Improved Bone Regeneration Using Biodegradable Polybutylene Succinate Artificial Scaffold with BMP-2 Protein in a Rabbit Model
Abstract
:1. Introduction
2. Materials and Methods
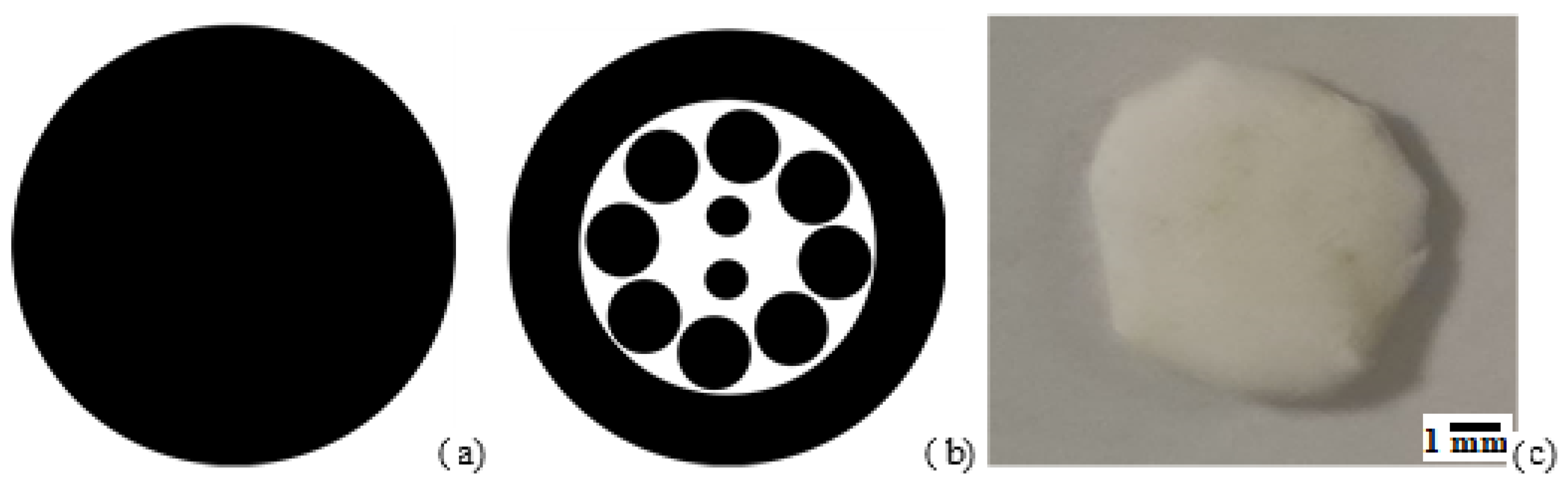
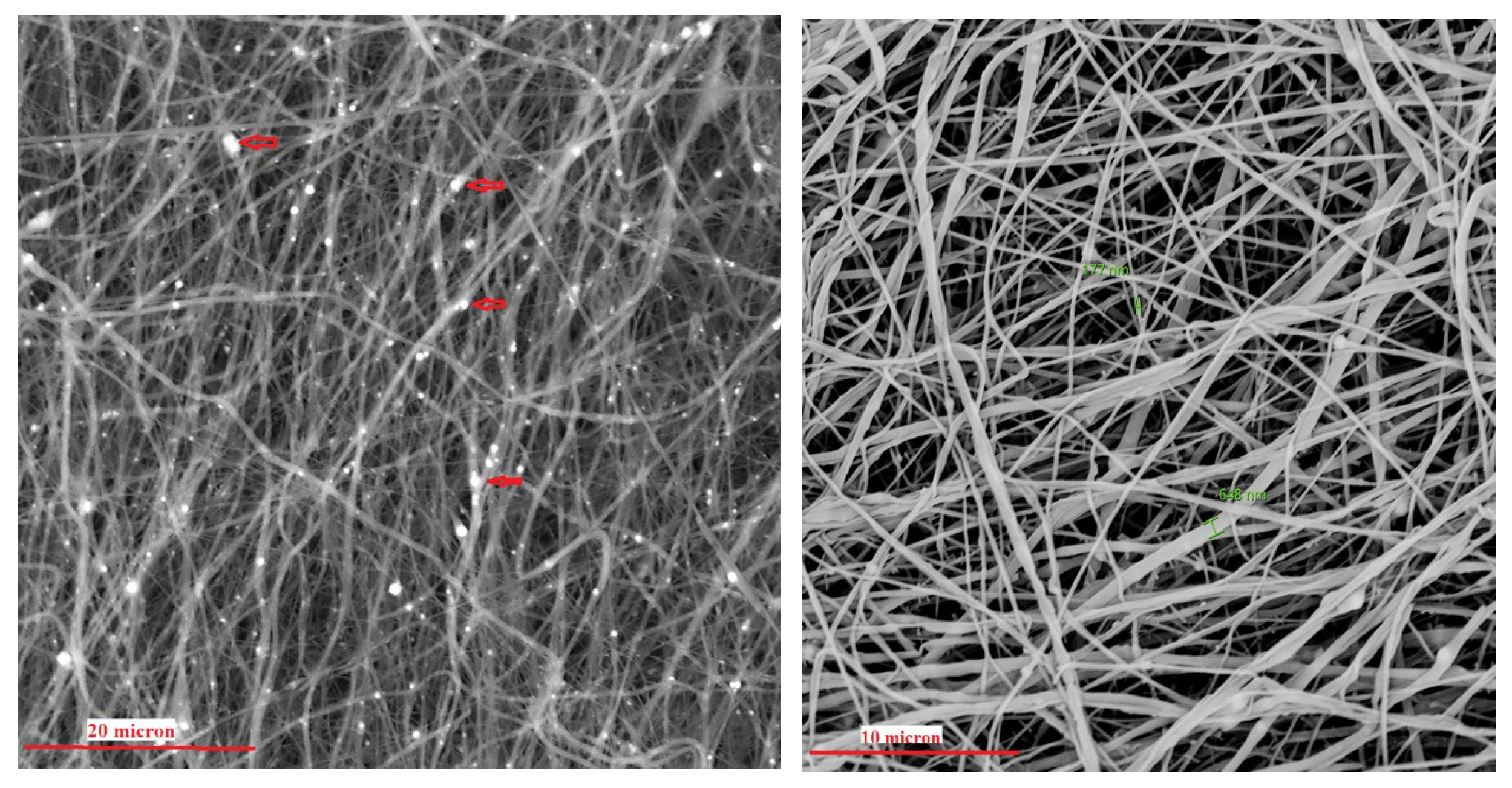
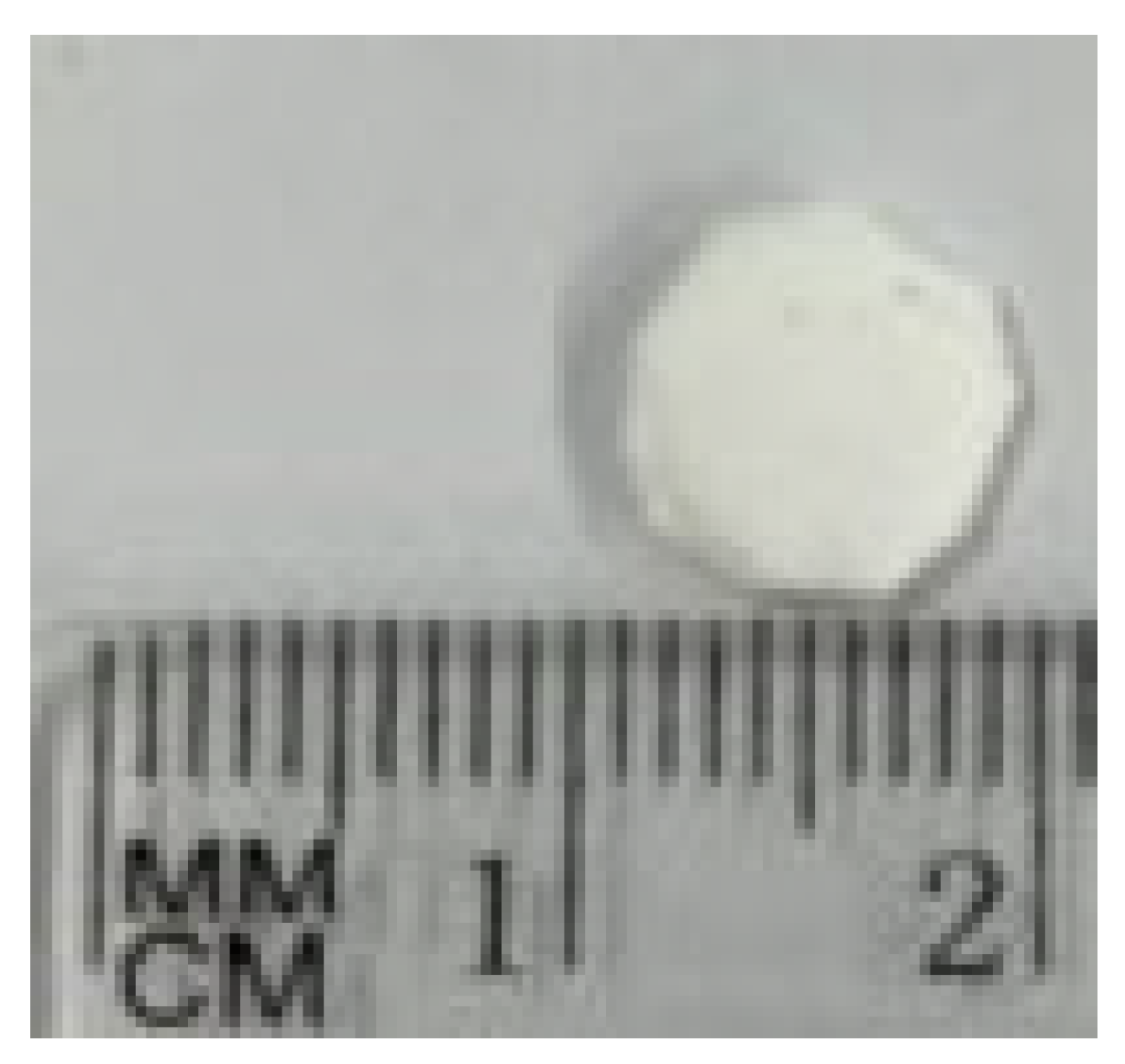
2.1. Scaffold Preparation and Characterization
2.2. Loading of Bone Morphogenetic Protein (BMP2)
2.3. Protein Deposition
2.4. Study Population
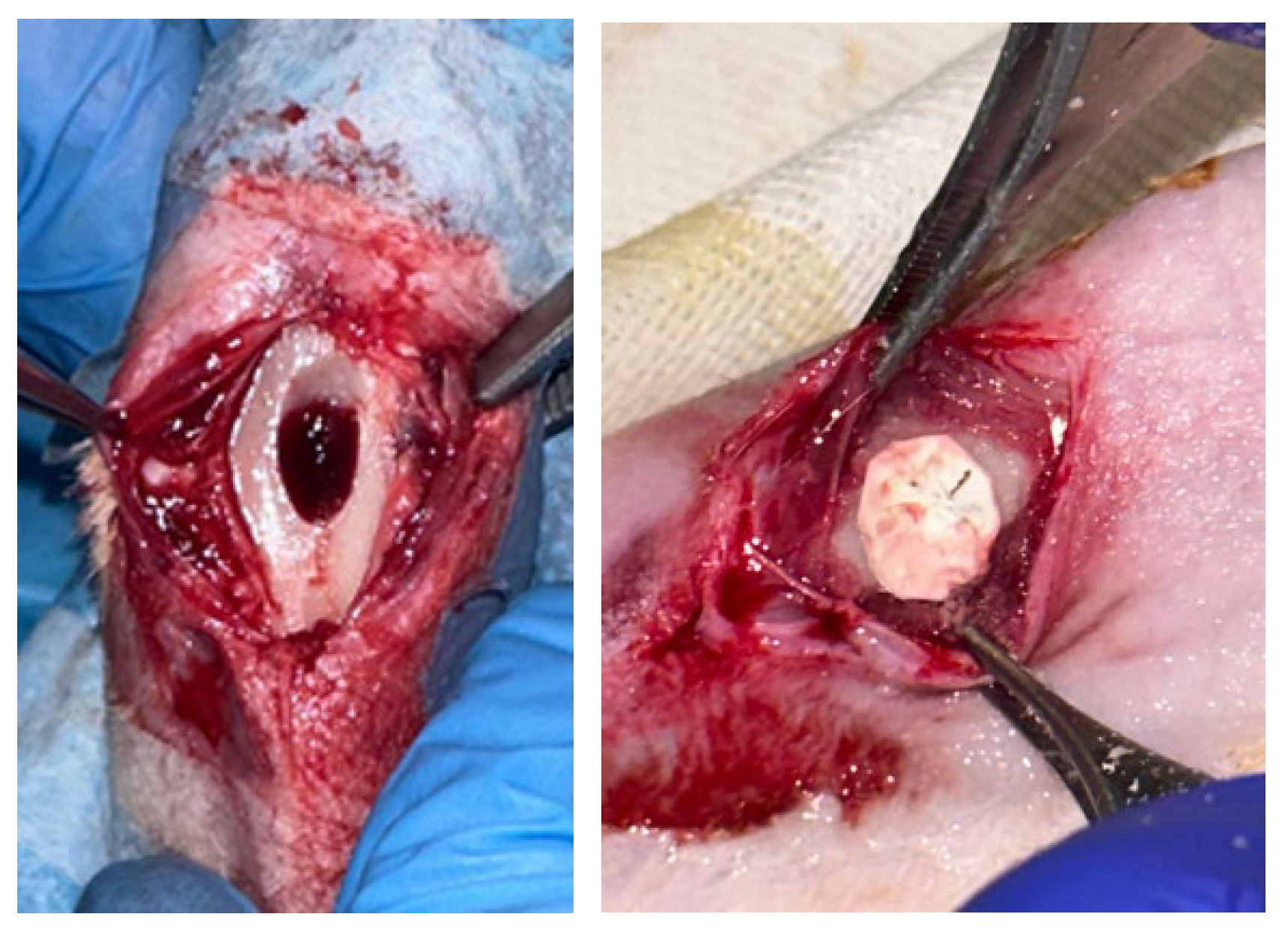
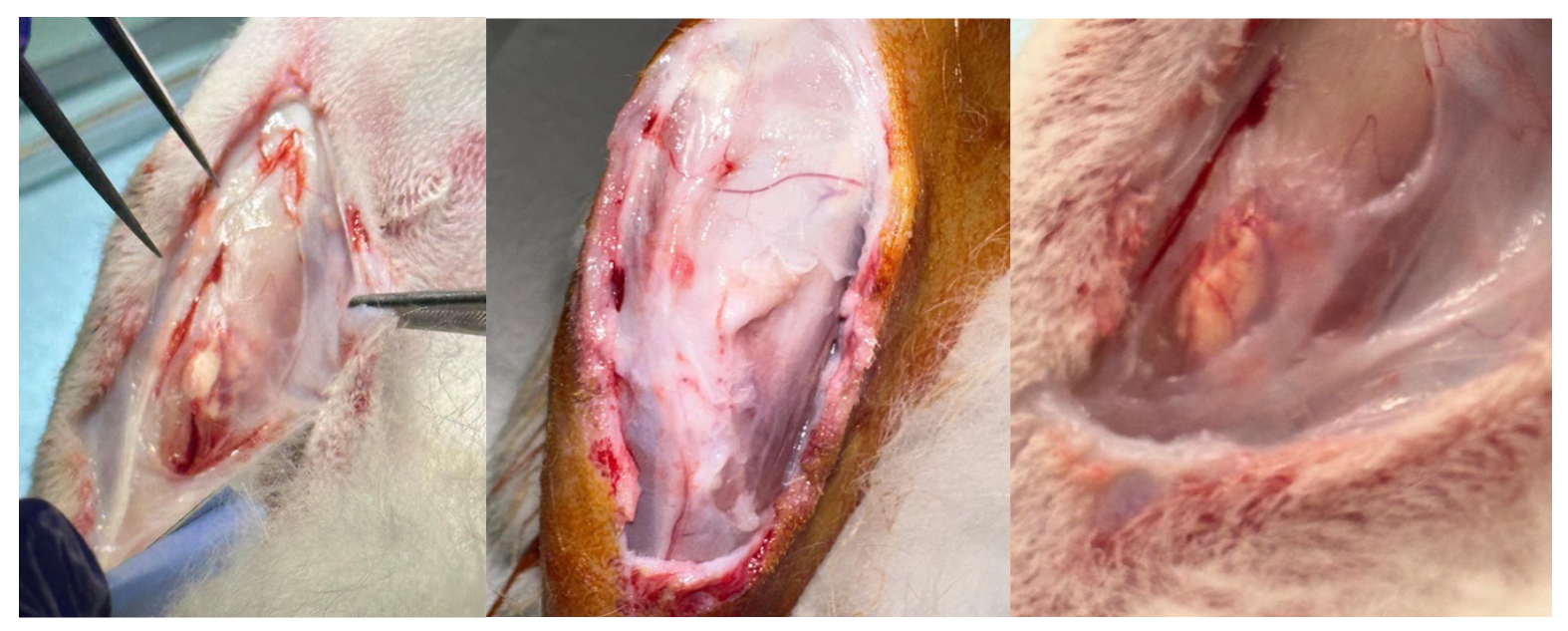
2.5. Surgical Procedure
2.6. Histology and Immunohistochemical Evaluation
2.7. Statistical Analysis
3. Results
3.1. Scaffold Characterization
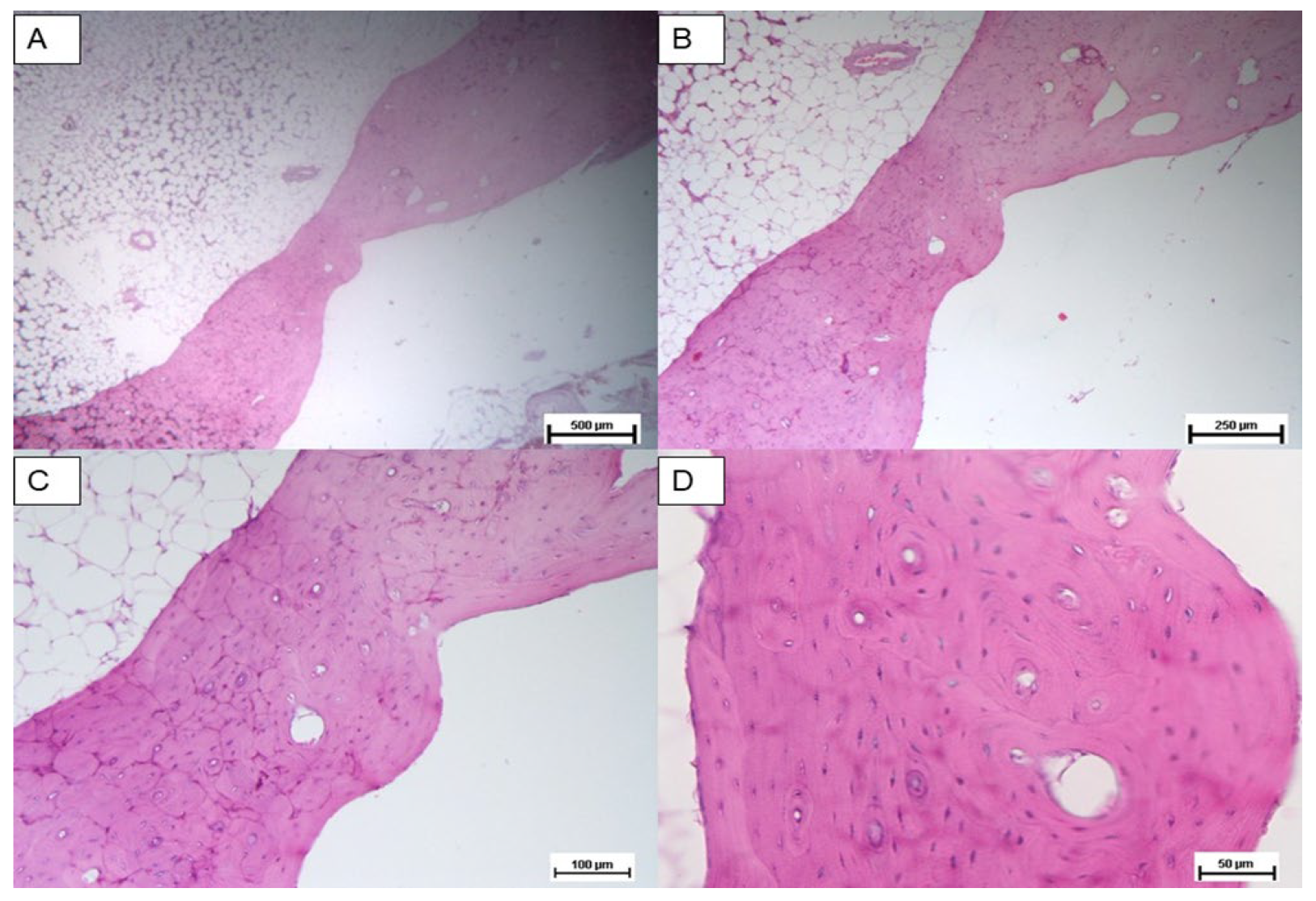
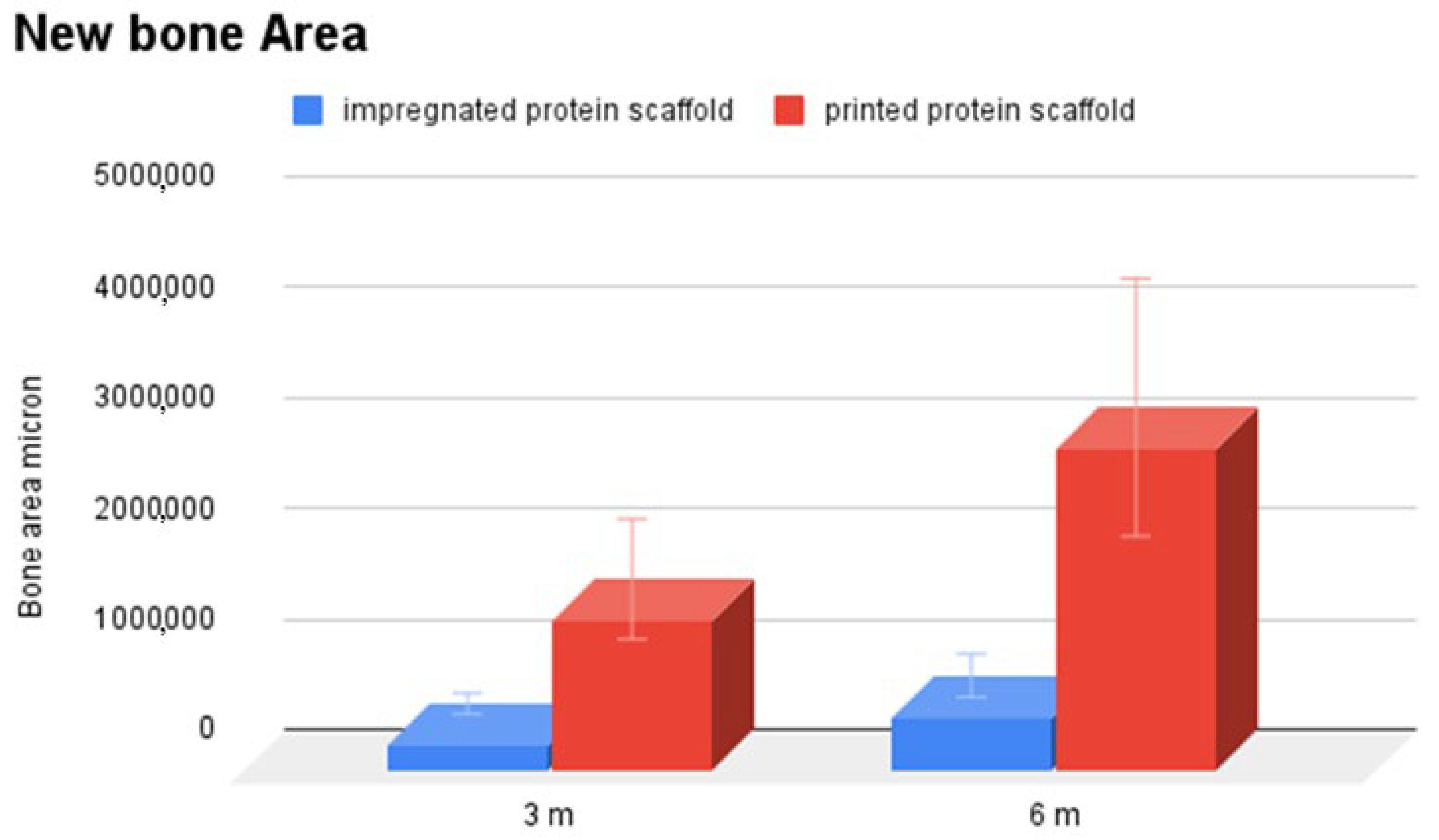
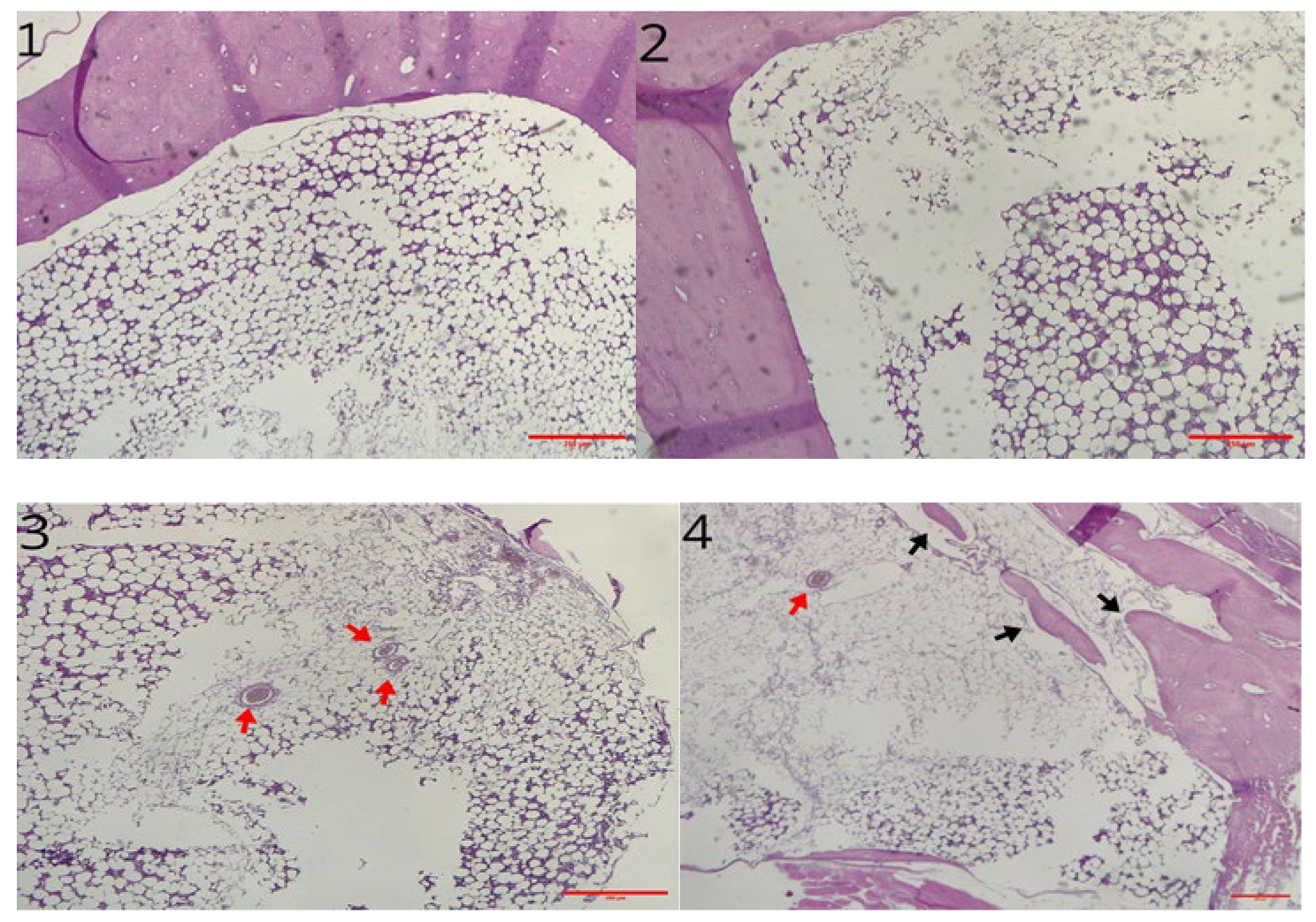
3.2. Histological Analysis
3.3. Immunohistochemical Analysis
3.4. Macroscopic Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, M.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38 (Suppl. S1), S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ao, Q.; Wang, A.; Gong, K.; Wang, X.