High-Temperature Mechanochemical Synthesis of Nano-ZrO2 for Enhanced Densification and Fracture Toughness in B4C Ceramics
Abstract
1. Introduction
2. Experiment
2.1. Reagents and Instruments
2.2. Sample Preparation
2.3. Sample Characterization
3. Results and Discussion
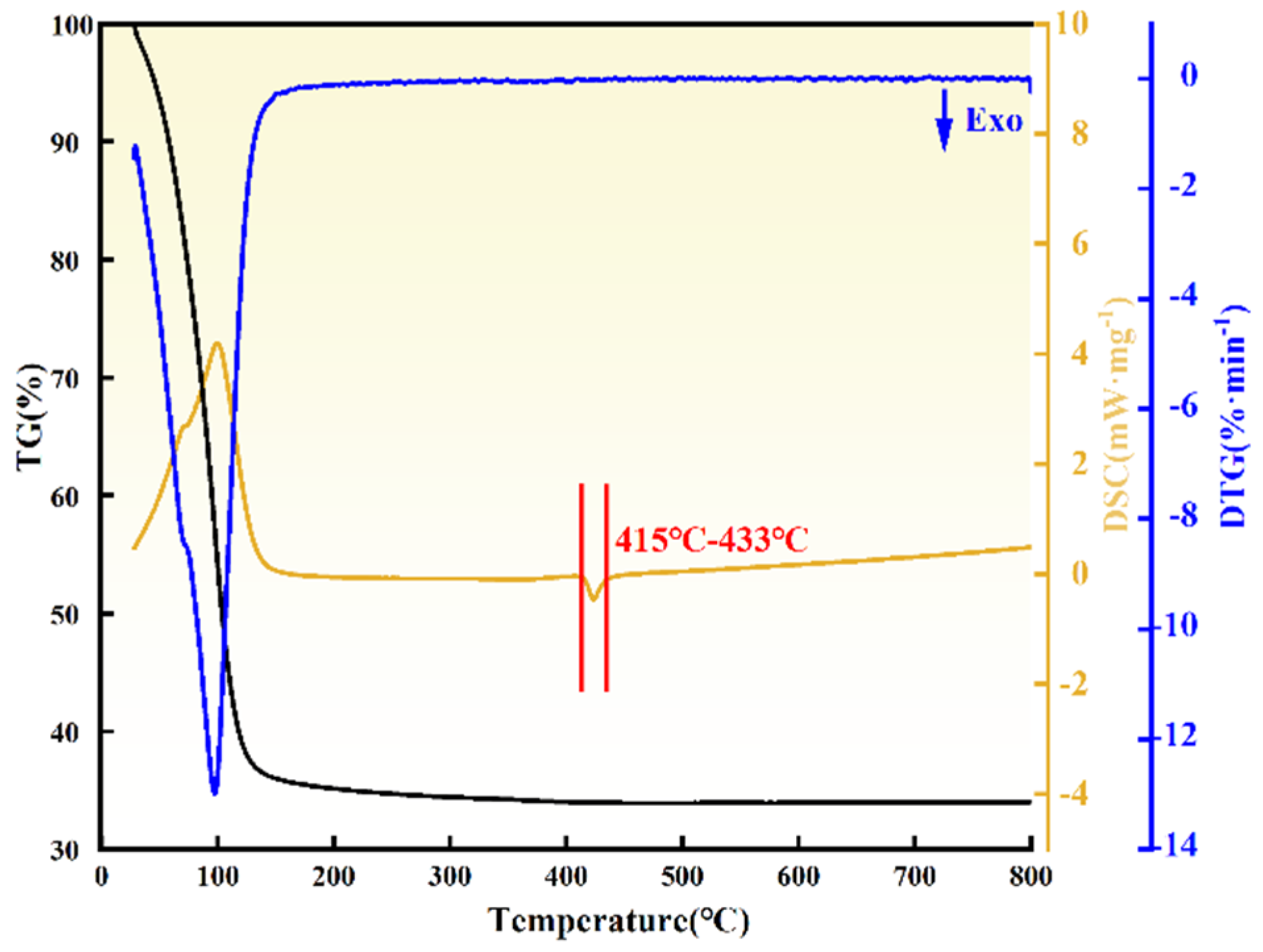
3.1. Thermogravimetric-Differential Thermal Analysis of ZrO(OH)2
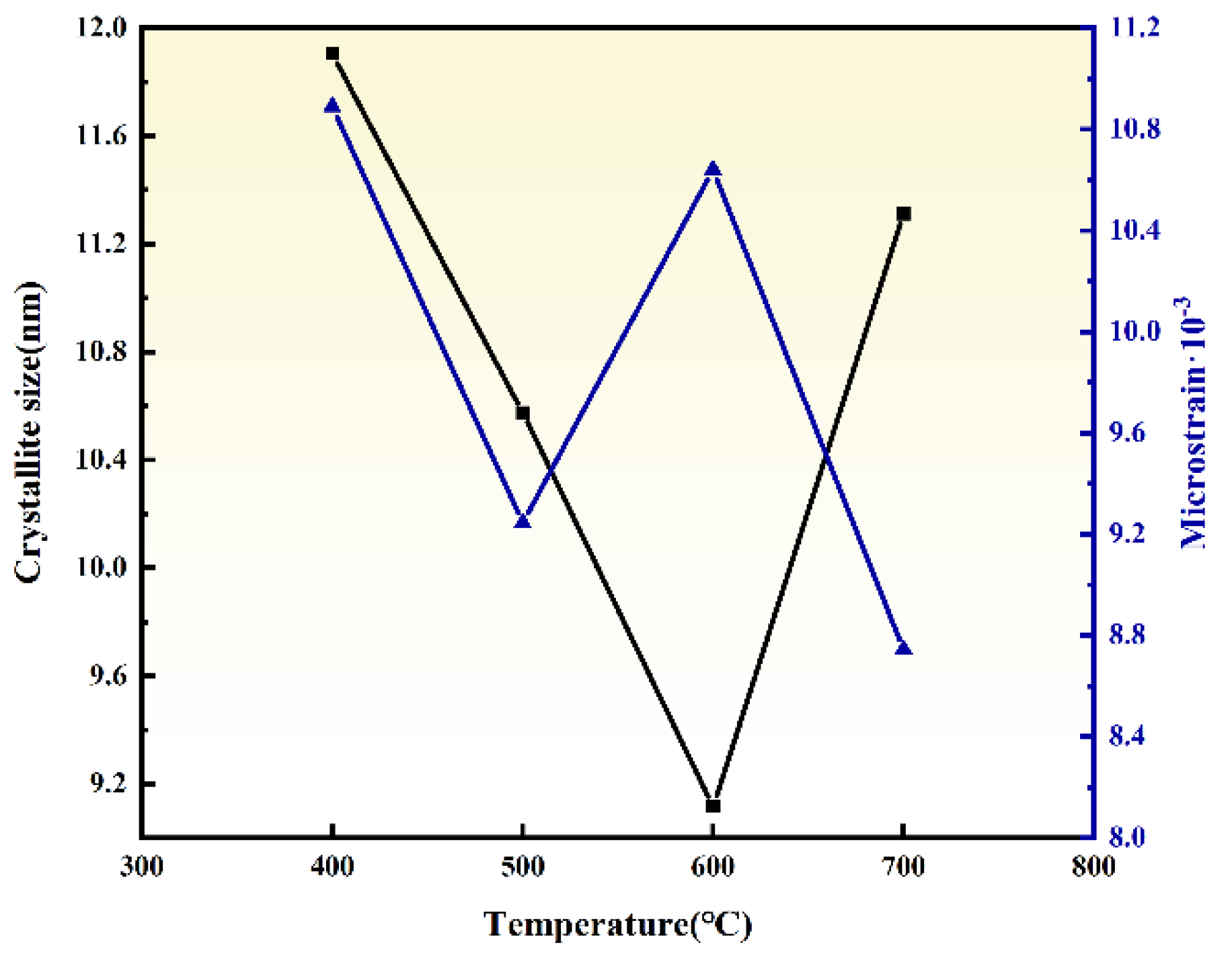
3.2. Effect of Ball Milling Temperature
