Poly(ε-Caprolactone)/Sodium Bicarbonate/β-Tricalcium Phosphate Composites: Surface Characterization and Early Biological Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. SEM and EDX Analyses
2.3. Contact Angle and Surface Free Energy
2.4. Nanoindentation
2.5. pH Evaluation
2.6. Protein Adsorption
2.7. Cellular Tests
2.7.1. Cell Adhesion and Cell Spreading
2.7.2. Cell Viability
2.8. Statistical Analysis
3. Results
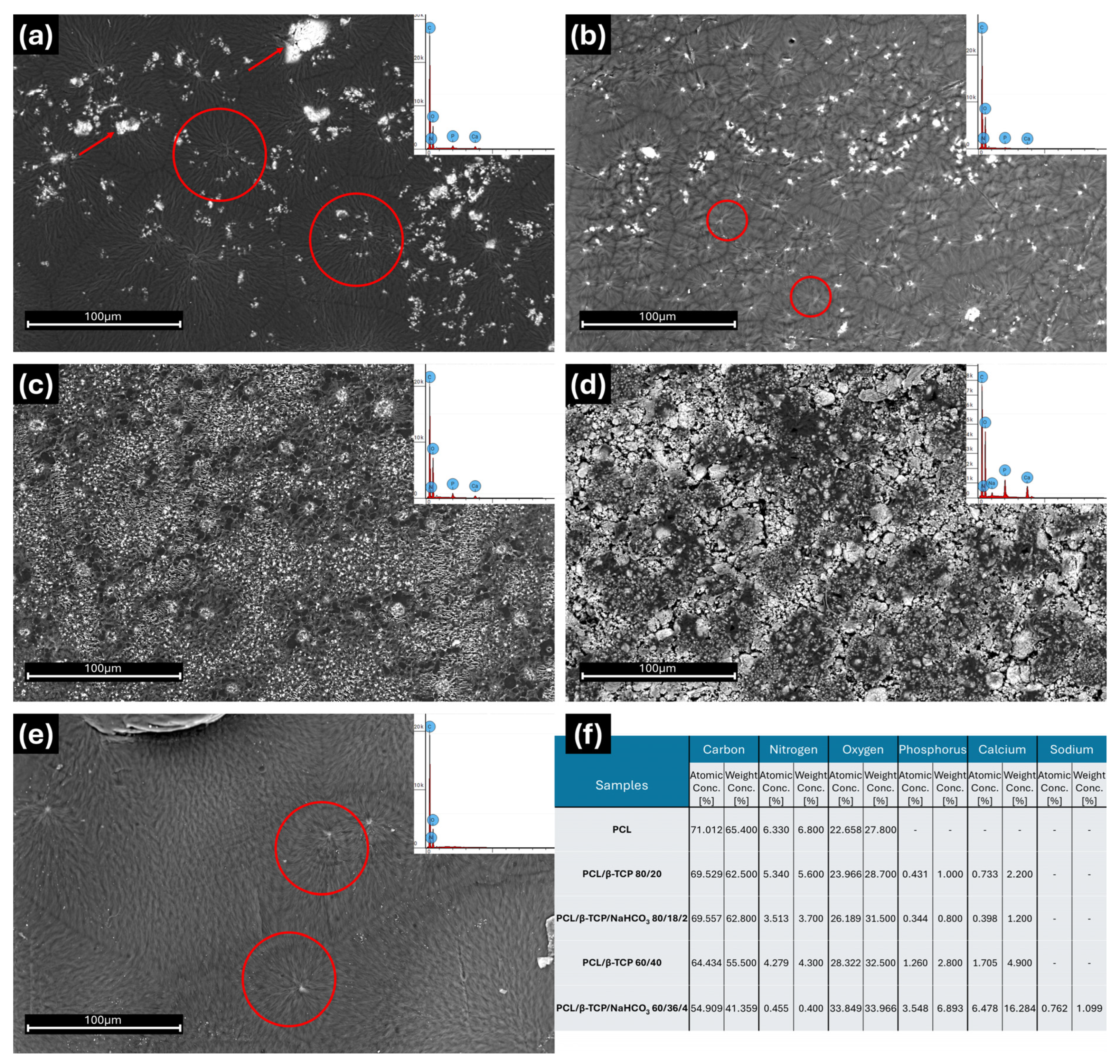
3.1. SEM and EDX Analyses
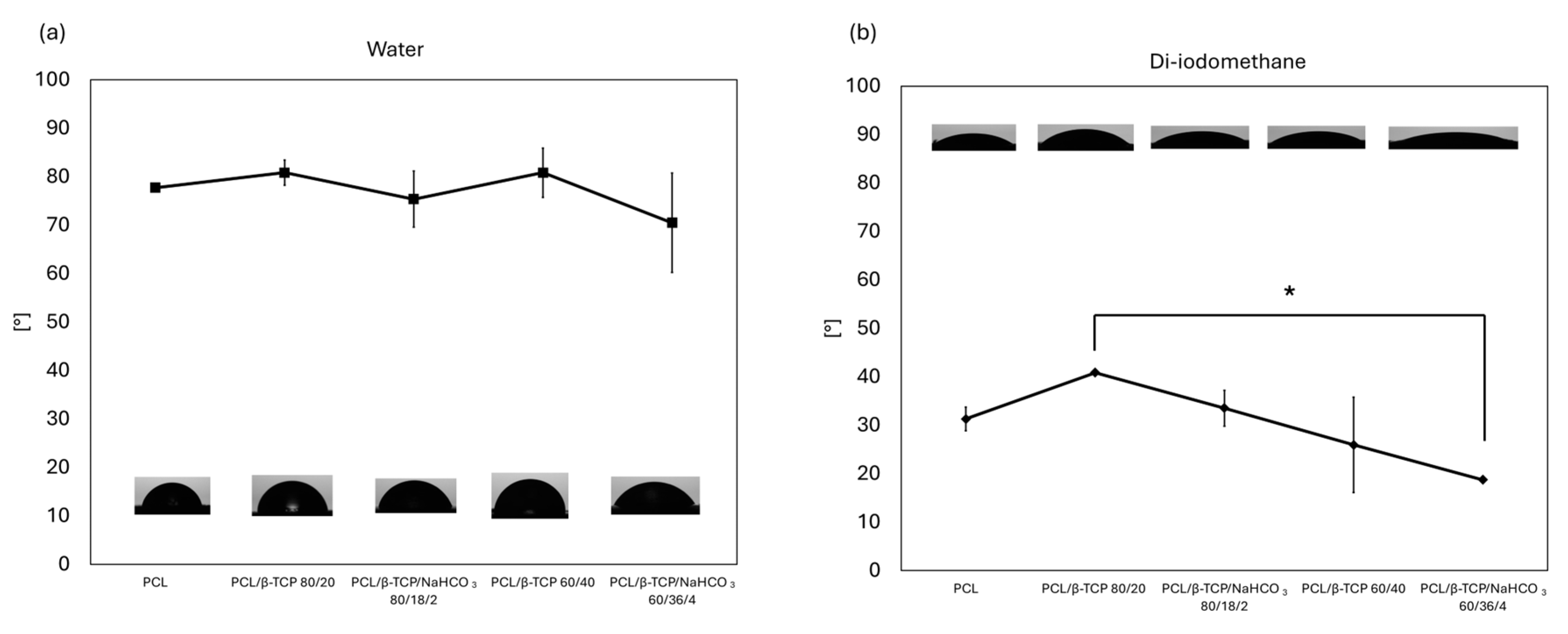
3.2. Contact Angle and Surface Free Energy
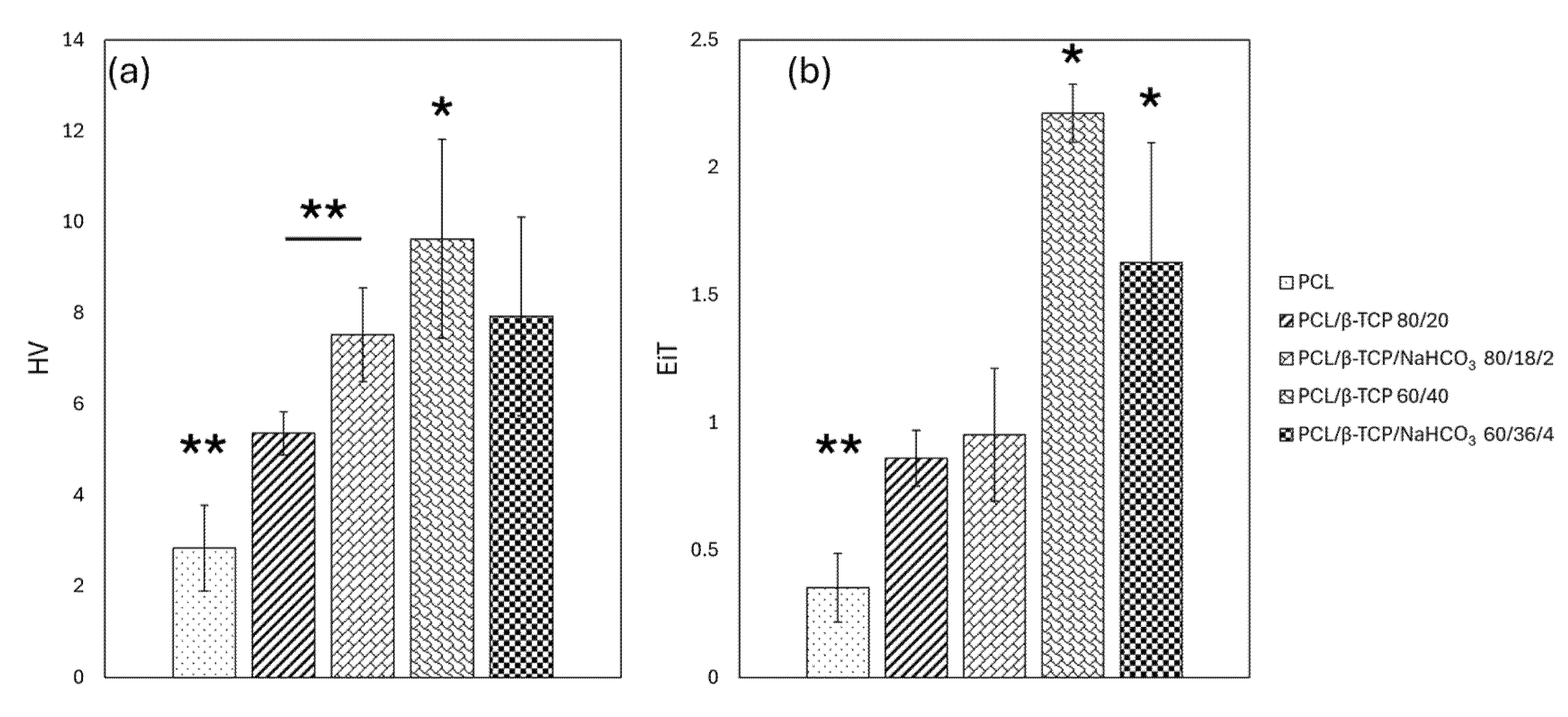
3.3. Nanoindentation
3.4. pH
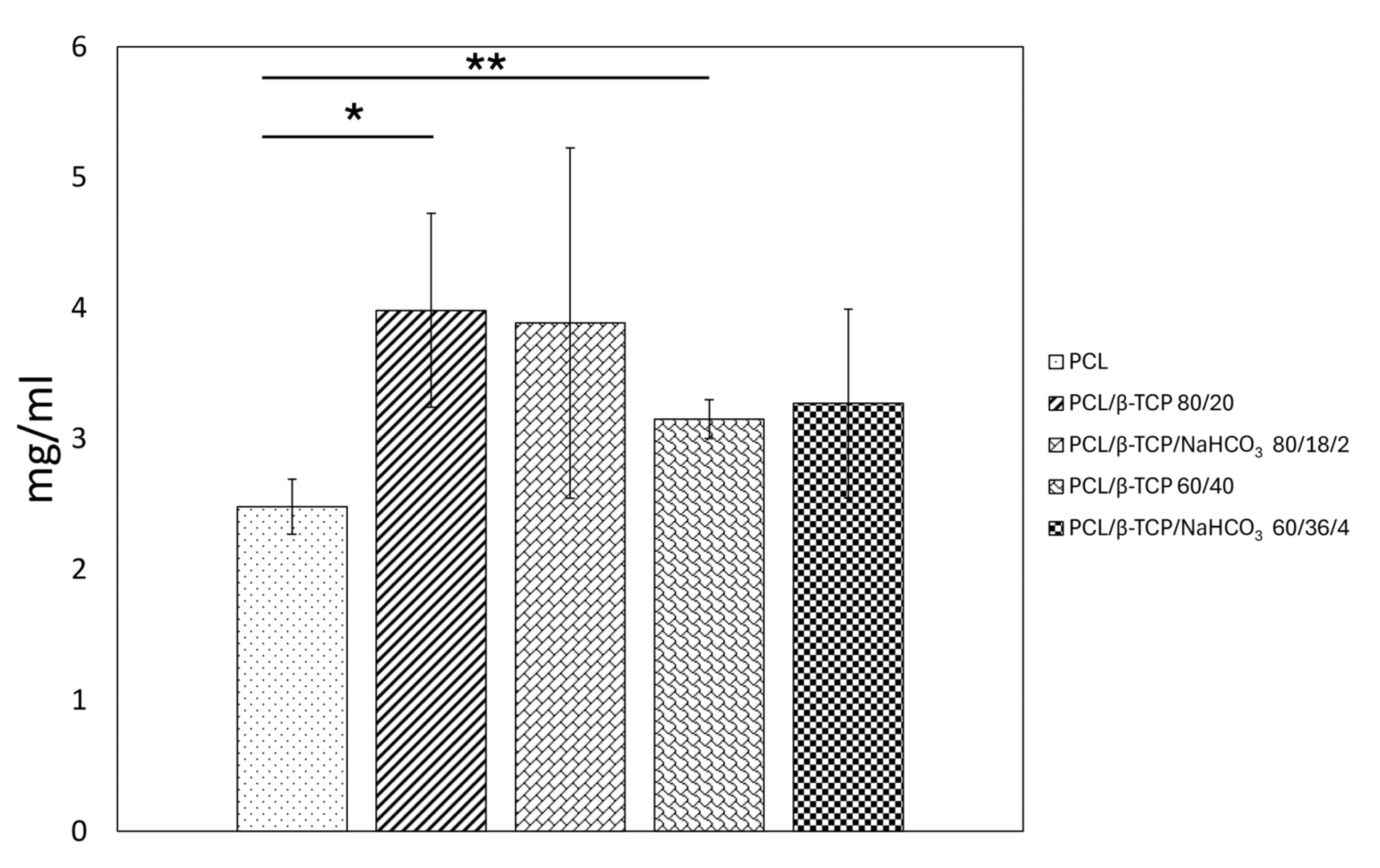
3.5. Protein Adsorption
3.6. Cellular Tests
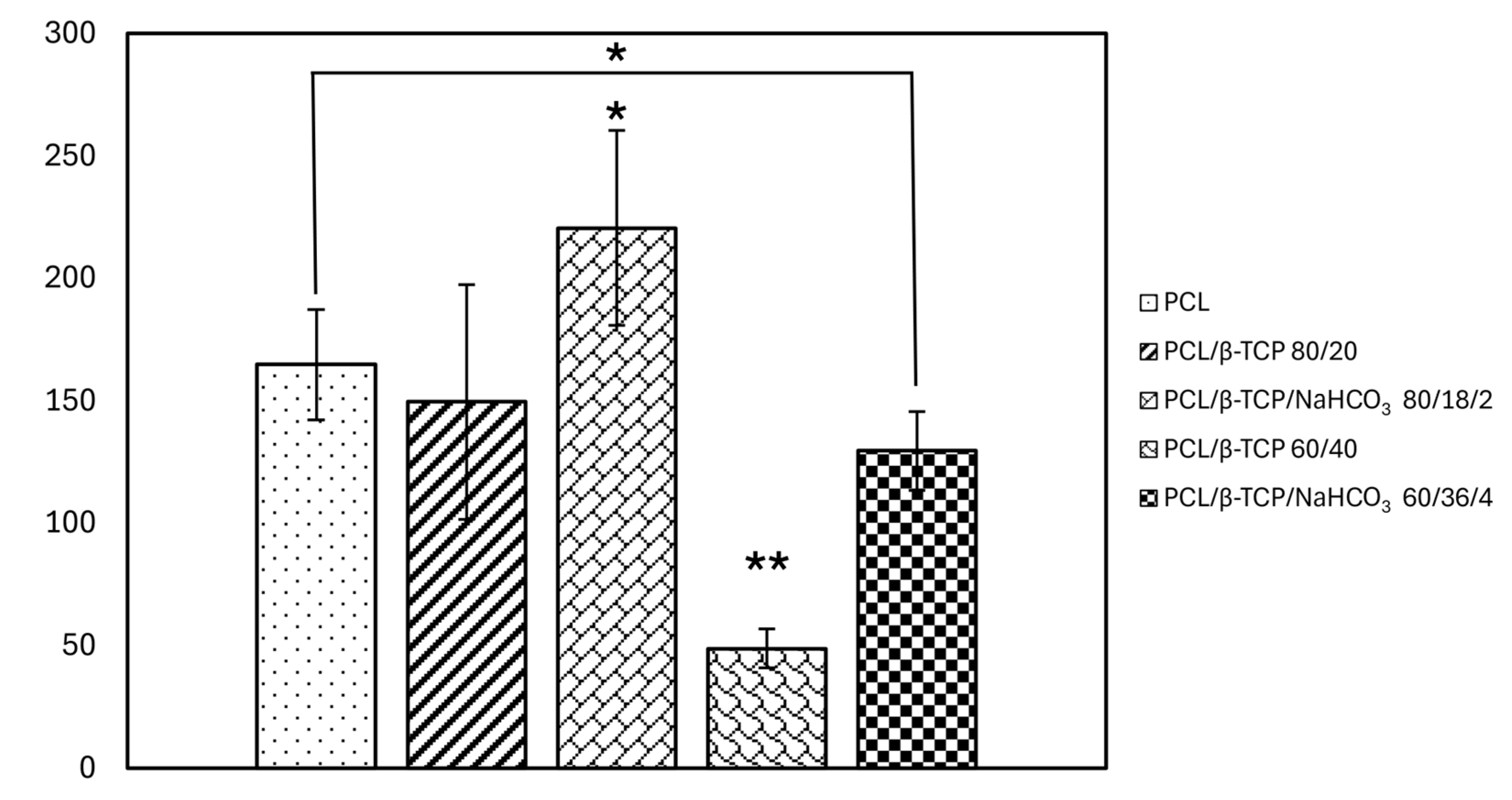
3.6.1. Cell Adhesion and Cell Spreading
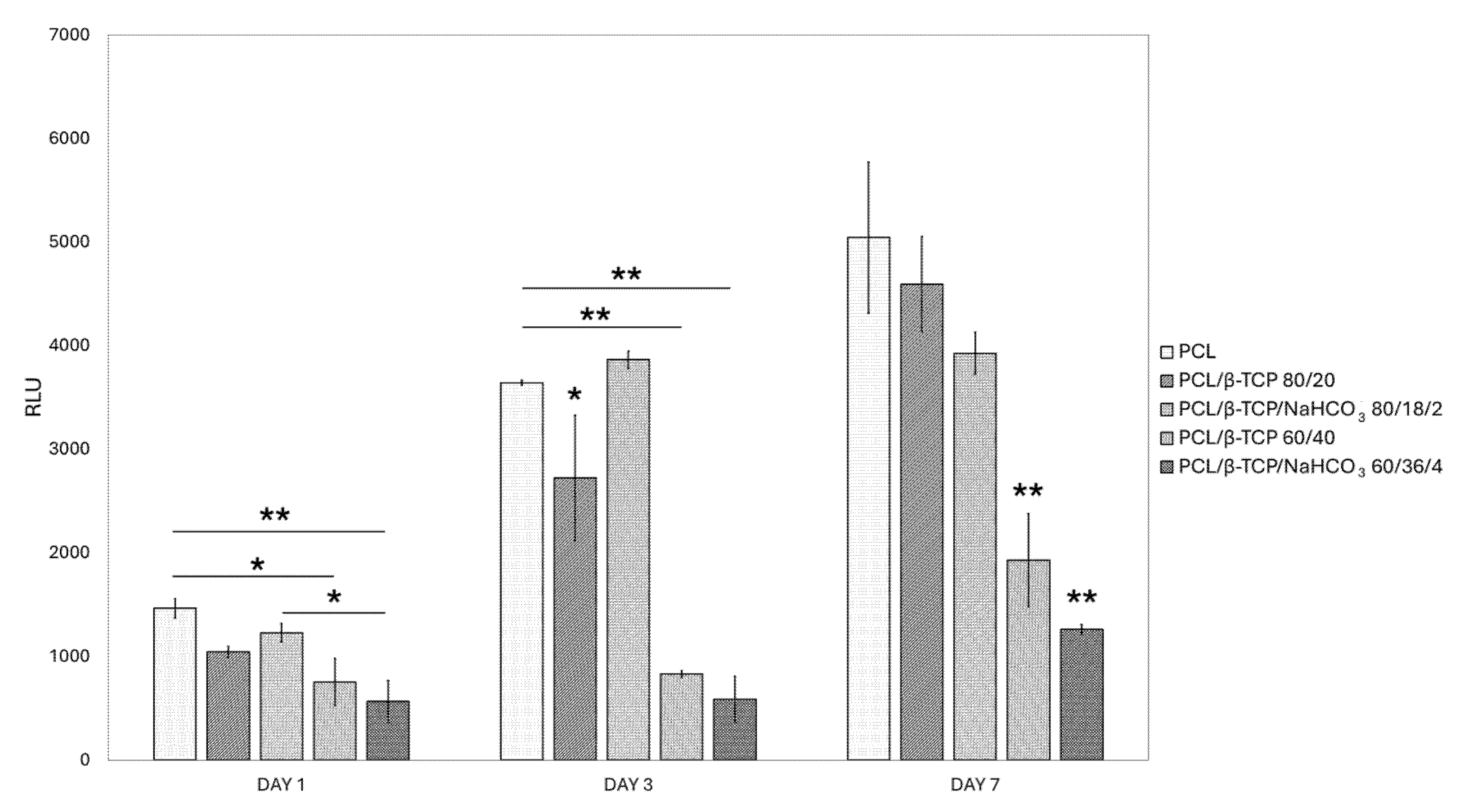
3.6.2. Viability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCL | Poly-ε-caprolactone |
| β-TCP | β-Tricalcium phosphate |
| CaPs | Calcium phosphate-based ceramics |
| HA | Hydroxyapatite |
| SEM | Scanning electron microscope |
| dH2O | Double-distilled water |
| CH2I2 | Diiodomethane |
| OWRK | Owens–Wendt–Rabel–Kaelbele |
| AC | Contact area |
| hC | Contact penetration depth |
| BSA | Bovine serum albumin |
| PBS | Phosphate-buffered saline |
| ASCs | Adipose stem cells ASC52hTert |
| FBS | Fetal bovine serum |
| BFE | Best fitting ellipse |
| RLUs | Relative light units |
| OCA | Contact angle |
| EiT | Young’s modulus |
