Visualizing Thermal Reduction in Graphene Oxide
Abstract
1. Introduction
2. Method
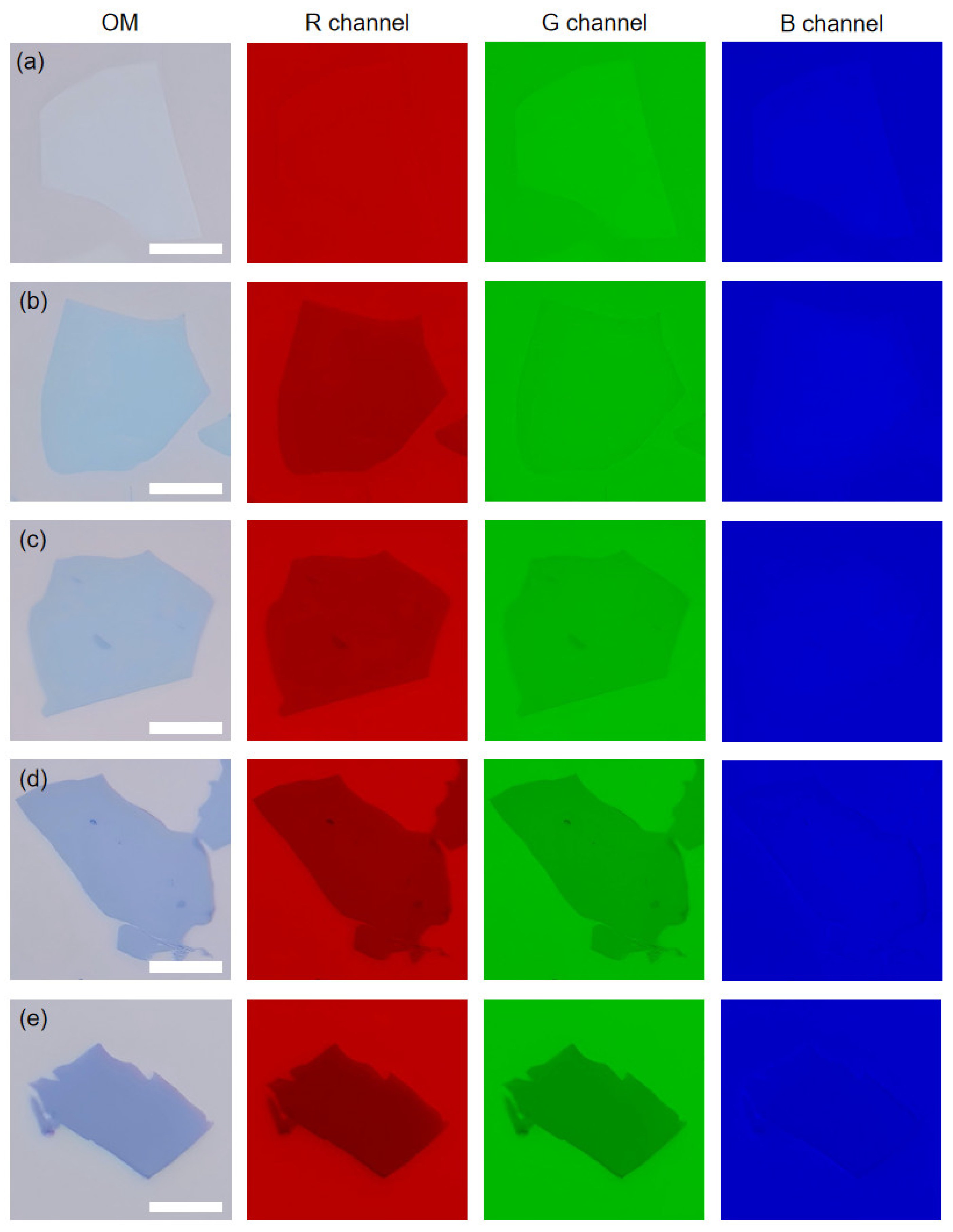
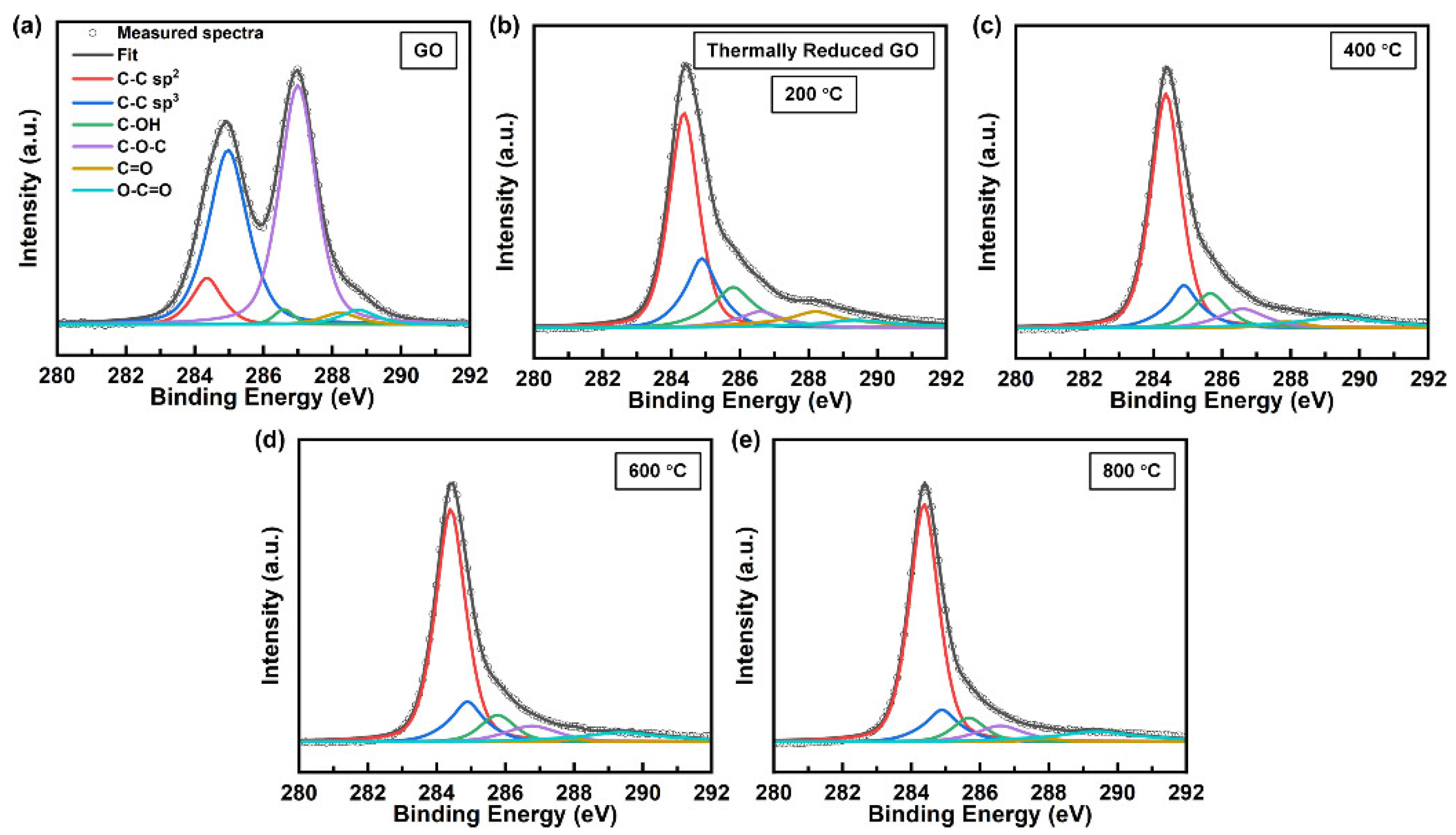
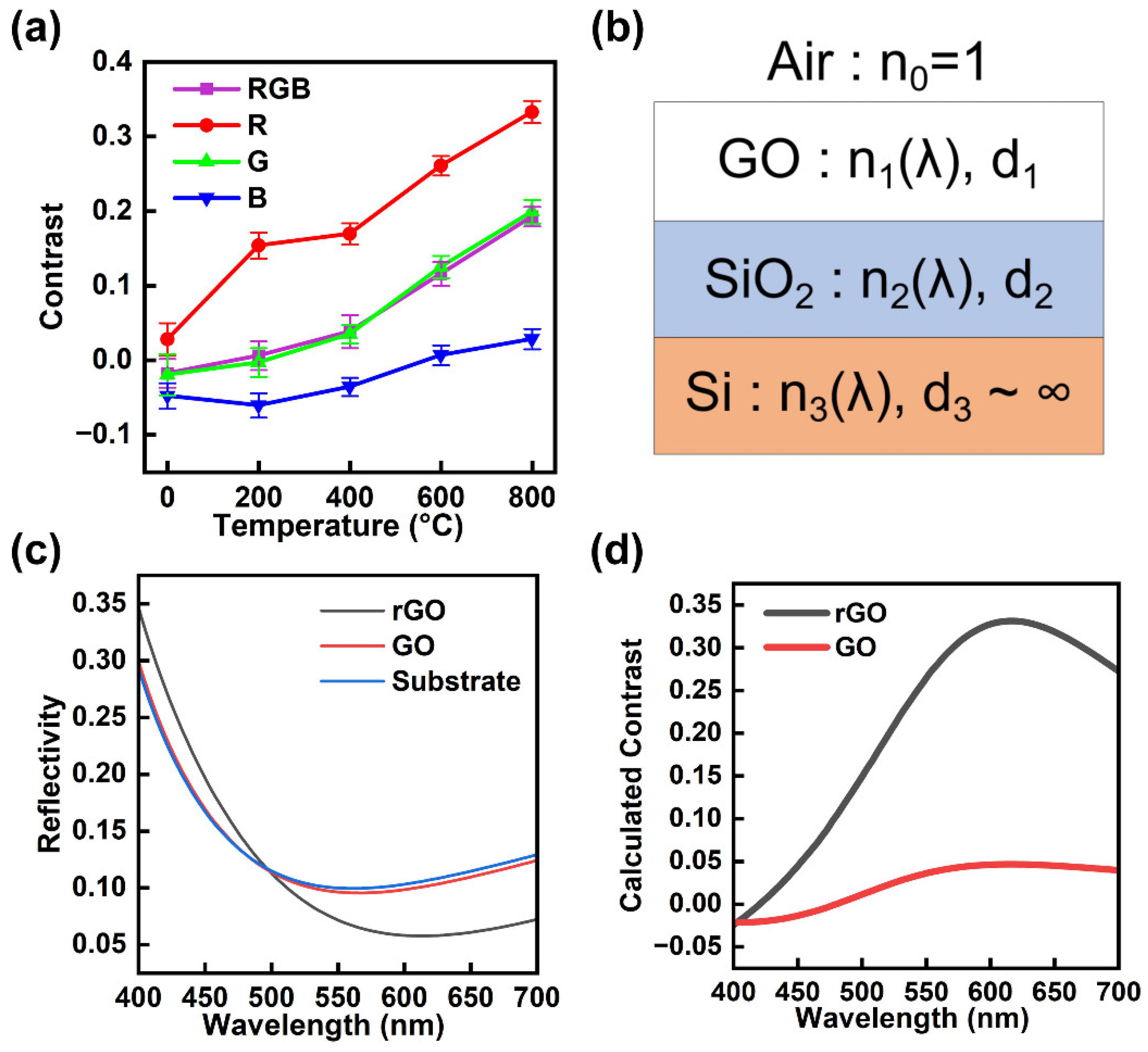
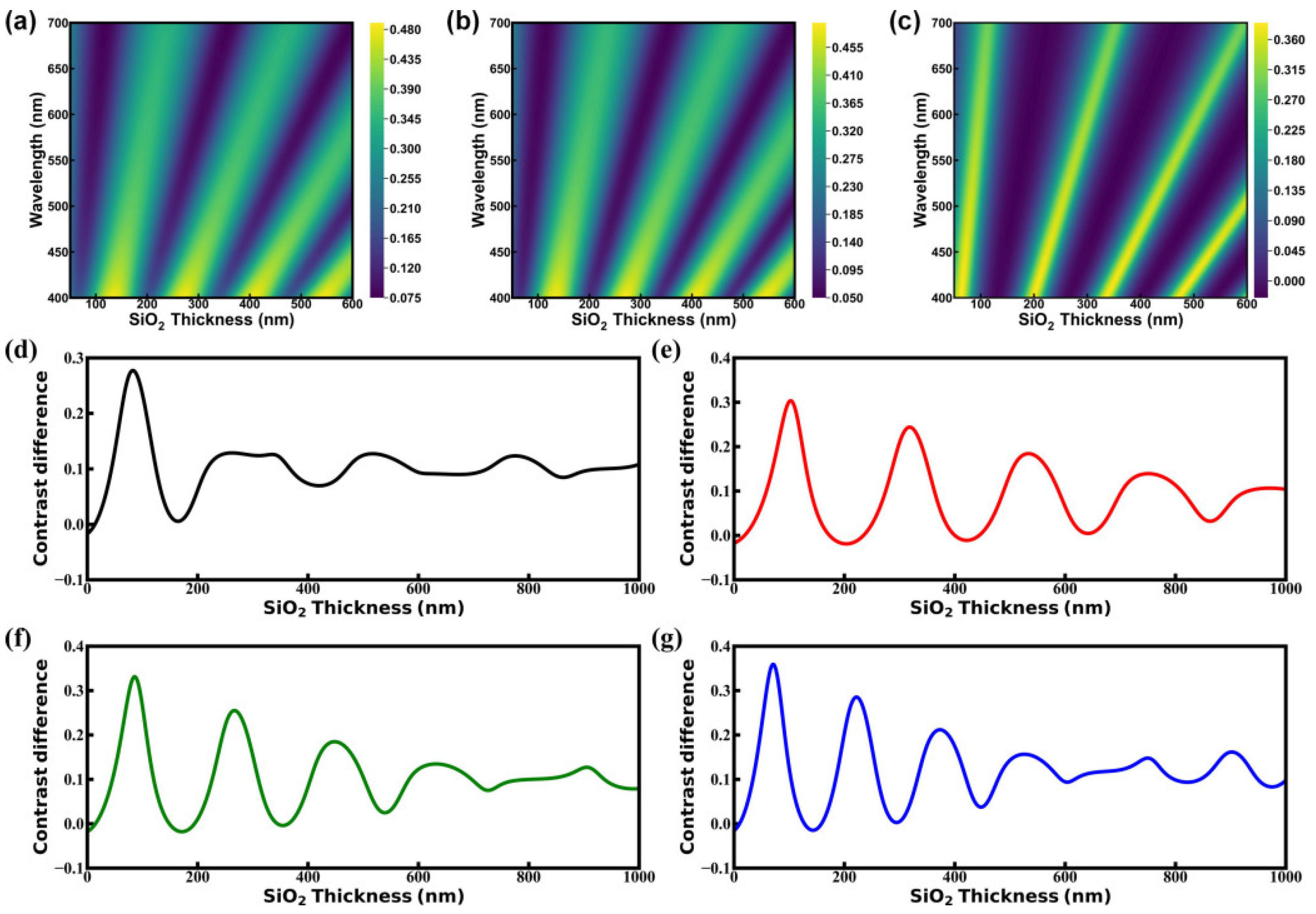
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Jia, B.; Lin, H.; Qiu, L.; Li, D.; Gu, M. Highly efficient and ultra-broadband graphene oxide ultrathin lenses with three-dimensional subwavelength focusing. Nat. Commun. 2015, 6, 8433. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Y.; Qu, Y.; Xu, X.; Liang, Y.; Chu, S.T.; Little, B.E.; Morandotti, R.; Jia, B.; Moss, D.J. Graphene Oxide Waveguide and Micro-Ring Resonator Polarizers. Laser Photonics Rev. 2019, 13, 1900056. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, B.; Li, S.; Lin, H.; Qiu, L.; Li, D.; Jia, B. Free-standing graphene oxide mid-infrared polarizers. Nanoscale 2020, 12, 11480–11488. [Google Scholar] [CrossRef]
- Gan, S.X.; Chew, J.W.; Ng, K.B.; Tey, L.S.; Chong, W.Y.; Goh, B.T.; Lai, C.K.; Choi, D.-Y.; Madden, S.; Ahmad, H. Single-mode fiber multi-level all-optical switching using GSST-graphene oxide hybrid thin film structure. J. Appl. Phys. 2024, 136, 063101. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef]
- Afroj, S.; Tan, S.; Abdelkader, A.M.; Novoselov, K.S.; Karim, N. Highly Conductive, Scalable, and Machine Washable Graphene-Based E-Textiles for Multifunctional Wearable Electronic Applications. Adv. Funct. Mater. 2020, 30, 2000293. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, G.; Yang, X.; Qiao, L.; Lu, M. MXene enhanced reduced graphene oxide aerogel for high-performance supercapacitors. J. Chem. Phys. 2024, 161, 074704. [Google Scholar] [CrossRef]