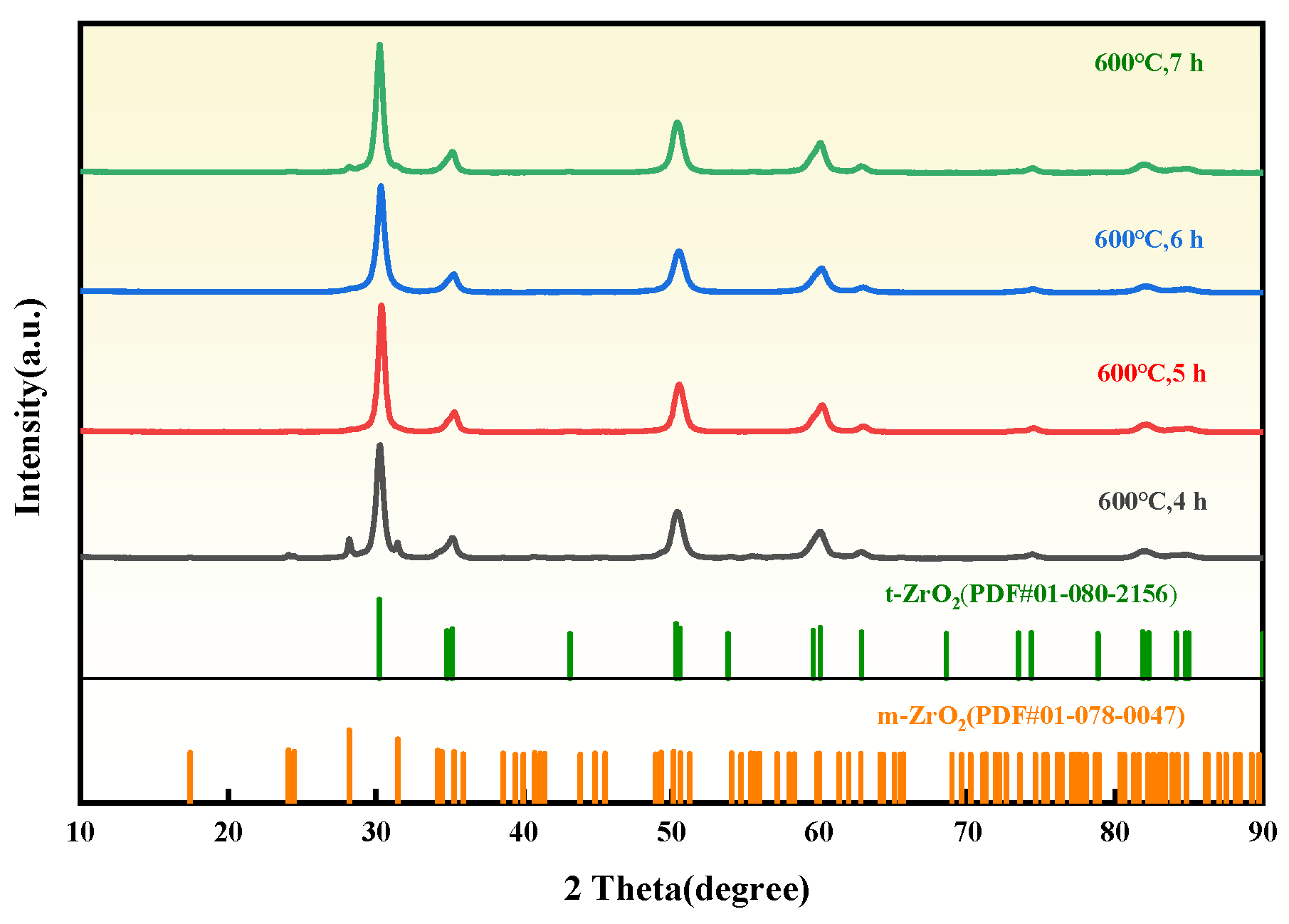
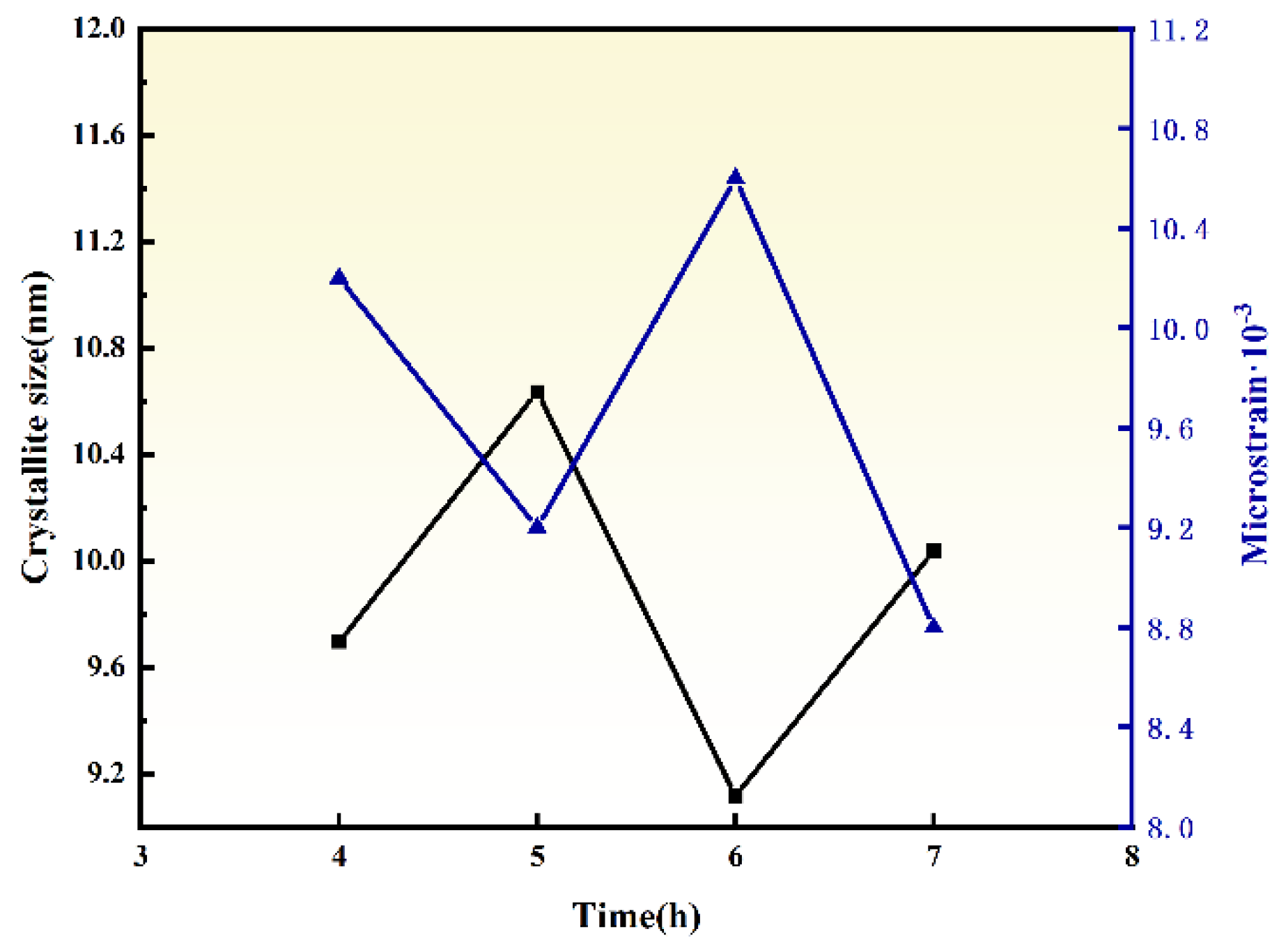
3.3. Effect of Ball Milling Time
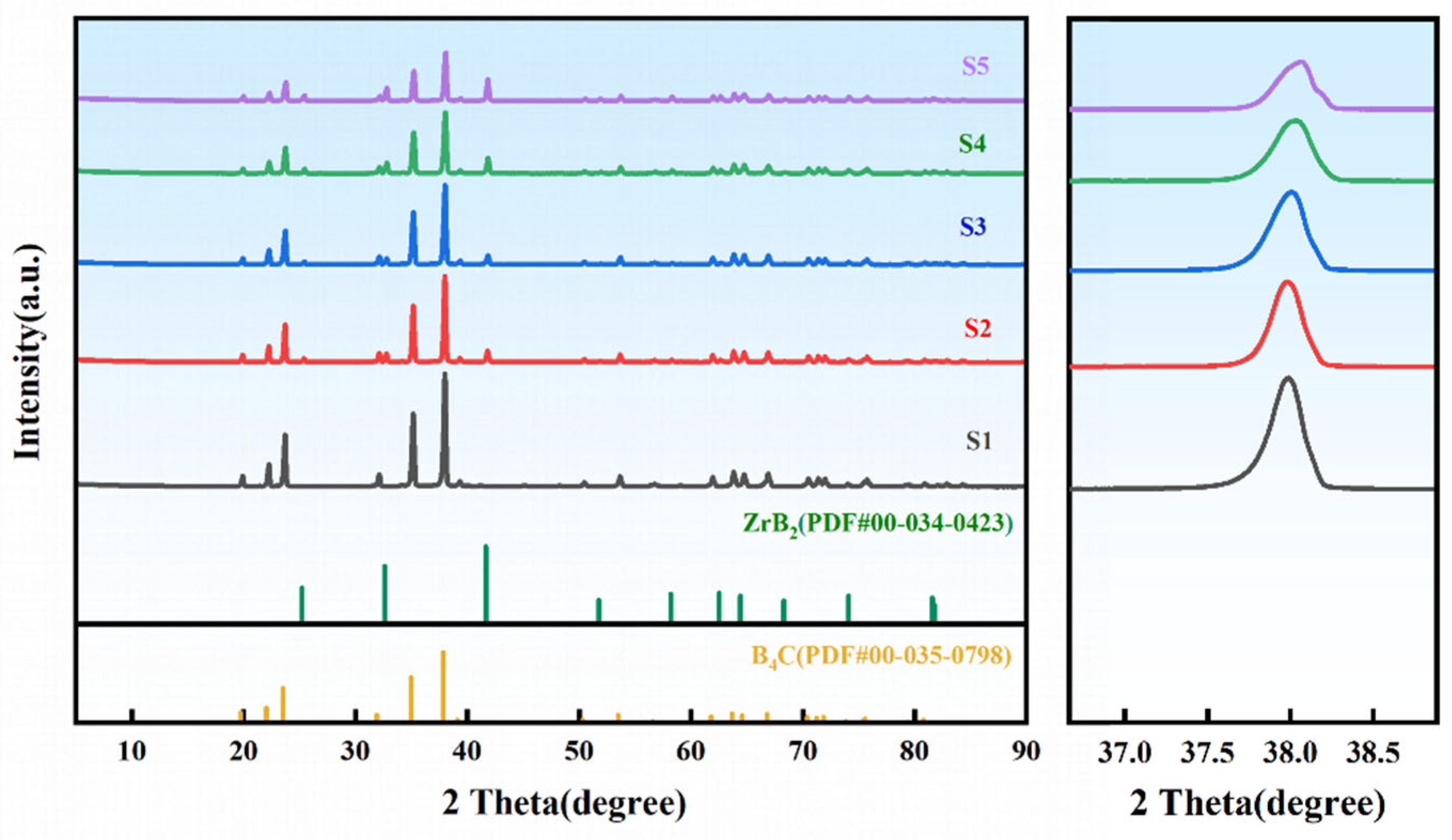
3.4. Phase Characterization of ZrO2-B4C Composites
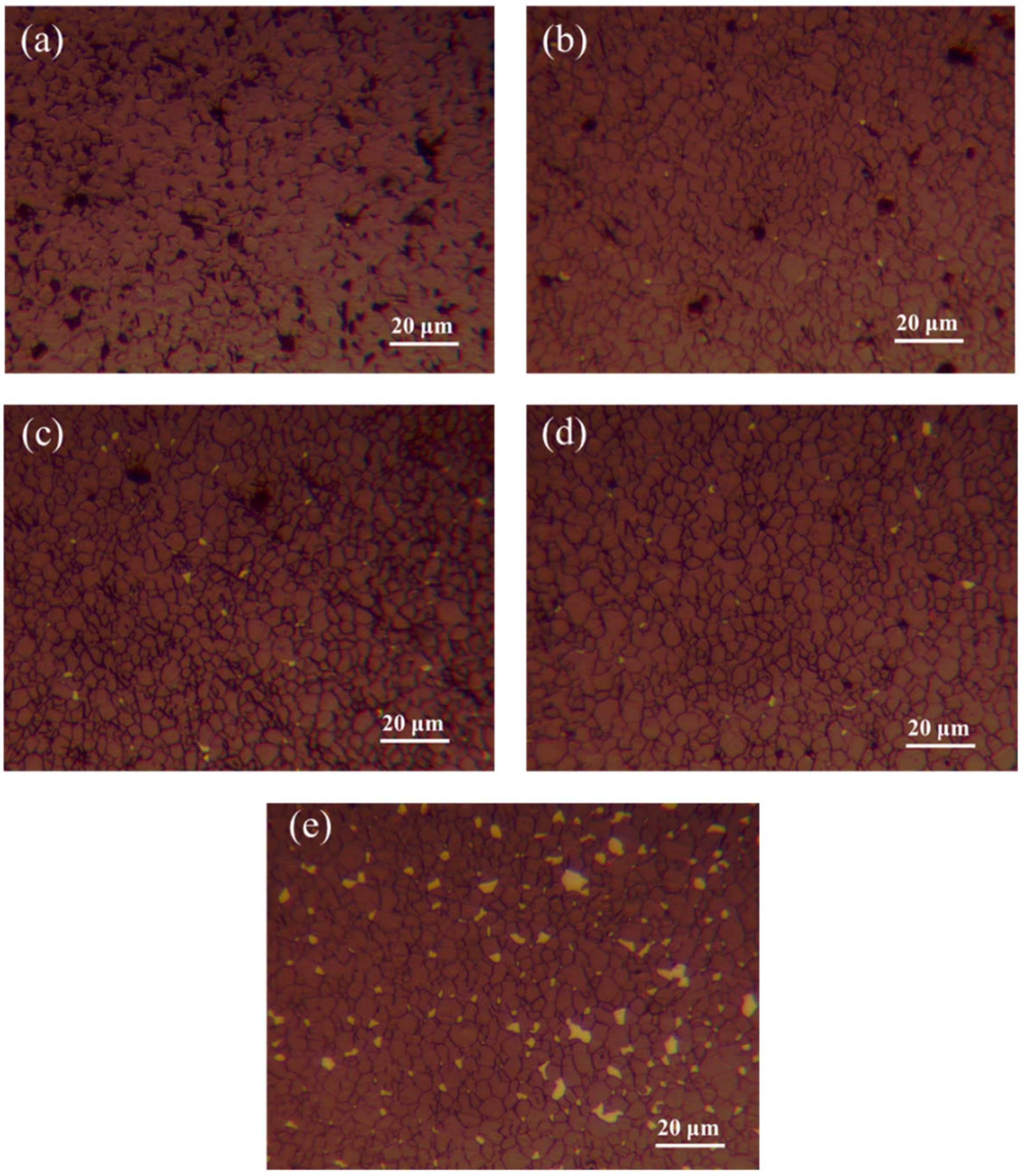
