3.2. Moulding Binders
Research was carried out on the selection of the optimal binder for the production of a ceramic mould for four types of binders used to produce sets of ceramic moulds. The first set, currently used, consists of a binder for the first layers, Ludox SK (contains colloidal silica), and a binder for the construction layers, hydrolysed ethyl silicate (HES). The set developed for new water-soluble binders, eliminating the HES binder from mass production, consists of a binder for the first layer, Keysol from Ransom and Randolph (Maumee, OH, USA), and a binder for construction layers, Matrixsol from Ransom and Randolph (Maumee, OH, USA).
Keysol is a two-component water-soluble binder, a concentrate based on an acrylic-vinyl copolymer with a weight content of 20–40% and the main aqueous solution containing SiO2 nanoparticles. Matrixsol is a two-component water-soluble binder, a concentrate of 2,2′,2″-(hexahydro-1,3,5-triazin-1,3,5-triyl)triethanol with a content of 70–100% by weight and a main aqueous solution containing SiO2 nanoparticles.
The research was based on the analysis of the properties of these binders, analysis of the impact on the environment (two water-based binders (K + M) including Keysol for the primary layer and Matrixsol for the backup layers), and determining whether they can replace existing standard binders based on ethyl alcohol. The main criterion for selecting these types of binders was to investigate which of these binders would be able to replace HES. The use of water-based binders is an interesting issue because they are more ecological materials and definitely more healthy for people working in the foundry. The use of binders and alcohol systems is harmful to the human body, causing dryness of the skin and conjunctiva. Replacement of HES with water-based binders will improve work safety, be more environmentally beneficial, and also create an opportunity to obtain moulding systems with better physical and mechanical properties compared to alcohol-based systems.
To characterise the nanoparticles present in the tested binders, SEM tests were performed, the results of which are presented in
Figure 5. No nanoparticles were observed in the HES binder (
Figure 5A). Analysis of the remaining binders showed the presence of SiO
2 oxide particles in the dried samples. They were characterised by a shape close to spherical (
Figure 5B–D). This is an important property of colloidal silica-based binders. When the ceramic moulds are dried, the binder undergoes the sol → gel transformation by the evaporation of water. As a result of the reaction between hydroxyl groups on the silica surface, SiO
2 oxide combines into three-dimensional structures (agglomerates) through –Si–O–Si– bonds. The reaction that takes place is irreversible. Thanks to this process, the ceramic mould has good strength properties.
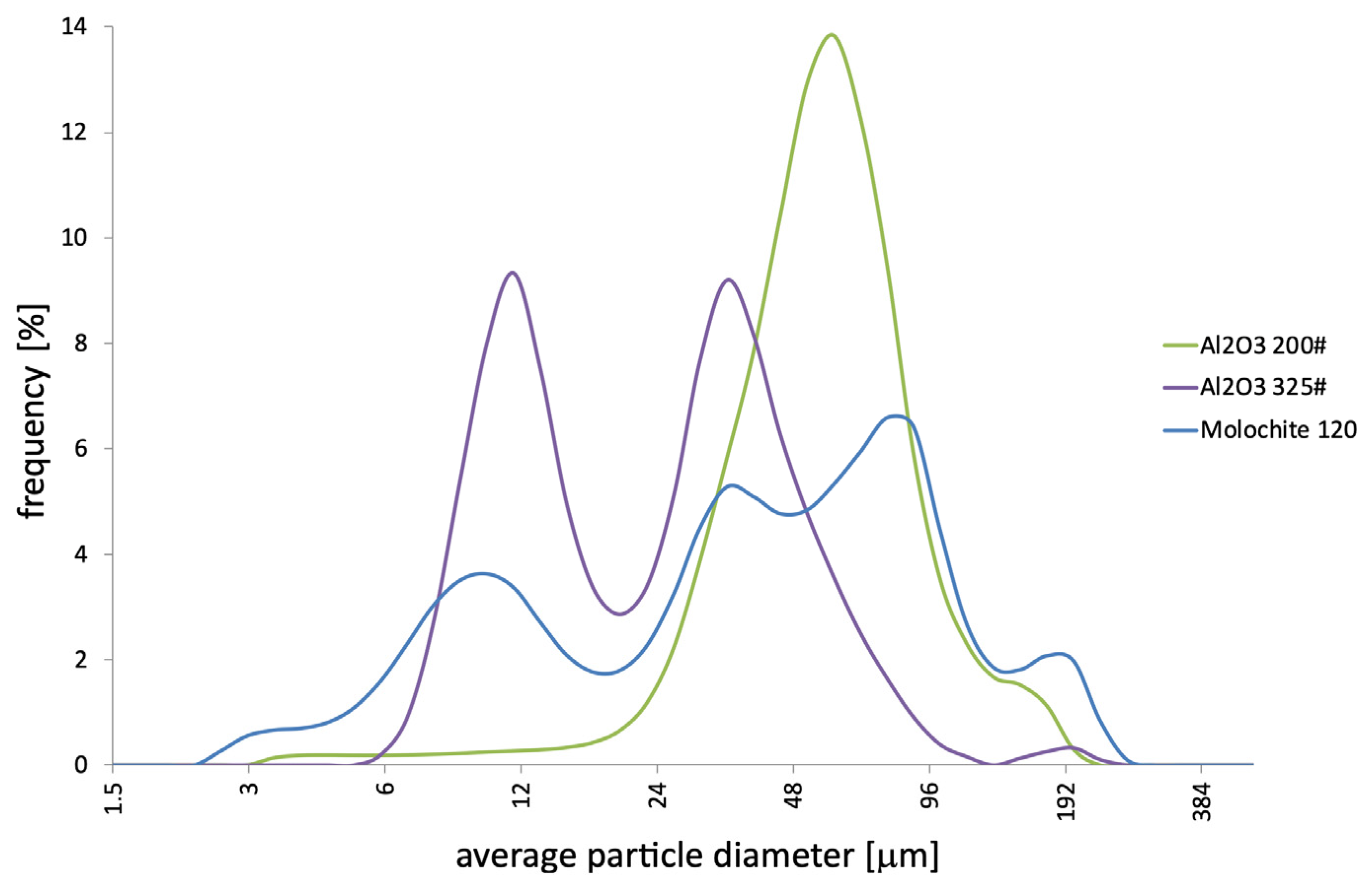
Measurements of the average particle diameter of the tested moulding binders were performed (
Figure 6). Because of the inability to determine the presence of nanoparticles in the HES binder, no measurement was performed for it. It was found that the nanopowders are characterised by a unimodal distribution of average diameter. The small difference between the minimum and maximum diameters indicates the high uniformity of the SiO
2 nanoparticles. Based on the analysis of microscopic examinations, it was shown that the SiO
2 nanopowder particles included in polymer binders have a spherical shape. The average diameter of all nano-SiO
2 particles does not exceed 18 nm. It should be noted that the study was conducted in an aqueous environment in which the agglomerates were dissolved. This makes it possible to determine the average diameter of the nanoparticles.
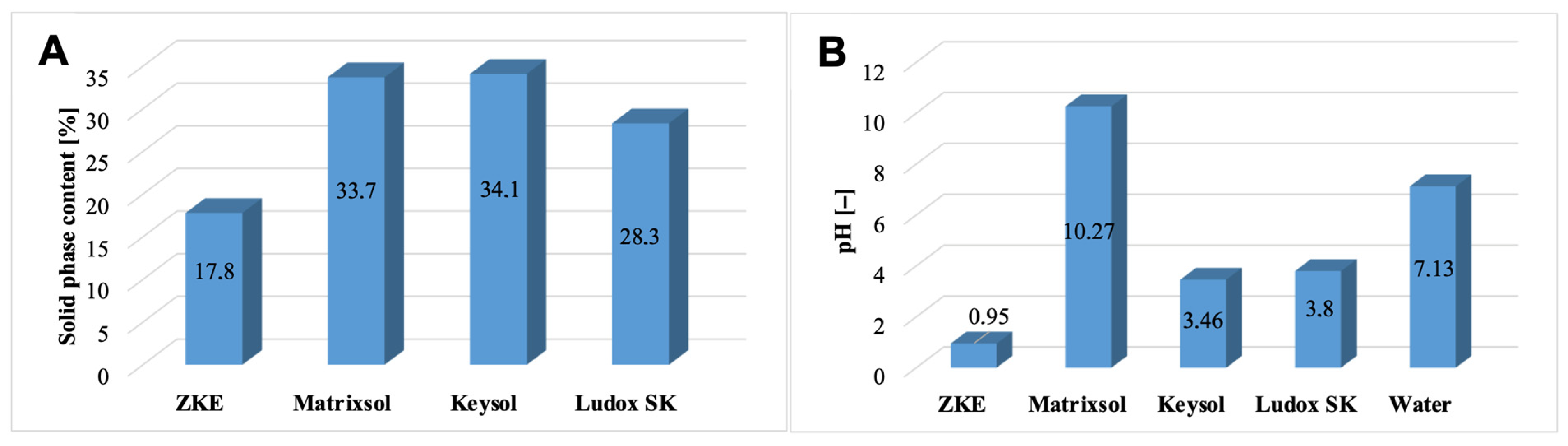
The solid-phase content in the tested moulding binders was also determined (
Figure 7A). The HES binder has the lowest solid-phase content. Water-soluble binders are characterised by a high solid-phase content in the form of colloidal silica >28% weight. It is almost twice as large compared to HES. However, Keysol binders have the highest nano-SiO
2 content, approximately 34.1% of weight, and Matrixsol, 33.7% of weight.
