Stabilization of Styrene Pickering Emulsions Using SiO2 Derived from Waste Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
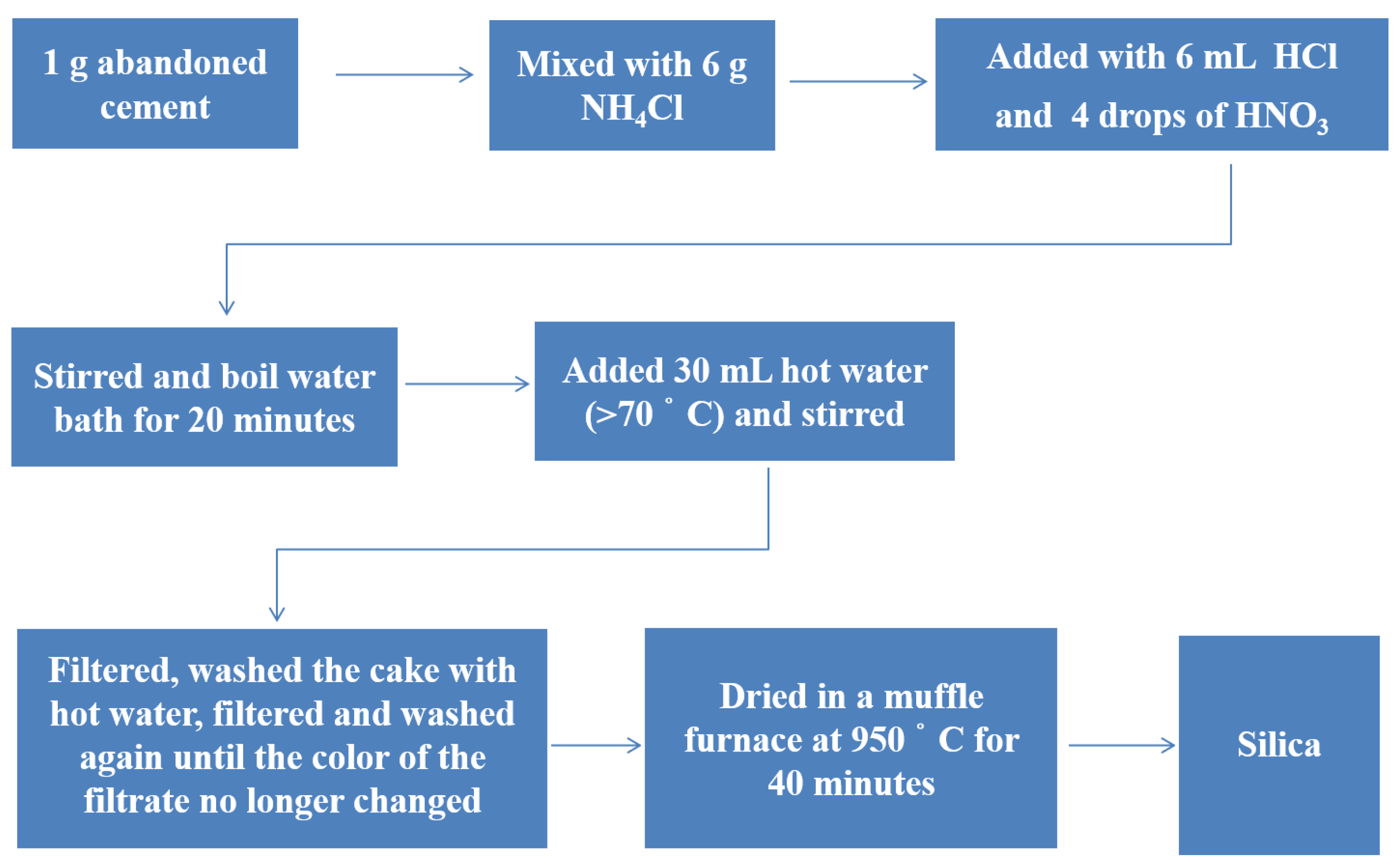
2.2. Extraction of Silica
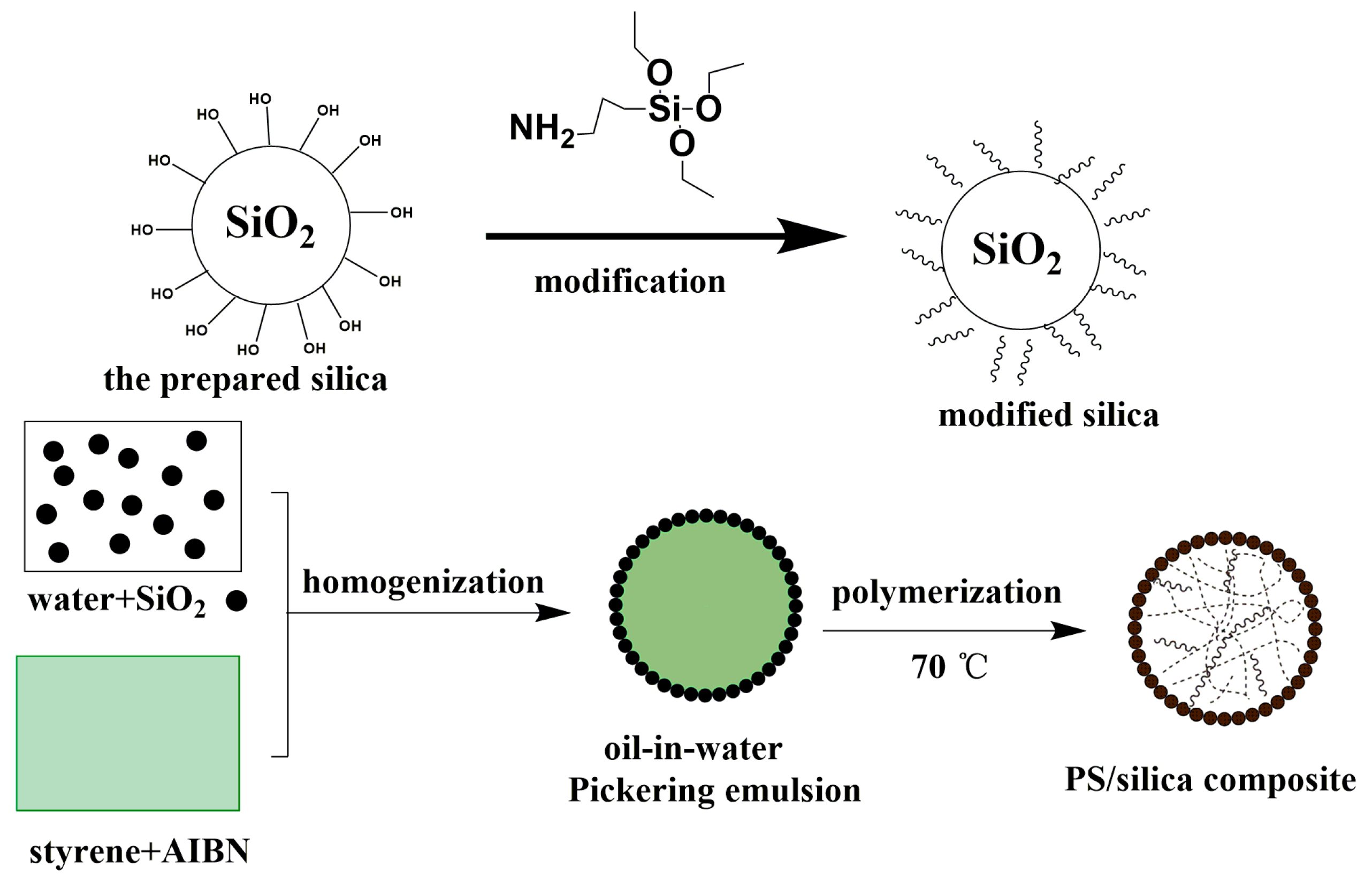
2.3. Modification of the Prepared Silica
2.4. Preparation of Pickering Emulsion and Polymerization
2.5. Measurements
3. Results
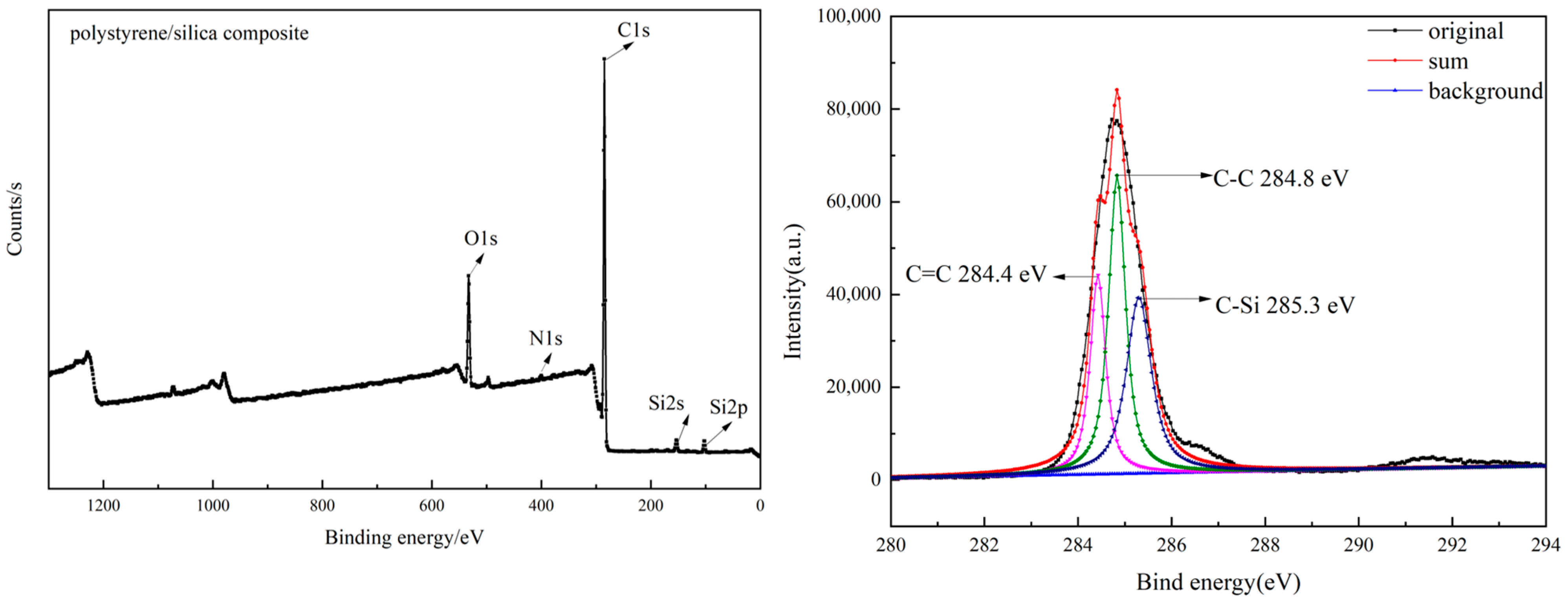
3.1. Formation of the Polystyrene/Silica Composite
3.2. Formation of the Pickering Emulsion Stabilized by the Prepared Silica
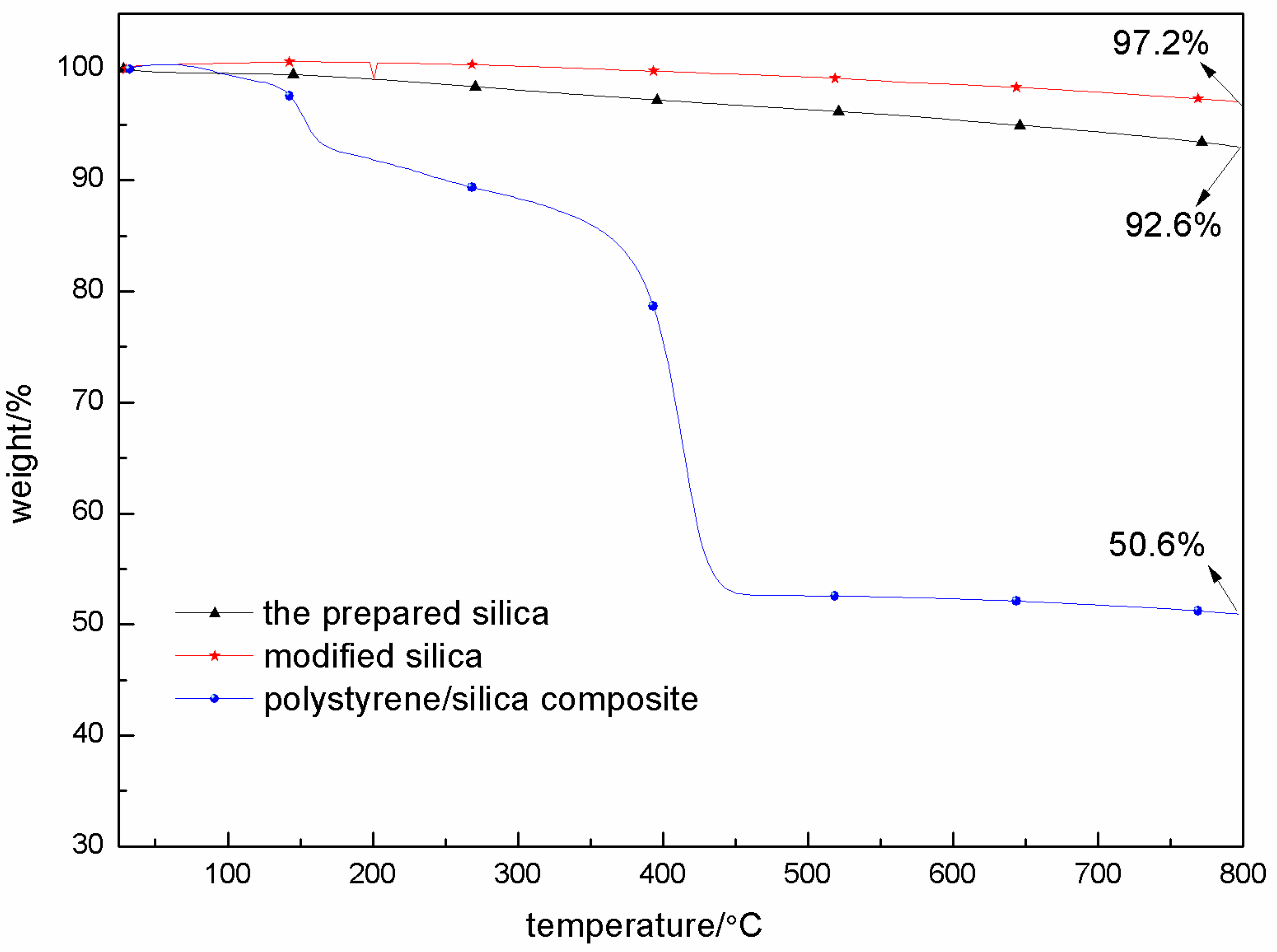
3.3. Thermal Stability
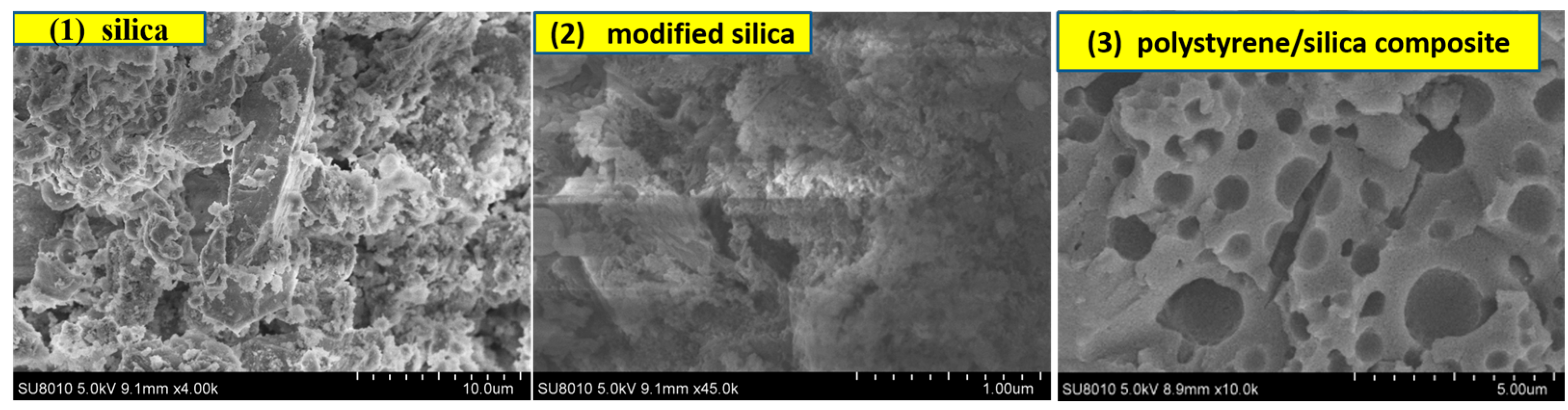
3.4. FE-SEM Image Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, M.; Luo, Q.; Liu, J.; Zhu, Z.; Dai, L.; Rong, Y.; Zhu, Y.; Fan, Z.; Han, W. Environmentally friendly zwitterionic fluorocarbon surfactant for high-efficiency emulsion polymerization of fluoropolymers. J. Coat. Technol. Res. 2024, 21, 1831–1842. [Google Scholar] [CrossRef]