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. Preparation and characterization of a multilayer biomimetic scaffold for bone tissue engineering. J. Biomater. Appl. 2007, 22, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.X.; He, M.; Chong, A.K.S. 3D-printed Poly-Lactic Co-Glycolic Acid (PLGA) scaffolds in non-critical bone defects impede bone regeneration in rabbit tibia bone. Biomed. Mater. Eng. 2021, 32, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.T.; Yoshimoto, M.; Allegrini, S., Jr.; Bressiani, A.H. Hydroxyapatite Dome for Bone Neoformation in Rabbit Tibia. Int. J. Oral. Maxillofac. Implants 2016, 31, 571–579. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Ma, J.; Wu, S.; Liu, J.; Liu, C.; Ni, S.; Dai, T.; Wu, X.; Zhang, Z.; Qu, J.; Zhao, H.; et al. Synergistic effects of nanoattapulgite and hydroxyapatite on vascularization and bone formation in a rabbit tibia bone defect model. Biomater. Sci. 2022, 10, 4635–4655. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Xie, Z.; Tang, S.; Huang, M.; Feng, J.; Wei, Y.; Shen, Z.; Zhou, R.; Feng, Y.; et al. An anti-infection and biodegradable TFRD-loaded porous scaffold promotes bone regeneration in segmental bone defects: Experimental studies. Int. J. Surg. 2024, 110, 3269–3284. [Google Scholar] [CrossRef]
- Guo, H.G.; Yao, F.L.; Ma, X.L.; Yao, K.D. An experimental study on rabbit’s radial bone defect healed by application of mimetic periosteum with tissue-engineered bone. Chin. J. Plast. Surg. 2008, 24, 63–67. [Google Scholar]
- Teotia, A.K.; Dienel, K.; Qayoom, I.; van Bochove, B.; Gupta, S.; Partanen, J.; Seppälä, J.; Kumar, A. Improved Bone Regeneration in Rabbit Bone Defects Using 3D Printed Composite Scaffolds Functionalized with Osteoinductive Factors. ACS Appl. Mater. Interfaces 2020, 12, 48340–48356. [Google Scholar] [CrossRef]
- Cicero, L.; Licciardi, M.; Cirincione, R.; Puleio, R.; Giammona, G.; Giglia, G.; Sardo, P.; Edoardo Vigni, G.; Cioffi, A.; Sanfilippo, A.; et al. Polybutylene succinate artificial scaffold for peripheral nerve regeneration. J. Biomed. Mat. Res. Part. B Appl. Biomater. 2022, 110, 125–134. [Google Scholar] [CrossRef]