It was also established that the Matrixsol, Keysol, and Ludox SK binders differ significantly in terms of pH (
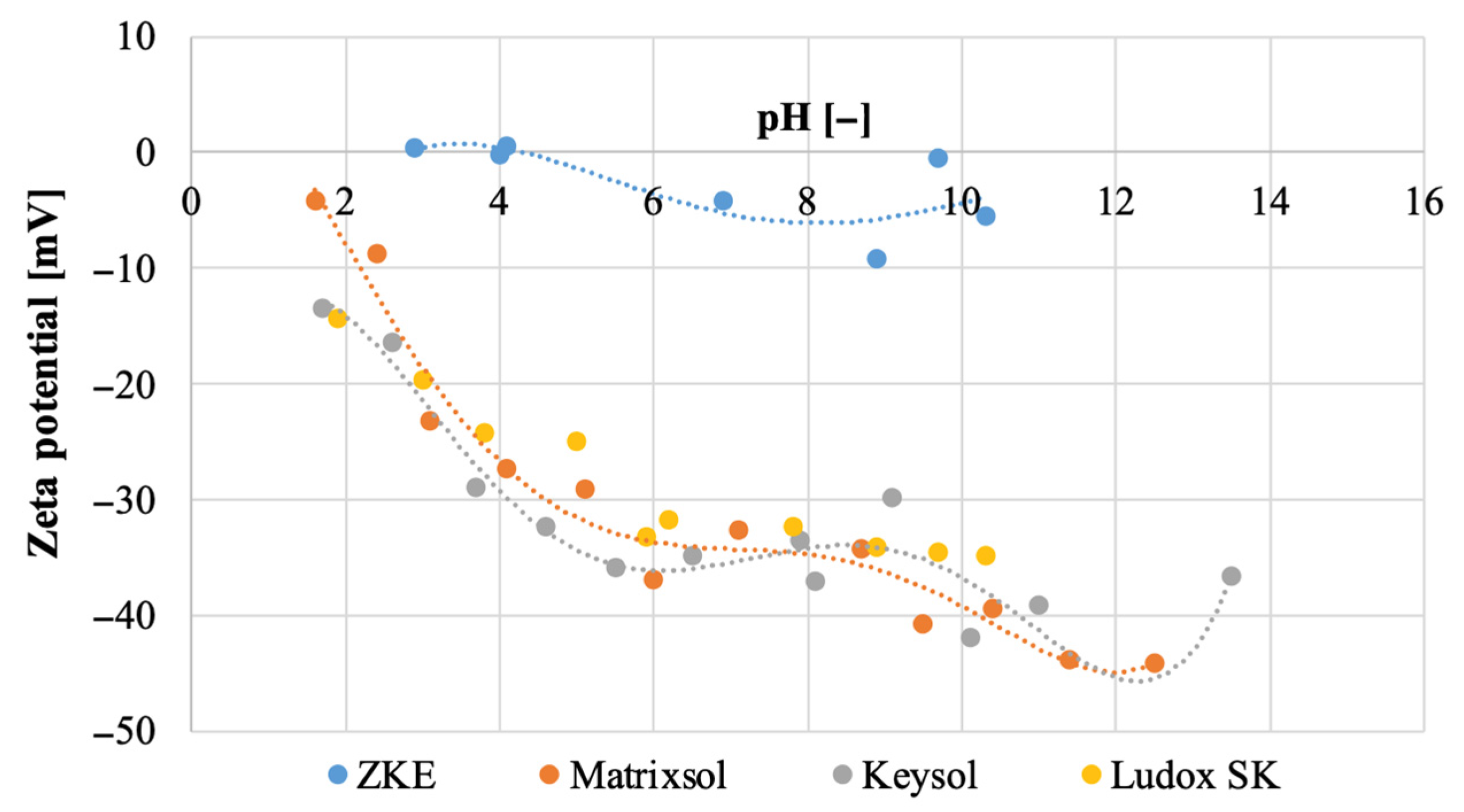
Figure 7B). The reference HES binder is characterised by a strong acidic reaction, pH = 0.95. The keysol binder (pH = 3.46) and the Ludox SK (pH = 3.80) are less acidic. However, the Matrixsol binder has an alkaline reaction with a pH = 10.27. Analysis of the potential values of the tested binder species showed that the reference HES binder has an isoelectric point at pH = 4.4 (
Figure 8). For pH > 4.4, the potential is negative and has a minimum value of −9.2 mV. Keysol, Matrixsol, and Ludox SK binders have a negative potential in the entire pH range ranging from −13.5 mV for pH = 1.7 to −41.9 mV for pH = 10.1; −4.25 mV for pH = 1.6 to 44.2 mV for pH = 12.5; and −14.3 mV for pH = 1.9 to −34.9 mV for pH = 10.3. These binders for pH > 4 show a high electrokinetic stability of the dispersion. Binder particles have a high force that causes them to repel each other; there is no tendency to form agglomerates [
18].
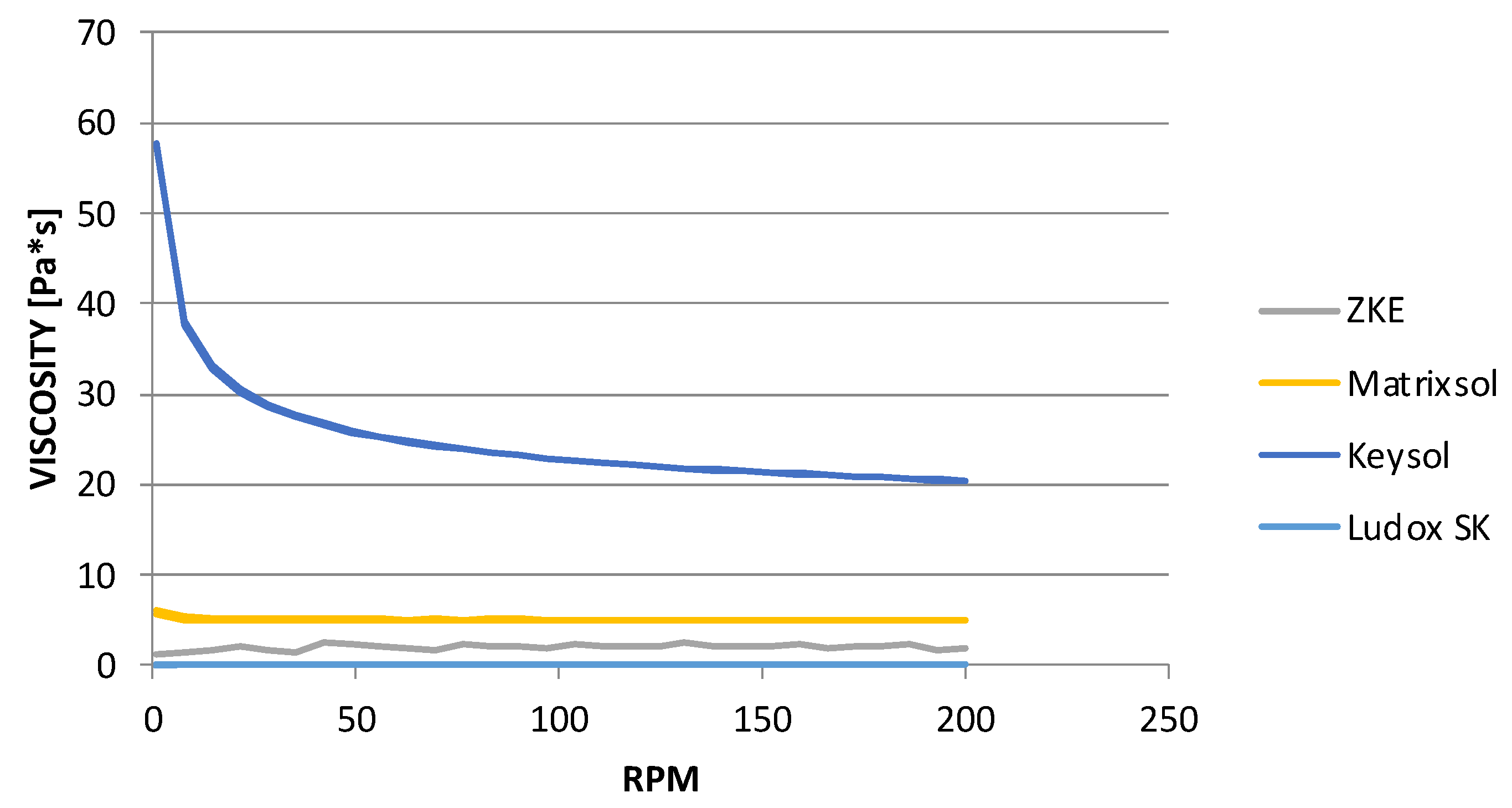
At the same time, the results of dynamic viscosity tests (
Figure 9) showed that the binders, Matrixsol and Ludox SK, belong to the rheologically unstable group. They are characterised by a low viscosity, and this is one of the reasons for their irregular characteristics. The second may be the inhomogeneity of the tested samples. The suspension could have sedimented during the preparation of the test. It can be stated that Matrixsol and Ludox SK binders belong to Newtonian fluids; their viscosity does not depend on shear. The highest viscosity is observed for the Keysol binder, which belongs to the group of pseudoplastic liquids; it thins out as the shear rate increases. It was also determined that increasing the spindle speed leads to a reduction in the viscosity of the Keysol binder from 57 to 20 mPas, that is, almost three times.
Analysis of the results of the wetting angle test (
Figure 10) showed that the binders have different wetting angle values. For HES, the wetting angle is 5 degrees. It can be assumed that this binder is a liquid that wets the surface well. Matrixsol, Keysol, and Ludox SK binders are characterised by hydrophobic properties.
Thermogravimetric analyses of the Matrixsol, Keysol, and Ludox SK (
Figure 11A–C) binders were performed. TGA curves show a rapid mass loss for all binder grades up to a temperature of approximately 190 °C. This causes the water to evaporate quickly. Subsequent peaks are characteristic of the decomposition of polymer compounds, components of the tested binders. According to the literature [
11,
12,
13], these values correspond to these substances with a slight change in temperature, probably caused by the presence of impurities or additives that help increase the stability of the binders.