- Chen, N.; Liu, W.; Huang, J.; Qiu, X. Preparation of octopus-like lignin-grafted cationic polyacrylamide flocculant and its application for water flocculation. Int. J. Biol. Macromol. 2020, 146, 9–17. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Zheng, C.; Lei, Q.; Dong, Y.; Zhao, X.; Yin, J. Pickering emulsion polymerization of poly(ionic liquid)s encapsulated nano-SiO2 composite particles with enhanced electro-responsive characteristic. Polymer 2018, 146, 109–119. [Google Scholar] [CrossRef]
- Harman, C.L.; Patel, M.A.; Guldin, S.; Davies, G.-L. Recent developments in Pickering emulsions for biomedical applications. Curr. Opin. Colloid Interface Sci. 2019, 39, 173–189. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Qazvini, N.T.; Deng, Y. Surfactant free Pickering emulsion polymerization of styrene in w/o/w system using cellulose nanofibrils. Eur. Polym. J. 2015, 64, 179–188. [Google Scholar] [CrossRef]
- Piao, X.; Guo, H.; Cao, Y.; Wang, Z.; Jin, C. Preparation and exploration of multifunctional wood coating based on an interpenetrating network system of CO2-polyurethane and natural bio-based benzoxazine. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129437. [Google Scholar] [CrossRef]
