Correlating the Effects of UV Aging on the Macro-Micro Behaviors of Asphalt with Its Molecular Mechanisms
Abstract
1. Introduction
2. Materials and Test Method
2.1. Asphalt Material
2.2. Ultraviolet Aging Test
2.3. Rheological Performance Test
2.4. Chemical Composition Test and Its Index Calculation
2.4.1. Chemical Composition Test
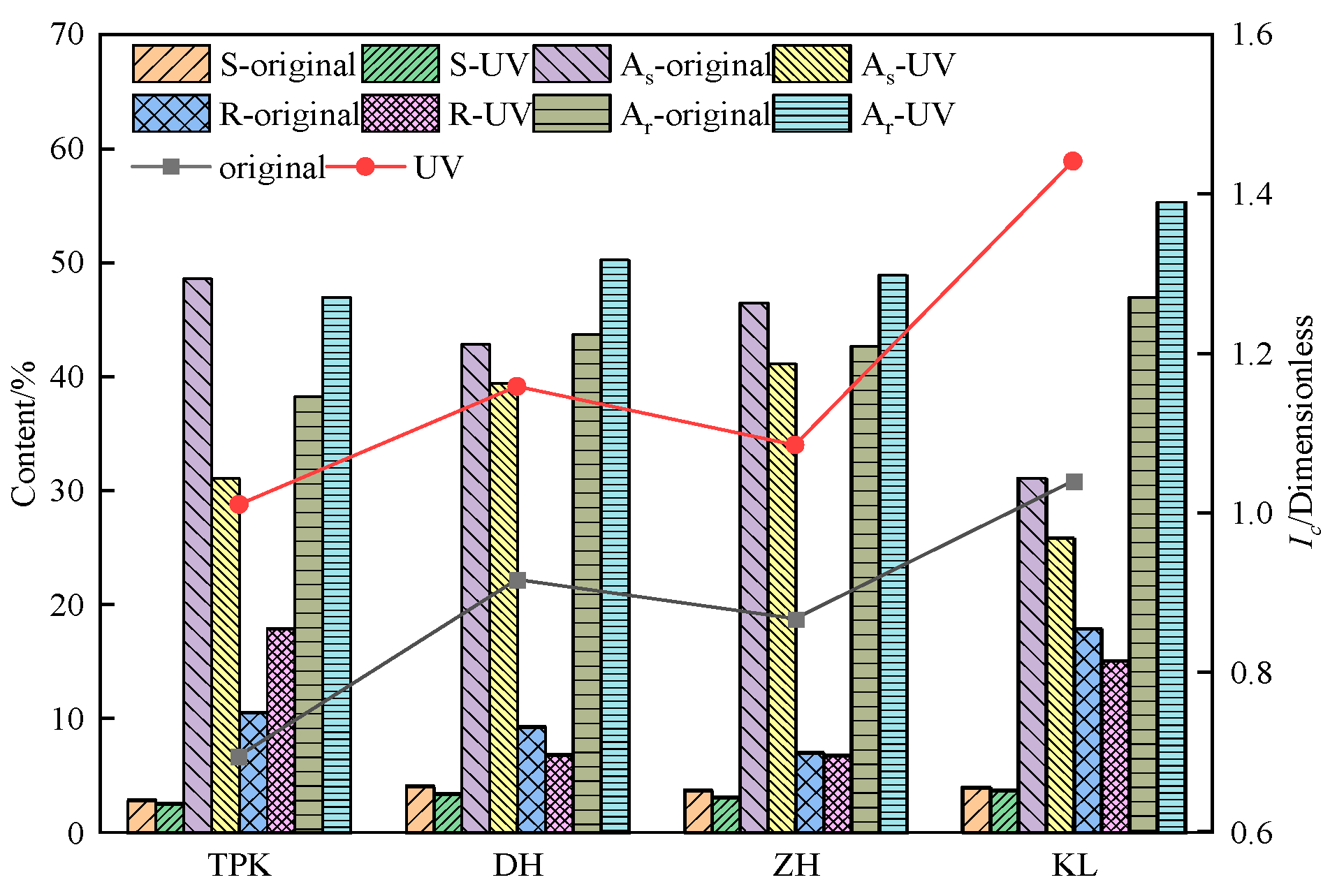
2.4.2. The Component Content and Colloidal Instability Index Calculation
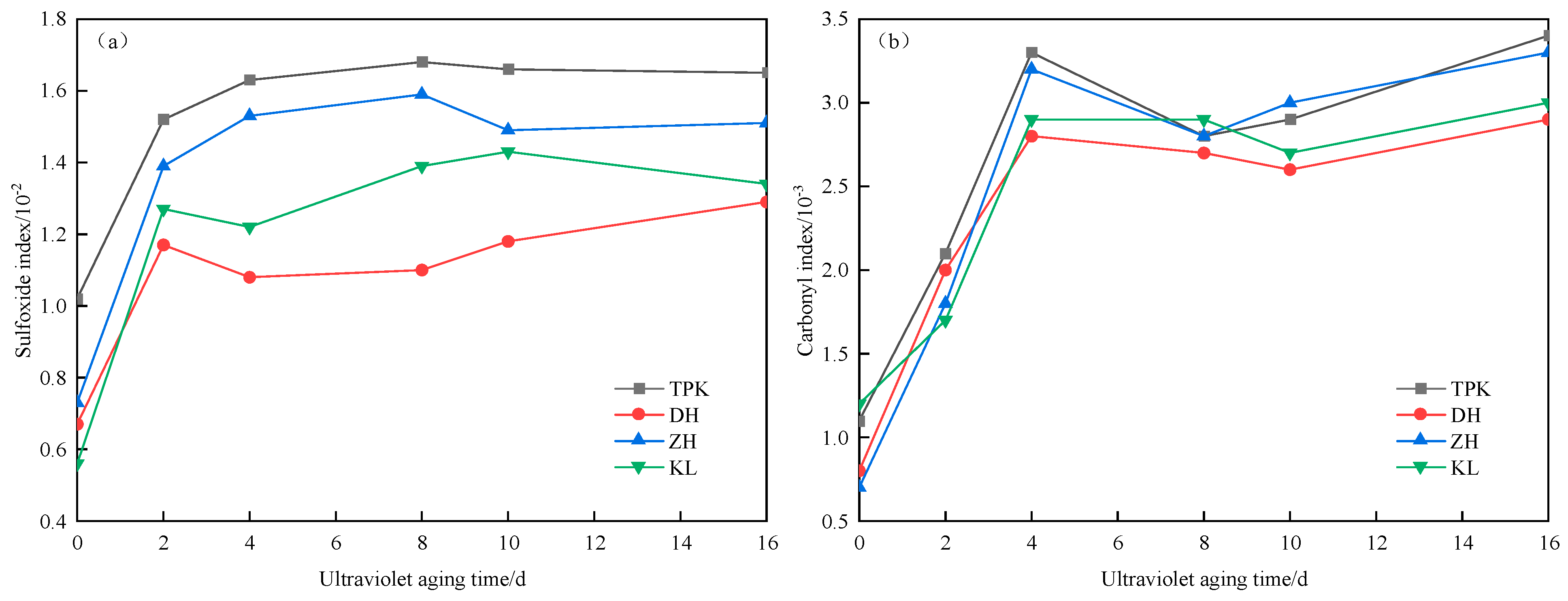
2.4.3. The Sulfoxide and Carbonyl Functional Group Indices Calculation