- Miceli, G.C.; Palumbo, F.S.; Bonomo, F.P.; Zingales, M.; Licciardi, M. Polybutylene succinate processing and evaluation as a micro fibrous graft for tissue engineering applications. Polymers 2022, 14, 4486. [Google Scholar] [CrossRef] [PubMed]
- Cicero, L.; Puleio, R.; Cassata, G.; Cirincione, R.; Camarda, L.; Caracappa, D.; D’Itri, L.; Licciardi, M.; Vigni, G.E. Peripheral nerve regeneration at 1 Year: Biodegradable polybutylene succinate artificial scaffold vs. conventional epineurial sutures. Polymers 2023, 15, 3398. [Google Scholar] [PubMed]
- Vigni, G.E.; Cassata, G.; Caldarella, G.; Cirincione, R.; Licciardi, M.; Miceli, G.C.; Puleio, R.; D’Itri, L.; Lo Coco, R.; Camarda, L.; et al. Improved bone regeneration using biodegradable polybutylene succinate artificial scaffold in a rabbit model. J. Funct. Biomater. 2023, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Terracina, F.; Scirè, S.; Puleo, G.; Catania, V.; Lo Monte, A.I.; Schillaci, D.; Licciardi, M. Polybutylene succinate-based electrospun scaffolds improving skin permeation of the antibacterial ciprofloxacin. J. Drug Deliv. Sci. Technol. 2025, 107, 106815. [Google Scholar] [CrossRef]
