio, M.; Bollino, F.; Catauro, M.; Barbieri, L.; Leonelli, C. Geopolymers: An option for the valorization of incinerator bottom ash derived “end of waste. Ceram. Int. 2015, 41, 2116–2123. [Google Scholar] [CrossRef]
- Margallo, M.; Taddei, M.B.M.; Hernández-Pellón, A.; Aldaco, R.; Irabien, A. Environmental sustainability assessment of the management of municipal solid waste incineration residues: A review of the current situation. Clean Technol. Environ. Policy 2015, 17, 1333–1353. [Google Scholar] [CrossRef]
- Poulesquen, A.; Rodrigues, D.G.; Keshavarz, B.; Courtois, N.; Ilavsky, J.; McKinley, G.H. Aluminosilicate colloidal gels: From the early age to the precipitation of zeolites. Soft Matter 2024, 20, 5538. [Google Scholar] [CrossRef]
- França, S.; Sousa, L.N.; Silva, M.V.d.M.S.; Borges, P.H.R.; Bezerra, A.C.d.S. Preliminary Reactivity Test for Precursors of Alkali-Activated Materials. Building 2023, 13, 693. [Google Scholar] [CrossRef]
- Lancellotti, I.; Dal Poggetto, G.; Barbieri, L.; Nguyen, H.; Leonelli, C. Chemical resistance in acidic environments of alkali-activated lightweight composites based on secondary raw materials. J. Sustain. Cem.-Based Mater. 2024, 13, 1631–1640. [Google Scholar] [CrossRef]
- Zhuravlev, L.; Potapov, V. Density of silanol groups on the surface of silica precipitated from a hydrothermal solution. Russ. J. Phys. Chem. 2006, 80, 1119–1128. [Google Scholar] [CrossRef]
- Tameni, G.; Carollo, F.; Cavazzini, A.M.; Forzan, M.; Bernardo, E. Microwave assisted cold consolidation of alkali activated suspension of glass waste powders. Mater. Lett. 2025, 389, 138354. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Zhang, S. Dispersion, properties, and mechanisms of nanotechnology-modified alkali-activated materials: A review. Renew. Sustain. Energy Rev. 2024, 192, 114215. [Google Scholar] [CrossRef]
- Lago, D.; Kraxner, J.; Galusek, D.; Bernardo, E. Novel glass-based membranes for Cu adsorption: From alkali activation to sintering. Heliyon 2023, 9, e18221. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, Z.; Wang, D.; Lv, Y.; Gao, C.; Xue, G. Effects of alkaline activators on pore structure and mechanical properties of ultrafine metakaolin geopolymers cured at room temperature. Constr. Build. Mater. 2022, 361, 129678. [Google Scholar] [CrossRef]
- Aschoff, J.; Partschefeld, S.; Schneider, J.; Osburg, A. Effect of Microwaves on the Rapid Curing of Metakaolin and Aluminum Orthophosphate-Based Geopolymers. Materials 2024, 17, 463. [Google Scholar] [CrossRef]
- Horvat, B.; Pavlin, M.; Ducman, V. Influence of microwaves in the early stage of alkali activation on the mechanical strength of alkali-activated materials. Ceram. Int. 2023, 49, 24246–24258. [Google Scholar] [CrossRef]
- Shi, S.; Li, H.; Zhou, Q.; Zhang, H.; Basheer, P.A.M.; Bai, Y. Alkali-activated fly ash cured with pulsed microwave and thermal oven: A comparison of reaction products, microstructure and compressive strength. Cem. Concr. Res. 2023, 166, 107104. [Google Scholar] [CrossRef]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- UNI EN 826; Isolanti Termici per Edilizia—Determinazione del Comportamento a Compressione. Ente Italiano di Normazione (UNI): Rome, Italy, 2013.
- EN 12457:2003; Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges. CEN (European Committee for Standardization): Bruxelles, Belgium, 2003.