2.5. Molecular Mechanism Test and Its Index Calculation
2.5.1. Molecular Composition Test
2.5.2. Molecular Weight Calculation and Molecular Relative Mass Distribution
- (1)
- Molecular weight calculation
- (2)
- Molecular relative mass distribution
2.5.3. Molecular Structure Calculation
- (1)
- Attribution of hydrogen and carbon atoms
- (2)
- Hydrogen spectra and carbon spectra
- (3)
- Carbon atom content
- (4)
- Molecular structure parameter
2.6. Relevance Calculation
- (1)
- Reference series and comparative series
- (2)
- Correlation degree calculation
- (3)
- Calculate the absolute difference
- (4)
- Calculate the correlation coefficient
- (5)
- Calculate the relative correlation degree
2.7. Experiment Method
3. Results and Analysis
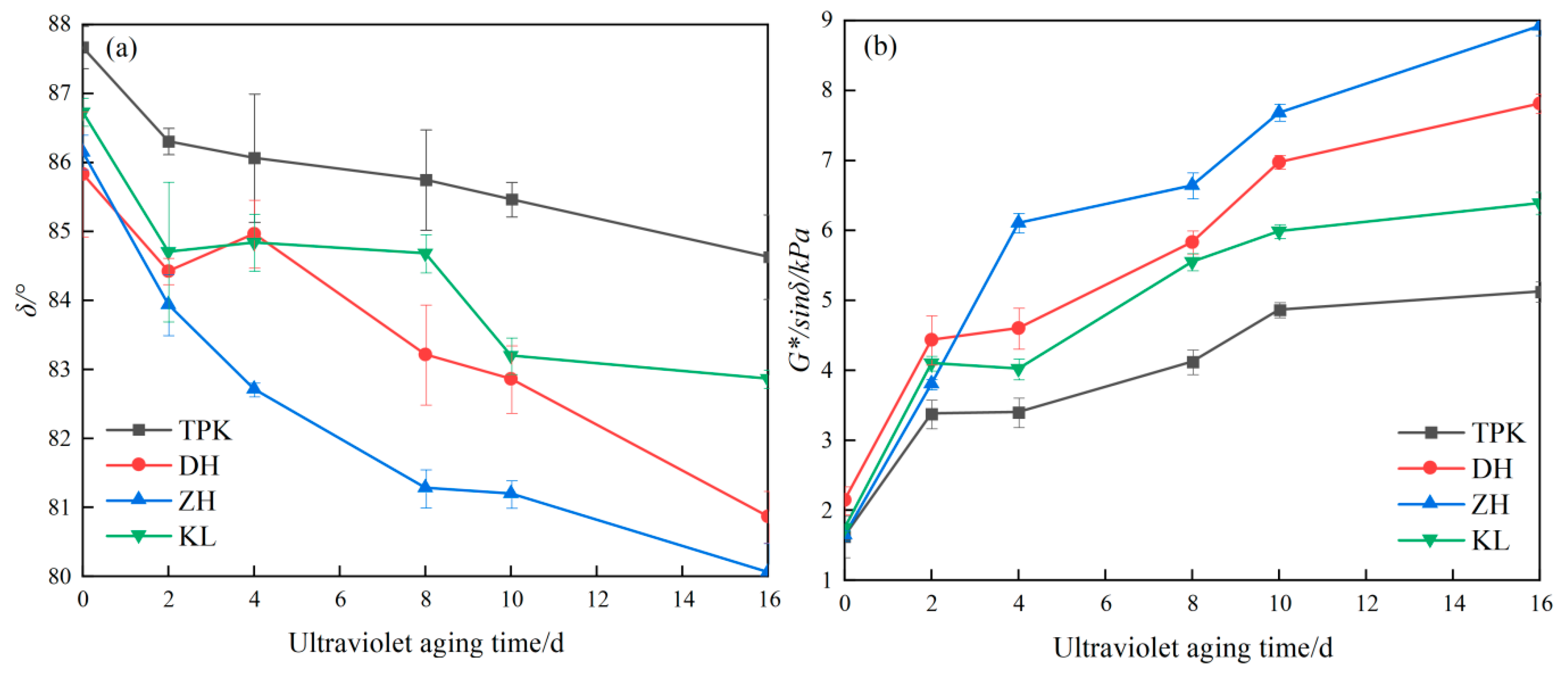
3.1. Rheology Index
3.1.1. Phase Angle and Rutting Factor Change
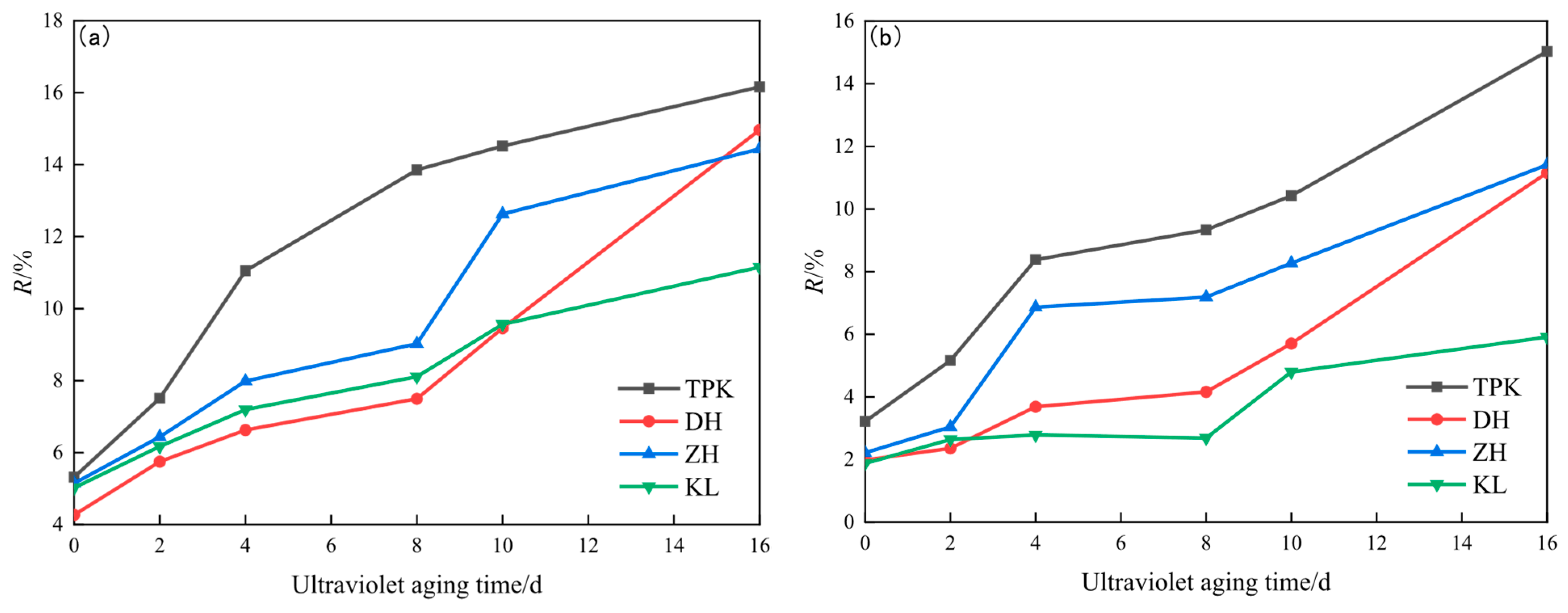
