Laser Nanostructuring of Titanium Surfaces for Enhanced Bioactive Applications
Abstract
1. Introduction
2. Experimental and Methods
2.1. Preparation of Nanostructured Titanium Surfaces
2.2. Bioactivity Test (Kokubo Method)
2.3. Atomic Force Microscopy (AFM)
2.4. X-Ray Photoelectron Spectroscopy (XPS)
2.5. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy (SEM–EDS)
3. Results and Discussion
3.1. Effect of Experimental Parameters on the LIPSS
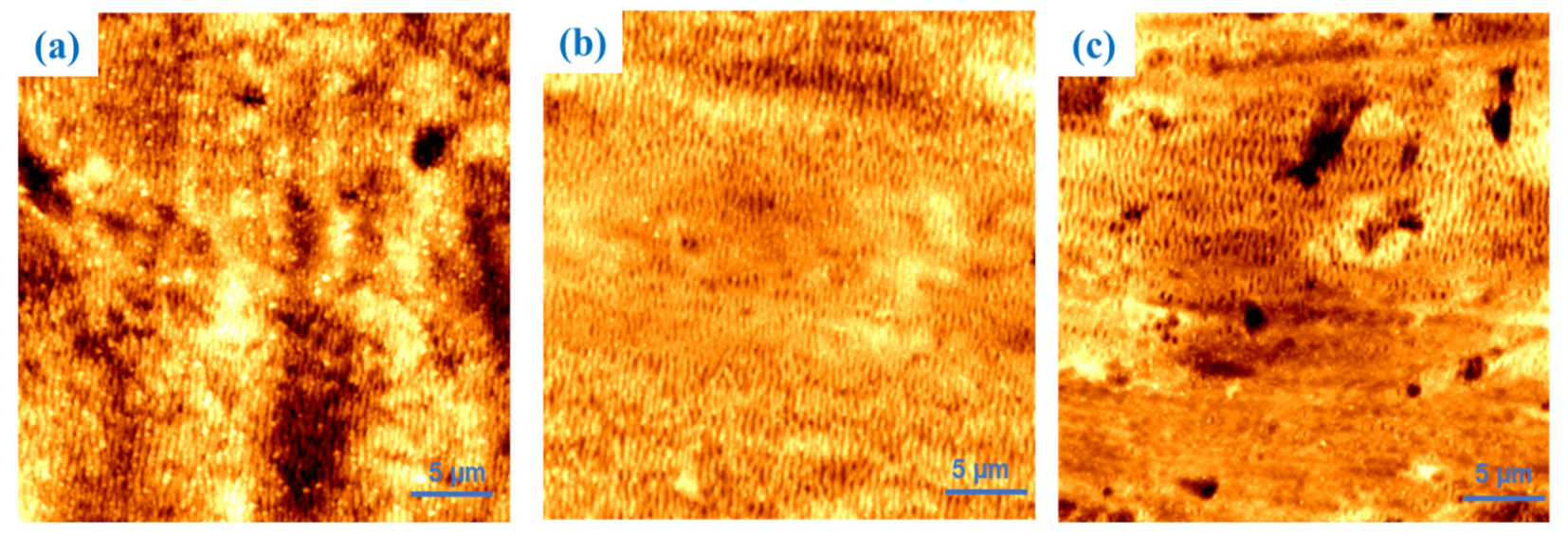
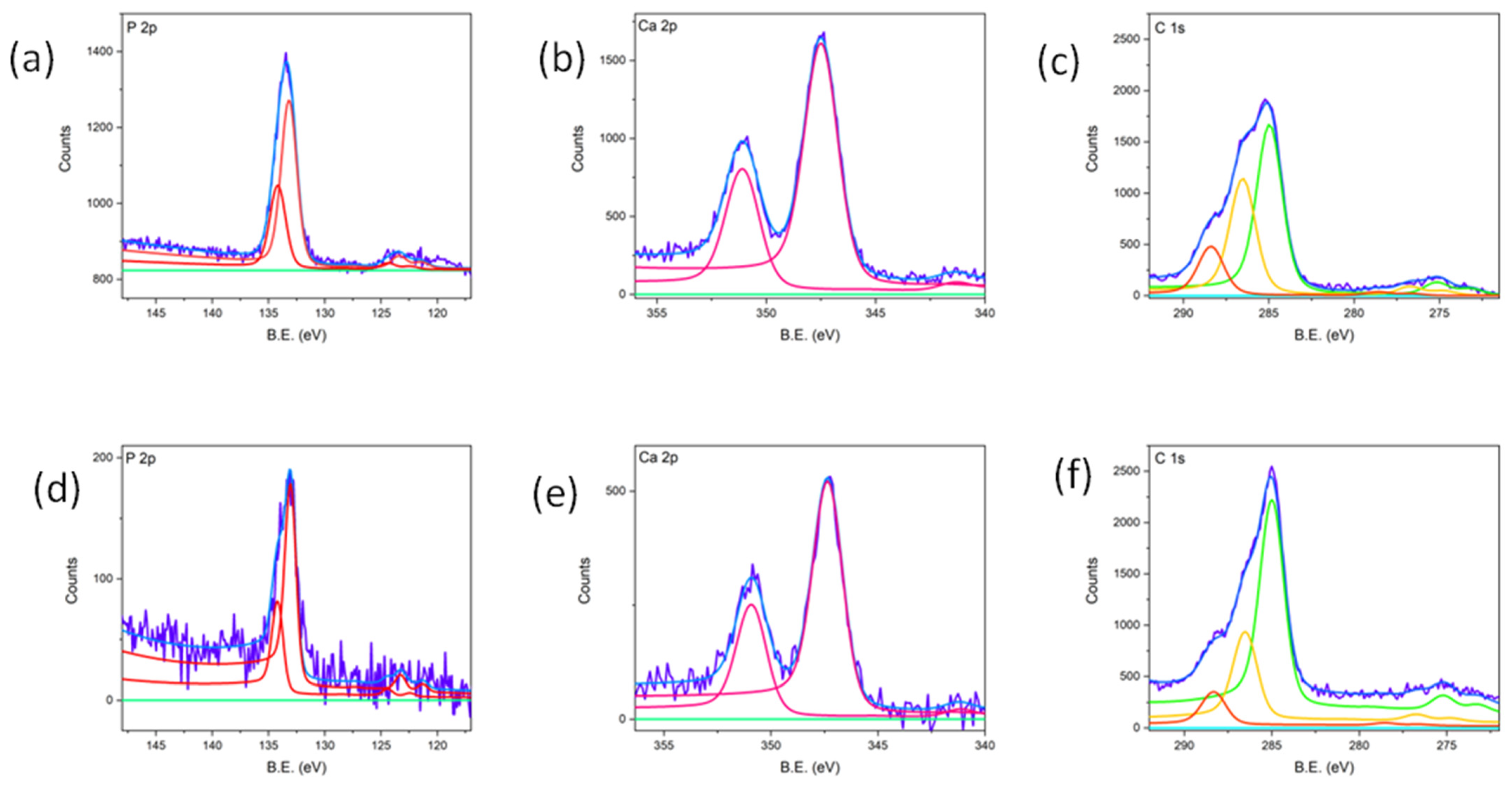
3.2. Topographic and Compositional Analysis
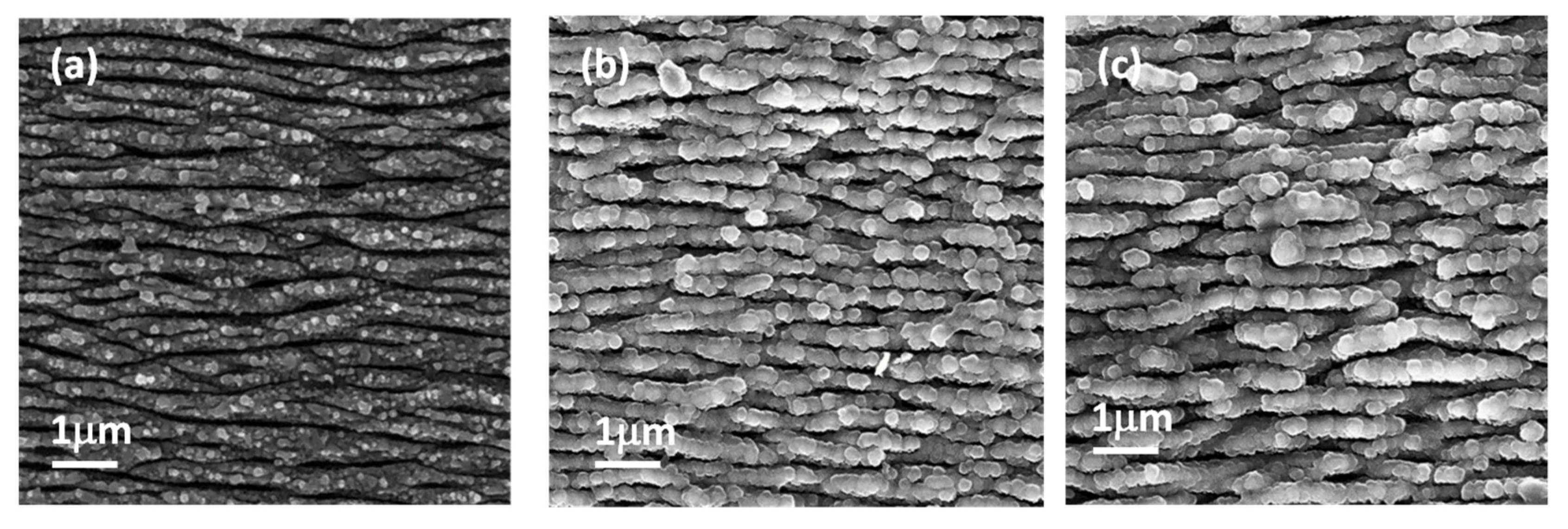
3.3. In Vitro Bioactivity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonse, J.; Krüger, J.; Höhm, S. Femtosecond Laser-Induced Periodic Surface Structures. J. Laser Appl. 2012, 24, 8. [Google Scholar] [CrossRef]
- Bonse, J.; Höhm, S.; Kirner, S.V.; Rosenfeld, A.; Krüger, J. Laser-Induced Periodic Surface Structures—A Scientific Evergreen. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 9000615. [Google Scholar] [CrossRef]
- Bonse, J.; Graef, S. Maxwell Meets Marangoni—A Review of Theories on Laser-Induced Periodic Surface Structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Emmony, D.; Howson, R.; Willis, L. Laser Mirror Damage In Germanium AT 10.6 MU. Appl. Phys. Lett. 1973, 23, 598–600. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct Femtosecond Laser Surface Nano/Microstructuring and Its Applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Bonse, J.; Rosenfeld, A.; Krüger, J. Implications of Transient Changes of Optical and Surface Properties of Solids during Femtosecond Laser Pulse Irradiation to the Formation of Laser-Induced Periodic Surface Structures. Appl. Surf. Sci. 2011, 257, 5420–5423. [Google Scholar] [CrossRef]
- Bonse, J.; Höhm, S.; Rosenfeld, A.; Krüger, J. Sub-100-Nm Laser-Induced Periodic Surface Structures upon Irradiation of Titanium by Ti:Sapphire Femtosecond Laser Pulses in Air. Appl. Phys. A 2013, 110, 547–551. [Google Scholar] [CrossRef]
- Bonse, J.; Baudach, S.; Krüger, J.; Kautek, W.; Lenzner, M. Femtosecond Laser Ablation of Silicon–Modification Thresholds and Morphology. Appl. Phys. A 2002, 74, 19–25. [Google Scholar] [CrossRef]
- Florian, C.; Déziel, J.-L.; Kirner, S.V.; Siegel, J.; Bonse, J. The Role of the Laser-Induced Oxide Layer in the Formation of Laser-Induced Periodic Surface Structures. Nanomaterials 2020, 10, 147. [Google Scholar] [CrossRef]
- Höhm, S.; Rosenfeld, A.; Krüger, J.; Bonse, J. Femtosecond Diffraction Dynamics of Laser-Induced Periodic Surface Structures on Fused Silica. Appl. Phys. Lett. 2013, 102, 054102. [Google Scholar] [CrossRef]