| HV | Vickers hardness |
| SFE | Surface free energy |
References
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Morris, M.T.; Tarpada, S.P.; Cho, W. Bone Graft Materials for Posterolateral Fusion Made Simple: A Systematic Review. Eur. Spine J. 2018, 27, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Buser, Z.; Brodke, D.S.; Youssef, J.A.; Meisel, H.-J.; Myhre, S.L.; Hashimoto, R.; Park, J.-B.; Tim Yoon, S.; Wang, J.C. Synthetic Bone Graft versus Autograft or Allograft for Spinal Fusion: A Systematic Review. J. Neurosurg. Spine 2016, 25, 509–516. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and Bone Healing. EFORT Open Rev. 2018, 3, 173–183. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; De Boer, J.; Barradas, A.M.C.; De Ruiter, A.; Walsh, W.R.; Van Blitterswijk, C.A.; De Bruijn, J.D. Osteoinductive Ceramics as a Synthetic Alternative to Autologous Bone Grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef]
- Hernigou, P.; Dubory, A.; Pariat, J.; Potage, D.; Roubineau, F.; Jammal, S.; Flouzat Lachaniette, C.H. Beta-Tricalcium Phosphate for Orthopedic Reconstructions as an Alternative to Autogenous Bone Graft. Morphologie 2017, 101, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Brochu, B.M.; Sturm, S.R.; Kawase De Queiroz Goncalves, J.A.; Mirsky, N.A.; Sandino, A.I.; Panthaki, K.Z.; Panthaki, K.Z.; Nayak, V.V.; Daunert, S.; Witek, L.; et al. Advances in Bioceramics for Bone Regeneration: A Narrative Review. Biomimetics 