3.4. Ceramic Moulds
The lack of sufficient data in the literature regarding the forming method, as well as the properties that the ceramic mould should have, and therefore the finished casting, was the basis for a comprehensive analysis of the process of producing ceramic moulds using hydrolysed ethyl silicate. The test results obtained make it possible to determine the predicted properties of the mould produced in the process using new water-soluble binders.
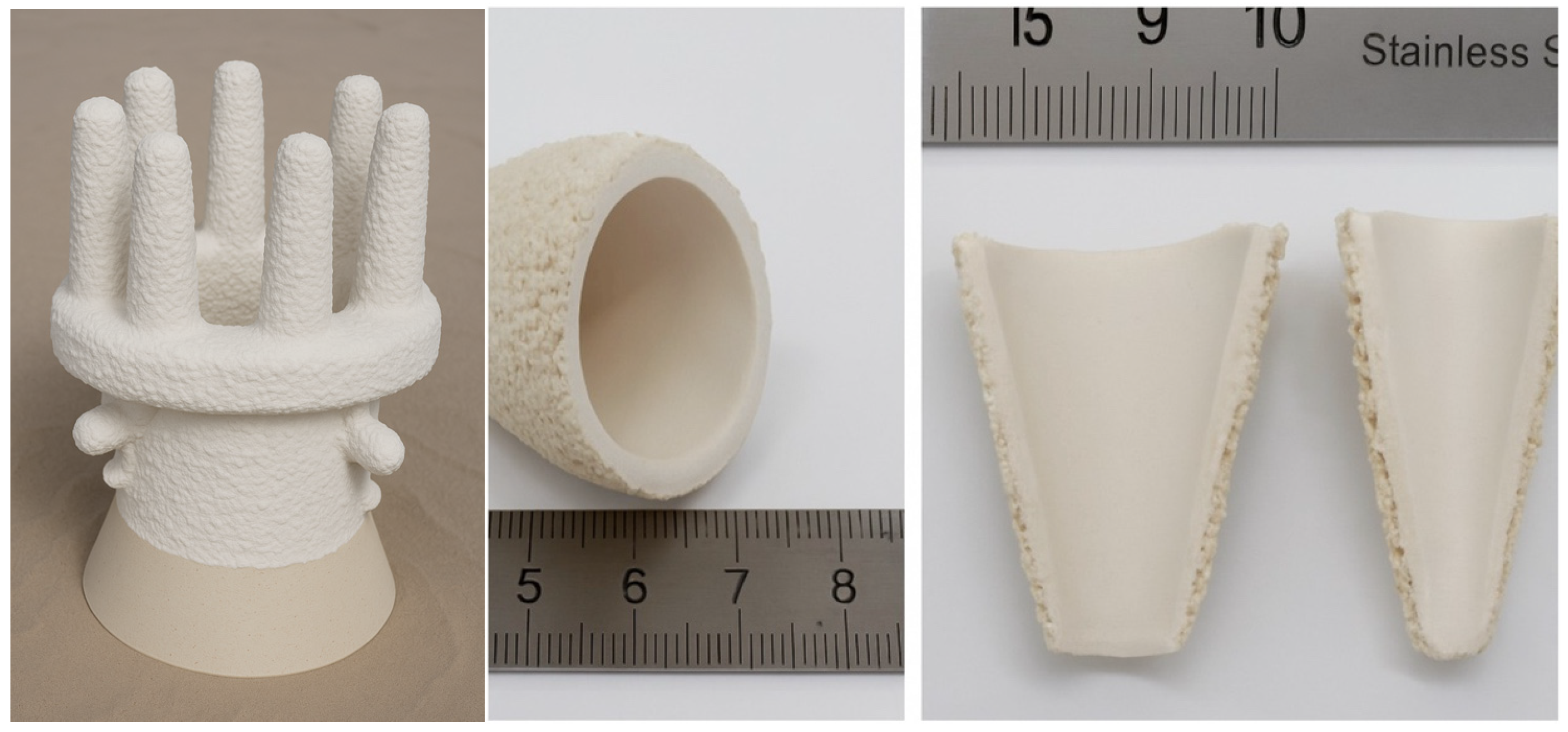
Multilayer ceramic moulds were made by applying successive layers to a wax model. The model was immersed in a ceramic mass and sprinkled with coarse ceramic powder. The prepared layer was left to dry under established conditions of temperature, humidity, and time. These activities were repeated until seven layers were created. The first two layers were made of a higher-viscosity moulding sand for better coverage and reproduction of the surface of the wax model. However, the structural layers were made of a lower-viscosity mass. The ceramic mould was then heat treated to remove wax and increase its mechanical strength. This process consisted of three stages:
melting wax—in an autoclave, in an atmosphere of superheated steam at a temperature of 170 °C and a pressure of 0.86 MPa;
wax firing—in an electric furnace, at a temperature of 760 °C, for 1 h, cooling with the furnace;
annealing the mould—in an electric chamber furnace, at a temperature of 1200 °C, for 2 h, cooling with the furnace.
Ceramic moulds (marked K + M) were made of Al2O3 + Keysol moulding sand for the first layer and Molochite 120 + Matrixsol for the construction layers, with controlled air circulation, humidity, and temperature conditions. The conditions used made it possible to reproduce the process of producing ceramic moulds using the previously used HES binder. The inability to dry experimental forms in an industrial dryer led to the development of other evaluation methods. The test results obtained for both the HES form and the newly developed form were the basis for a comparative analysis.
Microscopic examinations allowed the characterisation of the geometric structure of the HES (
Figure 13) and K + M (
Figure 14) ceramic moulds in three states: raw, after wax firing at 760 °C/1 h, and after annealing at 1200 °C/1 h. It was found that the surface of the tested forms was non-uniform and porous. Particles of moulding powders with sharp edges used as a matrix were observed. The fractures obtained were brittle. Increasing the temperature of heat treatment resulted in an increase in the number of pores.
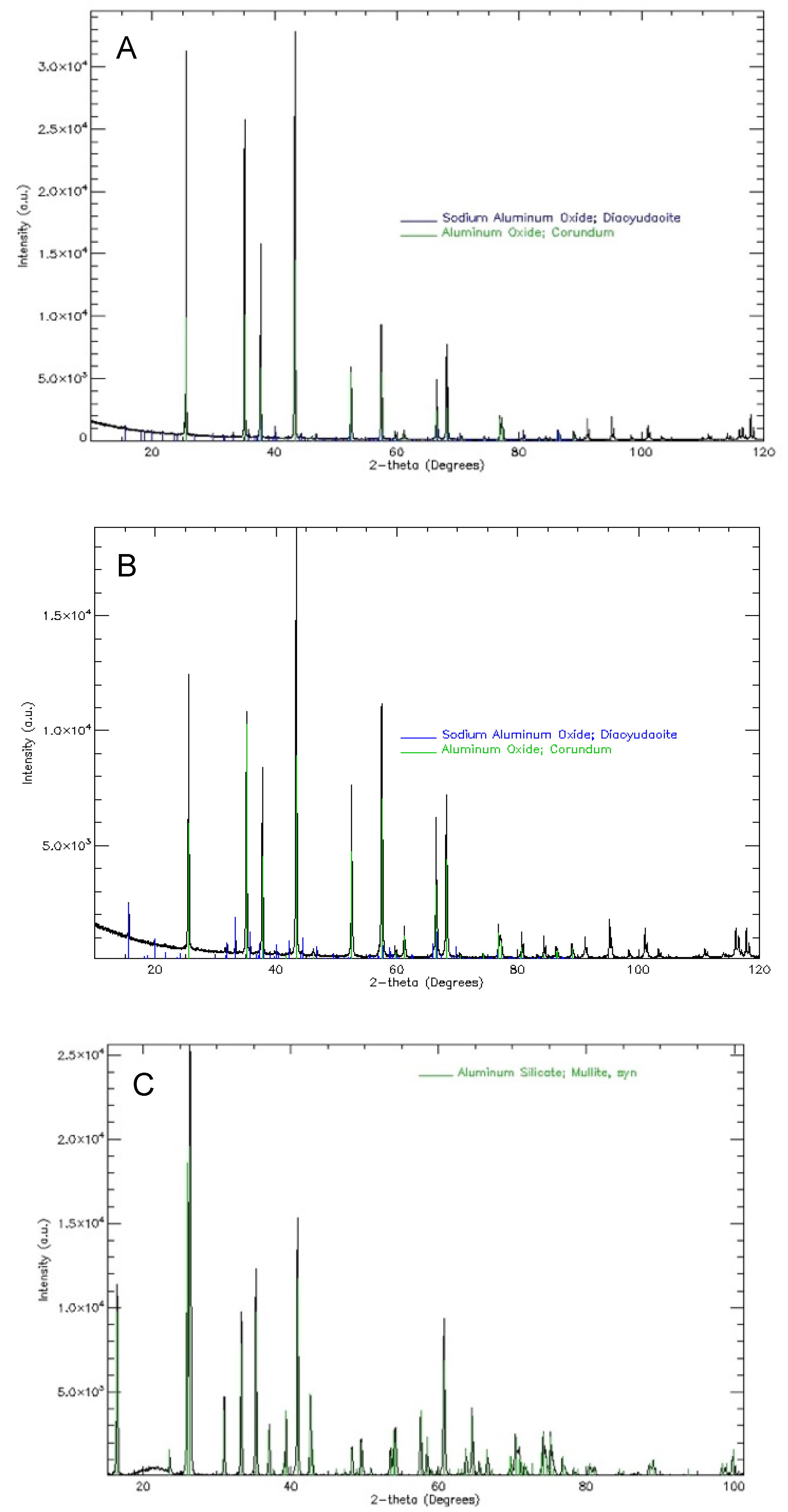
Analysis of the phase composition of the mould material after the process of melting and firing the wax mould model showed the presence of phase components:
Additionally, a low content of α cristobalite was found in the annealed forms. In the process of annealing the mould material, a phase transformation of amorphous SiO2 oxide occurs, which is a component of the binder and Molochite 120 powder. It should be noted that the relative volume of this phase component is small. Its presence is not important for technological reasons. However, crystalline SiO2 oxide, especially cristobalite, is dangerous to human health. Therefore, it is recommended to be particularly careful when creating the moulds. The tests also confirm the good thermal stability of the main materials used in the moulding of sands, corundum, zirconium silicate, cobalt aluminate, and mullite. This applies to all stages of the heat treatment of ceramic moulds.