- Knapic, D.; Mardare, A.I.; Voss, H.; Bonse, J.; Hassel, A.W. Corrosion Study of Picosecond-Laser Structured and Anodized Ti6Al4V for Bone Screws. Phys. Status Solidi-Appl. Mater. Sci. 2024, 221, 2300609. [Google Scholar] [CrossRef]
- Mueller, K.; Mirabella, F.; Knigge, X.; Mezera, M.; Weise, M.; Sahre, M.; Wasmuth, K.; Voss, H.; Hertwig, A.; Krueger, J.; et al. Chemical and Topographical Changes upon Sub-100-Nm Laser-Induced Periodic Surface Structure Formation on Titanium Alloy: The Influence of Laser Pulse Repetition Rate and Number of Over-Scans. Phys. Status Solidi-Appl. Mater. Sci. 2024, 221, 2300719. [Google Scholar] [CrossRef]
- Schwibbert, K.; Richter, A.M.; Krueger, J.; Bonse, J. Laser-Textured Surfaces: A Way to Control Biofilm Formation? Laser Photonics Rev. 2024, 18, 4477–4483. [Google Scholar] [CrossRef]
- Santagata, A.; Pace, M.L.; Bellucci, A.; Mastellone, M.; Bolli, E.; Valentini, V.; Orlando, S.; Sani, E.; Failla, S.; Sciti, D.; et al. Enhanced and Selective Absorption of Molybdenum Nanostructured Surfaces for Concentrated Solar Energy Applications. Materials 2022, 15, 8333. [Google Scholar] [CrossRef]