- Pförringer, D.; Harrasser, N.; Beirer, M.; Crönlein, M.; Stemberger, A.; van Griensven, M.; Lucke, M.; Burgkart, R.; Obermeier, A. Influence of absorbable calcium sulfate-based bone substitute materials on human haemostasis-in vitro biological behavior of antibiotic loaded implants. Materials 2018, 11, 935. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic materials and fabrication approaches for bone tissue engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The family of bone morphogenetic proteins. Kidney Int. 2000, 57, 2207–2214. [Google Scholar] [CrossRef]
- Mai, Z.; Peng, Z.; Wu, S.; Zhang, J.; Chen, L.; Liang, H.; Bai, D.; Yan, G.; Ai, H. Single bout short duration fluid shear stress induces osteogenic differentiation of MC3T3-E1 cells via integrin β1 and BMP2 signaling cross-talk. PLoS ONE 2013, 8, e61600. [Google Scholar] [CrossRef]
- Moutsatsos, I.K.; Turgeman, G.; Zhou, S.; Kurkalli, B.G.; Pelled, G.; Tzur, L.; Kelley, P.; Stumm, N.; Mi, S.; Müller, R.; et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol. Ther. 2001, 3, 449–461. [Google Scholar] [CrossRef]
- Ripamonti, U.; Crooks, J.; Matsaba, T.; Tasker, J. Induction of endochondral bone formation by recombinant human transforming growth factor-beta2 in the baboon (Papio ursinus). Growth Factors 2000, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C.A. Review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, B.J. Surgical reconstruction of canine nonunion fractures using bone morphogenetic protein-2-loaded alginate microbeads and bone allografts. Vivo 2024, 38, 611–619. [Google Scholar] [CrossRef]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Jt. J. 2013, 95-B, 583–597. [Google Scholar] [CrossRef]
- Jégoux, F.; Goyenvalle, E.; Cognet, R.; Malard, O.; Moreau, F.; Daculsi, G.; Aguado, E. Mandibular segmental defect regenerated with macroporous biphasic calcium phosphate, collagen membrane, and bone marrow graft in dogs. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 971–978. [Google Scholar] [CrossRef]