Figure 15 shows the average surface roughness of the HES and K + M moulds. Moulds that use the HES binder are characterised by an increase in the Ra parameter as a result of heat treatment. However, the moulds developed and manufactured using water-soluble binders show similar values of average surface roughness for samples in all states, i.e., in the raw state, after firing, and after annealing. The low Ra values are influenced by silica nanoparticles that fill the pores open at the surface. For aviation applications, Ra should not exceed 5 µm. As can be seen from the results obtained, the developed moulding systems meet the requirements. The silica nanoparticles (SiO
2) present in water-soluble binders such as Keysol and Matrixsol act as fillers by entering microvoids and surface irregularities during the drying and sintering stages. Upon gelation and subsequent thermal treatment, they form a continuous network that seals surface pores and reduces roughness. This results in a smoother surface finish, which is particularly advantageous for aerospace casting applications.
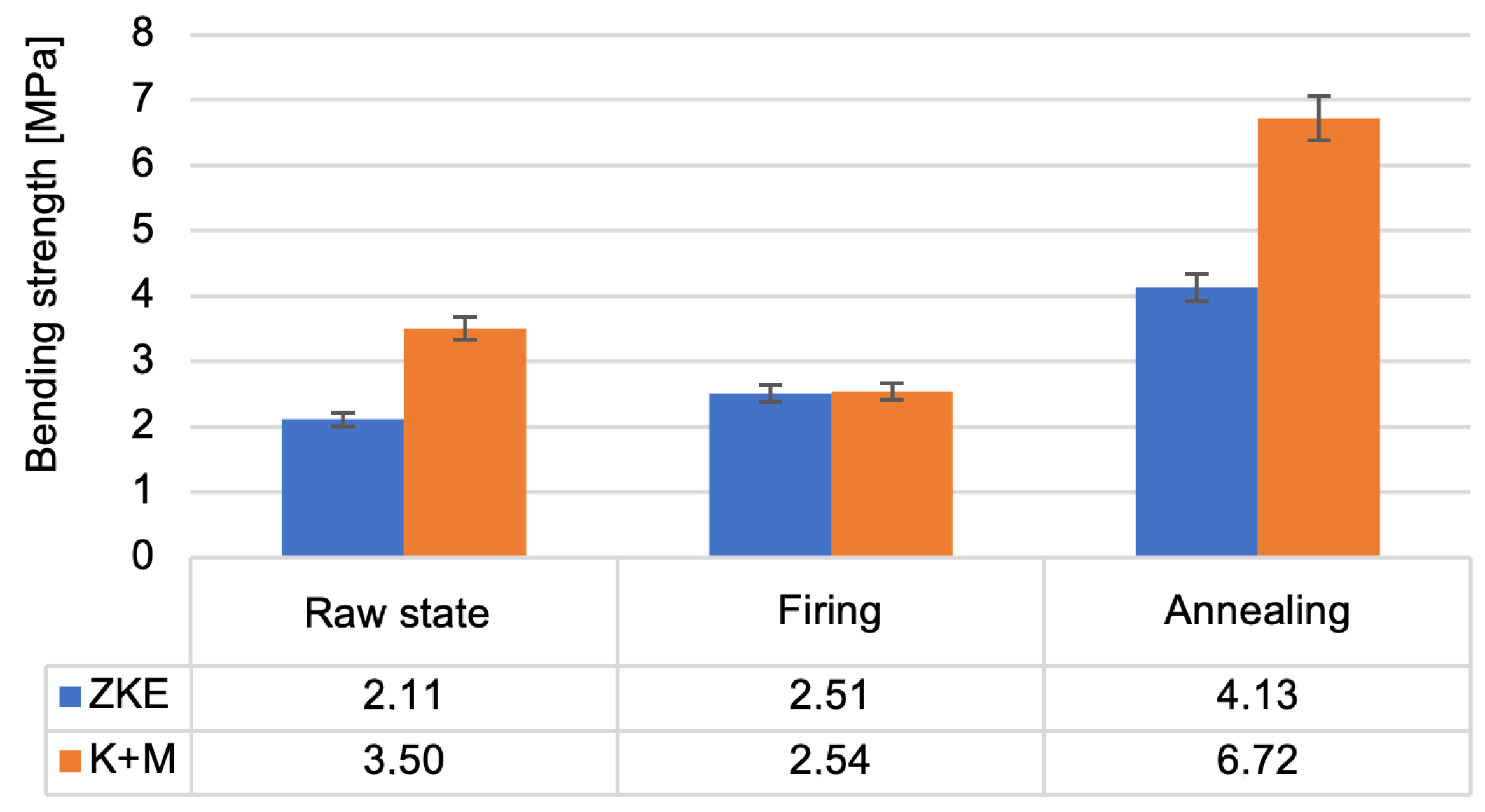
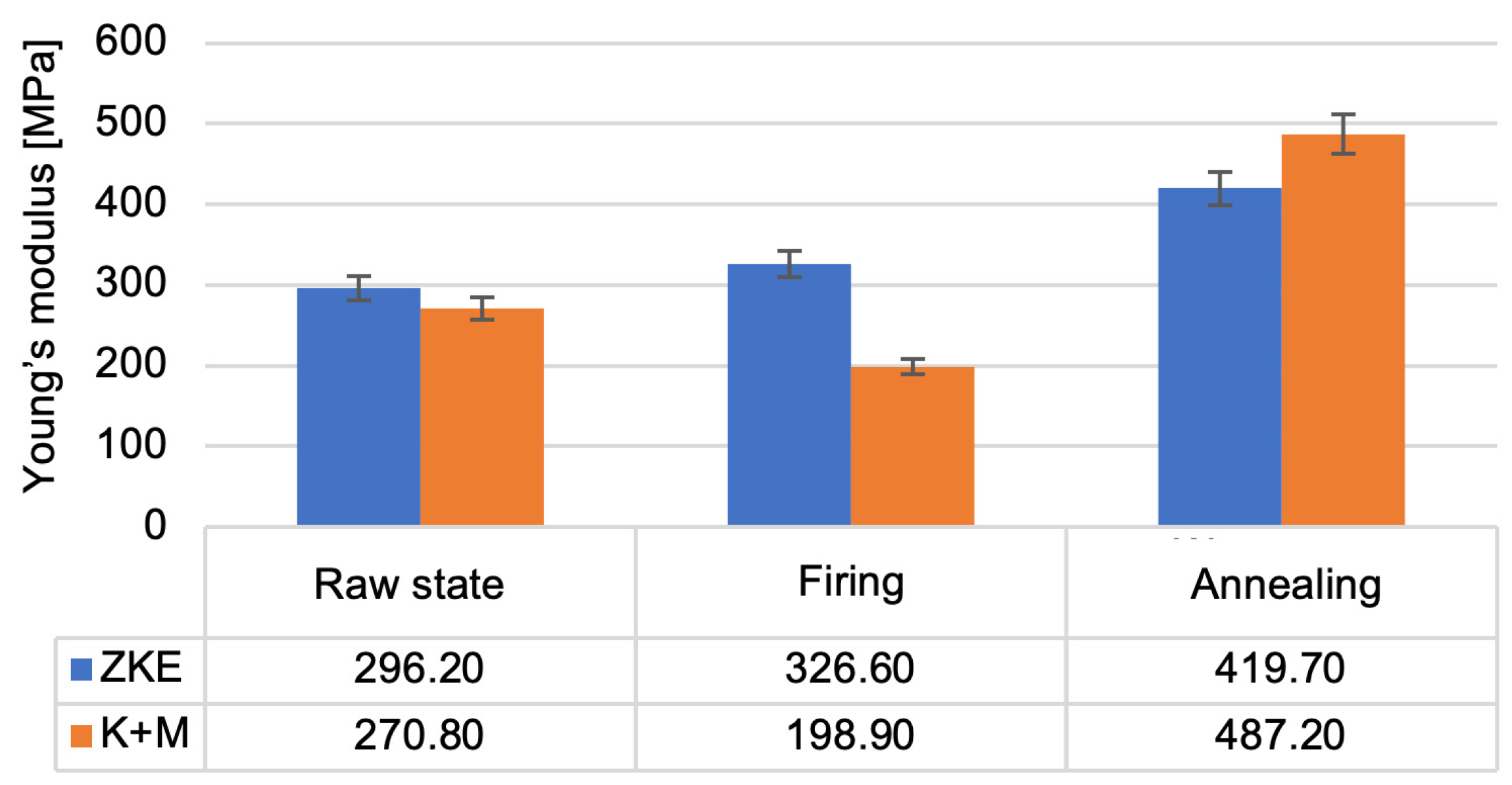
Analysis of the results of the testing of the mechanical properties of the created ceramic moulds showed that the highest bending strength (
Figure 16) and the Young modulus value (
Figure 17) were obtained after annealing. For bending strength, measurements were performed on ten specimens per group (HES and K + M) in three material states: raw, after wax firing (760 °C/1 h), and after final annealing (1200 °C/2 h). In the annealed state, the average bending strength for HES-based moulds was 11.3 MPa (±0.9 MPa), whereas the K + M moulds achieved 12.4 MPa (±0.7 MPa). A two-tailed Student’s
t-test showed that this difference was statistically significant (
p = 0.0037), indicating superior post-sintering mechanical integrity in the water-based system [
19].
