CeO2-Modified Ni2P/Fe2P as Efficient Bifunctional Electrocatalyst for Water Splitting
Abstract
1. Introduction
2. Experiment
2.1. Chemicals and Reagents
2.2. Nickel Foam Pretreatment
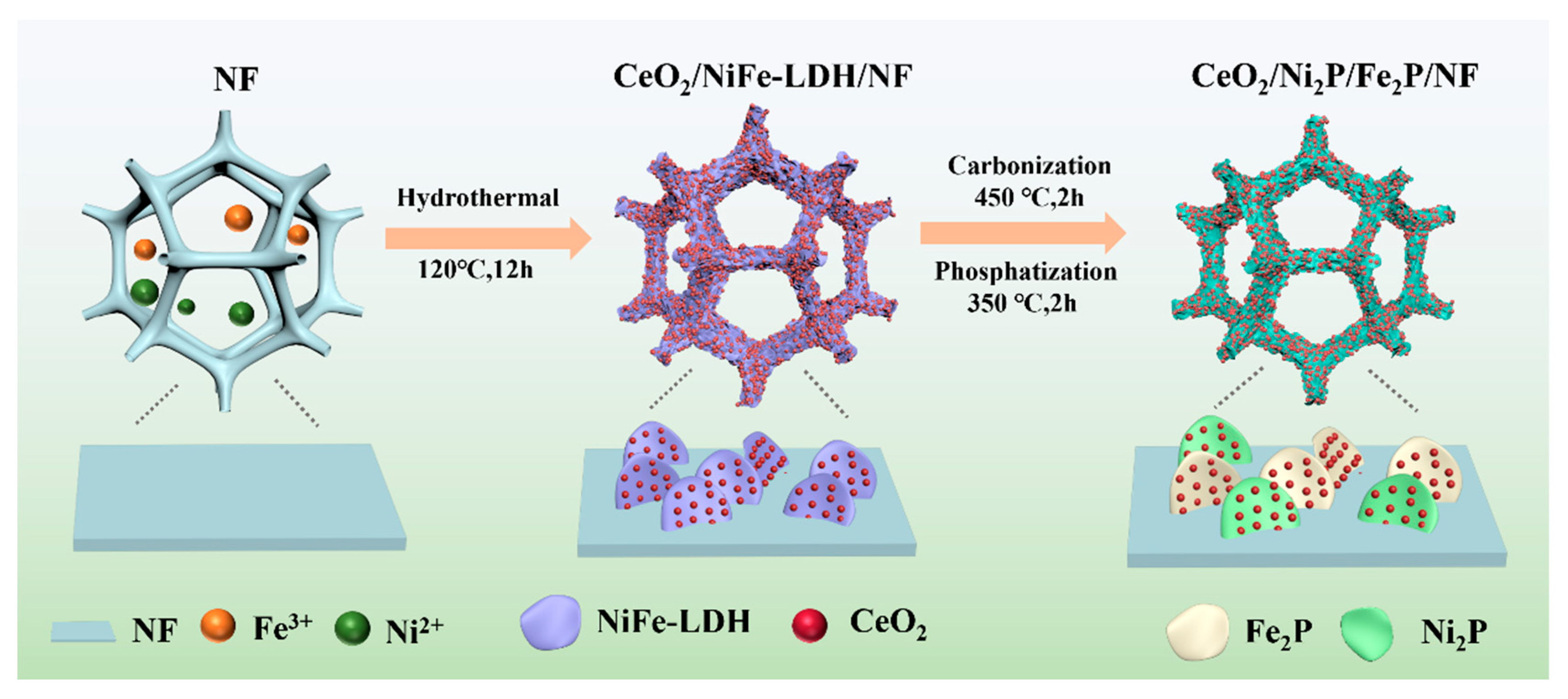
2.3. Preparation of CeO2/NiFe-LDH/NF Precursor
2.4. Preparation of CeO2/Ni2P/Fe2P/NF Catalyst
2.5. Characterization
2.6. Electrochemical Measurement
3. Results and Discussion
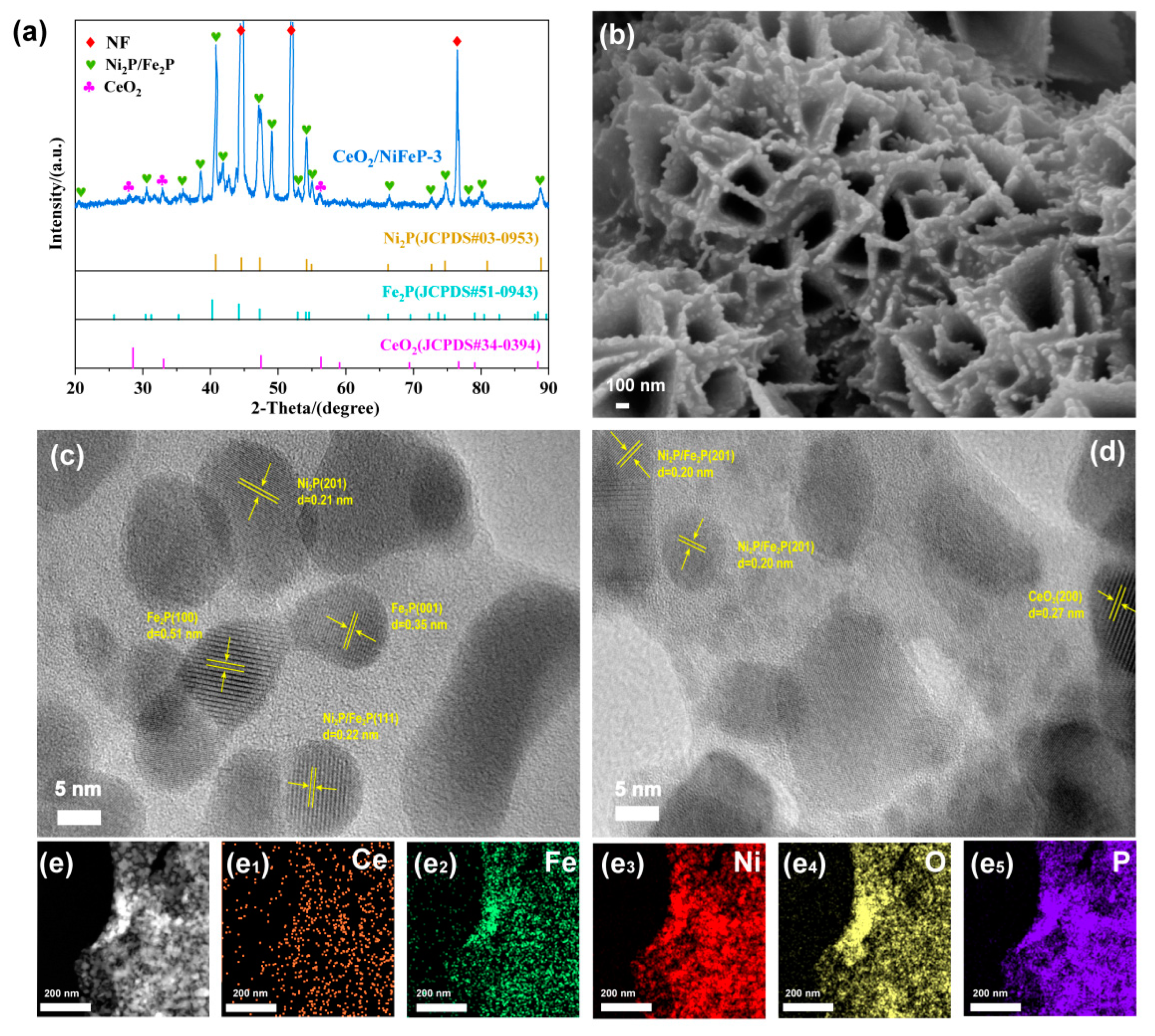
3.1. Structure and Morphology Characterization
3.2. Electrochemical HER Performance
3.3. Electrochemical OER Performance
3.4. Overall Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, W.X.; Wang, D.H.; Zhou, Q.; Zheng, X.P. Preparation of 3D Fe-Co-Ni-OH/NiCoP electrode as a highly efficient electrocatalyst in the oxygen evolution reactions. J. Alloys Compd. 2023, 941, 168578. [Google Scholar] [CrossRef]
- Fu, L.J.; Zhou, S.L.; Xiang, M.; Yang, J.J.; Fan, W.X.; Yang, Z.; Ou, J.F. Advances in anion vacancy for electrocatalytic oxygen evolution reaction. Electroanal. Chem. 2022, 921, 11650. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Yang, Y.; Pan, S.; Pan, W.; Tang, M.; Liu, K. Electronic modulation of MOF-derived CoxMnyB nanosheet arrays toward efficient bifunctional electrocatalysts for water splitting. Electroanal. Chem. 2024, 970, 118553. [Google Scholar] [CrossRef]
- Dong, Y.C.; Wang, H.X.; Wang, X.Y.; Wang, H.; Dong, Q.; Wang, W.S.; Wei, X.F.; Ren, J.W.; Liu, J.; Wang, R.F. Superhydrophilic/superaerophobic NiFe with internal bubble flow channels for electrocatalytic water splitting. Chem. Eng. J. 2024, 488, 150953. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Yi, L.; Wang, R.; Wei, Y.; Fang, C.; Sun, W.; Li, Y.; Hu, W. Ultrathin metal–organic framework hybrid nanosheets enabled active oxygen evolution electrocatalysis in alkaline media. Electroanal. Chem. 2022, 922, 116765. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Shi, Y.; Sun, Z.C.; Liu, R.P. Role of Co in the Electrocatalytic Activity of Monolayer Ternary NiFeCo-Double Hydroxide Nanosheets for Oxygen Evolution Reaction. Materuals 2021, 14, 207. [Google Scholar] [CrossRef]
- Young, H.L.; McCormick, C.R.; Butterfield, A.G.; Gomez, E.D.; Schaak, R.E. Postsynthetic Thiol-Induced Reshaping of Copper Sulfide Nanoparticles. Chem. Mater. 2022, 34, 11014–11025. [Google Scholar] [CrossRef]