- Alves, L.S.M.; Neves, M.F.F.d.; Benatto, L.; Ramos, M.K.; Eising, M.; de Oliveira, C.K.B.Q.M.; Zarbin, A.J.G.; Roman, L.S. Influence of Nanostructuring Sensors Based on Graphene Oxide and PEDOT:PSS for Methanol Detection. IEEE Sens. J. 2023, 23, 1845–1853. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, J.; Kang, S.; Cha, H.J.; Shin, H.; Park, J.; Jang, Y.-S.; Woo, J.-S.; Won, C.; Min, D.-H. Discovery of direct-acting antiviral agents with a graphene-based fluorescent nanosensor. Sci. Adv. 2020, 6, eaaz8201. [Google Scholar] [CrossRef]
- Kweon, D.H.; Baek, J.-B. Edge-Functionalized Graphene Nanoplatelets as Metal-Free Electrocatalysts for Dye-Sensitized Solar Cells. Adv. Mater. 2019, 31, 1804440. [Google Scholar] [CrossRef]
- Vaqueiro-Contreras, M.; Bartlam, C.; Bonilla, R.S.; Markevich, V.P.; Halsall, M.P.; Vijayaraghavan, A.; Peaker, A.R. Graphene oxide films for field effect surface passivation of silicon for solar cells. Sol. Energy Mater. Sol. Cells 2018, 187, 189–193. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Sengupta, I.; Kumar, S.S.S.S.; Pal, S.K.; Chakraborty, S. Characterization of structural transformation of graphene oxide to reduced graphene oxide during thermal annealing. J. Mater. Res. 2020, 35, 1197–1204. [Google Scholar] [CrossRef]
- Pelaez-Fernandez, M.; Bermejo, A.; Benito, A.M.; Maser, W.K.; Arenal, R. Detailed thermal reduction analyses of graphene oxide via in-situ TEM/EELS studies. Carbon 2021, 178, 477–487. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman study of D* band in graphene oxide and its correlation with reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution In Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Vidano, R.; Fischbach, D.B. New Lines in the Raman Spectra of Carbons and Graphite. J. Am. Ceram. Soc. 1978, 61, 13–17. [Google Scholar] [CrossRef]
