One-Step Synthesis of Nitrogen-Doped TiO2 Heterojunctions and Their Visible Light Catalytic Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of N-TiO2
2.3. Sample Characterization
2.4. Photocatalytic Degradation of TC
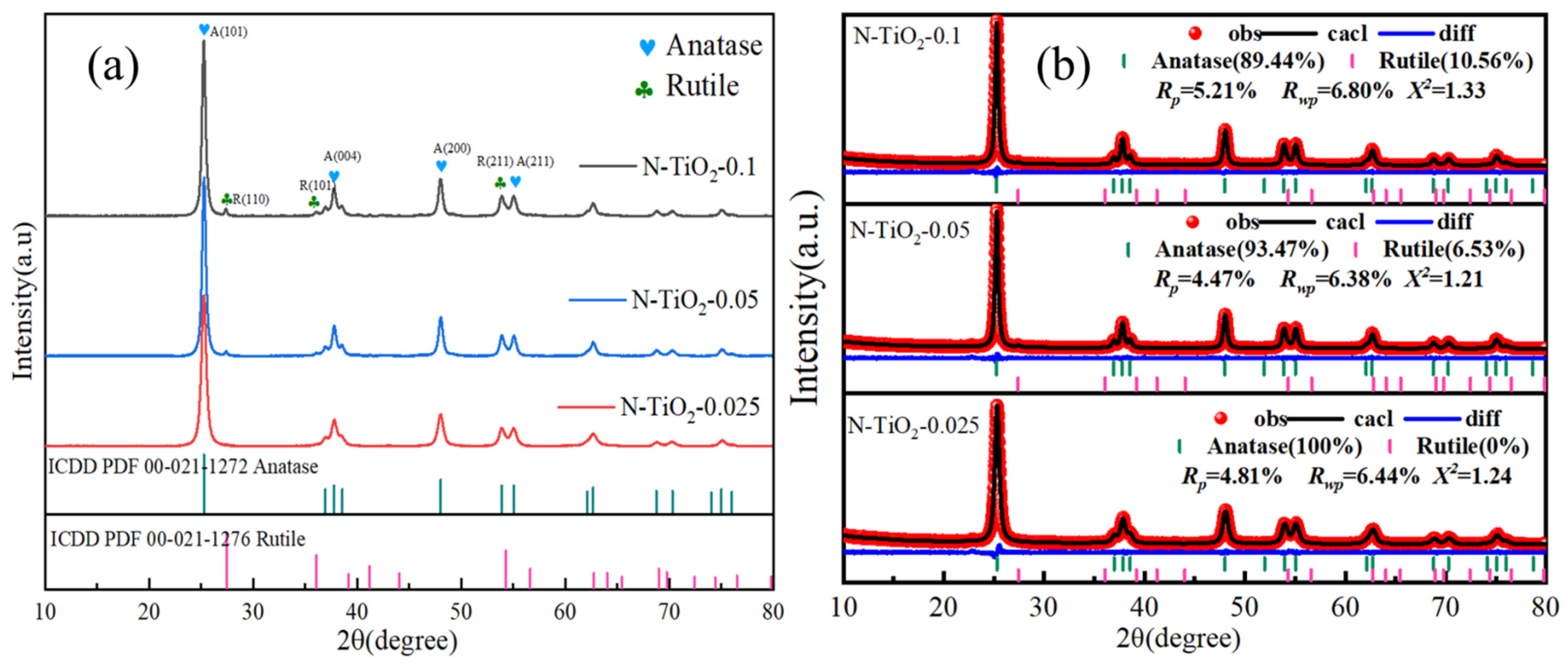
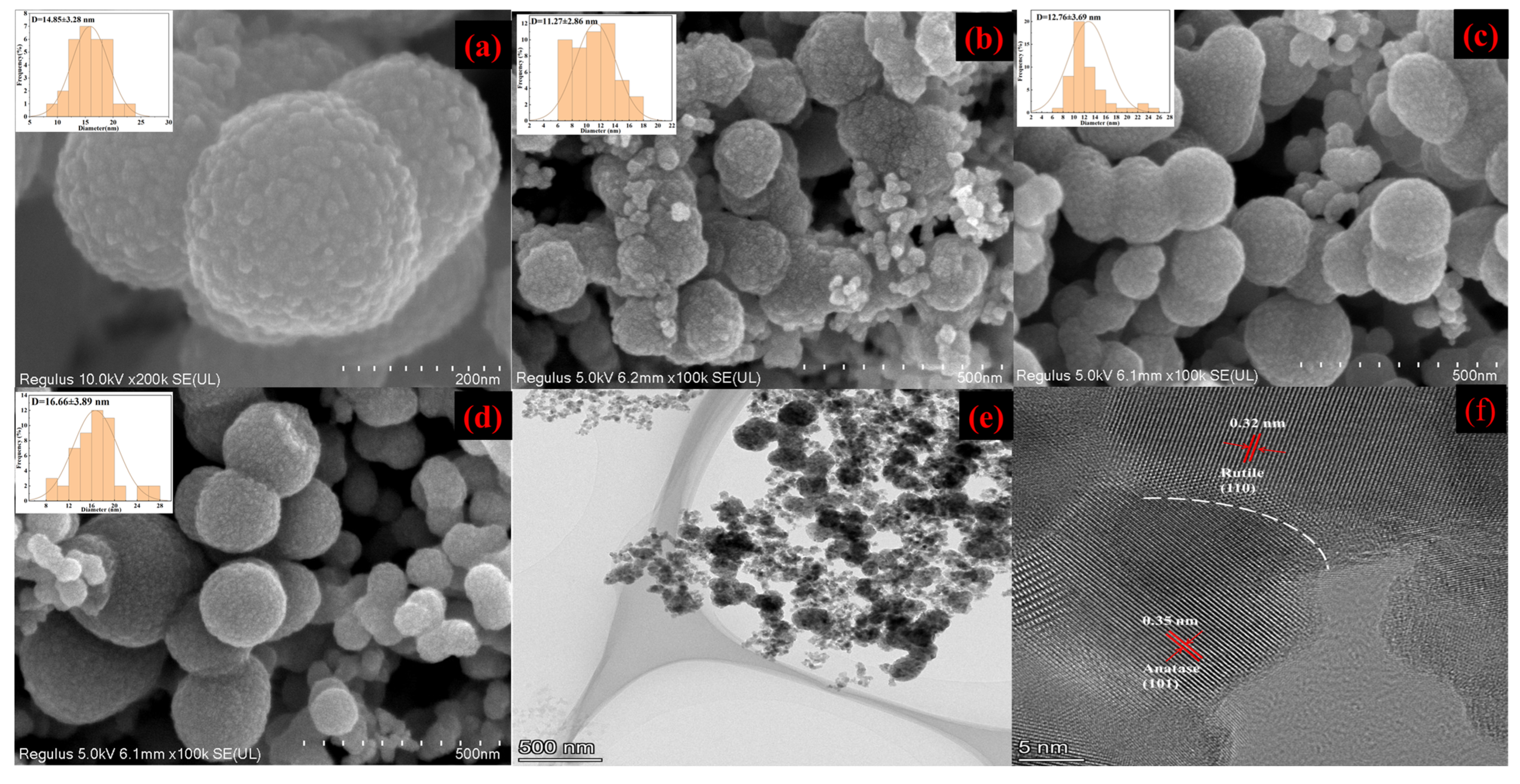
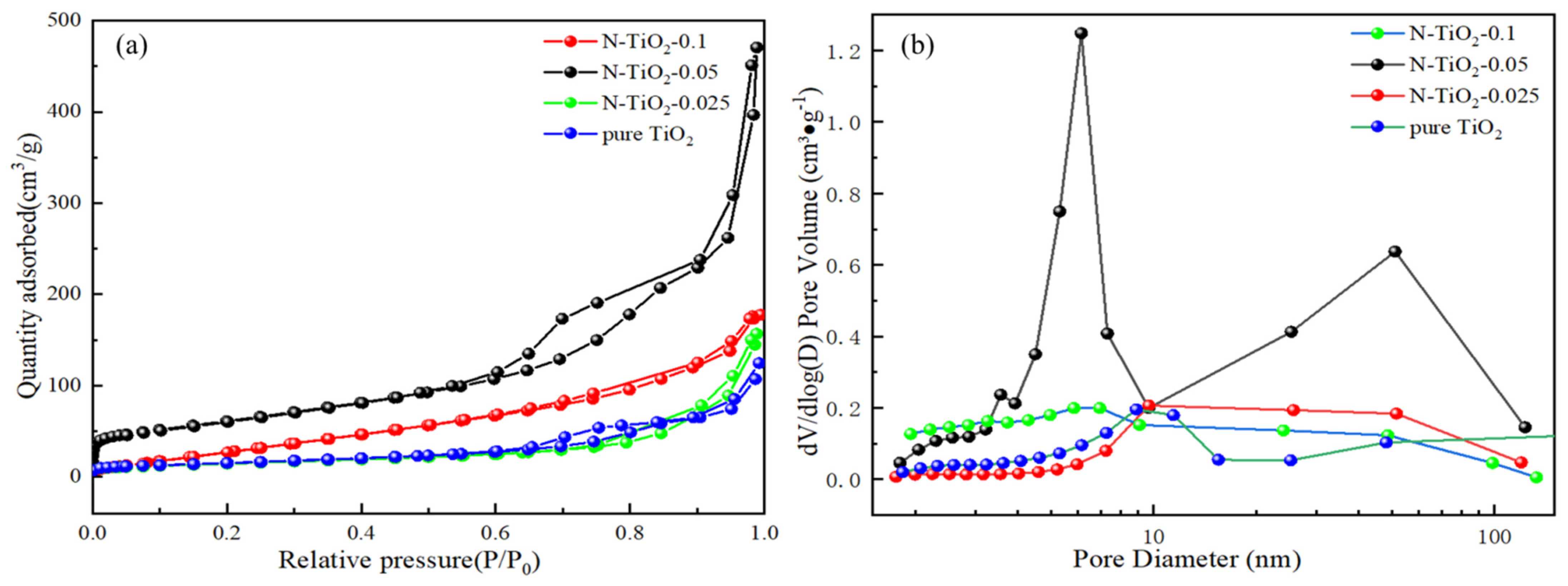
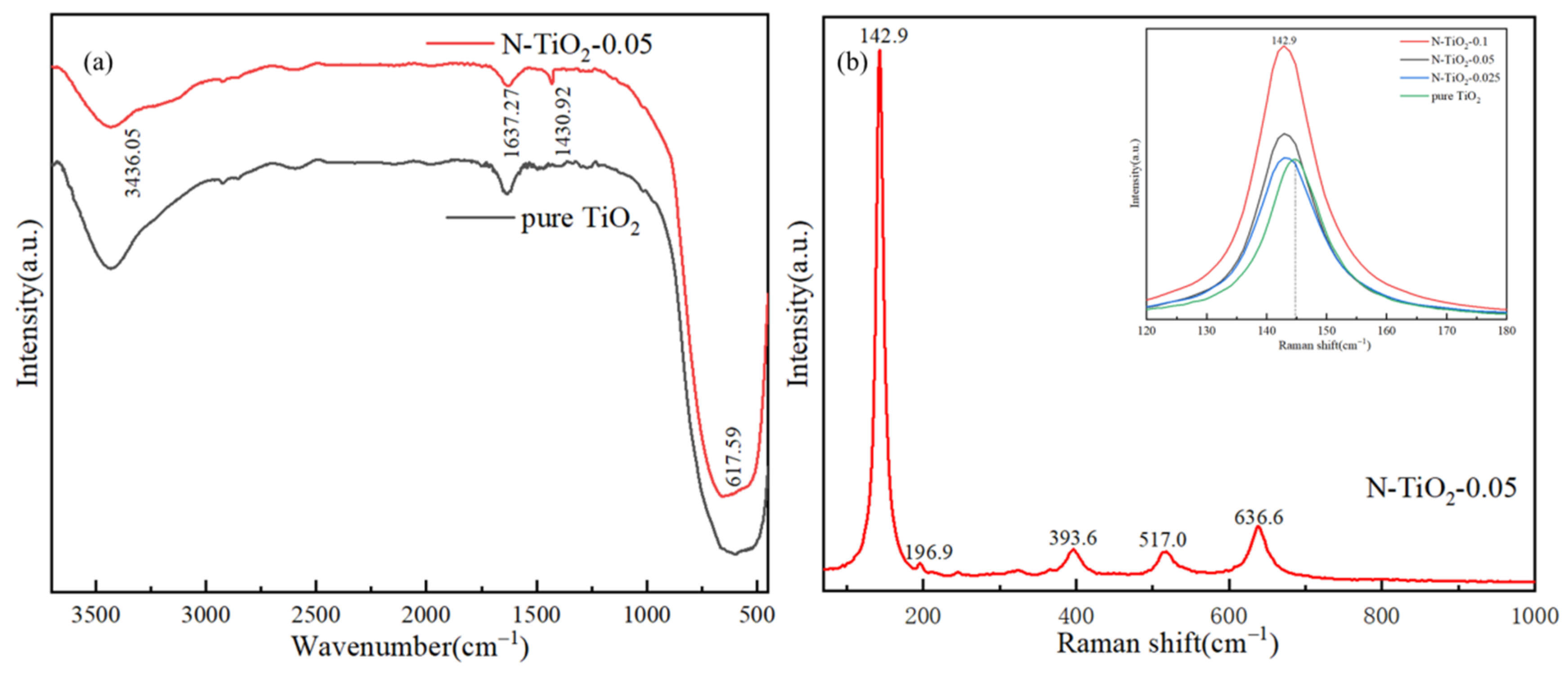
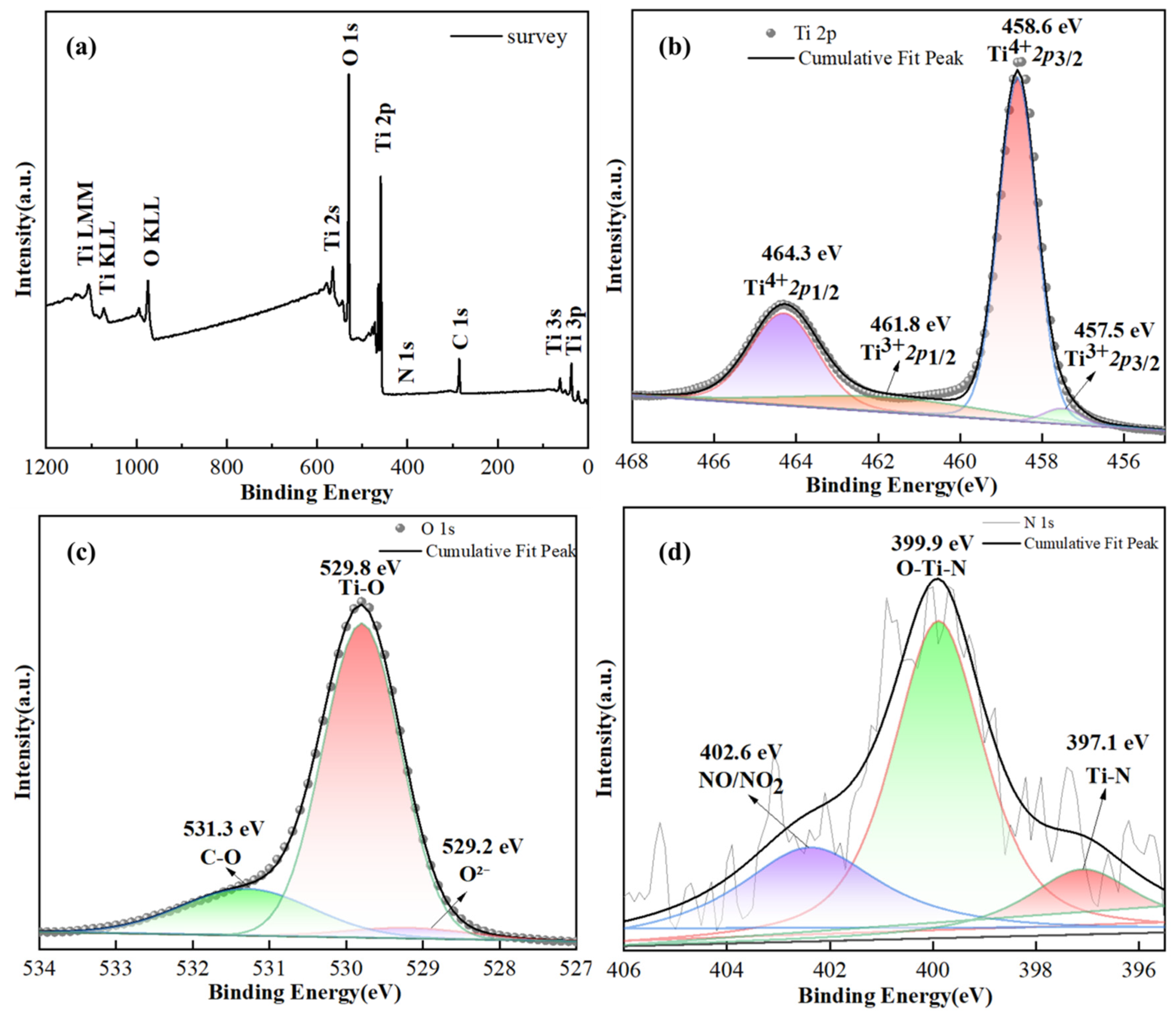
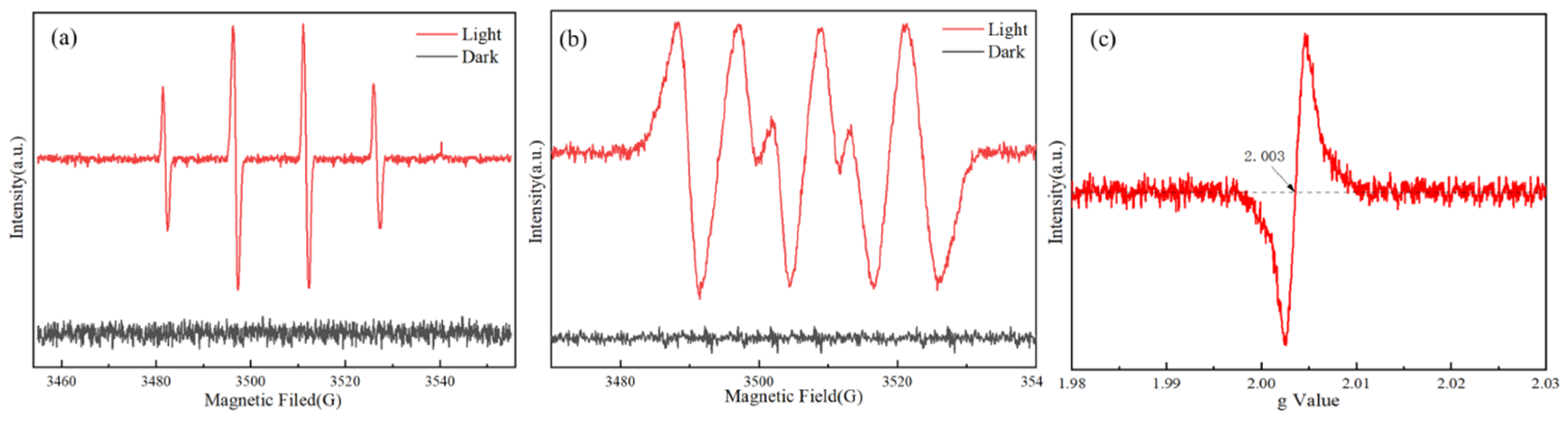
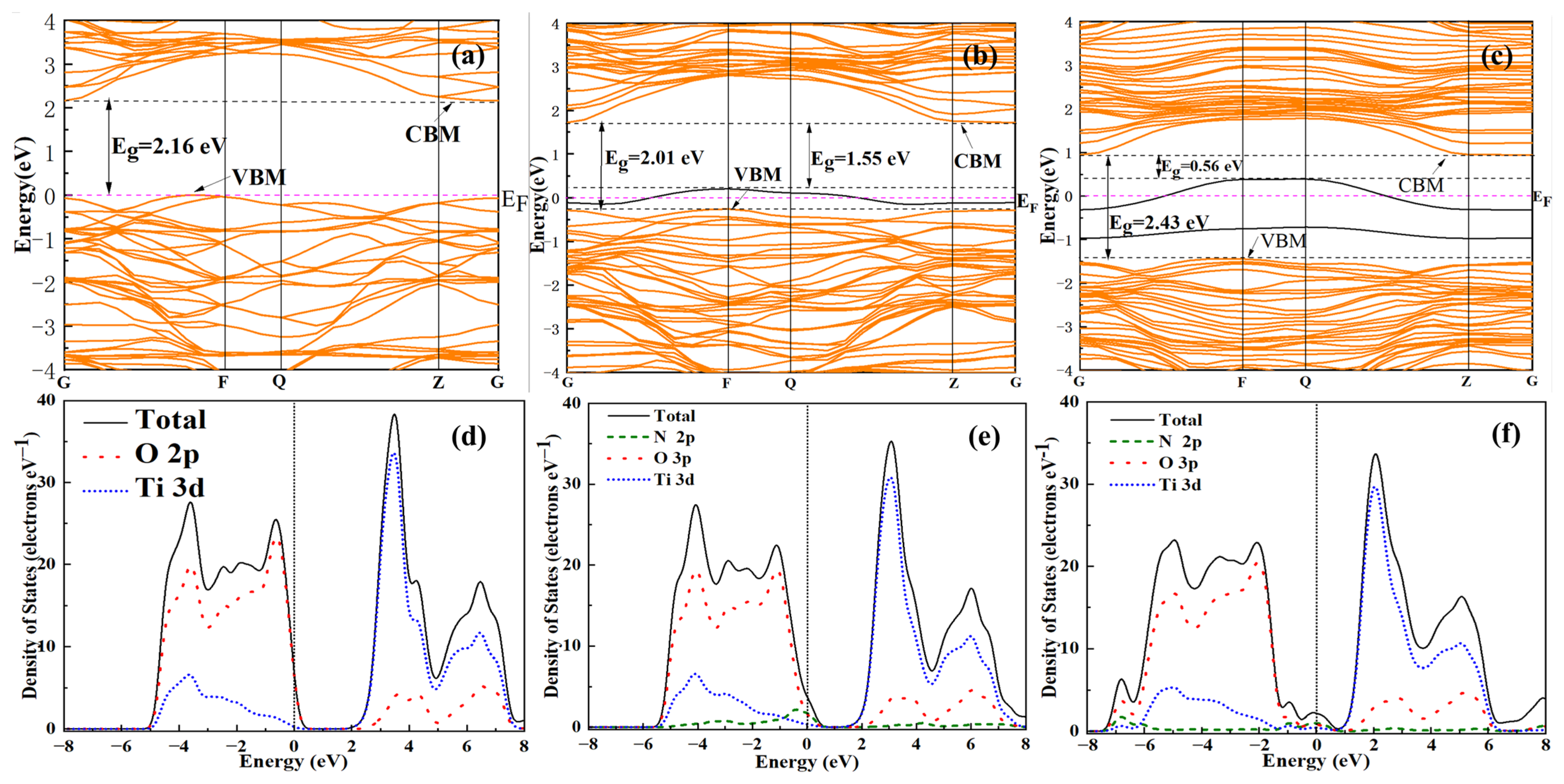
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Lian, J.; Zhang, F. Visible light-responsive N-doped TiO2 photocatalysis: Synthesis, characterizations, and applications. Trans. Tianjin Univ. 2021, 28, 33–52. [Google Scholar] [CrossRef]
- Jalloul, G.; Hijazi, N.; Boyadjian, C.; Awala, H.; Albadarin, A.B.; Ahmad, M.N. Titania-zeolite composite for tetracycline photocatalytic degradation under visible light: A comparison between doping and ion exchange. Heliyon 2024, 10, 31854. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Li, Y.; Sun, X.; Xu, L.; Huang, W. Facile defect construction of TiO2 nanotube for excellent photocatalytic degradation of tetracycline under visible light. J. Photochem. Photobiol. A Chem. 2023, 437, 114475. [Google Scholar] [CrossRef]
- Karaca, M.; Eroğlu, Z.; Açışlı, Ö.; Metin, Ö.; Karaca, S. Boosting tetracycline degradation with an s-scheme heterojunction of N-doped carbon quantum dots-decorated TiO2. ACS Omega 2023, 8, 26597–26609. [Google Scholar] [CrossRef]
- Pang, D.; Liu, Y.; Song, H.; Chen, D.; Zhu, W.; Liu, R.; Yang, H.; Li, A.; Zhang, S. Trace Ti3+ and N-codoped TiO2 nanotube array anode for significantly enhanced electrocatalytic degradation of tetracycline and metronidazole. Chem. Eng. J. 2021, 405, 126982. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen doped titanium dioxide (N-TiO2): Synopsis of synthesis methodologies, doping mechanisms, property evaluation and visible light photocatalytic applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N-and S-doped TiO2 photocatalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Gao, R.; Zhao, Q.; Yang, Z.; Gao, X.; Wang, Z.; Zhang, F.; Wu, W. Enhanced visible light photoelectrocatalytic degradation of tetracycline hydrochloride by I and P co-doped TiO2 photoelectrode. J. Hazard. Mater. 2021, 406, 124309. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, X.; Sun, P.; Lin, S.; Cao, W.; Li, Z.; Liu, W. Photocatalytic degradation of norfloxacin using N-doped TiO2: Optimization, mechanism, identification of intermediates and toxicity evaluation. Chemosphere 2019, 237, 124433. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, X.; Wang, Y. Photocatalytic degradation of flumequine by N-doped TiO2 catalysts under simulated sunlight. Environ. Eng. Res. 2020, 26, 200524. [Google Scholar] [CrossRef]
- Jiang, A.; Chen, X.; Xu, Y.; Shah, K.J.; You, Z. One-step hydrothermal generation of oxygen-deficient N-doped blue TiO2–Ti3C2 for degradation of pollutants and antibacterial properties. Environ. Res. 2023, 235, 116657. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Ganguli, S.; Sabur, A. Nitrogen doped titanium dioxide (N-TiO2): Electronic band structure, visible light harvesting and photocatalytic applications. J. Water Process Eng. 2023, 55, 104183. [Google Scholar] [CrossRef]
- Tzeng, J.-H.; Weng, C.-H.; Yen, L.-T.; Gaybullaev, G.; Chang, C.-J.; de Luna, M.D.G.; Lin, Y.-T. Inactivation of pathogens by visible light photocatalysis with nitrogen-doped TiO2 and tourmaline-nitrogen co-doped TiO2. Sep. Purif. Technol. 2021, 274, 118979. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, X.; Liu, Y.; Zeng, Y.; Wang, T.; Yu, H. One-pot synthesis of C-modified and N-doped TiO2 for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2022, 902, 163677. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Soleimani, M.; Ghasemi, J.B.; Badiei, A. Black titania; novel researches in synthesis and applications. Inorg. Chem. Commun. 2022, 135, 109092. [Google Scholar] [CrossRef]
- Ren, R.; Wen, Z.; Cui, S.; Hou, Y.; Guo, X.; Chen, J. Controllable synthesis and tunable photocatalytic properties of Ti3+-doped TiO2. Sci. Rep. 2015, 5, 10714. [Google Scholar] [CrossRef]
- Lian, P.; Qin, A.; Liu, Z.; Ma, H.; Liao, L.; Zhang, K.; Li, N. Facile synthesis to porous TiO2 nanostructures at low temperature for efficient visible-light degradation of tetracycline. Nanomaterials 2024, 14, 943. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A. Synthesis, characterisation, and applications of TiO and other black titania nanostructures species (Review). Crystals 2024, 14, 647. [Google Scholar] [CrossRef]
- Mirzaei, A.; Eddah, M.; Roualdes, S.; Ma, D.; Chaker, M. Multiple-homojunction gradient nitrogen doped TiO2 for photocatalytic degradation of sulfamethoxazole, degradation mechanism, and toxicity assessment. Chem. Eng. J. 2021, 422, 130507. [Google Scholar] [CrossRef]
- Wafi, A.; Roza, L.; Timuda, G.E.; Aji, D.; Khaerudini, D.S.; Darsono, N.; Yudasari, N.; Szabó-Bárdos, E.; Horváth, O.; Khan, M.M. N-doped TiO2 for photocatalytic degradation of colorless and colored organic pollutants under visible light irradiation. Transit. Met. Chem. 2024, 49, 305–317. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Song, H.; Guo, Y.; Hu, S.; Zheng, H.; Zhang, S.; Li, X.; Gao, Q.; Li, C.; et al. Photocatalytic hydrogen under visible light by nitrogen-doped rutile titania graphitic carbon nitride composites: An experimental and theoretical study. Adv. Compos. Hybrid Mater. 