- Rasheed, T.; Rasheed, A.; Alzahrani, F.M.A.; Ajmal, S.; Warsi, M.F.; Al-Buriahi, M.S.; Dastgeer, G.; Lee, S.G. Bifunctional electrocatalytic water splitting augmented by cobalt-nickel-ferrite NPs-supported fluoride-free MXene as a novel electrocatalyst. Fuel 2023, 346, 128305. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.H.; Fu, L.Y.; Wang, M.L.; Zhou, J.D. Inorganic crystal-supported precious metal single-atom catalysts for photo/electrocatalysis. Nano Energy 2024, 128, 109869. [Google Scholar] [CrossRef]
- Teng, L.; Zhao, L.F. Cobalt-regulated NiFe-LDH for efficient electrocatalytic oxygen evolution in alkaline simulated industrial sewage and natural seawater. J. Electroanal. Chem. 2023, 949, 117824. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, Y.H. Effect of constituent cations on the electrocatalytic oxygen evolution reaction in high-entropy oxide (MgFeCoNiCu)O. Electroanal. Chem. 2022, 922, 116737. [Google Scholar] [CrossRef]
- Hao, X.; Wang, R.; Tan, X.; Zhang, X.; Liu, X.; Wu, Z.; Yuan, D. Fabricating Spinel-Type High-Entropy Oxides of (Co, Fe, Mn, Ni, Cr)3O4 for Efficient Oxygen Evolution Reaction. Materials 2024, 17, 3415. [Google Scholar] [CrossRef] [PubMed]
- Anne Acedera, R.; Theresse Dumlao, A.; Donn Matienzo, D.J.; Divinagracia, M.; Anne Paraggua, J.; Abel Chuang, P.-Y.; Ocon, J. Templated synthesis of transition metal phosphide electrocatalysts for oxygen and hydrogen evolution reactions. J. Energy Chem. 2024, 89, 646–669. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B.; Lin, T.W. Electrocatalytic hydrogen and oxygen evolution reactions: Role of two-dimensional layered materials and their composites. Electrochim. Acta 2023, 447, 142119. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, M.Y.; Zhao, H.M.; Zeng, Z.Q.; Xia, C.Q.; Yang, T. In Situ Growth of Nano-MoS2 on Graphite Substrates as Catalysts for Hydrogen Evolution Reaction. Materials 2023, 16, 4627. [Google Scholar] [CrossRef]
- Aziz, T.; Haque, M.A.; Saha, S.; Mondal, B.; Jain, S.; Dutta, A. A Review of Nanostructured Transition Metal Phosphide-Driven Electrocatalytic Oxygen Evolution Reaction. Energy Fuels 2023, 37, 18291–18309. [Google Scholar] [CrossRef]