- Ammar, M.R.; Galy, N.; Rouzaud, J.N.; Toulhoat, N.; Vaudey, C.E.; Simon, P.; Moncoffre, N. Characterizing various types of defects in nuclear graphite using Raman scattering: Heat treatment, ion irradiation and polishing. Carbon 2015, 95, 364–373. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Verzhbitskiy, I.; Davey, R.; Casiraghi, C. Raman study on defective graphene: Effect of the excitation energy, type, and amount of defects. Phys. Rev. B 2013, 88, 035426. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Perrozzi, F.; Prezioso, S.; Donarelli, M.; Bisti, F.; De Marco, P.; Santucci, S.; Nardone, M.; Treossi, E.; Palermo, V.; Ottaviano, L. Use of Optical Contrast To Estimate the Degree of Reduction of Graphene Oxide. J. Phys. Chem. C 2013, 117, 620–625. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Carvalho, A.; Costa, M.C.F.; Marangoni, V.S.; Ng, P.R.; Nguyen, T.L.H.; Castro Neto, A.H. The Degree of Oxidation of Graphene Oxide. Nanomaterials 2021, 11, 560. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.-B.; Chen, X.-D.; Xing, F.; Jiang, W.-S.; Dong, B.; Xin, W.; Tian, J.-G. Accurate layers determination of graphene on transparent substrate based on polarization-sensitive absorption effect. Appl. Phys. Lett. 2013, 103, 181902. [Google Scholar] [CrossRef]