2023, 6, 83. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Shah, N.S.; Khan, S.; Khan, A.A.; Sultan, M.; Tighezza, A.M.; Iqbal, J.; Boczkaj, G. Synthesis of N-doped TiO2 nanoparticles with enhanced photocatalytic activity for 2,4-dichlorophenol degradation and H2 production. J. Environ. Chem. Eng. 2023, 11, 111308. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Dai, X.; Zhang, J.; Li, J.; Ma, Y.; Han, Q.; Dong, Y. Research on the adsorption-photocatalytic synergistic degradation of tetracycline by Au nanoparticles/TiO2 nanorods/biochar. J. Alloys Compd. 2024, 976, 172985. [Google Scholar] [CrossRef]
- Divakaran, K.; Baishnisha, A.; Balakumar, V.; Perumal, K.N.; Meenakshi, C.; Kannan, R.S. Photocatalytic degradation of tetracycline under visible light using TiO2@sulfur doped carbon nitride nanocomposite synthesized via in-situ method. J. Environ. Chem. Eng. 2021, 9, 105560. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, C.; Duan, Y.; Wang, C.; Zhao, Z.; Wang, H.; Gao, Y. Phosphorus-doped TiO2 for visible light-driven oxidative coupling of benzyl amines and photodegradation of phenol. Appl. Surf. Sci. 2020, 527, 146693. [Google Scholar] [CrossRef]
- Sun, S.; Sun, M.; Ming, W.; Ma, Y.; Li, H.; Xu, G. Amorphous titanium dioxide with synergistic effect of nitrogen doping and oxygen vacancies by photoexcited sol-gel preparation for enhanced photodegradation of tetracycline. J. Photochem. Photobiol. A Chem. 2025, 461, 116165. [Google Scholar] [CrossRef]
- El-Rahman, A.M.A.; Rabia, M.; Mohamed, S.H. Nitrogen doped TiO2 films for hydrogen generation and optoelectronic applications. J. Mater. Sci. Mater. Electron. 2023, 34, 1149. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; Kr, S.D. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 10063. [Google Scholar] [CrossRef]