In the raw state, HES moulds exhibited slightly higher bending strength (8.7 MPa vs. 8.2 MPa), which is attributed to the strong initial cohesion provided by the alcohol-based binder [
21]. However, this difference was not statistically significant (
p = 0.087), and the values converged after heat treatment. Notably, K + M moulds demonstrated better thermal resilience, retaining higher mechanical performance after each processing stage, consistent with findings in recent work on water-based ceramic slurries [
16,
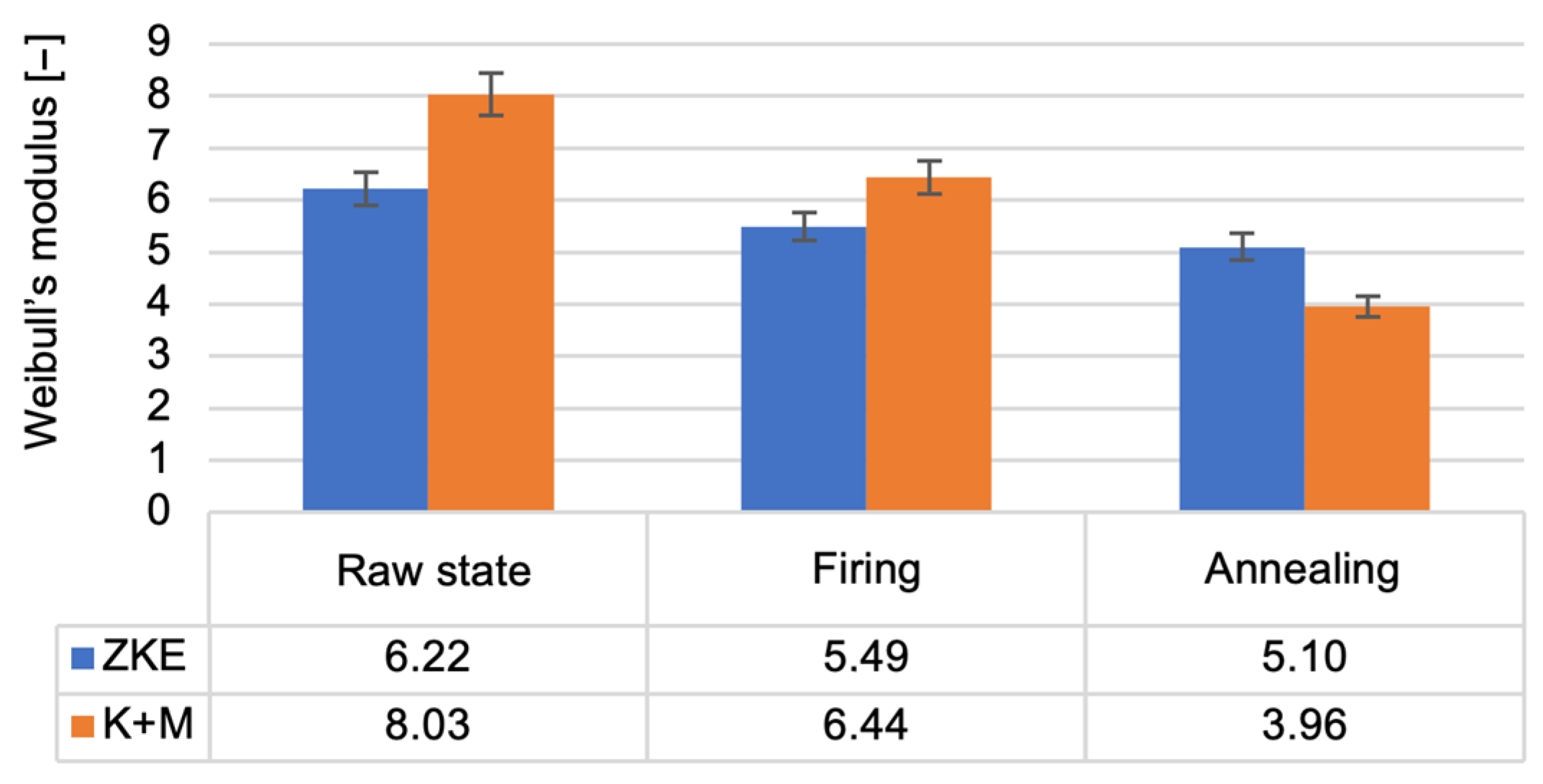
24]. The reduction in bending strength after wax firing is caused by the decomposition of the polymer contained in the binder; it provides greater bending strength in the raw state. The increase in bending strength after annealing is related to the sintering process of the ceramic matrix and colloidal silica. The value of the Weibull modulus (
Figure 18) decreases as the annealing temperature of the ceramic moulds increases. This is due to the high porosity of the samples, which resulted in low Weibull modulus values for all samples. The m modulus for the K + M samples is smaller than that for the HES samples, which could be due to the lower value of the wetting angle of the hydrolysed ethyl silicate, which resulted in good mixing of the composition and a smaller number of surface defects.
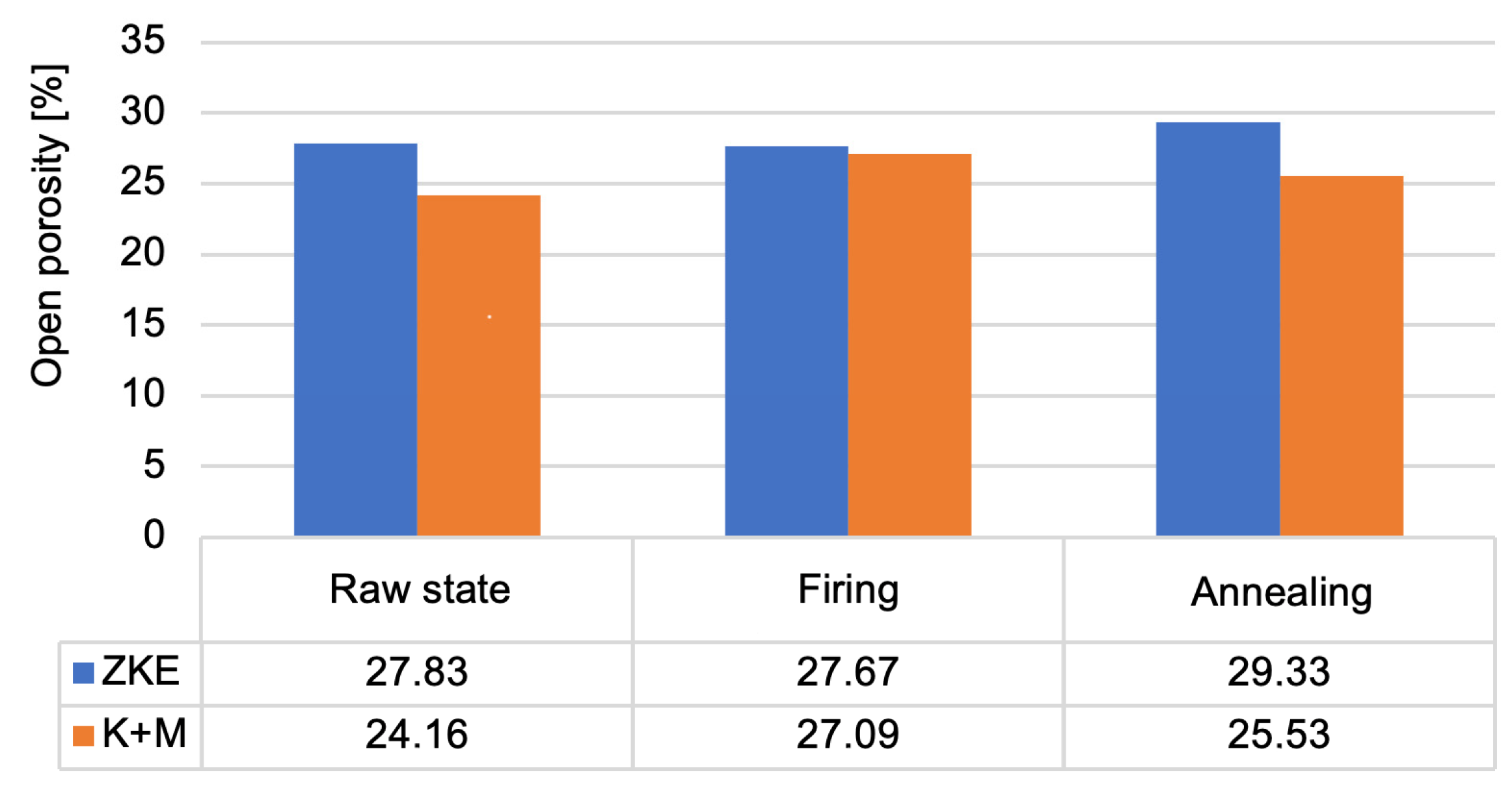
In terms of porosity, mercury intrusion porosimetry revealed that the average open porosity in annealed HES moulds was 26.8%, while in K + M moulds it was slightly higher at 29.1%. Although this increase may appear unfavorable at first glance, further analysis showed that the gas permeability of K + M moulds was significantly enhanced—5.6 × 10
−9 cm
2 at 1100 °C versus 4.1 × 10
−9 cm
2 for HES moulds—leading to improved venting during casting [
13,
25]. This effect is aligned with the beneficial role of porosity in degassing described in advanced investment casting systems [
17,
20]. The increased porosity in the K + M system also had no measurable negative impact on casting surface quality, as evidenced by the Ra values (<5 µm) and the absence of gas-related defects in pilot trials.
For surface roughness, the average Ra of K + M moulds after annealing was 4.2 µm, compared to 5.1 µm in the HES group. This difference was statistically significant (
p = 0.012), confirming that silica nanoparticle infiltration in the K + M binder contributed to improved surface quality by partially sealing surface pores during drying and sintering [
12,
14].
The improved mechanical, thermal, and surface characteristics of the K + M system strongly support its practical replacement of the traditional HES-based solution, in line with sustainable material trends described in the recent literature [
16,
26,
27].