- Yin, H.; Rong, F.; Xie, Y. A review of typical transition metal phosphides electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 52, 350–375. [Google Scholar] [CrossRef]
- Fu, L.; Xv, R.; Shang, X.; Li, Z. Nanoflower-like NiCoFe layered hydroxide by in-situ electrochemical corrosion as highly efficient electrocatalyst for water oxidation. J. Alloys Compd. 2025, 1010, 177244. [Google Scholar] [CrossRef]
- Tian, L.; Wo, H.X.; Wang, K.; Wang, X.; Zhuang, W.C.; Li, T.X.; Du, X.H. Ultrathin wrinkled NiFeP nanosheets enable efficient oxygen evolution electrocatalysis. J. Taiwan Inst. Chem. Eng. 2019, 97, 200–206. [Google Scholar] [CrossRef]
- Yue, R.M.; Mo, Z.L.; Shuai, C.; He, S.M.; Liu, W.T.; Liu, G.G.; Du, Y.X.; Dong, Q.B.; Ding, J.X.; Zhu, X.L.; et al. N-doped bimetallic NiFeP nanocubic clusters derived from Prussian blue analogues as a high-efficiency and durable water splitting electrocatalyst. Electroanal. Chem. 2022, 918, 116427. [Google Scholar] [CrossRef]
- Guo, Z.; Bi, M.; He, H.; Liu, Z.; Duan, Y.; Cao, W. Defect engineering associated with cationic vacancies for promoting electrocatalytic water splitting in iron-doped Ni2P nanosheet arrays. J. Colloid Interface Sci. 2024, 654, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Liu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Self-Supported Cobalt Phosphide Mesoporous Nanorod Arrays: A Flexible and Bifunctional Electrode for Highly Active Electrocatalytic Water Reduction and Oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Zhang, X.; Guo, J. Engineering P-doped Ni3S2-NiS hybrid nanorod arrays for efficient overall water electrolysis. J. Alloys Compd. 2021, 862, 158391. [Google Scholar] [CrossRef]
- Saleem, M.K.; Niaz, N.A.; Fawy, K.F.; Abdelmohsen, S.A.M.; Alanazi, M.M.; Hussain, F.; Ashiq, M.N.; Rasheed, U.; Abbas, Y.; Khan, M.S. Electrocatalytic behavior of Ni-Co-Fe3O4 nanospheres for efficient oxygen evolution reaction. Electroanal. Chem. 2023, 940, 117503. [Google Scholar] [CrossRef]