- D’Angelo, A.; Viola, V.; Fiorentino, M.; Dal Poggetto, G.; Blanco, I. Use of natural dyes to color metakaolin-based geopolymer materials. Ceram. Int. 2025, 51, 5528–5535. [Google Scholar] [CrossRef]
- Gravina, C.; Formato, M.; Piccolella, S.; Fiorentino, M.; Stinca, A.; Pacifico, S.; Esposito, A. Lavandula austroapennina (Lamiaceae): Getting Insights into Bioactive Polyphenols of a Rare Italian Endemic Vascular Plant. Int. J. Mol. Sci. 2023, 24, 8038. [Google Scholar] [CrossRef]
- Haddad, M.A.; Ofer-Rozovsky, E.; Bar-Nes, G.; Borojovich, E.J.C.; Nikolski, A.; Mogiliansky, D.; Katz, A. Formation of zeolites in metakaolin-based geopolymers and their potential application for Cs immobilization. J. Nucl. Mater. 2017, 493, 168–179. [Google Scholar] [CrossRef]
- Dinh, H.L.; Doh, J.H.; Liu, J.; Lu, L.; Song, H.; Park, D. Comprehensive assessment of geopolymer concrete mechanical and environmental performance with glass cullet fine aggregates. J. Build. Eng. 2023, 76, 107094. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
- Rivera, J.F.; Cuarán-Cuarán, Z.I.; Vanegas-Bonilla, N.; Mejía de Gutiérrez, R. Novel use of waste glass powder: Production of geopolymeric tiles. Adv. Powder Technol. 2018, 29, 3448–3454. [Google Scholar] [CrossRef]
- Ulugol, H.; Kul, A.; Yıldırım, G.; Sahmaran, M.; Aldemir, A.; Figueira, D.; Ashour, A. Mechanical and microstructural charac-terization of geopolymers from assorted construction and demolition waste-based masonry and glass. J. Clean. Prod. 2021, 280, 124358. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J.; Separovic, F.; Gan, Z.H. 39K NMR of free potassium in geopolymers. Ind. Eng. Chem. Res. 2006, 45, 9208–9210. [Google Scholar] [CrossRef]
- Ahmed Khan, H.; Kanwal Baig, F.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Chen, T.Y.; Rautiyal, P.; Vaishnav, S.; Gupta, G.; Schlegl, H.; Dawson, R.J.; Evans, A.W.; Kamali, S.; Johnson, J.A.; Johnson, C.E.; et al. Composition-structure-property effects of antimony in soda-lime-silica glasses. J. Non-Cryst. Solids 2020, 544, 120184. [Google Scholar] [CrossRef]
- Nnaji, N.D.; Anyanwu, C.U.; Miri, T.; Onyeaka, H. Mechanisms of Heavy Metal Tolerance in Bacteria: A Review. Sustainability 2024, 16, 11124. [Google Scholar] [CrossRef]
- Gravina, C.; Fiorentino, M.; Formato, M.; Pecoraro, M.T.; Piccolella, S.; Stinca, A.; Pacifico, S.; Esposito, A. LC-HR/MS Analysis of Lipophilic Extracts from Calendula arvensis (Vaill.) L. Organs: An Unexplored Source in Cosmeceuticals. Molecules 2022, 27, 8905. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, K.; Komori, R.; Ishikawa, S.; Ueno, T.; Suzuki, Y.; Iwami, Y.; Takahashi, N. Resistance to acidic and alkaline environments in the endodontic pathogen Enterococcus faecalis. Oral Microbiol. Immunol. 2006, 21, 283–288. [Google Scholar] [CrossRef]
- Cimmino, G.; De Nisco, M.; Alonso, C.; Gravina, C.; Piscopo, V.; Lemos, R.; Coderch, L.; Piccolella, S.; Pacifico, S.; Pedatella, S. Novel synthesized seleno-glycoconjugates as cosmeceutical ingredients: Antioxidant activity and in vitro skin permeation. Eur. J. Med. Chem. Rep. 2024, 12, 100240. [Google Scholar] [CrossRef]