2024, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Di Maro, M.; Faga, M.G.; Malucelli, G.; Mussano, F.D.; Genova, T.; Morsi, R.E.; Hamdy, A.; Duraccio, D. Influence of Chitosan on the Mechanical and Biological Properties of HDPE for Biomedical Applications. Polym. Test. 2020, 91, 106610. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.-J.; Yang, C.-J.; Lai, J.-Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Schäler, K.; Achilles, A.; Bärenwald, R.; Hackel, C.; Saalwächter, K. Dynamics in Crystallites of Poly(ε-Caprolactone) as Investigated by Solid-State NMR. Macromolecules 2013, 46, 7818–7825. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Kruft, M.-A.; Kellomäki, M. Degradation Mechanisms of Bioresorbable Polyesters. Part 1. Effects of Random Scission, End Scission and Autocatalysis. Acta Biomater. 2014, 10, 2223–2232. [Google Scholar] [CrossRef]
- Prasad, A.; Kandasubramanian, B. Fused Deposition Processing Polycaprolactone of Composites for Biomedical Applications. Polym.-Plast. Technol. Mater. 2019, 58, 1365–1398. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E.G. 3D Printing of Hybrid Biomaterials for Bone Tissue Engineering: Calcium-Polyphosphate Microparticles Encapsulated by Polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef]