Analysis of the results obtained from the porosity test (
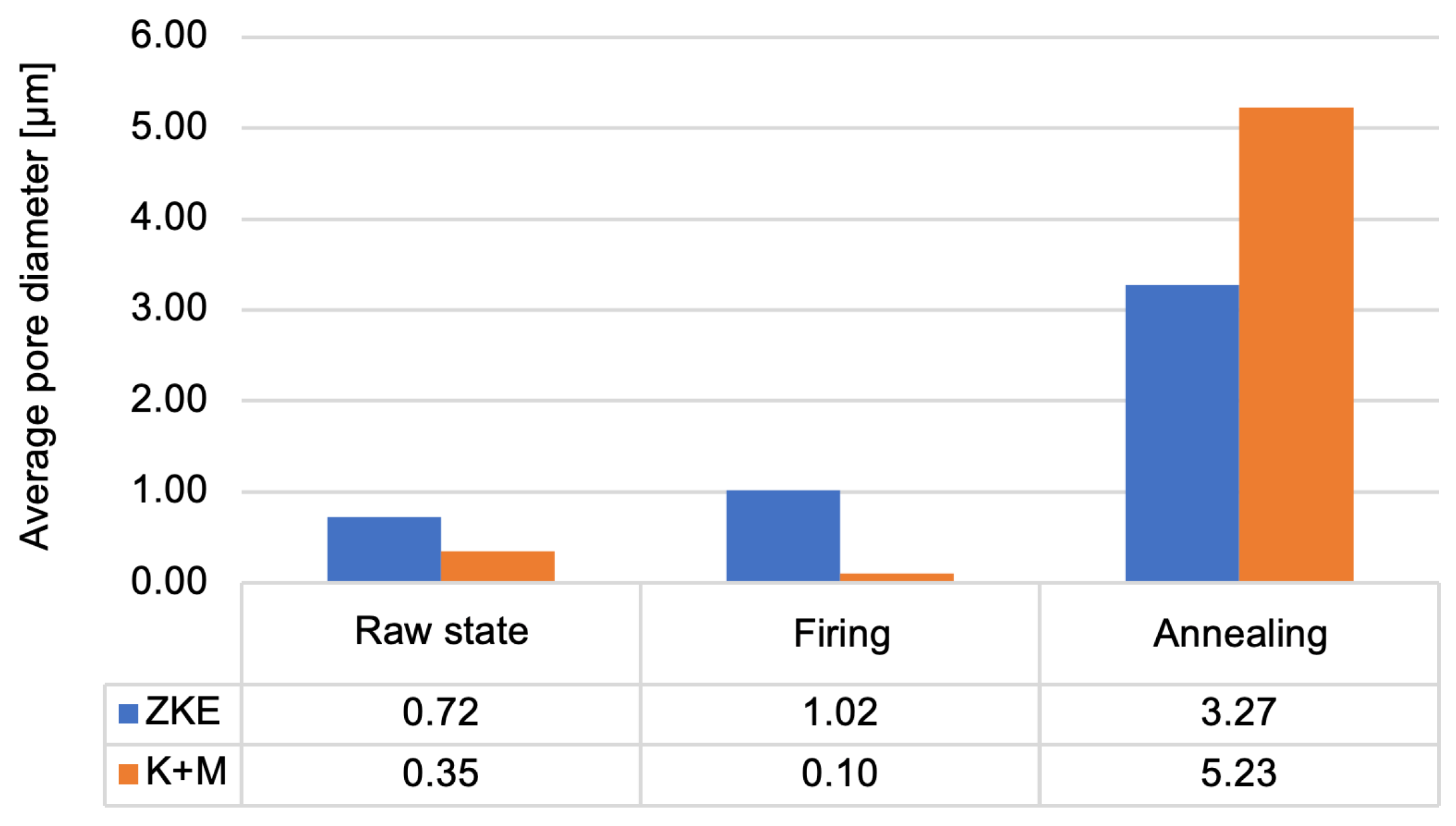
Figure 19) allows us to conclude that the porosity of the moulds ranges from 24% to 29%. Annealed forms are characterised by the highest porosity. This is caused by the sintering process. Up to a temperature of 760 °C, the entire binder does not undergo thermal decomposition. More is decomposed at 1000 °C, which also results in greater porosity. This parameter is also influenced by the rapid sintering process of 10 °C/min, which causes faster gas escape, the formation of voids, and the inability to close pores. Wax firing and annealing cause the evaporation of water and the degradation of organic compounds. After annealing, the average pore diameter increases significantly (
Figure 20) in the tested ceramic moulds. This proves the coalescence of pores and the sintering of the fine-grained fraction of the matrix ceramic powders. This is also evidenced by the distribution of the average pore diameter. The annealing process eliminates the occurrence of small pores. The high porosity of the mould in the annealed condition and the large average pore diameter are advantageous. Porous ceramic moulds are characterised by good gas permeability when poured with liquid metal and are also brittle when finishing castings.
Figure 20 shows that, in the case of the K + M system, the fired form has the highest porosity. For the K + M moulds, firing at 760 °C does not fully decompose organic additives, and partial sintering begins only after annealing at 1200 °C. Thus, larger pores are formed during annealing due to extensive sintering and agglomeration of ceramic particles.
The increased porosity observed in the K + M moulds, particularly after annealing (up to ~29.1%), indeed plays a dual role in the performance of the ceramic-casting system. On one hand, higher open porosity enhances gas permeability, which is a critical factor in avoiding casting defects such as gas porosity, blowholes, and oxide inclusions during metal pouring [
13]. This is especially beneficial in the casting of nickel superalloy components, where complete and rapid evacuation of air and residual volatiles through the mould wall is essential to ensure high surface quality and structural integrity of thin-walled blades [
4,
16,
24]. The gas permeability of K + M moulds increased with temperature, reaching a peak value of 5.6 × 10
−9 cm
2 at 1100 °C, compared to 4.1 × 10
−9 cm
2 for the HES system, which demonstrates superior outgassing capability [
13]. This enhanced permeability, in combination with good wettability (moderate wetting angles) and phase stability, contributed to the absence of internal defects or misruns in pilot castings, confirming the functional viability of the developed solution. On the other hand, excessive or uncontrolled porosity may compromise surface finish or reduce local mechanical strength of the mould, particularly in areas of high thermal gradient or metallostatic pressure [
20,
27]. Surface roughness may increase due to micro-cavities formed during binder burnout and sintering, especially if the pore structure is irregular or contains interconnected macrovoids. However, in our tests, surface roughness (Ra) remained below the critical threshold of 5 µm, and no crack propagation or surface degradation was observed after annealing or during pilot casting trials.
To ensure control over porosity and mitigate potential negative effects, the following strategies should be applied:
Optimization of binder-to-powder ratio to reduce excess binder phase and control pore evolution during drying and sintering;
Use of fine silica nanoparticle-rich sealant coatings for the final layer (without dusting), which partially closes surface pores and minimizes Ra increase during firing [
12,
14];
Tuning of the sintering schedule, e.g., by incorporating a pre-sintering step at 900–1000 °C to stabilize the pore structure before final densification at 1200 °C;
Inclusion of pore modifiers or sacrificial pore-forming agents (e.g., fugitive organics or microballoons) that decompose at lower temperatures, leading to more uniform porosity distribution [
17,
25];
Post-casting leaching or finishing processes, such as water jet or mechanical polishing, to eliminate residual ceramic that may have penetrated into micro-defects.
Overall, while increased porosity is an inherent consequence of using water-based systems with high SiO
2 content, it has been managed effectively through material and process design. The resulting moulds maintained both functional integrity and surface quality comparable or superior to conventional HES-based systems, which supports their practical implementation in aerospace casting technologies [
27].
The results of the gas permeability tests of the tested ceramic moulds are shown in
Figure 21. An increase in gas permeability with increasing temperature was found for the ceramic moulds using a water-soluble binder. However, for forms that used HES, the opposite phenomenon was observed. This is caused by the difference in the linear expansion coefficient of the mould ceramic material. The reduction in gas permeability at temperatures > 1100 °C is caused by the effect of sintering and shrinkage of the ceramic metal.