3.1.2. Changes in Non-Recoverable Creep Compliance and Creep Recovery Rate
3.2. Chemical Composition Index
3.2.1. Component Composition Change
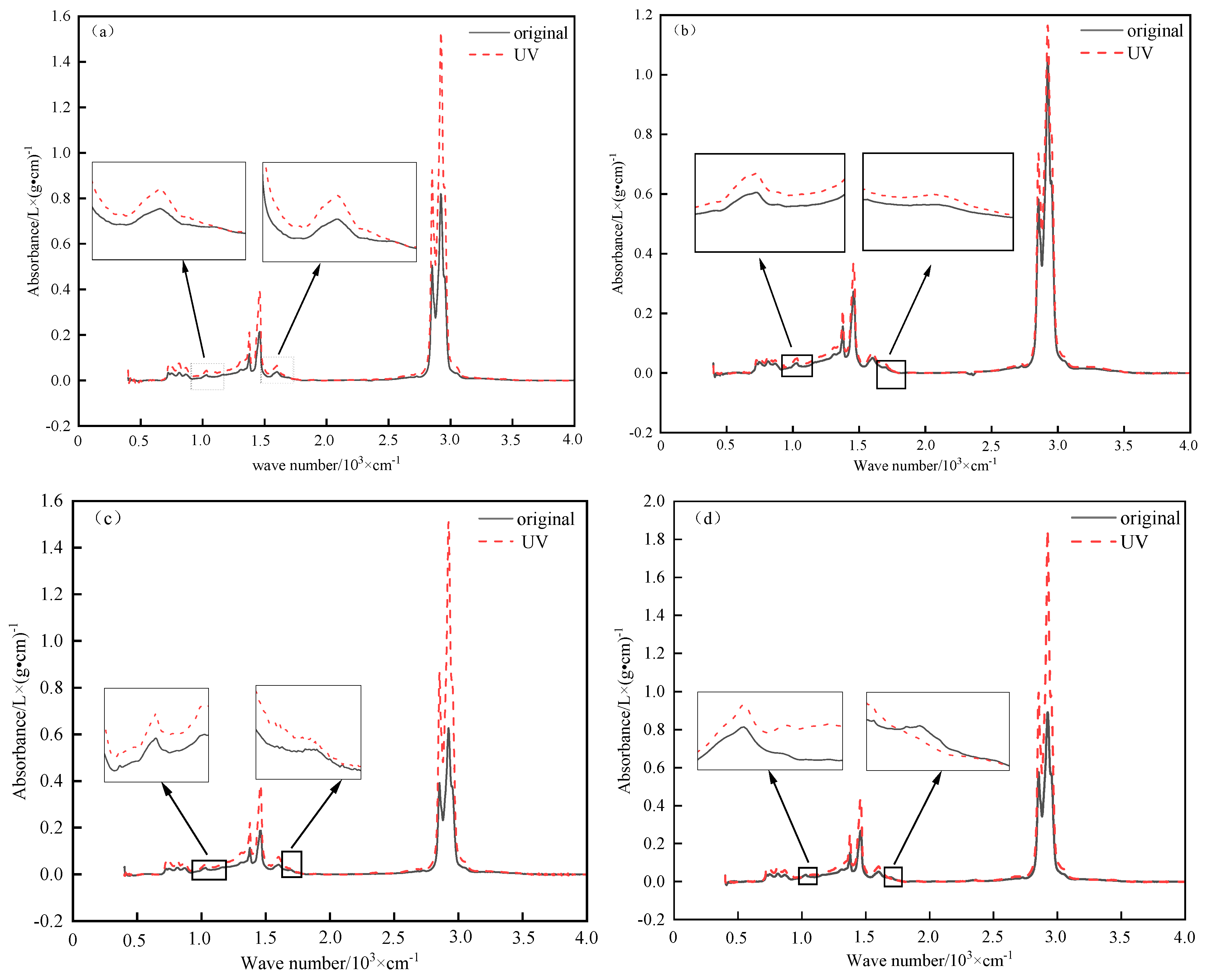
3.2.2. Characteristic Peaks and Functional Groups
- (1)
- Characteristic peaks analysis
- (2)