- Burr, D.B.; Allen, M.R. Basic and Applied Bone Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Pedraza, R.; Mosca Balma, A.; Roato, I.; Orrico, C.; Genova, T.; Baima, G.; Berta, G.N.; Giura, A.; Ribotta, L.; Duraccio, D.; et al. Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application. Polymers 2024, 16, 2521. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A Generalist Algorithm for Cellular Segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.; Pachitariu, M. Cellpose3: One-Click Image Restoration for Improved Cellular Segmentation. Nat. Methods 2025, 22, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F. Uncertainty, Calibration and Probability: The Statistics of Scientific and Industrial Measurement, 1st ed.; Routledge: New York, NY, USA, 2017; ISBN 978-0-203-73475-9. [Google Scholar]
- Hile, D.D.; Sonis, S.T.; Doherty, S.A.; Tian, X.Y.; Zhang, Q.; Jee, W.S.S.; Trantolo, D.J. Dimensional Stability of the Alveolar Ridge After Implantation of a Bioabsorbable Bone Graft Substitute: A Radiographic and Histomorphometric Study in Rats. J. Oral Implantol. 2005, 31, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hesaraki, S.; Zamanian, A.; Moztarzadeh, F. The Influence of the Acidic Component of the Gas-foaming Porogen Used in Preparing an Injectable Porous Calcium Phosphate Cement on Its Properties: Acetic Acid versus Citric Acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86B, 208–216. [Google Scholar] [CrossRef]
- Cimatti, B.; Santos, M.A.D.; Brassesco, M.S.; Okano, L.T.; Barboza, W.M.; Nogueira-Barbosa, M.H.; Engel, E.E. Safety, Osseointegration, and Bone Ingrowth Analysis of PMMA—Based Porous Cement on Animal Metaphyseal Bone Defect Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 649–658. [Google Scholar] [CrossRef]