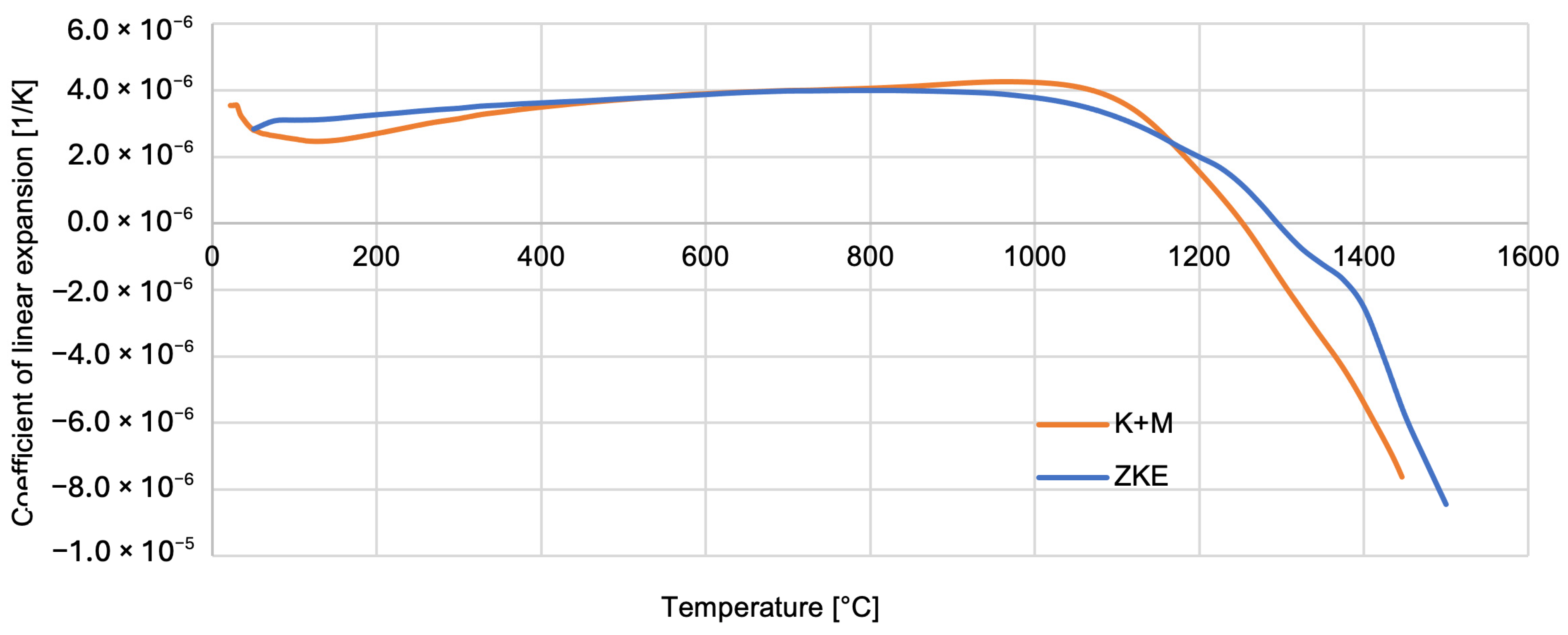
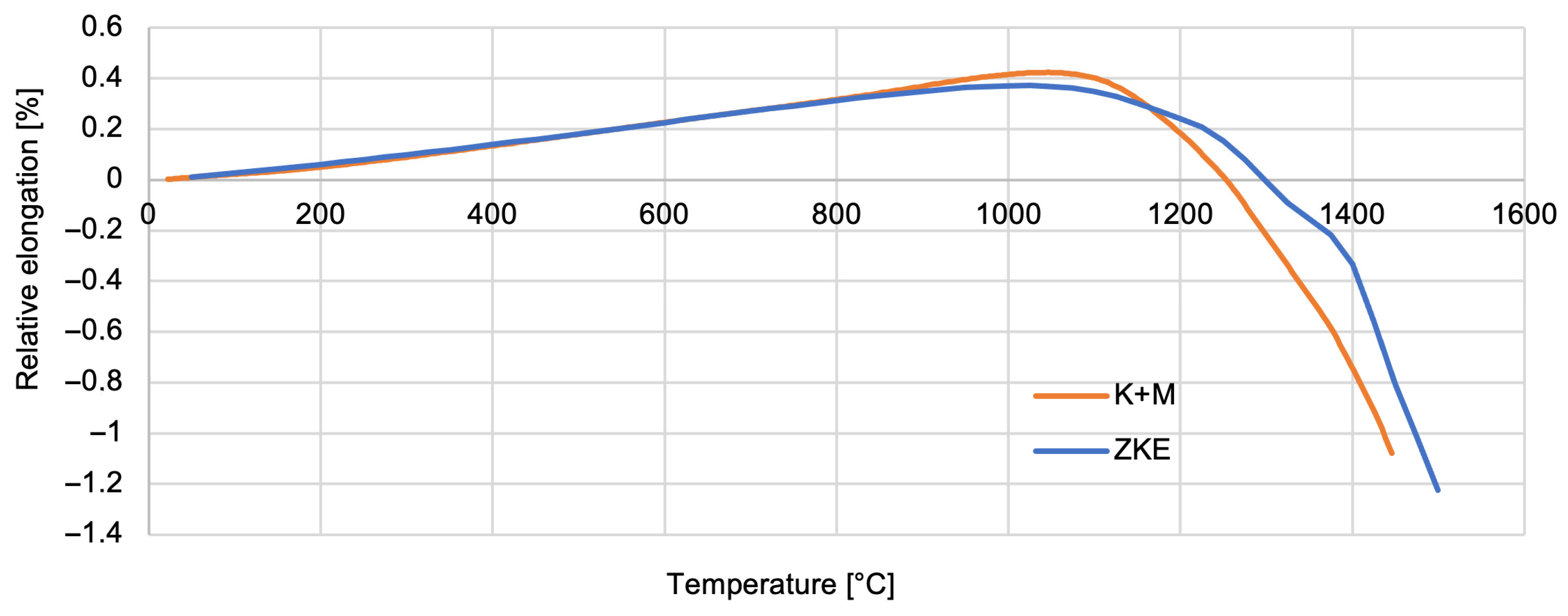
Thermal properties of the reference mould material were tested using HES, and that of the newly developed one was tested using K + M. The analysis of the results obtained indicates that the relative elongation increases slightly and reaches a maximum at a temperature of approximately 1100 °C—0.5% (
Figure 22). Above the temperature of 1100 °C, the mould material shrinks to a maximum value of 1.5%. It is caused by the ceramic sintering process. Similar dependencies were found for the linear expansion factor of the material of the tested forms (
Figure 23).
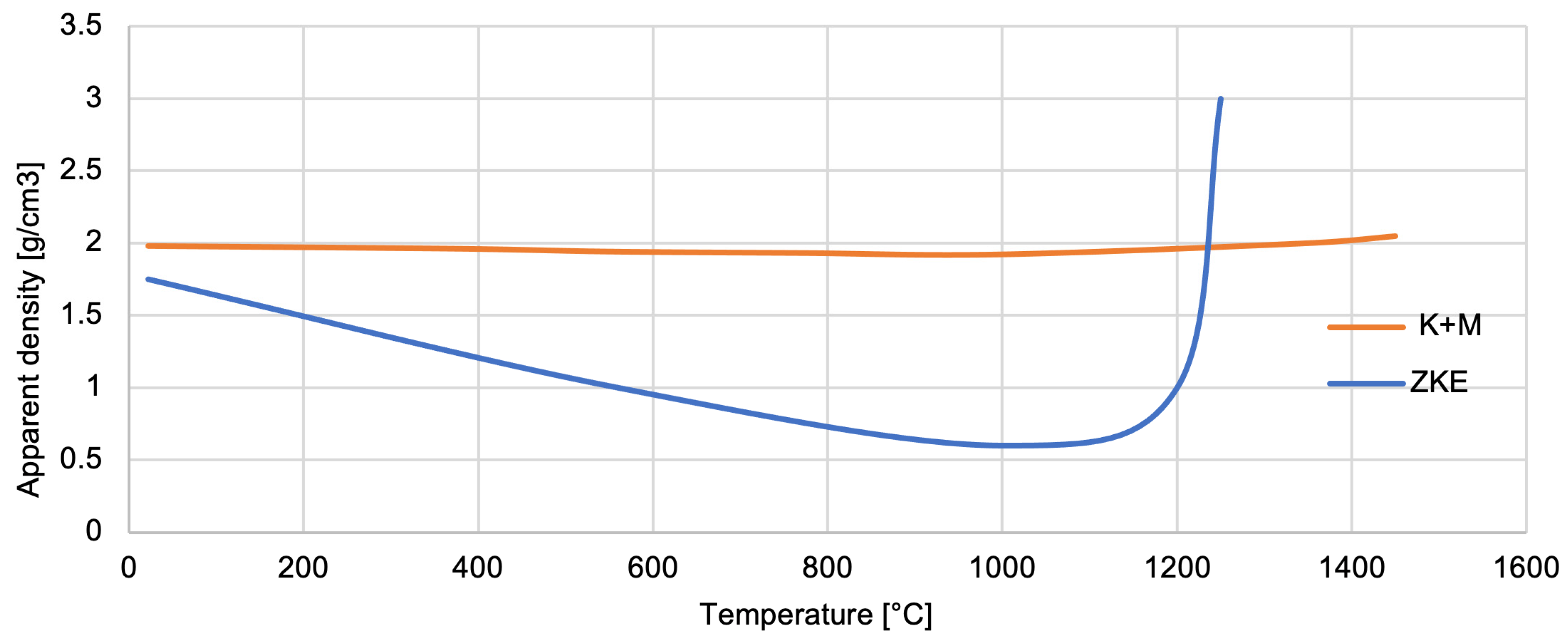
Analysis of the measurement of the apparent density of the mould material (
Figure 24) shows that it decreases as the temperature increases to its critical value—approximately 1100 °C. The formation of new phases then causes the material to thicken and increase its density [
17]. The density increase after phase transformation and sintering improves mould mechanical strength and thermal shock resistance. A denser mould reduces gas entrapment and enhances casting accuracy. However, excessive densification could limit gas permeability. In the K + M system, we observed an optimal balance, leading to improved mould integrity without sacrificing permeability.