- Ni, Z.H.; Wang, H.M.; Kasim, J.; Fan, H.M.; Yu, T.; Wu, Y.H.; Feng, Y.P.; Shen, Z.X. Graphene thickness determination using reflection and contrast spectroscopy. Nano Lett. 2007, 7, 2758–2763. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.-L.; Zhang, X.; Wu, J.-B.; Tan, P.-H. Optical contrast determination of the thickness of SiO2 film on Si substrate partially covered by two-dimensional crystal flakes. Sci. Bull. 2015, 60, 806–811. [Google Scholar] [CrossRef]
- Bayle, M.; Reckinger, N.; Felten, A.; Landois, P.; Lancry, O.; Dutertre, B.; Colomer, J.-F.; Zahab, A.-A.; Henrard, L.; Sauvajol, J.-L.; et al. Determining the number of layers in few-layer graphene by combining raman spectroscopy and optical contrast. J. Raman Spectrosc. 2018, 49, 36–45. [Google Scholar] [CrossRef]
- Dong, X.; Yetisen, A.K.; Tian, H.; Güler, İ.; Stier, A.V.; Li, Z.; Köhler, M.H.; Dong, J.; Jakobi, M.; Finley, J.J.; et al. Line-scan hyperspectral imaging microscopy with linear unmixing for automated two-dimensional crystals identification. ACS Photonics 2020, 7, 1216–1225. [Google Scholar] [CrossRef]
- Li, Y.; Dong, N.; Zhang, S.; Wang, K.; Zhang, L.; Wang, J. Optical identification of layered MoS2 via the characteristic matrix method. Nanoscale 2015, 8, 1210–1215. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Li, S.; Shi, W.; Han, W.; Zhou, C.; Zhao, X.; Liang, B. Layer-number dependent reflection spectra of MoS2 flakes on SiO2/Si substrate. Opt. Mater. Express 2018, 8, 3082–3091. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Matte, H.S.S.R.; Rao, C.N.R.; Dravid, V.P. Rapid characterization of ultrathin layers of chalcogenides on SiO2/Si substrates. Adv. Funct. Mater. 2012, 22, 1894–1905. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Quereda, J.; Meulen, H.P.v.d.; Agraït, N.; Rubio-Bollinger, G. Spatially resolved optical absorption spectroscopy of single- and few-layer MoS2 by hyperspectral imaging. Nanotechnology 2016, 27, 115705. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Agraït, N.; Rubio-Bollinger, G. Optical identification of atomically thin dichalcogenide crystals. Appl. Phys. Lett. 2010, 96, 213116. [Google Scholar] [CrossRef]
- Aslan, B.; Chenet, D.A.; van der Zande, A.M.; Hone, J.C.; Heinz, T.F. Linearly polarized excitons in single- and few-layer ReS2 crystals. ACS Photonics 2016, 3, 96–101. [Google Scholar] [CrossRef]
- Golla, D.; Chattrakun, K.; Watanabe, K.; Taniguchi, T.; LeRoy, B.J.; Sandhu, A. Optical thickness determination of hexagonal boron nitride flakes. Appl. Phys. Lett. 2013, 102, 161906. [Google Scholar] [CrossRef]
- Zhao, Q.; Puebla, S.; Zhang, W.; Wang, T.; Frisenda, R.; Castellanos-Gomez, A. Thickness identification of thin InSe by optical microscopy methods. Adv. Photonics Res. 2020, 1, 2000025. [Google Scholar] [CrossRef]
- Puebla, S.; Mariscal-Jiménez, A.; Galán, R.S.; Munuera, C.; Castellanos-Gomez, A. Optical-based thickness measurement of MoO3 nanosheets. Nanomaterials 2020, 10, 1272. [Google Scholar] [CrossRef]
- Huang, L.; Shang, Z.; Gao, M.; Miao, C.; Cheng, Y.; Huang, W. Optimized parameters for identifying the layer number of few layer chromium tri-iodide from a theoretical perspective: Implications for two-dimensional spintronics. ACS Appl. Nano Mater. 2020, 3, 8382–8388. [Google Scholar] [CrossRef]
- Mao, N.; Tang, J.; Xie, L.; Wu, J.; Han, B.; Lin, J.; Deng, S.; Ji, W.; Xu, H.; Liu, K.; et al. Optical anisotropy of black phosphorus in the visible regime. J. Am. Chem. Soc. 2016, 138, 300–305. [Google Scholar] [CrossRef]