- Cohen, R.; Mani, K.A.; Pirmatova, M.; Jacobi, G.; Zelinger, E.; Belausov, E.; Fallik, E.; Banin, E.; Mechrez, G. A green formulation for superhydrophobic coatings based on Pickering emulsion templating for anti-biofilm applications. Colloids Surf. B Biointerfaces 2023, 227, 113355. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Nikfarjam, N.; Kazemi, S.H. Hollow molecularly imprinted microspheres made by w/o/w double Pickering emulsion polymerization stabilized by graphene oxide quantum dots targeted for determination of l-cysteine concentration. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125978. [Google Scholar] [CrossRef]
- Heidari, F.; Jafari, S.M.; Ziaiifar, A.M.; Anton, N. Surface modification of silica nanoparticles by chitosan for stabilization of water-in-oil Pickering emulsions. Carbohydr. Polym. Technol. Appl. 2023, 6, 100381. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Monteiro, S.S.; Santos, N.C.; Rios, N.S.; dos Santos, E.S. Starch modification and its application in Pickering emulsion stabilization: A review. J. Food Meas. Charact. 2024, 18, 4984–5003. [Google Scholar] [CrossRef]
- Bakera, A.T.; Alexander, M.G. In situ concrete sewer performance: Comparison of Portland cement, calcium sulfoaluminate cement, and calcium aluminate cement. Mater. Struct. 2024, 57, 114. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Lu, H. Modified Janus silica-based sheets stable stimulus-responsive Pickering emulsion: An excellent recycling platform for tandem reaction. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134485. [Google Scholar] [CrossRef]
- Sertsoongnern, P.; Ayawanna, J.; Kingnoi, N.; Chaiyaput, S. Utilization of asphalt waste dust with fly ash in mixed cement materials for sustainable construction. Sustain. Chem. Pharm. 2024, 41, 101699. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Lai, L. Surface activity, micellization, and application of nano-surfactants—Amphiphilic carbon dots. Carbon 2023, 202, 398–413. [Google Scholar] [CrossRef]