- Hao, X.D.; Zhang, X.S.; Xu, Y.; Zhou, Y.H.; Wei, T.T.; Hu, Z.Z.; Wu, L.; Feng, X.Y.; Zhang, J.; Liu, Y.; et al. Atomic-scale insights into the interfacial charge transfer in a NiO/CeO2 heterostructure for electrocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 643, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hai, G.; Zhao, G.; Li, R.; Huang, X.; Lu, Y.; Wang, G. CeO2 as an “electron pump” to boost the performance of Co4N in electrocatalytic hydrogen evolution, oxygen evolution and biomass oxidation valorization. Appl. Catal. B-Environ. 2023, 325, 122364. [Google Scholar] [CrossRef]
- Zeng, S.; Zeng, X.; Jiang, L.; Ding, Z.; Shao, J.-J.; Zhao, J. β-Mo2C nanoparticles/carbon nanotubes Mott-Schottky nanoarray on carbon cloth as freestanding cathodes for efficient hydrogen evolution reaction. J. Alloys Compd. 2024, 996, 174868. [Google Scholar] [CrossRef]
- Ai, L.H.; Luo, Y.; Huang, W.J.; Tian, Y.; Jiang, J. Cobalt/cerium-based metal-organic framework composites for enhanced oxygen evolution electrocatalysis. Int. J. Hydrogen Energy 2022, 47, 12893–12902. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.; Zhang, G. A general strategy for constructing transition metal Oxide/CeO2 heterostructure with oxygen vacancies toward hydrogen evolution reaction and oxygen evolution reaction. J. Power Sources 2021, 512, 230514. [Google Scholar] [CrossRef]
- Yin, L.L.; Zhang, S.; Huang, Y.K.; Yan, C.H.; Du, Y.P. Cerium contained advanced materials: Shining star under electrocatalysis. Coord. Chem. Rev. 2024, 518, 216111. [Google Scholar] [CrossRef]
- Li, G.; Wang, P.; He, M.; Yuan, X.L.; Tang, L.L.; Li, Z.X. Cerium-based nanomaterials for photo/electrocatalysis. Sci. China Chem. 2023, 66, 2204–2220. [Google Scholar] [CrossRef]
- Chen, J.W.; Ling, Y.C.; Qu, D.Q.; Huang, L.A.; Li, J.J.; Tang, P.J.; He, A.P.; Jin, X.; Zhou, Y.; Xu, M.X.; et al. Enhanced electrocatalysis of NiMnIn Heusler alloy films for hydrogen evolution reaction by magnetic field. J. Alloys Compd. 2021, 877, 160271. [Google Scholar] [CrossRef]
- Li, J.; Kang, Y.; Lei, Z.; Liu, P. Well-controlled 3D flower-like CoP3/CeO2/C heterostructures as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Appl. Catal. B-Environ. 2023, 321, 122029. [Google Scholar] [CrossRef]
- Zhao, D.D.; Pi, Y.C.; Shao, Q.; Feng, Y.G.; Zhang, Y.; Huang, X.Q. Enhancing Oxygen Evolution Electrocatalysis the Intimate Hydroxide-Oxide Interface. ACS Nano 2018, 12, 6245–6251. [Google Scholar] [CrossRef]