- Taylor, E.A.; Donnelly, E. Raman and Fourier Transform Infrared Imaging for Characterization of Bone Material Properties. Bone 2020, 139, 115490. [Google Scholar] [CrossRef]
- Taylor, E.A.; Mileti, C.J.; Ganesan, S.; Kim, J.H.; Donnelly, E. Measures of Bone Mineral Carbonate Content and Mineral Maturity/Crystallinity for FT-IR and Raman Spectroscopic Imaging Differentially Relate to Physical–Chemical Properties of Carbonate-Substituted Hydroxyapatite. Calcif. Tissue Int. 2021, 109, 77–91. [Google Scholar] [CrossRef]
- Chuang, K.-W.; Liu, Y.-C.; Balaji, R.; Chiu, Y.-C.; Yu, J.; Liao, Y.-C. Enhancing Stability of High-Concentration β-Tricalcium Phosphate Suspension for Biomedical Application. Materials 2022, 16, 228. [Google Scholar] [CrossRef]
- Puszka, A.; Sikora, J.W. New Segmented Poly(Thiourethane-Urethane)s Based on Poly(ε-Caprolactone)Diol Soft Segment: Synthesis and Characterization. Materials 2022, 15, 4940. [Google Scholar] [CrossRef]
- Gao, J.; Wu, S.; Rogers, M.A. Harnessing Hansen Solubility Parameters to Predict Organogel Formation. J. Mater. Chem. 2012, 22, 12651–12658. [Google Scholar] [CrossRef]
- Di Maro, M.; Pedraza, R.; Mosca Balma, A.; Gomez d’Ayala, G.; Poggetto, G.D.; Malucelli, G.; Roato, I.; Duraccio, D.; Mussano, F.; Faga, M.G. Influence of Dry-Mixing and Solvent Casting Blending Techniques on the Mechanical and Biological Behavior of Novel Biocompatible Poly (ε-Caprolactone)/Alumina-Toughened Zirconia Scaffolds Obtained by 3D Printing. J. Compos. Sci. 2024, 8, 194. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Hossein Siadati, M.; Fallah, A.; Zarrabi, A.; Afghah, F.; Koc, B.; Dalir Abdolahinia, E.; Omidi, Y.; Barar, J.; Akbari-Fakhrabadi, A.; et al. Effect of Zinc-Doped Hydroxyapatite/Graphene Nanocomposite on the Physicochemical Properties and Osteogenesis Differentiation of 3D-Printed Polycaprolactone Scaffolds for Bone Tissue Engineering. Chem. Eng. J. 2021, 426, 131321. [Google Scholar] [CrossRef]
- Juan, P.-K.; Fan, F.-Y.; Lin, W.-C.; Liao, P.-B.; Huang, C.-F.; Shen, Y.-K.; Ruslin, M.; Lee, C.-H. Bioactivity and Bone Cell Formation with Poly-ε-Caprolactone/Bioceramic 3D Porous Scaffolds. Polymers 2021, 13, 2718. [Google Scholar] [CrossRef]
- Lei, Y.; Rai, B.; Ho, K.H.; Teoh, S.H. In Vitro Degradation of Novel Bioactive Polycaprolactone—20% Tricalcium Phosphate Composite Scaffolds for Bone Engineering. Mater. Sci. Eng. C 2007, 27, 293–298. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. Regulation of Mesenchymal Stem Cell Attachment and Spreading on Hydroxyapatite by RGD Peptides and Adsorbed Serum Proteins. Biomaterials 2005, 26, 1467–1475. [Google Scholar] [CrossRef]
- Dos Santos, E.A.; Farina, M.; Soares, G.A.; Anselme, K. Surface Energy of Hydroxyapatite and β-Tricalcium Phosphate Ceramics Driving Serum Protein Adsorption and Osteoblast Adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2307–2316. [Google Scholar] [CrossRef]