- Ip, H.T.; Liu, L.; Hong, L.; Ngai, T. Synthesis of polystyrene/silica and poly(styrene-co-butyl acrylate)/silica nanocomposite particles by Pickering emulsion polymerization with non-functionalized silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130104. [Google Scholar] [CrossRef]
- Yu, D.; Li, G.; Liu, W.; Li, Y.; Song, Z.; Wang, H.; Guan, F.; Chen, X. A fluorescent pickering-emulsion stabilizer prepared using carbon nitride quantum dots and laponite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 310–317. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, D.; Wang, Y.; Xie, P.; Li, M.; Zhou, L.; Chen, Y.; Liu, H. Pickering emulsion strategy for high compressive carbon aerogel for use as insulation material and dual-mode pressure/magnetic sensor. Ceram. Int. 2023, 49, 8121–8131. [Google Scholar] [CrossRef]
- Saini, S.; Gupta, A.; Mehta, A.J.; Pramanik, S. Rice husk-extracted silica reinforced graphite/aluminium matrix hybrid composite. J. Therm. Anal. Calorim. 2022, 147, 1157–1166. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Li, C.; Han, Y.; Bai, Y.; Xu, K.; Chi, H.; Liu, Y.; Huang, X.; Wang, C.; et al. Oil-in-water high-internal-phase poly(styrene-acrylate) Pickering emulsions and their applications as waterborne damping coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128783. [Google Scholar] [CrossRef]
- Gao, J.; Dong, C.; Zhao, Y.; Liang, Y.; Ning, K.; Yang, L.; He, Y. Removal of zinc ions from aqueous solution using zinc extractant-encapsulated hydrogels formed by Pickering emulsion technique. Environ. Dev. Sustain. 2023, 26, 8359–8375. [Google Scholar] [CrossRef]
- Jing, J.; Liu, H.; Wang, X. Multifunctional BiOI/SiO2/Fe3O4@nDocosane Phase-Change Microcapsules for Waste Heat Recovery and Wastewater Treatment. Materials 2023, 16, 1656. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Liu, J.; Song, W.; Xu, D.; Wang, Z.; Xie, Y. CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis. Materials 2023, 16, 1675. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.; Zhao, W.; Zhang, K. Bio-benzoxazine structural design strategy toward highly thermally stable and intrinsically flame-retardant thermosets. Chem. Eng. J. 2023, 457, 141232. [Google Scholar] [CrossRef]