- Chen, H.; Fei, W.; Zhou, J.; Miao, C.; Guo, W. Layer identification of colorful black phosphorus. Small 2017, 13, 1602336. [Google Scholar] [CrossRef]
- Naylor, C.H.; Parkin, W.M.; Gao, Z.; Kang, H.; Noyan, M.; Wexler, R.B.; Tan, L.Z.; Kim, Y.; Kehayias, C.E.; Streller, F.; et al. Large-Area Synthesis of High-Quality Monolayer 1T’-WTe2 Flakes. 2D Mater. 2017, 4, 021008. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, S.; Li, A.; Yu, Y.; Zhang, H.; Hu, P.; Zhou, W.; Sheng, L.; Jiang, T.; Cheng, H.; et al. Structural Evolution of Atomically Thin 1T′-MoTe2 Alloyed in Chalcogen Atmosphere. Small Struct. 2022, 3, 2200025. [Google Scholar] [CrossRef]
- Simona, P.; Leonardo, M.; Domenica, C.; Dong, K.; Stiven, F.; Sergio, P.; Filippo, F.; Vaidotas, M.; Camilla, C. Synthesis of Large-Scale Monolayer 1T′-MoTe2 and Its Stabilization via Scalable hBN Encapsulation. ACS Nano. 2021, 15, 4213–4225. [Google Scholar]
- Guo, X.; Li, J.; Yu, Y.; Zafar, A.; Ni, Z. Thermally enhanced optical contrast of graphene oxide for thickness identification. Nanotechnology 2019, 30, 295704. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano. 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Mathew, S.; Chan, T.; Zhan, D.; Gopinadhan, K.; Barman, A.-R.; Breese, M.; Dhar, S.; Shen, Z.; Venkatesan, T.; Thong, J.T. The Effect of Layer Number and Substrate on the Stability of Graphene under MeV Proton Beam Irradiation. Carbon 2011, 49, 1720–1726. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L. Graphite Oxide under High Pressure: A Raman Spectroscopic Study. J. Nanomater. 2013, 2013, 731875. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ioni, Y.; Timur, K.; Ivan, S.; Vasiliy, B.; Ayrat, M.D. Revealing the Effect of Graphite Source on the Properties of Synthesized Graphene Oxide. Carbon Lett. 2024, 34, 1219–1228. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Park, H.; Lim, S.; Nguyen, D.D.; Suk, J.W. Electrical Measurements of Thermally Reduced Graphene Oxide Powders under Pressure. Nanomaterials 2019, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Huang, J.-Q.; Zhang, Q.; Gong, W.-Z.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 2012, 50, 659–667. [Google Scholar] [CrossRef]
- Wang, M.; Duong, L.D.; Oh, J.-S.; Mai, N.T.; Kim, S.; Hong, S.; Hwang, T.; Lee, Y.; Nam, J.-D. Large-Area, Conductive and Flexible Reduced Graphene Oxide (RGO) Membrane Fabricated by Electrophoretic Deposition (EPD). ACS Appl. Mater. Interfaces 2014, 6, 1747–1753. [Google Scholar] [CrossRef]
- Perrozzi, F.; Croce, S.; Treossi, E.; Palermo, V.; Santucci, S.; Fioravanti, G.; Ottaviano, L. Reduction dependent wetting properties of graphene oxide. Carbon 2014, 77, 473–480. [Google Scholar] [CrossRef]
- Larciprete, R.; Fabris, S.; Sun, T.; Lacovig, P.; Baraldi, A.; Lizzit, S. Dual Path Mechanism in the Thermal Reduction of Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 17315–17321. [Google Scholar] [CrossRef]
- Jung, I.; Vaupel, M.; Pelton, M.; Piner, R.; Dikin, D.A.; Stankovich, S.; An, J.; Ruoff, R.S. Characterization of Thermally Reduced Graphene Oxide by Imaging Ellipsometry. J. Phys. Chem. C 2008, 112, 8499–8506. [Google Scholar] [CrossRef]
- Yang, H.; Hu, H.; Wang, Y.; Yu, T. Rapid and non-destructive identification of graphene oxide thickness using white light contrast spectroscopy. Carbon 2013, 52, 528–534. [Google Scholar] [CrossRef]