- Erisken, C.; Kalyon, D.M.; Wang, H. Functionally Graded Electrospun Polycaprolactone and β-Tricalcium Phosphate Nanocomposites for Tissue Engineering Applications. Biomaterials 2008, 29, 4065–4073. [Google Scholar] [CrossRef]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive Development of the Rat Osteoblast Phenotype in Vitro: Reciprocal Relationships in Expression of Genes Associated with Osteoblast Proliferation and Differentiation during Formation of the Bone Extracellular Matrix. J. Cell. Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.A.; Kang, Y.G.; Shin, J.W.; Park, Y.S.; Gu, S.R.; Wu, Y.R.; Wei, J.; Shin, J.-W. PCL/β-TCP Composite Scaffolds Exhibit Positive Osteogenic Differentiation with Mechanical Stimulation. Tissue Eng. Regen. Med. 2017, 14, 349–358. [Google Scholar] [CrossRef] [PubMed]


| Sample | PCL [g] | β-TCP [g] | NaHCO3 [g] |
|---|---|---|---|
| PCL | 5 | / | / |
| PCL/β-TCP 80/20 | 4 | 1 | / |
| PCL/β-TCP/NaHCO3 80/18/2 | 4 | 0.9 | 0.1 |
| PCL/β-TCP 60/40 | 3 | 2 | / |
| PCL/β-TCP/NaHCO3 60/36/4 | 3 | 1.8 | 0.2 |
| Liquid | γ [mN/m] | γP [mN/m] | γD [mN/m] |
|---|---|---|---|
| Water | 72.8 | 43.7 | 29.1 |
| Diiodomethane | 50 | 2.6 | 47.4 |
| Sample | Surface Energy: Total [mN/m] | Surface Energy: Polar [mN/m] | Surface Energy: Dispersive [mN/m] |
|---|---|---|---|
| PCL | 42.97 | 2.14 | 40.83 |
| PCL/β-TCP 80/20 | 38.55 | 2.12 | 36.43 |
| PCL/β-TCP/NaHCO3 80/18/2 | 42.10 | 3.27 | 38.84 |
| PCL/β-TCP 60/40 | 45.11 | 0.92 | 44.19 |