- Huang, J.; Meng, A.; Zhang, Z.; Xiao, S.; Guo, X.; Wu, X.; Huang, S.; Ma, G.; Han, P.; He, B. Enhanced visible-light-driven photoelectrochemical activity in nitrogen-doped TiO2/boron-doped diamond heterojunction electrodes. ACS Appl. Energy Mater. 2022, 5, 7144–7156. [Google Scholar] [CrossRef]
- Kim, N.; Raj, M.R.; Lee, G. Nitrogen-doped TiO2(B) nanobelts enabling enhancement of electronic conductivity and efficiency of lithium-ion storage. Nanotechnology 2020, 31, 415401. [Google Scholar] [CrossRef]
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple preparation of nitrogen-doped TiO2 and its performance in selective oxidation of benzyl alcohol and benzylamine under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125743. [Google Scholar] [CrossRef]
- Assayehegn, E.; Solaiappan, A.; Chebude, Y.; Alemayehu, E. Fabrication of tunable anatase/rutile heterojunction N/TiO2 nanophotocatalyst for enhanced visible light degradation activity. Appl. Surf. Sci. 2020, 515, 145966. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S. Shirsat, Influence of N sources on the photocatalytic activity of N-doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651. [Google Scholar] [CrossRef]
- Calisir, M.D.; Gungor, M.; Demir, A.; Kilic, A.; Khan, M.M. Nitrogen-doped TiO2 fibers for visible-light-induced photocatalytic activities. Ceram. Int. 2020, 46, 16743–16753. [Google Scholar] [CrossRef]
- Mehdizadeh, P.; Tavangar, Z.; Shabani, N.; Hamadanian, M. Visible light activity of nitrogen-doped TiO2 by sol-gel method using various nitrogen sources. J. Nanostruct. 2020, 10, 307–316. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. Energy 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Lim, C.; An, H.