- Bolli, E.; Fornari, A.; Bellucci, A.; Mastellone, M.; Valentini, V.; Mezzi, A.; Polini, R.; Santagata, A.; Trucchi, D.M. Room-Temperature O3 Detection: Zero-Bias Sensors Based on ZnO Thin Films. Crystals 2024, 14, 90. [Google Scholar] [CrossRef]
- Bolli, E.; Bellucci, A.; Mastellone, M.; Mezzi, A.; Orlando, S.; Polini, R.; Salerno, R.; Santagata, A.; Valentini, V.; Trucchi, D.M. Engineered SnO2-Based Thin Films for Efficient CO2 Gas Sensing at Room Temperature. Appl. Surf. Sci. 2025, 683, 161795. [Google Scholar] [CrossRef]
- Mastellone, M.; Pace, M.L.; Curcio, M.; Caggiano, N.; De Bonis, A.; Teghil, R.; Dolce, P.; Mollica, D.; Orlando, S.; Santagata, A.; et al. LIPSS Applied to Wide Bandgap Semiconductors and Dielectrics: Assessment and Future Perspectives. Materials 2022, 15, 1378. [Google Scholar] [CrossRef]
- Mastellone, M.; Bellucci, A.; Girolami, M.; Serpente, V.; Polini, R.; Orlando, S.; Santagata, A.; Sani, E.; Hitzel, F.; Trucchi, D.M. Deep-Subwavelength 2D Periodic Surface Nanostructures on Diamond by Double-Pulse Femtosecond Laser Irradiation. Nano Lett. 2021, 21, 4477–4483. [Google Scholar] [CrossRef]
- Mastellone, M.; Bolli, E.; Bellucci, A.; Valentini, V.; Orlando, S.; Lettino, A.; Santagata, A.; Pace, M.L.; Sani, E.; Polini, R.; et al. Enhancing Anti-Reflection Properties of Laser Nanotextured Indium Phosphide. Surf. Interfaces 2025, 60, 106056. [Google Scholar] [CrossRef]