- Functional group index analysis
3.3. Molecular Composition Index
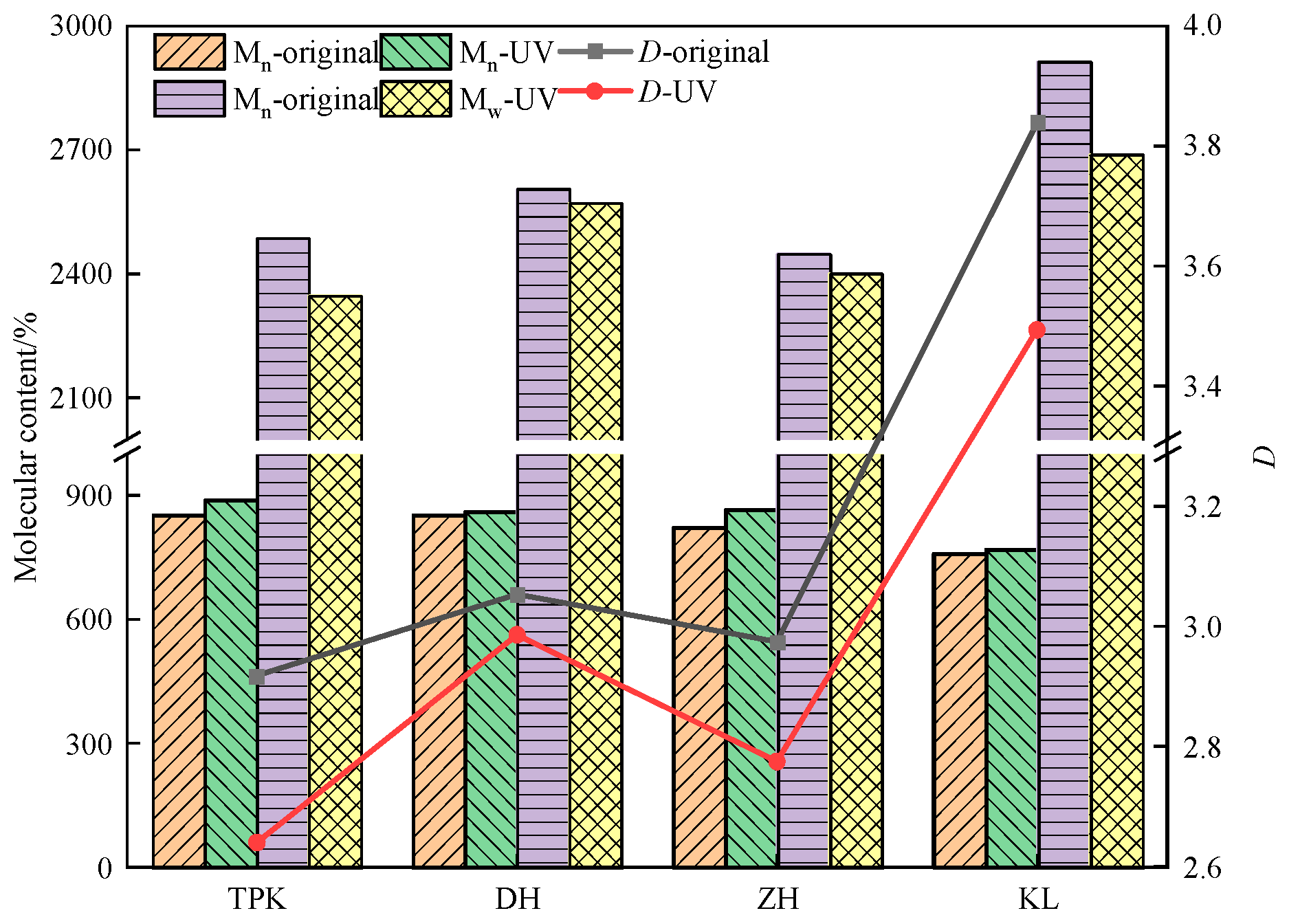
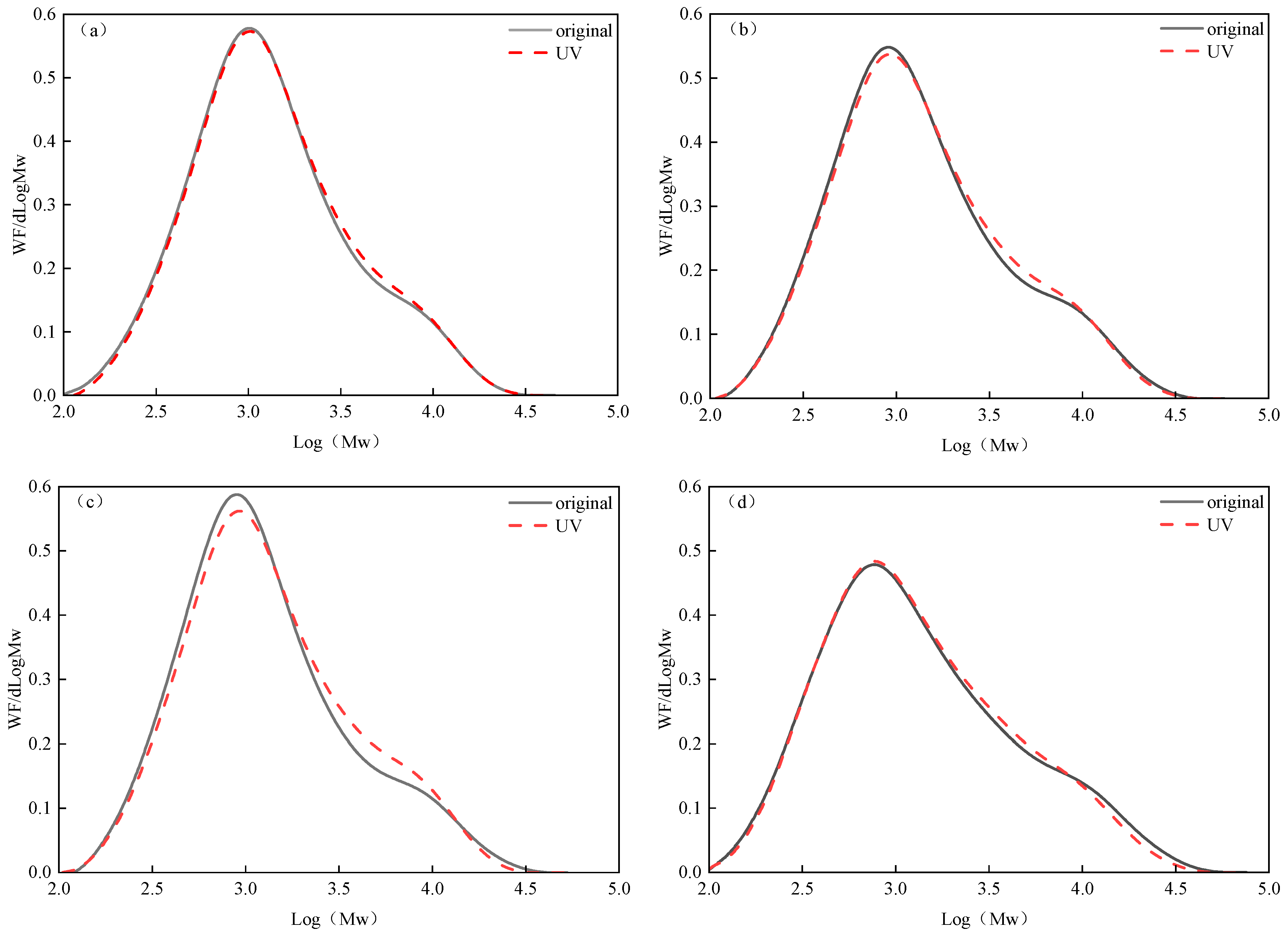
3.3.1. Molecular Weight and Its Distribution Change
- (1)
- Change in molecular weight
- (2)
- Changes in the distribution of molecular relative mass.