| PCL/β-TCP/NaHCO3 60/36/4 | 47.52 | 3.72 | 43.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca Balma, A.; Pedraza, R.; Orrico, C.; Meinardi, S.; Genova, T.; Gautier di Confiengo, G.; Faga, M.G.; Roato, I.; Mussano, F. Poly(ε-Caprolactone)/Sodium Bicarbonate/β-Tricalcium Phosphate Composites: Surface Characterization and Early Biological Response. Materials 2025, 18, 2600. https://doi.org/10.3390/ma18112600
Mosca Balma A, Pedraza R, Orrico C, Meinardi S, Genova T, Gautier di Confiengo G, Faga MG, Roato I, Mussano F. Poly(ε-Caprolactone)/Sodium Bicarbonate/β-Tricalcium Phosphate Composites: Surface Characterization and Early Biological Response. Materials. 2025; 18(11):2600. https://doi.org/10.3390/ma18112600
Chicago/Turabian StyleMosca Balma, Alessandro, Riccardo Pedraza, Clarissa Orrico, Sara Meinardi, Tullio Genova, Giovanna Gautier di Confiengo, Maria Giulia Faga, Ilaria Roato, and Federico Mussano. 2025. "Poly(ε-Caprolactone)/Sodium Bicarbonate/β-Tricalcium Phosphate Composites: Surface Characterization and Early Biological Response" Materials 18, no. 11: 2600. https://doi.org/10.3390/ma18112600
APA StyleMosca Balma, A., Pedraza, R., Orrico, C., Meinardi, S., Genova, T., Gautier di Confiengo, G., Faga, M. G., Roato, I., & Mussano, F. (2025). Poly(ε-Caprolactone)/Sodium Bicarbonate/β-Tricalcium Phosphate Composites: Surface Characterization and Early Biological Response. Materials, 18(11), 2600. https://doi.org/10.3390/ma18112600