- Jamnongpak, W.; Tiptipakorn, S.; Arumugam, H.; Charoensuk, K.; Karagiannidis, P.; Rimdusit, S. Development of NIR light-responsive shape memory composites based on bio-benzoxazine/bio-urethane copolymers reinforced with graphene. Nanoscale Adv. 2024, 6, 499–510. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Wan, F.; Sun, Y. Carbon quantum dots-stabilized Pickering emulsion to prepare NIR light-responsive PLGA drug delivery system. Mater. Today Commun. 2020, 23, 100951. [Google Scholar] [CrossRef]



| Samples | Modified SiO2 (wt%) | Deionized Water (mL) | Styrene (mL) | AIBN (mg) |
|---|---|---|---|---|
| 1 | 2 | 15 | 3 | 3 |
| 2 | 6 | 15 | 3 | 3 |
| 3 | 10 | 15 | 3 | 3 |
| Samples | Element Analysis on the Surface (wt%) | |||
|---|---|---|---|---|
| C | Si | O | N | |
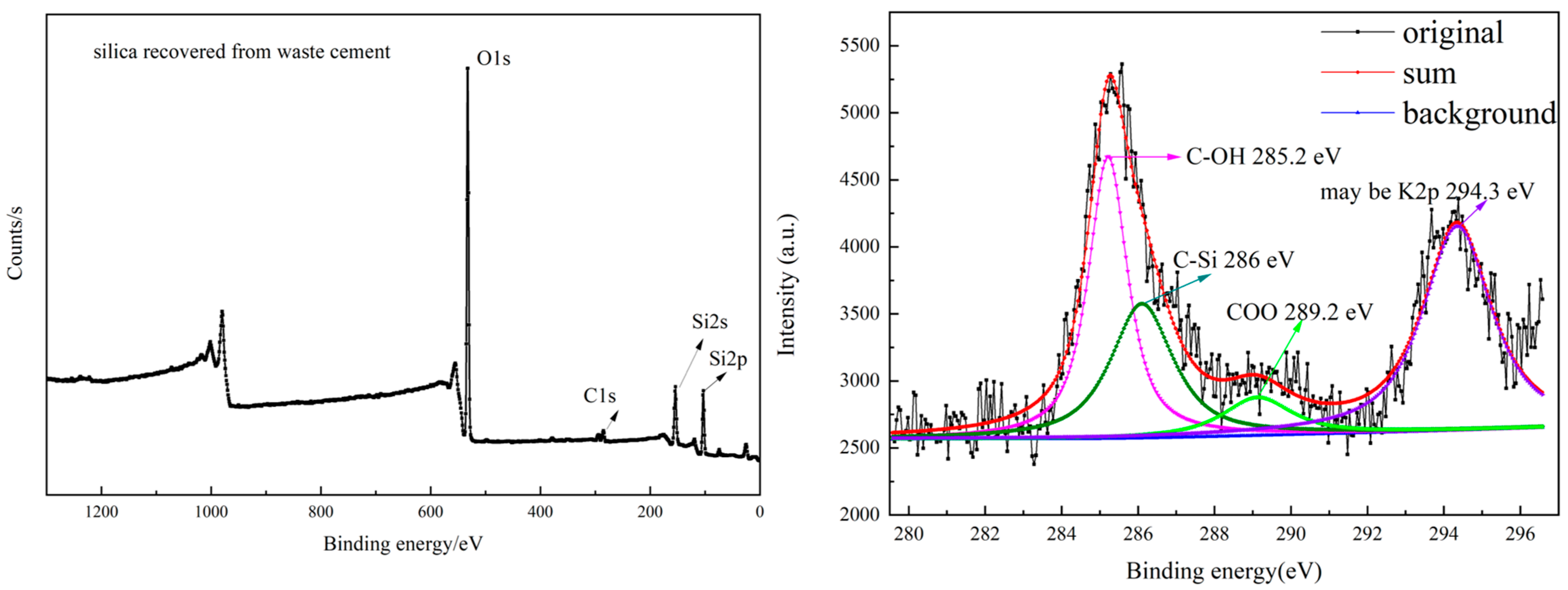
| The prepared silica | 4.14 | 26.66 | 63.59 | 0 |