- Huang, Y.X.; Ding, X.D.; Huang, B.B.; Xie, Z.L. CeO2-decorated Fe-doped Ni2P nanosheets for efficient electrocatalytic overall water splitting at high current densities. J. Alloys Compd. 2024, 981, 173672. [Google Scholar] [CrossRef]
- Yu, Y.; Peng, X.; Ali, U.; Liu, X.; Xing, Y.; Xing, S. Facile route to achieve bifunctional electrocatalysts for oxygen reduction and evolution reactions derived from CeO2encapsulated by the zeolitic imidazolate framework-67. Inorg. Chem. Front. 2019, 6, 3255–3263. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, X.; Mei, Y.; Ye, J.; Li, T.-T. CeO2 decorated bimetallic phosphide nanowire arrays for enhanced oxygen evolution reaction electrocatalysis via interface engineering. Dalton. Trans. 2022, 51, 2923–2931. [Google Scholar] [CrossRef]
- Dao, D.V.; Le, T.D.; Adilbish, G.; Lee, I.-H.; Yu, Y.-T. Pt-loaded Au@CeO2 core–shell nanocatalysts for improving methanol oxidation reaction activity. J. Mater. Chem. A 2019, 7, 26996–27006. [Google Scholar] [CrossRef]
- Tan, S.F.; Ouyang, W.M.; Ji, Y.J.; Hong, Q.W. Carbon wrapped bimetallic NiCo nanospheres toward excellent HER and OER performance. J. Alloys Compd. 2021, 889, 161528. [Google Scholar] [CrossRef]
- Mandari, K.K.; Pandey, S.; Kang, M.S. Highly efficient ternary NiO/MoS2/BiVO4 heterostructure for electrocatalytic HER/OER applications. Int. J. Hydrogen Energy 2024, 52, 275–287. [Google Scholar] [CrossRef]
- Wen, S.T.; Huang, J.; Li, T.T.; Chen, W.; Chen, G.L.; Zhang, Q.; Zhang, X.H.; Qian, Q.Y.; Ostrikov, K. Multiphase nanosheet-nanowire cerium oxide and nickel-cobalt phosphide for highly-efficient electrocatalytic overall water splitting. Appl. Catal. B-Environ. 2022, 316, 121678. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B-Environ. 2021, 297, 120386. [Google Scholar] [CrossRef]
- Li, D.; Guo, Z.; Zhao, R.; Ren, H.; Huang, Y.; Yan, Y.; Cui, W.; Yao, X. An efficient cerium dioxide incorporated nickel cobalt phosphide complex as electrocatalyst for All-pH hydrogen evolution reaction and overall water splitting. J. Colloid Interface Sci. 2024, 653, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Z.; Zhang, T.; Zhao, Y.-H.; Ni, J.-C.; Pan, Y.; Tan, Z.; Wang, X.-F. Heterostructure Interface Engineering in CoP/FeP/CeOx with a Tailored d-Band Center for Promising Overall Water Splitting Electrocatalysis. Inorg. Chem. 2023, 62, 8347–8356. [Google Scholar] [CrossRef]