- Li, Y.; Singh, A.; Krylyuk, S.; Davydov, A.; Jaramillo, R. Near-infrared photonic phase-change properties of transition metal ditellurides. In Low-Dimensional Materials and Devices 2019; Kobayashi, N.P., Talin, A.A., Davydov, A.V., Eds.; SPIE: San Diego, CA, USA, 2019; p. 28. [Google Scholar] [CrossRef]
- Palik, E.D. Handbook of Optical Constants of Solids, 1st ed.; Academic Press: San Diego, CA, USA, 1997; ISBN 978-0-12-544423-1. [Google Scholar]
- Blake, P.; Hill, E.W.; Castro Neto, A.H.; Novoselov, K.S.; Jiang, D.; Yang, R.; Booth, T.J.; Geim, A.K. Making graphene visible. Appl. Phys. Lett. 2007, 91, 063124. [Google Scholar] [CrossRef]
- Xu, X.; Su, Y.; Miao, G.; Huang, J.; Lin, G.; Zhu, T.; Xu, Y.; Huang, C.; Zhou, Y.; Zhang, Y.; et al. Visualizing Oxidation in Monolayer 1T′-MoTe2. J. Phys. D Appl. Phys. 2025, 58, 175302. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Huang, J.; Miao, G.; Yan, B.; Chen, Y.; Zhou, Y.; Zhang, Y.; Zhang, X.; Cai, W. Visualizing Thermal Reduction in Graphene Oxide. Materials 2025, 18, 2222. https://doi.org/10.3390/ma18102222
Xu X, Huang J, Miao G, Yan B, Chen Y, Zhou Y, Zhang Y, Zhang X, Cai W. Visualizing Thermal Reduction in Graphene Oxide. Materials. 2025; 18(10):2222. https://doi.org/10.3390/ma18102222
Chicago/Turabian StyleXu, Xiangrui, Junjie Huang, Gesong Miao, Bo Yan, Yangbo Chen, Yinghui Zhou, Yufeng Zhang, Xueao Zhang, and Weiwei Cai. 2025. "Visualizing Thermal Reduction in Graphene Oxide" Materials 18, no. 10: 2222. https://doi.org/10.3390/ma18102222
APA StyleXu, X., Huang, J., Miao, G., Yan, B., Chen, Y., Zhou, Y., Zhang, Y., Zhang, X., & Cai, W. (2025). Visualizing Thermal Reduction in Graphene Oxide. Materials, 18(10), 2222. https://doi.org/10.3390/ma18102222