-R.; Ha, S.; Myeong, S.; Min, C.G.; Chung, H.-J.; Son, B.; Kim, C.-Y.; Park, J.-I.; Kim, H.; et al. Highly visible-light-responsive nanoporous nitrogen-doped TiO2 (N-TiO2) photocatalysts produced by underwater plasma technology for environmental and biomedical applications. Appl. Surf. Sci. 2023, 638, 158123. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Di Camillo, D.; Ruggieri, F.; Santucci, S.; Lozzi, L. N-Doped TiO2 nanofibers deposited by electrospinning. J. Phys. Chem. C 2012, 116, 18427–18431. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. Photoelectron spectroscopic investigation of nitrogen-doped titania nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Tao, F.-T.; Hu, C.; Wu, J.C.S.; Nguyen, V.-H.; Tung, K.-L. Influence of nitrogen sources on N-doped reduced TiO2 prepared using atmospheric plasma spraying for photocatalytic tetracycline and ciprofloxacin degradation. Sep. Purif. Technol. 2023, 326, 124784. [Google Scholar] [CrossRef]
- Ouyang, H.; Huang, H.; Wang, H.; Zheng, X. The morphology evolution of nitrogen-doped carbon quantum dots/hollow TiO2 composites and their applications in photocatalysis. J. Mater. Sci. 2019, 55, 976–989. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef]
- Cipagauta-Díaz, S.; Estrella-González, A.; Navarrete-Magaña, M.; Gómez, R. N doped -TiO2 coupled to BiVO4 with high performance in photodegradation of ofloxacin antibiotic and rhodamine B dye under visible light. Catal. Today 2022, 394, 445–457. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, T.; Liu, S.; Liu, Y.; Chen, H.; Li, Z.; Du, J.; Lei, Z.; Peng, H. N-doped magnetic three-dimensional carbon microspheres@TiO2 with a porous architecture for enhanced degradation of tetracycline and methyl orange via adsorption/photocatalysis synergy. Chem. Eng. J. 2021, 411, 128615. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Q. Mechanism of higher photocatalytic activity of anatase TiO2 doped with nitrogen under visible-light irradiation from density functional theory calculation. J. Phys. D Appl. Phys. 2008, 41, 025105. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, X.; Tian, B.; Zhou, S.; Li, Y.; Du, Z. The effect of electronegative difference on the electronic structure and visible light photocatalytic activity of N-doped anatase TiO2 by first-principles calculations. Appl. Phys. Lett. 2011, 98, 162107. [Google Scholar] [CrossRef]
- Long, R.; English, N.J. First-principles calculation of nitrogen-tungsten codoping effects on the band structure of anatase-titania. Appl. Phys. Lett. 2009, 94, 132102. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. Modified N-doped TiO2 anatase nanoparticle as an ideal O3 gas sensor: Insights from density functional theory calculations. Comput. Theor. Chem. 2016, 1095, 15–28. [Google Scholar] [CrossRef]
- Wu, H.-C.; Lin, S.-W.; Wu, J.-S. Effects of nitrogen concentration on N-doped anatase TiO2: Density functional theory and hubbard U analysis. J. Alloy. Compds. 2012, 522, 46–50. [Google Scholar] [CrossRef]
- Navarra, W.; Ritacco, I.; Sacco, O.; Caporaso, L.; Camellone, M.F.; Venditto, V.; Vaiano, V. Density functional theory study and photocatalytic activity of ZnO/N-doped TiO2 heterojunctions. J. Phys. Chem. C 2022, 126, 7000–7011. [Google Scholar] [CrossRef]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; Walczak, L.; Gopinath, C.S. A Study on doped heterojunctions in TiO2 nanotubes: An efficient photocatalyst for solar water splitting. Sci. Rep. 2017, 7, 14314. [Google Scholar] [CrossRef]