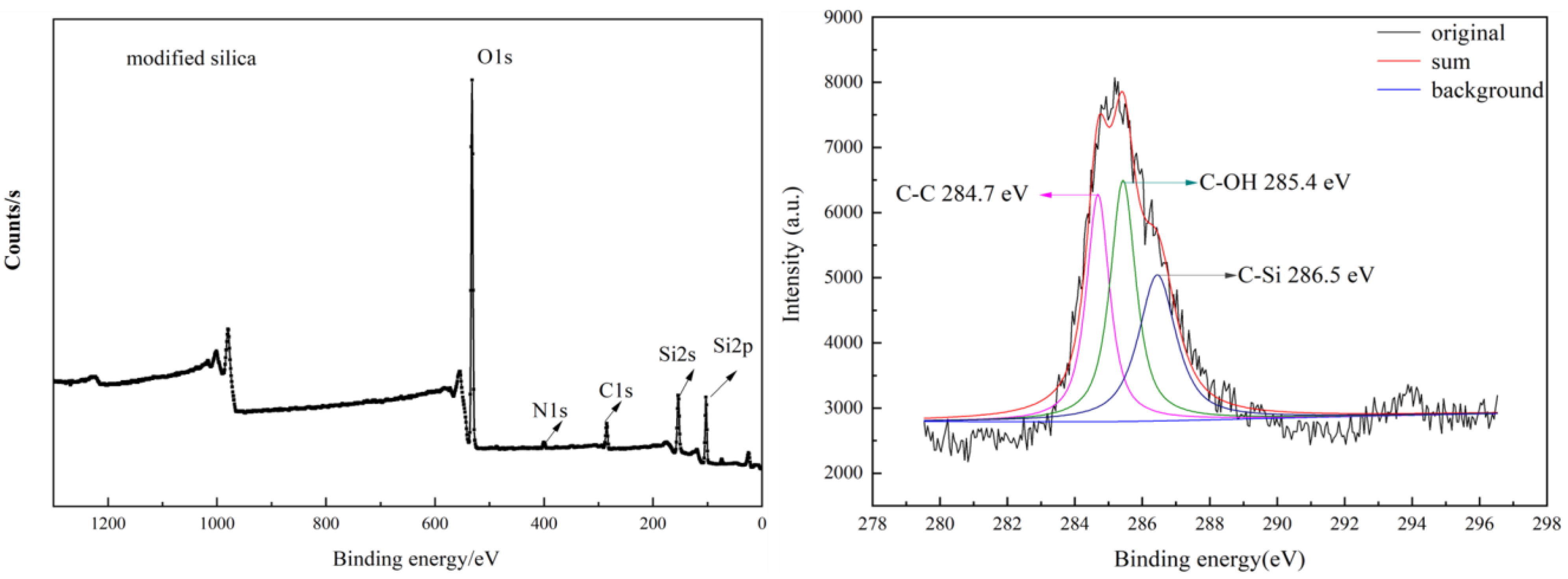
| Modified silica | 10.97 | 27.1 | 59.87 | 1.91 |
| Polystyrene/silica composites | 83.76 | 3.09 | 11.72 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Zhang, J.; Long, D.; Wang, H.; Ying, H.; Xie, H. Stabilization of Styrene Pickering Emulsions Using SiO2 Derived from Waste Cement. Materials 2025, 18, 2281. https://doi.org/10.3390/ma18102281
Xu G, Zhang J, Long D, Wang H, Ying H, Xie H. Stabilization of Styrene Pickering Emulsions Using SiO2 Derived from Waste Cement. Materials. 2025; 18(10):2281. https://doi.org/10.3390/ma18102281
Chicago/Turabian StyleXu, Guomei, Jihua Zhang, Defei Long, Huayang Wang, Hanjie Ying, and Hongxue Xie. 2025. "Stabilization of Styrene Pickering Emulsions Using SiO2 Derived from Waste Cement" Materials 18, no. 10: 2281. https://doi.org/10.3390/ma18102281
APA StyleXu, G., Zhang, J., Long, D., Wang, H., Ying, H., & Xie, H. (2025). Stabilization of Styrene Pickering Emulsions Using SiO2 Derived from Waste Cement. Materials, 18(10), 2281. https://doi.org/10.3390/ma18102281