- Ma, G.Y.; Du, X.Q.; Zhang, X.S. Controlled phosphating: A novel strategy toward NiP2@CeO2 interface engineering for efficient oxygen evolution electrocatalysis. Dalton. Trans. 2020, 49, 12581–12585. [Google Scholar] [CrossRef]
- Yu, J.; Du, X.J.; Liu, H.Z.; Qiu, C.; Yu, R.X.; Li, S.M.; Ren, J.Z.; Yang, S.H. Mini Review on Active Sites in Ce-Based Electrocatalysts for Alkaline Water Splitting. Energy Fuels 2021, 35, 19000–19011. [Google Scholar] [CrossRef]
- Yao, Y.C.; Sun, S.J.; Zhang, H.; Li, Z.X.; Yang, C.X.; Cai, Z.W.; He, X.; Dong, K.; Luo, Y.L.; Wang, Y.; et al. Enhancing the stability of NiFe-layered double hydroxide nanosheet array for alkaline seawater oxidation by Ce doping. J. Energy Chem. 2024, 91, 306–312. [Google Scholar] [CrossRef]
- Kim, S.; Sa, Y.J. Ce4+/Ce3+ Redox-Promoted Electron Transfer for Efficient Neutral H2O2 Electrosynthesis from Two-Electron Oxygen Reduction. ACS Catal. 2024, 14, 6842–6855. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; An, S.; Zhang, J.; Zhou, G.; Guo, L. Effect of cerium oxide prepared under different hydrothermal time on electrocatalytic performance of Pt-based anode catalysts. J. Rare Earth 2020, 38, 384–394. [Google Scholar] [CrossRef]
- Bao, W.T.; Tang, Y.; Yu, J.; Yan, W.X.; Wang, C.X.; Li, Y.Y.; Wang, Z.M.; Yang, J.F.; Zhang, L.L.; Yu, F. Si-doped ZnAl-LDH nanosheets by layer-engineering for efficient photoelectrocatalytic water splitting. Appl. Catal. B-Environ. Eenrgy 2024, 346, 123706. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, B.; Xu, G.; Zhao, T.; Ding, H.; Feng, Y.; Li, Y.; Zhang, L. Electron regulation of CeO2 on CoP multi-shell hetero-junction micro-sphere towards highly efficient water oxidation. J. Colloid Interface Sci. 2024, 668, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Zhang, X.; Sun, M.; Huang, B.; Zhang, N.; Da, P.; Yang, R.; He, Z.; Wang, W.; Xi, P.; et al. Uncovering the Promotion of CeO2/CoS1.97 Heterostructure with Specific Spatial Architectures on Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2102593. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, Y.; Sun, L.; Wang, C.; Xin, H.; Song, J.; Li, D.; Ma, F. Interface oxygen vacancy enhanced alkaline hydrogen evolution activity of cobalt-iron phosphide/CeO2 hollow nanorods. Chem. Eng. J. 2022, 437, 135376. [Google Scholar] [CrossRef]