- Mastellone, M.; Bellucci, A.; Girolami, M.; Serpente, V.; Polini, R.; Orlando, S.; Valentini, V.; Santagata, A.; Paci, B.; Generosi, A.; et al. Temperature-Dependent Electrical and Structural Characterization of Laser-Induced Graphitic Microwires in CVD Diamond. Diam. Relat. Mater. 2022, 128, 109294. [Google Scholar] [CrossRef]
- Mastellone, M.; Bolli, E.; Valentini, V.; Bellucci, A.; Orlando, S.; Santagata, A.; Polini, R.; Lettino, A.; Sani, E.; Trucchi, D.M. Two-Dimensional Periodic Surface Nanotexturing of 6H-SiC by Ultrashort Laser Pulses. Surf. Interfaces 2024, 46, 104006. [Google Scholar] [CrossRef]
- Sipe, J.E.; Young, J.F.; Preston, J.S.; van Driel, H.M. Laser-Induced Periodic Surface Structure. I. Theory. Phys. Rev. B 1983, 27, 1141–1154. [Google Scholar] [CrossRef]
- van Driel, H.M.; Sipe, J.E.; Young, J.F. Laser-Induced Periodic Surface Structure on Solids: A Universal Phenomenon. Phys. Rev. Lett. 1982, 49, 1955–1958. [Google Scholar] [CrossRef]
- Young, J.F.; Sipe, J.E.; van Driel, H.M. Laser-Induced Periodic Surface Structure. III. Fluence Regimes, the Role of Feedback, and Details of the Induced Topography in Germanium. Phys. Rev. B 1984, 30, 2001–2015. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- De Bonis, A.; Curcio, M.; Fosca, M.; Cacciotti, I.; Santagata, A.; Teghil, R.; Rau, J.V. RBP1 Bioactive Glass-Ceramic Films Obtained by Pulsed Laser Deposition. Mater. Lett. 2016, 175, 195–198. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Biocompatibility of Plasma Electrolytic Oxidation Coated Titanium Alloy for Biomedical Applications. Bionanoscience 2025, 15, 232. [Google Scholar] [CrossRef]
- Curcio, M.; Bochicchio, B.; Pepe, A.; Laezza, A.; De Stefanis, A.; Rau, J.V.; Teghil, R.; De Bonis, A. Mn-Doped Glass-Ceramic Bioactive (Mn-BG) Thin Film to Selectively Enhance the Bioactivity of Electrospun Fibrous Polymeric Scaffolds. Coatings 2022, 12, 1427. [Google Scholar] [CrossRef]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; De Bonis, A.; Boccaccini, A.R. Cu-Releasing Bioactive Glass Coatings and Their in Vitro Properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef]
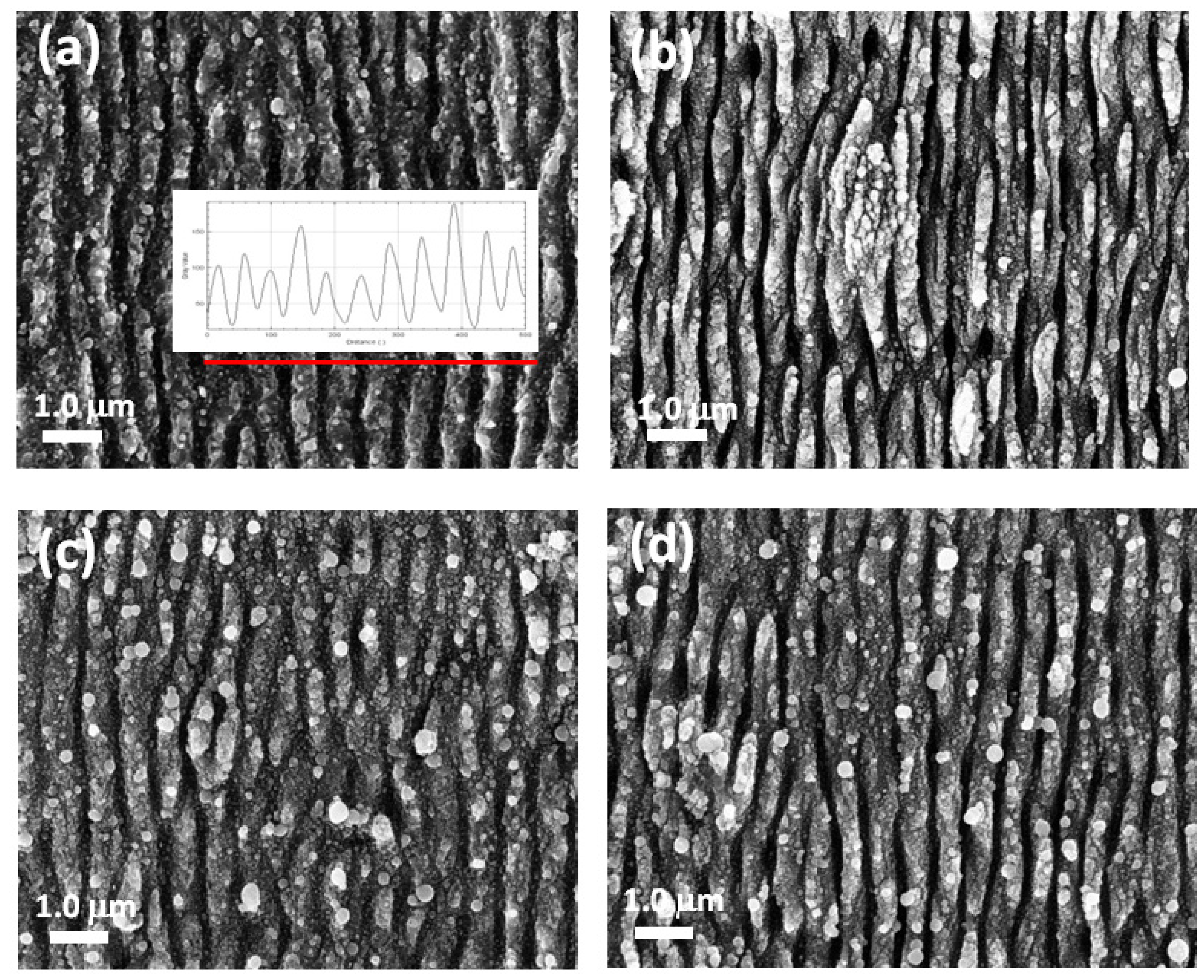
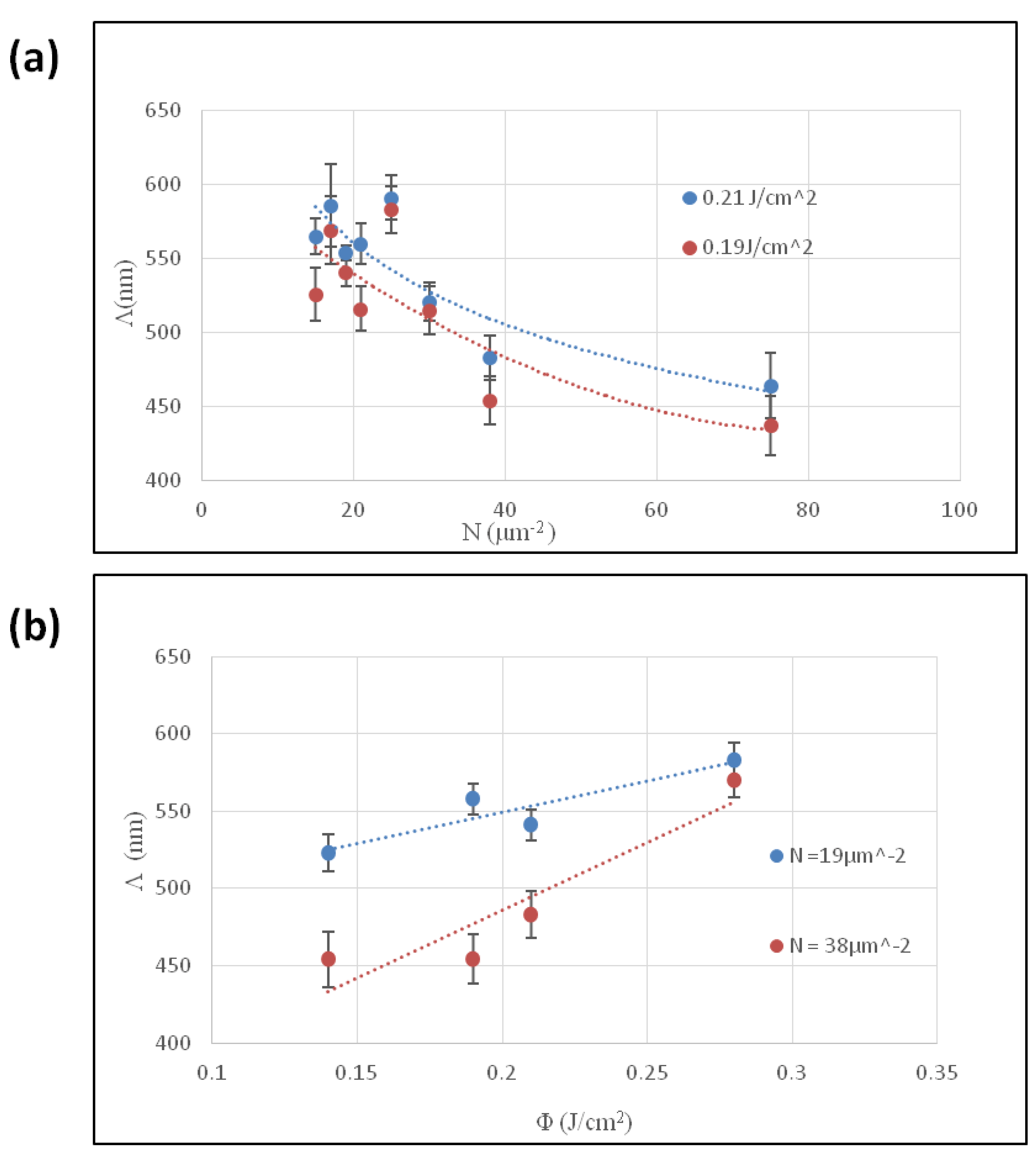


| Sample | Φ (J/cm2) | l (μm) | N(μm−2) |
|---|---|---|---|
| 1 | 0.19 | 25 | 75 |
| 2 | 0.19 | 25 | 19 |
| 3 | 0.19 | 50 | 15 |
| Sample | 30 × 30 μm | 5 × 5 μm |
|---|---|---|
| 1 | 22.1 | 47.9 |
| 2 | 25.5 | 76.0 |
| 3 | 10.4 | 47.9 |
| % | Before LIPPS | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|---|