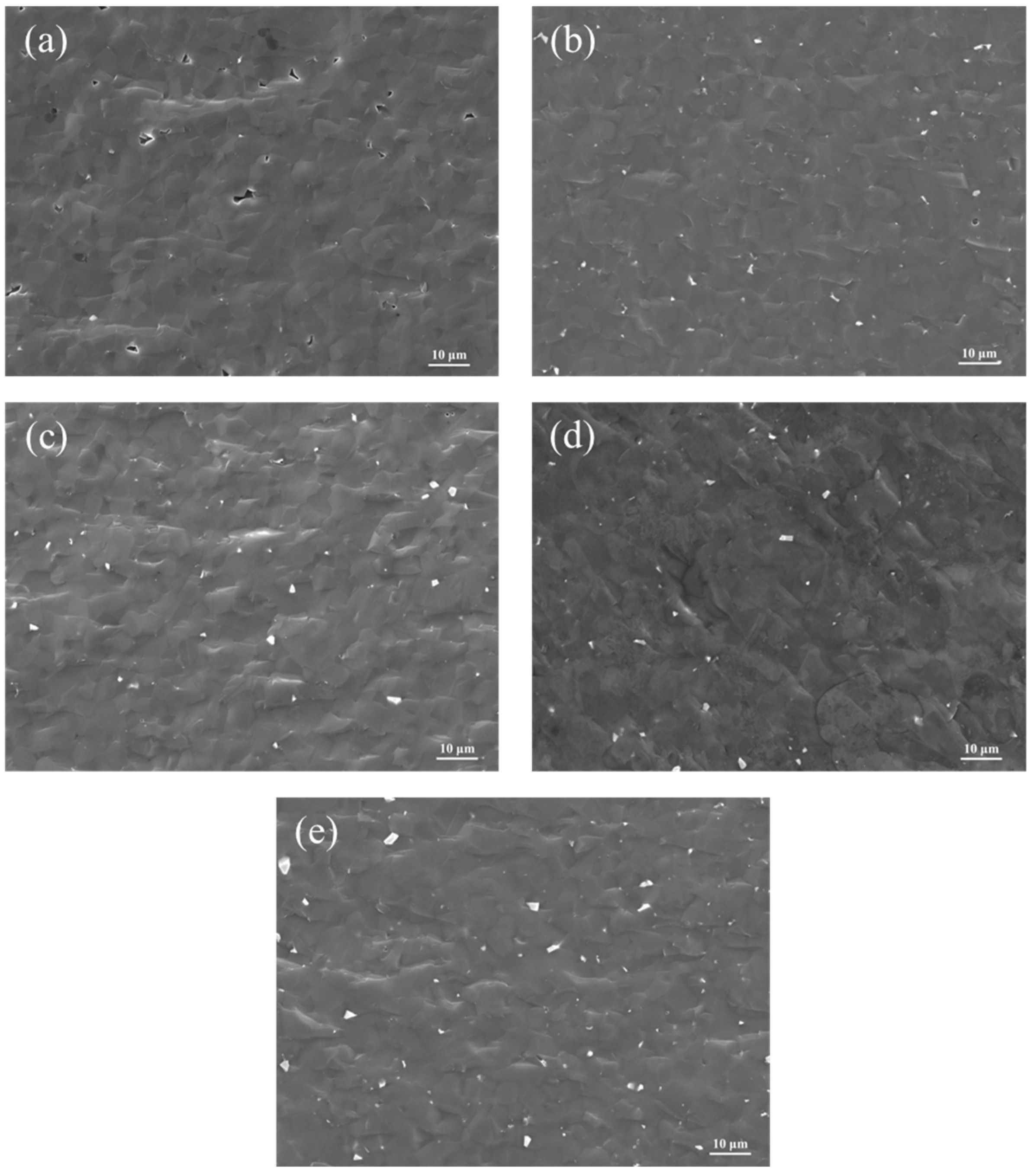
3.5. Effect of ZrO2 Content on the Microstructure of B4C Ceramics
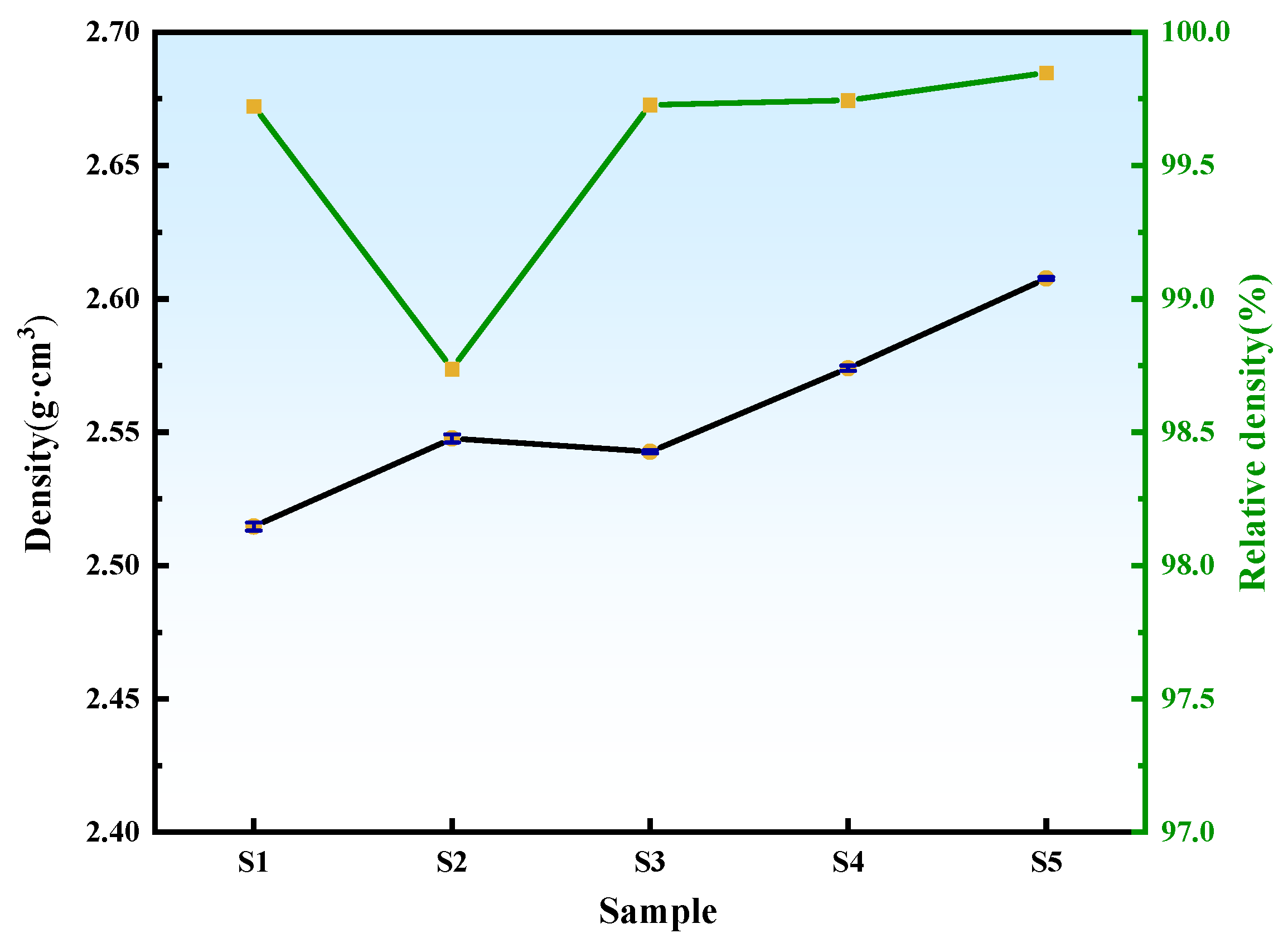
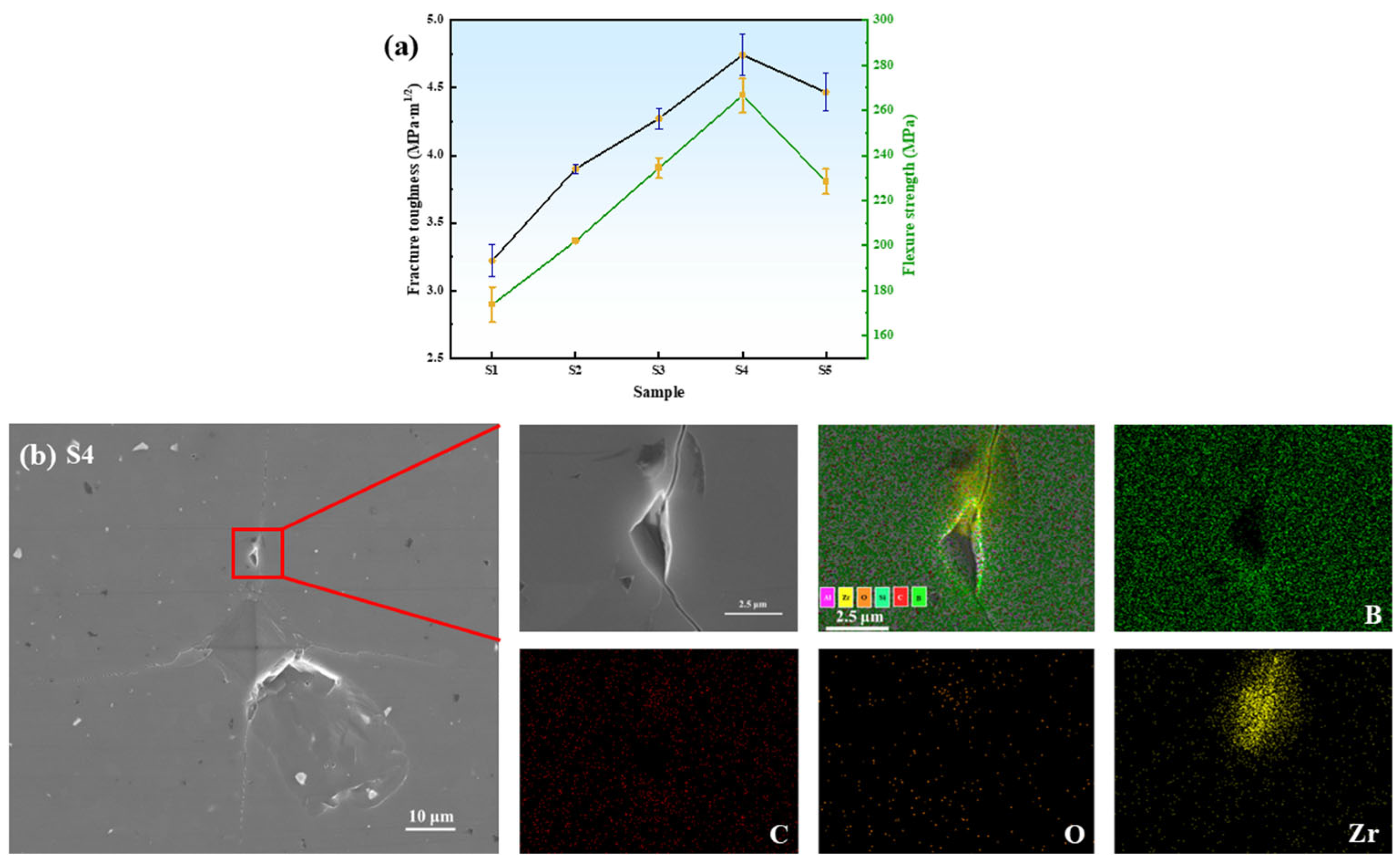
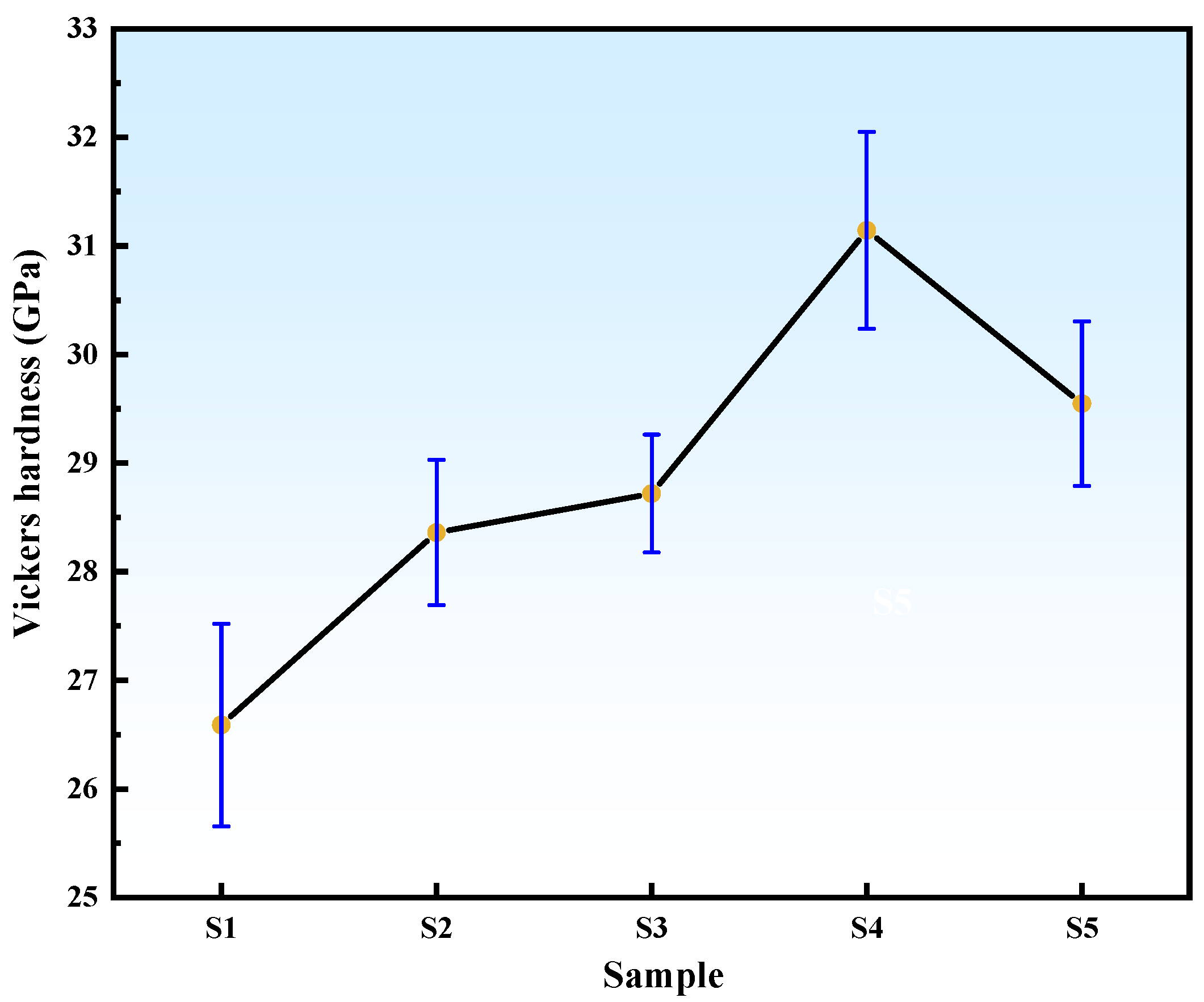
3.6. Effect of ZrO2 Content on the Mechanical Properties of B4C Ceramics
4. Conclusions
- (1)
- HTMT Process Optimization: The ZrO2 particle size exhibited a non-monotonic trend (initial decrease followed by increase) with rising ball milling temperature (400–700 °C) and prolonged duration (4–7 h). Optimal conditions were identified as 600 °C for 6 h, yielding ZrO2 powders with narrow size distribution (9.12 nm), low microstrain (10.64 × 10−3), high crystallinity, and excellent dispersion.