- Min, K.; Kim, M.; Min, S.; Kim, H.; Baeck, S.H. Facile synthesis of CeO2-decorated Co4N nanoparticles as a highly active and durable electrocatalyst for the oxygen evolution reaction. Appl. Surf. Sci. 2023, 624, 157117. [Google Scholar] [CrossRef]
- Zhang, J.F.; Gong, W.B.; Yin, H.J.; Wang, D.D.; Zhang, Y.X.; Zhang, H.M.; Wang, G.Z.; Zhao, H.J. In Situ Growth of Ultrathin Ni(OH)2 Nanosheets as Catalyst for Electrocatalytic Oxidation Reactions. Chemsuschem 2021, 14, 2935–2942. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, Y.; Li, Q.; Liu, F.; Ji, X.; Liu, J. Facile construction of heterostructural Ni3(NO3)2(OH)4/CeO2 bifunctional catalysts for boosted overall water splitting. Int. J. Hydrogen Energy 2022, 47, 23221–23229. [Google Scholar] [CrossRef]
- Jiang, J.; Li, F.; Su, H.; Gao, Y.; Li, N. Flower-like NiCo2S4/NiFeP/NF composite material as an effective electrocatalyst with high overall water splitting performance. Chin. Chem. Lett. 2022, 33, 4367–4374. [Google Scholar] [CrossRef]
- Diao, F.; Huang, W.; Ctistis, G.; Wackerbarth, H.; Yang, Y.; Si, P.; Zhang, J.; Xiao, X. Bifunctional and Self-Supported NiFeP-Layer-Coated NiP Rods for Electrochemical Water Splitting in Alkaline Solution. ACS Appl. Mater. Interfaces 2021, 13, 23702–23713. [Google Scholar] [CrossRef]
- Dhandapani, H.N.; Mahendiran, D.; Karmakar, A.; Devi, P.; Nagappan, S.; Madhu, R.; Bera, K.; Murugan, P.; Babu, B.R.; Kundu, S. Boosting of overall water splitting activity by regulating the electron distribution over the active sites of Ce doped NiCo–LDH and atomic level understanding of the catalyst by DFT study. J. Mater. Chem. A 2022, 10, 17488–17500. [Google Scholar] [CrossRef]
- Shen, L.; Tang, S.; Yu, L.; Huang, Q.; Zhou, T.; Yang, S.; Yu, H.; Xiong, H.; Xu, M. Efficient Ternary Cefecop Bifunctional Electrocatalyst for Overall Water Splitting. J. Solid State Chem. 2022, 314, 123434. [Google Scholar] [CrossRef]