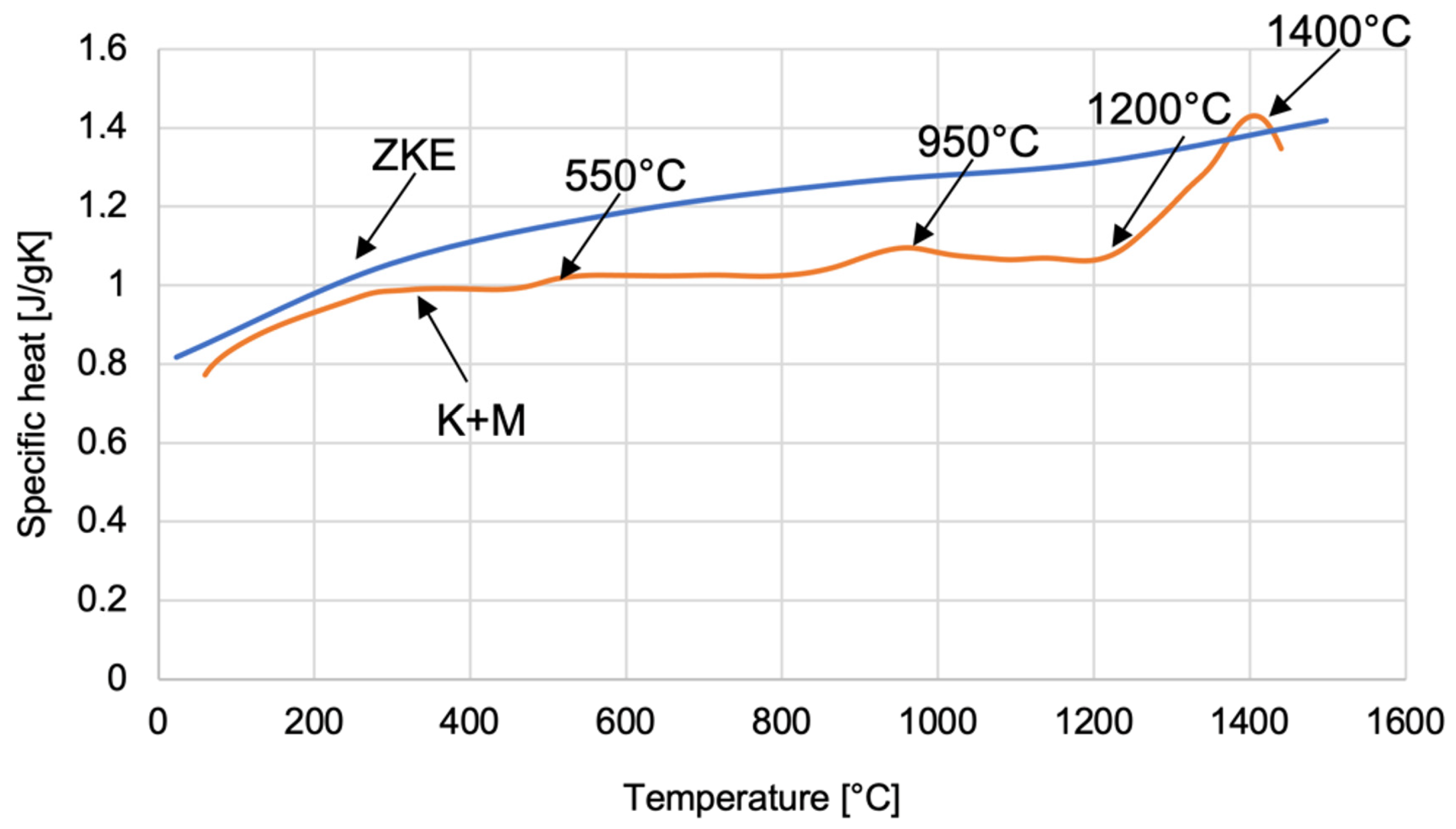
The dependence of the specific heat on temperature was determined for ceramic mould materials using HES and K + M binders (
Figure 25). The curve obtained allows one to determine four characteristic temperature values related to the polymorphic transformation of SiO
2 oxide. At a temperature of approximately 550 °C, α-quartz transforms into the β variety. Then, at a temperature of approximately 960 °C, the transformation of β-quartz → β-tridymite takes place. The β-quartz variety is transformed into β-cristobalite at a temperature of 1200 °C. However, at a temperature of approximately 1400 °C, β-tridymite → β-cristobalite. The specified polymorphic transformation temperatures differ slightly from the theoretically determined polymorphic transformation temperatures of SiO
2 oxide. This is caused by the presence of various chemical additives in SiO
2, which shift the temperatures of the polymorphic transformation.
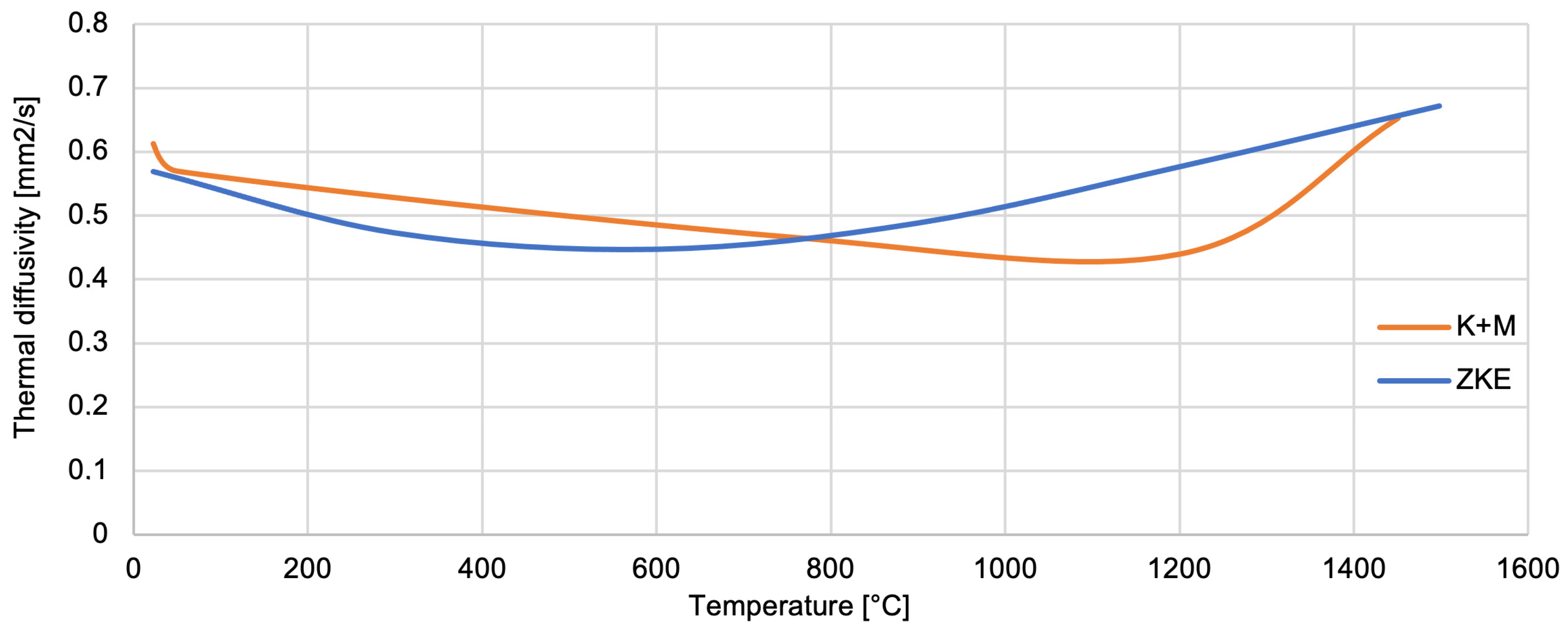
The thermal conductivity of SiO
2 oxide was determined (
Figure 26). The thermal conductivity coefficient ranges from 0.3–1 W/mK up to a temperature of approximately 1100 °C. The thermal diffusivity of SiO
2 oxide also decreases from 0.62 mm
2/s to 0.43 mm
2/s, also up to a temperature of 1100 °C (
Figure 27). SiO
2 oxide is characterised by low conductivity and thermal diffusivity due to high porosity. A particularly important factor is increasing the temperature value to 1100 °C. It causes the sintering effect of ceramics, shrinkage, and thickening of the mould material, hence the increase in thermal conductivity to 2 W/mK and thermal diffusivity to 0.67 mm
2/s at a temperature of 1400 °C. The analysis of the results of the thermal properties test indicates significant changes that are caused by the polymorphic transformation characteristic of the SiO
2 oxide. A particularly large difference in values was found at a temperature of approximately 1100 °C, which is the beginning of the ceramic sintering process. In addition, at this temperature, large changes in relative elongation, increased density, and thermal conductivity of the mould material were demonstrated. This indicates both shrinkage and compaction of the mould material. Additionally, characteristic values of the temperature of the polymorphic transformation of SiO
2 oxide can be distinguished in the curves of specific heat versus temperature.
In terms of binder systems, our findings support and extend previous work by Ismael et al. [
17], who investigated colloidal silica as a nanostructured binder for refractory castables. While they confirmed the potential of colloidal systems for improved thermal stability, our study goes further by integrating a dual-component water-soluble binder system (Keysol + Matrixsol) and demonstrating its full industrial viability for multilayer ceramic moulds in aerospace applications. Similarly, Wang et al. [
24] studied ceramic shell–metal interactions and gas permeability in yttria-based moulds; our work complements theirs by focusing on silica-alumina-based moulds but introduces a comparable porosity–permeability balance critical for thin-walled casting. Regarding mechanical properties, our annealed K + M moulds achieved bending strengths exceeding 12 MPa, which are higher than the values reported in earlier work by Maity and Maity [
14], where ceramic moulds reinforced with zircon/silica layers reached strengths of only 8–10 MPa under comparable heat treatment conditions. Furthermore, our observed Young’s modulus and Weibull modulus trends are consistent with the mechanical behavior described by Donachie et al. [
18] in superalloy-compatible mould systems, yet we extend this by correlating these values with binder microstructure (e.g., nanoparticle dispersion and sol-gel kinetics). In terms of thermal behavior, our thermal conductivity results (rising from 0.3 to 2.0 W/mK with temperature) align with those obtained by Szeliga et al. [
13], who measured similar trends in investment casting shell moulds. However, unlike their work, we incorporate a detailed thermal diffusivity analysis and correlate it with porosity and phase transformations (e.g., quartz → tridymite, cristobalite transitions) observed via DSC, thus offering a more complete thermophysical characterisation. Concerning surface quality, our surface roughness (Ra < 5 µm) after sintering matches the performance benchmark established by Chen et al. [
27] for alumina-based, laser-fused ceramic shells, despite the fundamentally different forming method. Our study thus demonstrates that water-based slurry systems can match or outperform more technologically intensive fabrication methods when appropriately optimized. From an environmental perspective, Cunha et al. [
27] demonstrated the feasibility of using waste ceramic moulds and paraffin wax in mortar composites, highlighting the lifecycle aspect of foundry materials. Our work contributes to this direction by proving that alcohol-free binder systems not only reduce VOC emissions by over 85% but also generate safer solid waste for downstream recycling, in line with circular economy goals. Finally, the present work is, to the best of our knowledge, the first comprehensive experimental validation of the Keysol/Matrixsol system for precision aerospace moulds that includes mechanical, thermal, microstructural, and gas permeability performance comparisons against conventional HES systems—thereby closing an existing gap in the literature on water-based binder substitution under production-simulating conditions [
27].