- Abdel-Gawwad, A.A.; Mohammed, M.S.; Heikal, M. Ultra-lightweight porous materials fabrication and hazardous lead-stabilization through alkali-activation/sintering of different industrial solid wastes. J. Clean. Prod. 2020, 244, 118742. [Google Scholar] [CrossRef]
- Refaat, M.; Mohsen, A.; Nasr, E.S.A.R.; Kohail, M. Utilization of optimized microwave sintering to produce safe and sustainable one-part alkali-activated materials. Sci. Rep. 2023, 13, 4611. [Google Scholar] [CrossRef]
- Bădănoiu, A.I.; Abood Al-Saadi, T.H.; Voicu, G. Synthesis and properties of new materials produced by alkaline activation of glass cullet and red mud. Int. J. Miner. Process. 2015, 135, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Chen, Y.; Li, Z.; Ye, G. Degradation of alkali-activated slag subjected to water immersion. Cem. Concr. Compos. 2023, 142, 105157. [Google Scholar] [CrossRef]
- Joseph, A.M.; Snellings, R.; Van den Heede, P.; Matthys, S.; De Belie, N. The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M.A.W.; Abdullah, M.M.A.B.; Jamil, N.H.; Mohamad, H.; Salleh, M.A.A.M.; Sandu, A.V.; Vizureanu, P.; Baltatu, M.S.; Sukmak, P. Alkaline-Activation Technique to Produce Low-Temperature Sintering Activated-HAp Ceramic. Appl. Sci. 2023, 13, 2643. [Google Scholar] [CrossRef]
- Pederson, F.; Ellersick, L.; Kim, H.J. A review of lunar regolith based alkali activated materials and sintered regolith for use as a construction material. Acta Astronaut. 2025, 232, 502–515. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, S. A review of glass ceramic foams prepared from solid wastes: Processing, heavy-metal solidification and volatilization, applications. Sci. Total Environ. 2021, 781, 146727. [Google Scholar] [CrossRef]


| Oxides | Al2O3 | BaO | CaO | Fe2O3 | K2O | MgO | Na2O | SiO2 | TiO2 | B2O3 | Traces |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BASG clean | 5.7 ± 0.3 | 0.08 ± 0.01 | 1.5 ± 0.3 | 0.04 ± 0.01 | 0.14 ± 0.2 | - | 7.5 ± 0.4 | 76.1 ± 0.5 | - | 8.9 ± 0.05 | 0.04 |
| BASG waste | 6.3 ± 0.3 | 0.54 ± 0.05 | 1 ± 0.1 | 3.6 ± 0.5 | 1.1 ± 0.1 | 0.11 ± 0.02 | 6.8 ± 0.5 | 72 ± 1 | 0.03 ± 0.01 | 8.5 ± 0.03 | 0.02 |
| Samples Name | Glass | Alkaline Activator | Consolidation Treatment |
|---|---|---|---|
| Waste 2.5 M CC | BASG waste | NaOH 2.5 M (50 wt%) | 7 days at 40 °C |
| Waste 2.5 M MW | BASG waste | NaOH 2.5 M (50 wt%) | 1 day at 40 °C + 5′ MW (450 W) |
| Waste 5 M MW | BASG waste | NaOH 5 M (50 wt%) | 1 day at 40 °C + 5′ MW (450 W) |
| Clean 2.5 M MW | BASG clean | NaOH 2.5 M (50 wt%) | 1 day at 40 °C + 5′ MW (450 W) |
| Clean 5 M MW | BASG clean | NaOH 5 M (50 wt%) | 1 day at 40 °C + 5′ MW (450 W) |
| Sample Type | Mass [g] | Bulk Density [g/cm3] | Compressive Strength [MPa] |
|---|---|---|---|
| Waste 2.5 M CC (dried) | 0.864 ± 0.023 | 1.265 ± 0.017 | 10.3 ± 1.8 |
| Waste 2.5 M MW dried | 0.896 ± 0.015 | 1.251 ± 0.018 | 18.2 ± 2.4 |
| boiled 1st cycle | 0.819 ± 0.020 | 1.124 ± 0.028 | 4.2 ± 1.1 |
| boiled 2nd cycle | 0.789 ± 0.021 | 1.101 ± 0.014 | 4.6 ± 1.2 |