- Kalay, E.; Ermutlu, C.; Yenigül, A.E.; Yalçınkaya, U.; Sarısözen, B. Effect of bone morphogenic protein-2 and desferoxamine on distraction osteogenesis. Injury 2022, 53, 1854–1857. [Google Scholar] [CrossRef]
- Saninggar, K.E.; Abe, F.; Nakano, A.; Kato, K. Collagen-binding bone morphogenetic protein-2 designed for use in bone tissue engineering. Dent. Mater. J. 2024, 43, 718–728. [Google Scholar] [CrossRef]
- Edelmayer, M.; Wehner, C.; Ulm, C.; Zechner, W.; Shafer, D.; Agis, H. Which substances loaded onto collagen scaffolds influence oral tissue regeneration?-an overview of the last 15 years. Clin. Oral. Investig. 2020, 24, 3363–3394. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigni, G.E.; Licciardi, M.; D’itri, L.; Terracina, F.; Scirè, S.; Arrabito, G.; Pignataro, B.; Camarda, L.; Cassata, G.; Puleio, R.; et al. Improved Bone Regeneration Using Biodegradable Polybutylene Succinate Artificial Scaffold with BMP-2 Protein in a Rabbit Model. Materials 2025, 18, 2234. https://doi.org/10.3390/ma18102234