| Ti | 19 | - | 6 | 11 |
| TiO | 5 | - | - | - |
| Ti2O3 | 18 | 2 | 10 | 12 |
| TiO2 | 58 | 98 | 84 | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonis, A.D.; Curcio, M.; Galasso, A.; Caggiano, N.; Lettino, A.; Dolce, P.; Mollica, D.; Pace, M.L.; Santagata, A. Laser Nanostructuring of Titanium Surfaces for Enhanced Bioactive Applications. Materials 2025, 18, 2362. https://doi.org/10.3390/ma18102362
Bonis AD, Curcio M, Galasso A, Caggiano N, Lettino A, Dolce P, Mollica D, Pace ML, Santagata A. Laser Nanostructuring of Titanium Surfaces for Enhanced Bioactive Applications. Materials. 2025; 18(10):2362. https://doi.org/10.3390/ma18102362
Chicago/Turabian StyleBonis, Angela De, Mariangela Curcio, Agostino Galasso, Nicola Caggiano, Antonio Lettino, Patrizia Dolce, Donato Mollica, Maria Lucia Pace, and Antonio Santagata. 2025. "Laser Nanostructuring of Titanium Surfaces for Enhanced Bioactive Applications" Materials 18, no. 10: 2362. https://doi.org/10.3390/ma18102362
APA StyleBonis, A. D., Curcio, M., Galasso, A., Caggiano, N., Lettino, A., Dolce, P., Mollica, D., Pace, M. L., & Santagata, A. (2025). Laser Nanostructuring of Titanium Surfaces for Enhanced Bioactive Applications. Materials, 18(10), 2362. https://doi.org/10.3390/ma18102362