- Wang, S.; Ning, X.; Cao, Y.; Chen, R.; Lu, Z. Construction of an Advanced NiFe-LDH/MoS2–Ni3S2/NF Heterostructure Catalyst toward Efficient Electrocatalytic Overall Water Splitting. Inorg. Chem. 2023, 62, 6428–6438. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zheng, X.; Peng, L.; Wei, Z. Heteroatom Modification of Nanoporous Nickel Surfaces for Electrocatalytic Water Splitting. ACS Appl. Nano Mater. 2020, 3, 11295–11306. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Hou, Y. Phosphorus-doped nickel sulfides/nickel foam as electrode materials for electrocatalytic water splitting. Int. J. Hydrogen Energy 2018, 43, 19002–19009. [Google Scholar] [CrossRef]




| Electrocatalysts | Overpotential (mV)/HER | Overpotential (mV)/OER | Overall Voltage (V) | Reference |
|---|---|---|---|---|
| CeO2/Ni2P/Fe2P | 87@10 mA cm−2 | 228@150 mA cm−2 | 1.501@10 mA cm−2 | This work |
| Ni3(NO3)2(OH)4/CeO2/NF | 120@10 mA cm−2 | 330@50 mA cm−2 | 1.64@10 mA cm−2 | [56] |
| NiCo2S4/Ni2P/Fe2P/NF | 205@50 mA cm−2 | 293@100 mA cm−2 | 1.56@10 mA cm−2 | [57] |
| Ni2P/Fe2P@NiP@NF | 105@10 mA cm−2 | 252@100 mA cm−2 | 1.57@10 mA cm−2 | [58] |
| Ce@NiCo-LDH | 134@50 mA cm−2 | 250@50 mA cm−2 | 1.68@10 mA cm−2 | [59] |
| CeFeCoP/NF | 97@10 mA cm−2 | 298@50 mA cm−2 | 1.55@10 mA cm−2 | [60] |
| NiFe-LDH/MoS2–Ni3S2/NF | 79@10 mA cm−2 | 220@50 mA cm−2 | 1.50@10 mA cm−2 | [61] |
| Ni/Ni-N0.28/NF | 63@10 mA cm−2 | 320@20 mA cm−2 | / | [62] |
| PNi3S2/NF | 137@10 mA cm−2 | 306@100 mA cm−2 | 1.47@10 mA cm−2 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Wang, D.; Ren, Y.; Zhang, H.; Yin, S.; Yan, M.; Li, Y.; Wei, S. CeO2-Modified Ni2P/Fe2P as Efficient Bifunctional Electrocatalyst for Water Splitting. Materials 2025, 18, 2221. https://doi.org/10.3390/ma18102221
Wu X, Wang D, Ren Y, Zhang H, Yin S, Yan M, Li Y, Wei S. CeO2-Modified Ni2P/Fe2P as Efficient Bifunctional Electrocatalyst for Water Splitting. Materials. 2025; 18(10):2221. https://doi.org/10.3390/ma18102221
Chicago/Turabian StyleWu, Xinyang, Dandan Wang, Yongpeng Ren, Haiwen Zhang, Shengyu Yin, Ming Yan, Yaru Li, and Shizhong Wei. 2025. "CeO2-Modified Ni2P/Fe2P as Efficient Bifunctional Electrocatalyst for Water Splitting" Materials 18, no. 10: 2221. https://doi.org/10.3390/ma18102221
APA StyleWu, X., Wang, D., Ren, Y., Zhang, H., Yin, S., Yan, M., Li, Y., & Wei, S. (2025). CeO2-Modified Ni2P/Fe2P as Efficient Bifunctional Electrocatalyst for Water Splitting. Materials, 18(10), 2221. https://doi.org/10.3390/ma18102221