- (2)
- Composite Performance: Compared to commercial nano-ZrO2, HTMT-ZrO2 demonstrated superior uniformity and dispersion, enabling enhanced densification of ZrB2-B4C composites. With increasing in situ-generated ZrB2 content, B4C crystallite size initially decreased (5.5 ± 0.3 μm at 2 wt%) and then increased (7.2 ± 0.4 μm at 6 wt%), while mechanical properties (fracture toughness: 4.74 MPa·m1/2; flexural strength: 266.61 MPa; Vickers hardness: 31.14 GPa) peaked at 4 wt% HTMT-ZrO2.
- (3)
- This mechanism prolongs the crack extension path, resulting in a 47.2% increase in fracture toughness over B4C ceramics without ZrO2 addition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Feng, Y.; Zhong, Z.; Guo, X.; Zhang, Z.; Li, J.; Zhao, S.; Wu, S.; Sun, H. Modified room temperature solid-state synthesis of yttria-stabilized zirconia (YSZ) nano-powders for solid oxide fuel cells. J. Rare Earths 2023, 41, 1385–1391. [Google Scholar] [CrossRef]
- Bugaeva, A.Y.; Nazarova, L.Y.; Tropnikov, E.M.; Shushkov, D.A.; Utkin, A.A.; Ryabkov, Y.I. Preparation, Microstructure, and Properties of a Ceramic Composite Based on Stabilized Zirconium Dioxide. Russ. J. Gen. Chem. 2023, 93, 2822–2830. [Google Scholar] [CrossRef]
- Ozsoy, M.; Tıkız, İ.; Pehlivan, H. Thermal analysis of a zirconium dioxide coated aluminum alloy piston. Int. J. Comput. Exp. Sci. Eng. Fail. Anal. 2018, 4, 43–50. [Google Scholar] [CrossRef]
- Ashok Kumaravel, V.K.; Elangovan, G. Influence of nano zirconia on the mechanical and durability properties of high-performance concrete containing nano-silica. Mater. Res. Express 2023, 10, 105012. [Google Scholar] [CrossRef]
- Yamagiwa, K.; Goudo, D. Synthesis of carbon nanotubes on carbon fiber substrates: Effects of nanozirconia dispersion on the growth of carbon nanotubes. Jpn. J. Appl. Phys. 2024, 63, 02SP04. [Google Scholar] [CrossRef]
- Song, Q.; Zha, X.; Gao, M.; Shi, J.; Ma, Y. Influence of ZrO2 on the phase composition and mechano-physical properties of MgO–ZrO2 refractories prepared by cold isostatic pressing. Ceram. Int. 2024, 50, 30474–30482. [Google Scholar] [CrossRef]
- Chakravarty, R.; Shukla, R.; Ram, R.; Tyagi, A.K.; Dash, A.; Venkatesh, M. Development of a nano-zirconia based 68Ge/68Ga generator for biomedical applications. Nucl. Med. Biol. 2011, 38, 575–583. [Google Scholar] [CrossRef]
- Bugaeva, A.Y.; Nazarova, L.Y.; Belyi, V.A.; Ryabkov, Y.I. Phase Transformations of Zirconium Dioxide and Crystal Growth During Heat Treatment of the ZrO2(CeO2,Y2O3)–La0.85Y0.15Al11O18–Al2O3 System. Russ. J. Gen. Chem. 2022, 92, 1488–1497. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, H.; Zhang, Y.; Nan, L.; Gao, L.; Chen, J.; Omran, M.; Chen, G. Preparation of nano zirconia by binary doping: Effect of controlled sintering on structure and phase transformation. Ceram. Int. 2022, 48, 25374–25381. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Yang, K.-H.; Chang, K.-M.; Yeh, S.-W.; Wang, M.-C. Synthesis and crystallization behavior of 3mol% yttria stabilized tetragonal zirconia polycrystals (3Y-TZP) nanosized powders prepared using a simple co-precipitation process. J. Alloys Compd. 2011, 509, 6864–6870. [Google Scholar] [CrossRef]
- Sarkar, D.; Mohapatra, D.; Ray, S.; Bhattacharyya, S.; Adak, S.; Mitra, N. Synthesis and characterization of sol–gel derived ZrO2 doped Al2O3 nanopowder. Ceram. Int. 2007, 33, 1275–1282. [Google Scholar] [CrossRef]
- Li, N.; Dong, B.; Yuan, W.; Gao, Y.; Zheng, L.; Huang, Y.; Wang, S. ZrO2 Nanoparticles Synthesized using Ionic Liquid Microemulsion. J. Dispers. Sci. Technol. 2007, 28, 1030–1033. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, B.; Jiang, G.; Yang, J.; Li, Y.; Liu, W.; Wang, J.; Kong, W. From modification to mechanism: Supercritical hydrothermal synthesis of nano-zirconia. Ceram. Int. 2022, 48, 4401–4423. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Gao, Y.; Wu, J.; Tang, B. Ultrafine nano zirconia as electrochemical pseudocapacitor material. Ceram. Int. 2015, 41, 2626–2630. [Google Scholar] [CrossRef]
- Puclin, T.; Kaczmarek, W.A.; Ninham, B.W. Mechanochemical processing of ZrSiO4. Mater. Chem. Phys. 1995, 40, 73–81. [Google Scholar] [CrossRef]