- Dehkordi, A.B.; Badiei, A. Insight into the activity of TiO2@nitrogen-doped hollow carbon spheres supported on g-C3N4 for robust photocatalytic performance. Chemosphere 2022, 288, 132392. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Wang, Y. Visible active N-doped TiO2/reduced graphene oxide for the degradation of tetracycline hydrochloride. Chem. Phys. Lett. 2018, 691, 408–414. [Google Scholar] [CrossRef]
- Tang, Y.; Li, T.; Xiao, W.; Huang, Z.; Wen, H.; Situ, W.; Song, X. Degradation mechanism and pathway of tetracycline in milk by heterojunction N-TiO2-Bi2WO6 film under visible light. Food Chem. 2023, 401, 134082. [Google Scholar] [CrossRef]
- Li, S.; Jiang, C.; Zhang, Y.; Tian, J.; Yang, H.; Wang, C. Synergistic effect of N doping and oxygen vacancies over TiO2 nanosheets with enhanced photocatalytic removal of tetracycline. Catal. Today 2024, 440, 114830. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, P.; Qin, A.; Liu, Z.; Ma, H.; Liao, L.; Zhang, K.; Qin, Y. One-Step Synthesis of Nitrogen-Doped TiO2 Heterojunctions and Their Visible Light Catalytic Applications. Materials 2025, 18, 2400. https://doi.org/10.3390/ma18102400
Lian P, Qin A, Liu Z, Ma H, Liao L, Zhang K, Qin Y. One-Step Synthesis of Nitrogen-Doped TiO2 Heterojunctions and Their Visible Light Catalytic Applications. Materials. 2025; 18(10):2400. https://doi.org/10.3390/ma18102400
Chicago/Turabian StyleLian, Peng, Aimiao Qin, Zhisen Liu, Hao Ma, Lei Liao, Kaiyou Zhang, and Yingxi Qin. 2025. "One-Step Synthesis of Nitrogen-Doped TiO2 Heterojunctions and Their Visible Light Catalytic Applications" Materials 18, no. 10: 2400. https://doi.org/10.3390/ma18102400
APA StyleLian, P., Qin, A., Liu, Z., Ma, H., Liao, L., Zhang, K., & Qin, Y. (2025). One-Step Synthesis of Nitrogen-Doped TiO2 Heterojunctions and Their Visible Light Catalytic Applications. Materials, 18(10), 2400. https://doi.org/10.3390/ma18102400