Vigni GE, Licciardi M, D’itri L, Terracina F, Scirè S, Arrabito G, Pignataro B, Camarda L, Cassata G, Puleio R, et al. Improved Bone Regeneration Using Biodegradable Polybutylene Succinate Artificial Scaffold with BMP-2 Protein in a Rabbit Model. Materials. 2025; 18(10):2234. https://doi.org/10.3390/ma18102234
Chicago/Turabian StyleVigni, Giulio Edoardo, Mariano Licciardi, Lorenzo D’itri, Francesca Terracina, Sergio Scirè, Giuseppe Arrabito, Bruno Pignataro, Lawrence Camarda, Giovanni Cassata, Roberto Puleio, and et al. 2025. "Improved Bone Regeneration Using Biodegradable Polybutylene Succinate Artificial Scaffold with BMP-2 Protein in a Rabbit Model" Materials 18, no. 10: 2234. https://doi.org/10.3390/ma18102234
APA StyleVigni, G. E., Licciardi, M., D’itri, L., Terracina, F., Scirè, S., Arrabito, G., Pignataro, B., Camarda, L., Cassata, G., Puleio, R., Di Silvestre, L., & Cicero, L. (2025). Improved Bone Regeneration Using Biodegradable Polybutylene Succinate Artificial Scaffold with BMP-2 Protein in a Rabbit Model. Materials, 18(10), 2234. https://doi.org/10.3390/ma18102234