- Ding, J.; Tsuzuki, T.; McCormick, P.G. Mechanochemical synthesis of ultrafine ZrO2 powder. Nanostruct. Mater. 1997, 8, 75–81. [Google Scholar] [CrossRef]
- Liu, M.; Wang, B.; Wang, Y.; Fu, D.; Chang, Y.; Li, B.; Liu, K.; He, X.; Chen, J.; Wei, S.; et al. Intercalation of AlCl3 in microcrystalline graphite via high-temperature mechanochemical method for electromagnetic wave absorption. Appl. Surf. Sci. 2024, 666, 160387. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Li, B.; Wang, B.; Wang, Y.; Han, Q.; Wei, S.; Liu, K.; He, X.; Sun, R.; et al. Electromagnetic wave absorption properties of Mn0.4Zn0.6Fe2O4 powders synthesized by high-temperature mechanochemical method. Mater. Sci. Eng. B 2024, 302, 117243. [Google Scholar] [CrossRef]
- Hou, Y.; Li, B.; Chen, J.; Shen, X.; Wang, B.; Liu, K.; Wei, S.; He, X.; Li, D.; Han, Q. Electromagnetic wave absorption properties of core double-shell structured α-Fe(Si)@Fe3O4@SiO2 composites. Appl. Surf. Sci. 2023, 615, 156345. [Google Scholar] [CrossRef]
- Jia, P.; Shao, Z.; Liu, K. Synthesis and electrochemical performance of Li4Ti5O12 by high temperature ball milling method. Mater. Lett. 2014, 125, 218–220. [Google Scholar] [CrossRef]
- Salim, E.T.; Ismail, R.A.; Halbos, H.T. Growth of Nb2O5 film using hydrothermal method: Effect of Nb concentration on physical properties. Mater. Res. Express 2019, 6, 116429. [Google Scholar] [CrossRef]
- Mondal, A.; Ram, S. Monolithic t-ZrO2 Nanopowder through a ZrO(OH)2·xH2O Polymer Precursor. J. Am. Ceram. Soc. 2004, 87, 2187–2194. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Garvie, R.C. The Occurrence of Metastable Tetragonal Zirconia as a Crystallite Size Effect. J. Phys. Chem. 1965, 69, 1238–1243. [Google Scholar] [CrossRef]
- Kim, H.-W.; Koh, Y.-H.; Kim, H.-E. Reaction sintering and mechanical properties of B4C with addition of ZrO2. J. Mater. Res. 2000, 15, 2431–2436. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, F.; Li, W.; Liu, J.A.; Gu, Y.; Zhang, F. Densification behavior and mechanical properties of spark plasma reaction sintered ZrB2–ZrC-B4C ceramics from B4C-Zr system. Ceram. Int. 2019, 45, 12122–12129. [Google Scholar] [CrossRef]
- Wang, X.-G.; Guo, W.-M.; Kan, Y.-M.; Zhang, G.-J. Hot-Pressed ZrB2 Ceramics With Composite Additives of Zr and B4C. Adv. Eng. Mater. 2010, 12, 893–898. [Google Scholar] [CrossRef]
- Xiong, Y.; Du, X.; Xiang, M.; Wang, H.; Wang, W.; Fu, Z. Densification mechanism during reactive hot pressing of B4C-ZrO2 mixtures. J. Eur. Ceram. Soc. 2018, 38, 4167–4172. [Google Scholar] [CrossRef]
- Sagar, J.S.; Kashyap, S.J.; Madhu, G.M. Investigation of mechanical, thermal and electrical parameters of gel combustion-derived cubic zirconia/epoxy resin composites for high-voltage insulation. Cerâmica 2020, 66, 186–196. [Google Scholar] [CrossRef]



| Sample | Characteristics (wt%) | |||
|---|---|---|---|---|
| B4C | Commercially Available Nano-ZrO2 | HTMT Nano-ZrO2 | SiO2 | |
| S1 | 99 | 0 | 0 | 1 |
| S2 | 97 | 2 | 0 | 1 |
| S3 | 97 | 0 | 2 | 1 |
| S4 | 95 | 0 | 4 | 1 |
| S5 | 93 | 0 | 6 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Jia, J.; Li, B.; Fu, D.; Wang, C.; Liu, K.; Wei, S.; Han, Q. High-Temperature Mechanochemical Synthesis of Nano-ZrO2 for Enhanced Densification and Fracture Toughness in B4C Ceramics. Materials 2025, 18, 2332. https://doi.org/10.3390/ma18102332
Xu J, Jia J, Li B, Fu D, Wang C, Liu K, Wei S, Han Q. High-Temperature Mechanochemical Synthesis of Nano-ZrO2 for Enhanced Densification and Fracture Toughness in B4C Ceramics. Materials. 2025; 18(10):2332. https://doi.org/10.3390/ma18102332
Chicago/Turabian StyleXu, Jingming, Jinchao Jia, Binchuan Li, Daxue Fu, Chunxin Wang, Kuiren Liu, Shicheng Wei, and Qing Han. 2025. "High-Temperature Mechanochemical Synthesis of Nano-ZrO2 for Enhanced Densification and Fracture Toughness in B4C Ceramics" Materials 18, no. 10: 2332. https://doi.org/10.3390/ma18102332
APA StyleXu, J., Jia, J., Li, B., Fu, D., Wang, C., Liu, K., Wei, S., & Han, Q. (2025). High-Temperature Mechanochemical Synthesis of Nano-ZrO2 for Enhanced Densification and Fracture Toughness in B4C Ceramics. Materials, 18(10), 2332. https://doi.org/10.3390/ma18102332