| boiled 3rd cycle | 0.761 ± 0.038 | 1.089 ± 0.024 | 3.6 ± 0.4 |
| Waste 5 M MW (dried) | 1.117 ± 0.012 | 1.309 ± 0.033 | 35.0 ± 3.1 |
| Clean 2.5 M MW (dried) | 0.981 ± 0.078 | 1.480 ± 0.024 | 19.3 ± 2.3 |
| Clean 5 M MW (dried) | 1.032 ± 0.045 | 1.493 ± 0.083 | 39.0 ± 5.9 |
| Element | Concentrations [ppm] | |||||
|---|---|---|---|---|---|---|
| Glass Clean | Clean 5 M | Clean 2.5 M | Waste 5 M | Waste 2.5 M | Waste Boiled | |
| Cr | 0.0055 ± 0.0001 | 0.0760 ± 0.0020 | 0.021 ± 0.001 | 0.6900 ± 0.0200 | 0.116 ± 0.003 | 0.0007 ± 0.0001 |
| Mn | 0.0023 ± 0.0000 | 0.0178 ± 0.0003 | 0.001 ± 0.000 | 0.1160 ± 0.0020 | 14.500 ± 0.300 | 0.0058 ± 0.0002 |
| Fe | 0.3824 ± 0.009 | 0.3120 ± 0.0050 | 0.073 ± 0.009 | 1.5600 ± 0.0300 | 88.000 ± 2.000 | 0.076 ± 0.004 |
| Ni | 0.0817 ± 0.002 | 0.1110 ± 0.0020 | 0.013 ± 0.001 | 0.0810 ± 0.0020 | 0.580 ± 0.010 | 0.0082 ± 0.0004 |
| Cu | 0.0241 ± 0.0004 | 0.2000 ± 0.0040 | 0.005 ± 0.001 | 0.1920 ± 0.0030 | 0.069 ± 0.001 | 0.0142 ± 0.0005 |
| Zn | 0.0482 ± 0.0006 | 0.1580 ± 0.0020 | 0.036 ± 0.002 | 0.3910 ± 0.0080 | 5.130 ± 0.090 | 0.144 ± 0.006 |
| As | 0.0618 ± 0.0008 | 0.6200 ± 0.0100 | 1.160 ± 0.050 | 2.0600 ± 0.0400 | 0.205 ± 0.003 | 0.057 ± 0.001 |
| Sb | 0.1622 ± 0.002 | 0.2220 ± 0.0030 | 0.072 ± 0.002 | 0.3710 ± 0.0060 | 0.077 ± 0.001 | 0.0129 ± 0.0008 |
| Ba | 0.2393 ± 0.010 | 2.0900 ± 0.0300 | 1.250 ± 0.030 | 2.0400 ± 0.0200 | 9.200 ± 0.200 | 0.300 ± 0.020 |
| Sample Type | ρ Geom [g/cm3] | ρ App [g/cm3] | ρ True [g/cm3] | TP [vol %] | OP [vol %] | CP [vol %] | Compressive Strength [MPa] |
|---|---|---|---|---|---|---|---|
| Fired-activated | 1.086 ± 0.015 | 1.22 ± 0.04 | 2.29 ± 0.07 | 52.40 ± 0.65 | 11.10 ± 1.22 | 41.38 ± 0.57 | 38.88 ± 6.23 |
| Fired-boiled | 1.809 ± 0.022 | 2.11 ± 0.04 | 2.33 ± 0.03 | 22.29 ± 0.97 | 14.23 ± 1.07 | 8.05 ± 1.10 | 40.16 ± 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, F.; Rienzo, E.D.; D’Angelo, A.; Sgarbossa, P.; Barbieri, L.; Leonelli, C.; Lancellotti, I.; Catauro, M.; Bernardo, E. Cold Consolidation of Waste Glass by Alkali Activation and Curing by Traditional and Microwave Heating. Materials 2025, 18, 2628. https://doi.org/10.3390/ma18112628
Carollo F, Rienzo ED, D’Angelo A, Sgarbossa P, Barbieri L, Leonelli C, Lancellotti I, Catauro M, Bernardo E. Cold Consolidation of Waste Glass by Alkali Activation and Curing by Traditional and Microwave Heating. Materials. 2025; 18(11):2628. https://doi.org/10.3390/ma18112628
Chicago/Turabian StyleCarollo, Francesco, Emanuele De Rienzo, Antonio D’Angelo, Paolo Sgarbossa, Luisa Barbieri, Cristina Leonelli, Isabella Lancellotti, Michelina Catauro, and Enrico Bernardo. 2025. "Cold Consolidation of Waste Glass by Alkali Activation and Curing by Traditional and Microwave Heating" Materials 18, no. 11: 2628. https://doi.org/10.3390/ma18112628
APA StyleCarollo, F., Rienzo, E. D., D’Angelo, A., Sgarbossa, P., Barbieri, L., Leonelli, C., Lancellotti, I., Catauro, M., & Bernardo, E. (2025). Cold Consolidation of Waste Glass by Alkali Activation and Curing by Traditional and Microwave Heating. Materials, 18(11), 2628. https://doi.org/10.3390/ma18112628