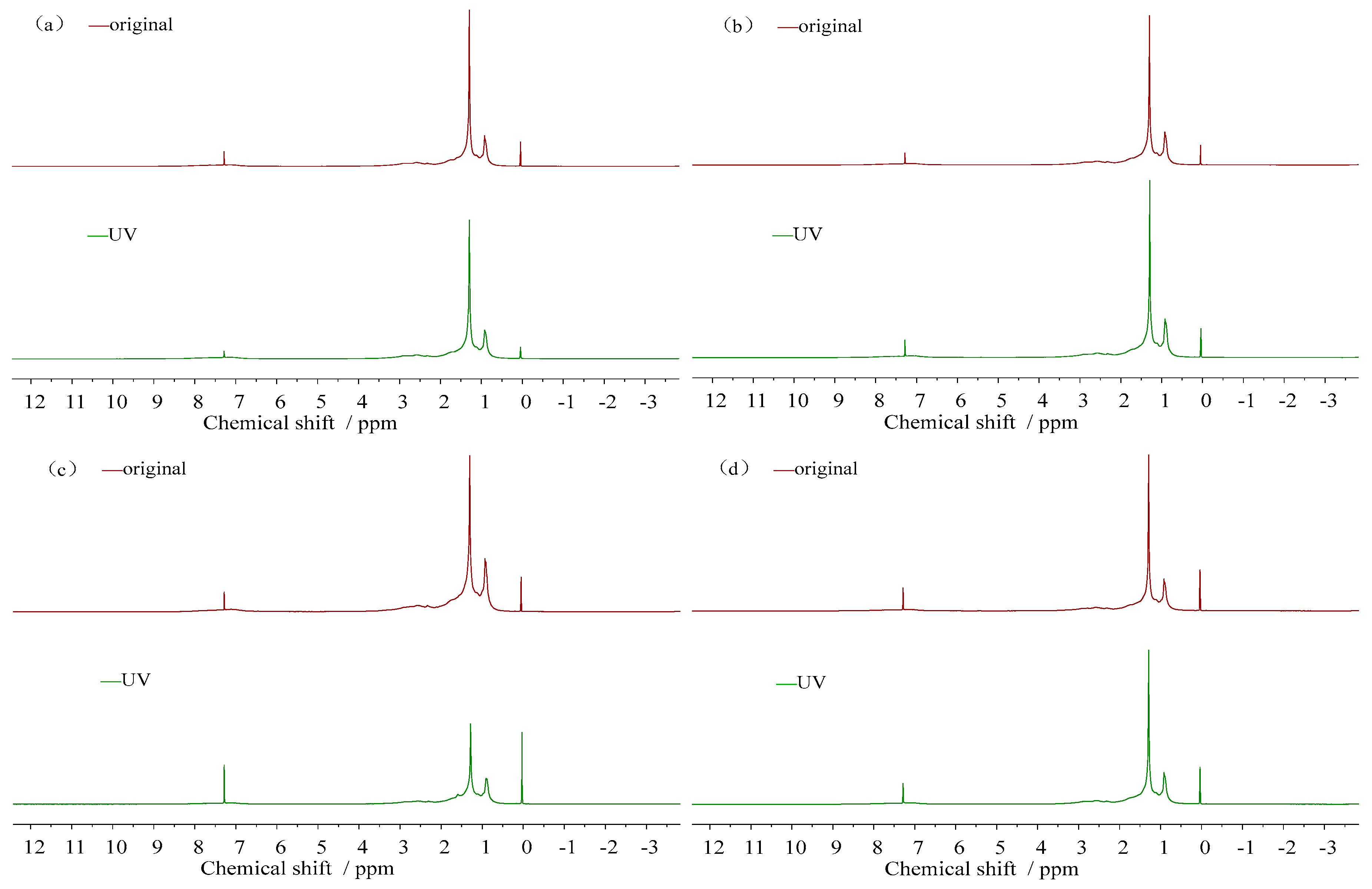
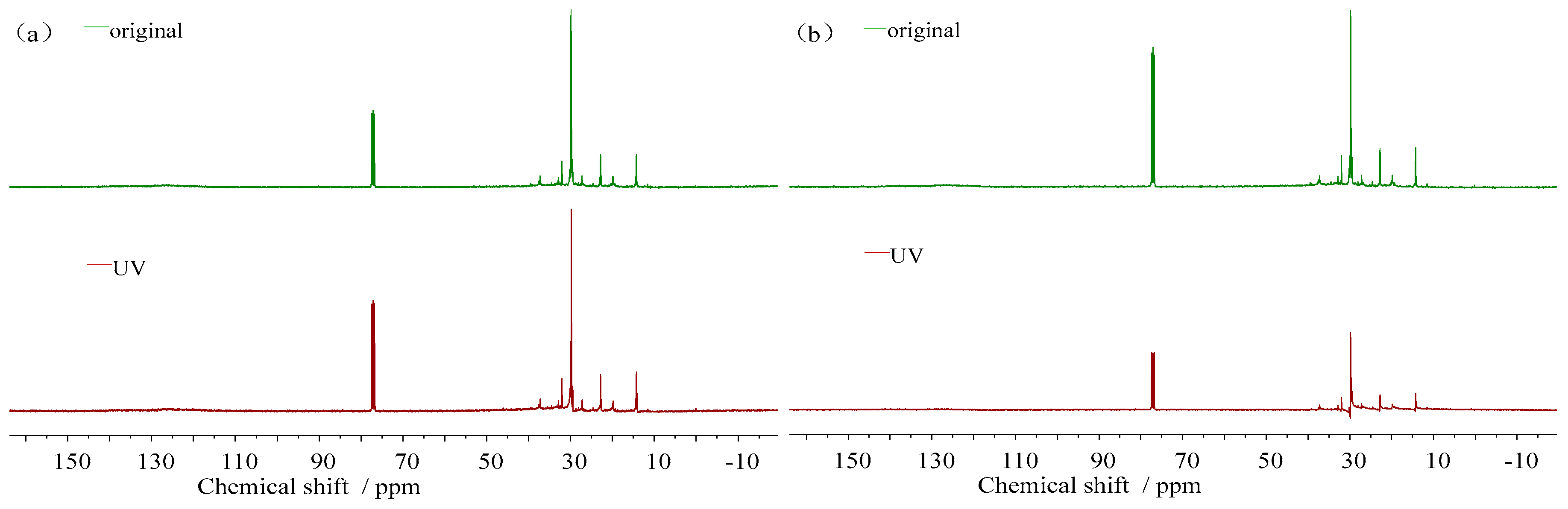
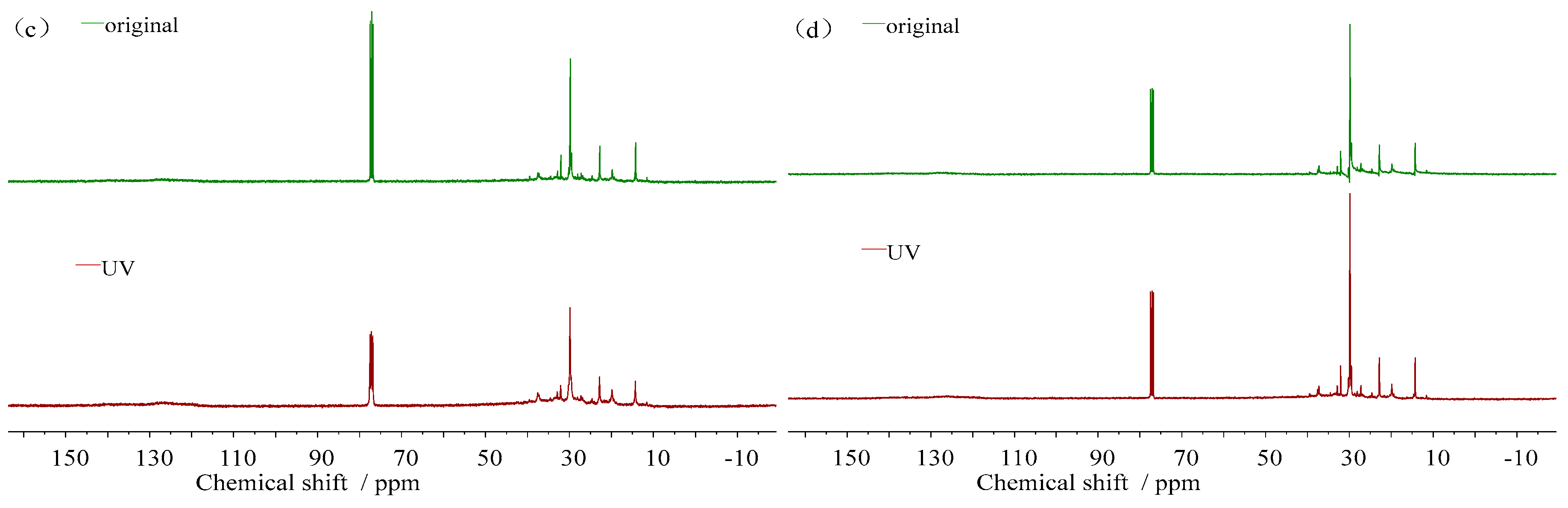
3.3.2. Molecular Structure Change
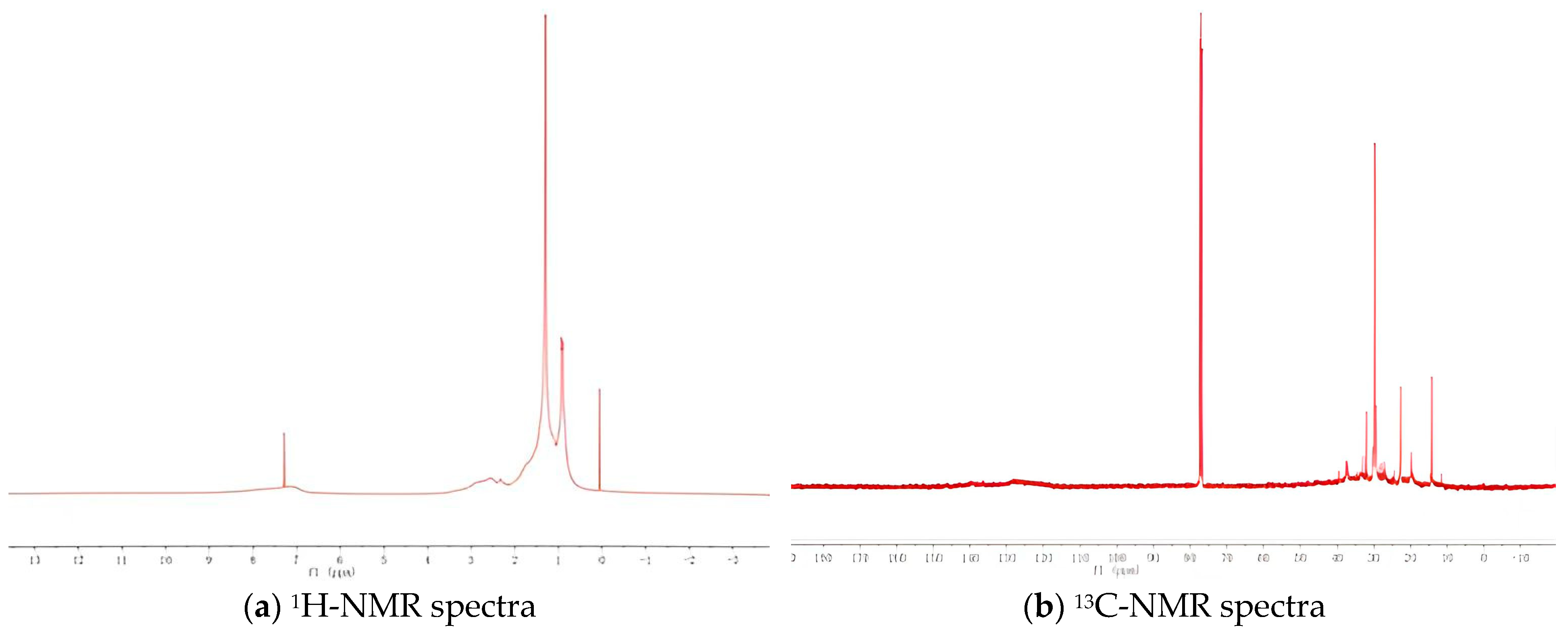
- (1)
- 1H-NMR spectra
- (2)
- Change of hydrogen content
- (3)
- 13C-NMR spectra
- (4)
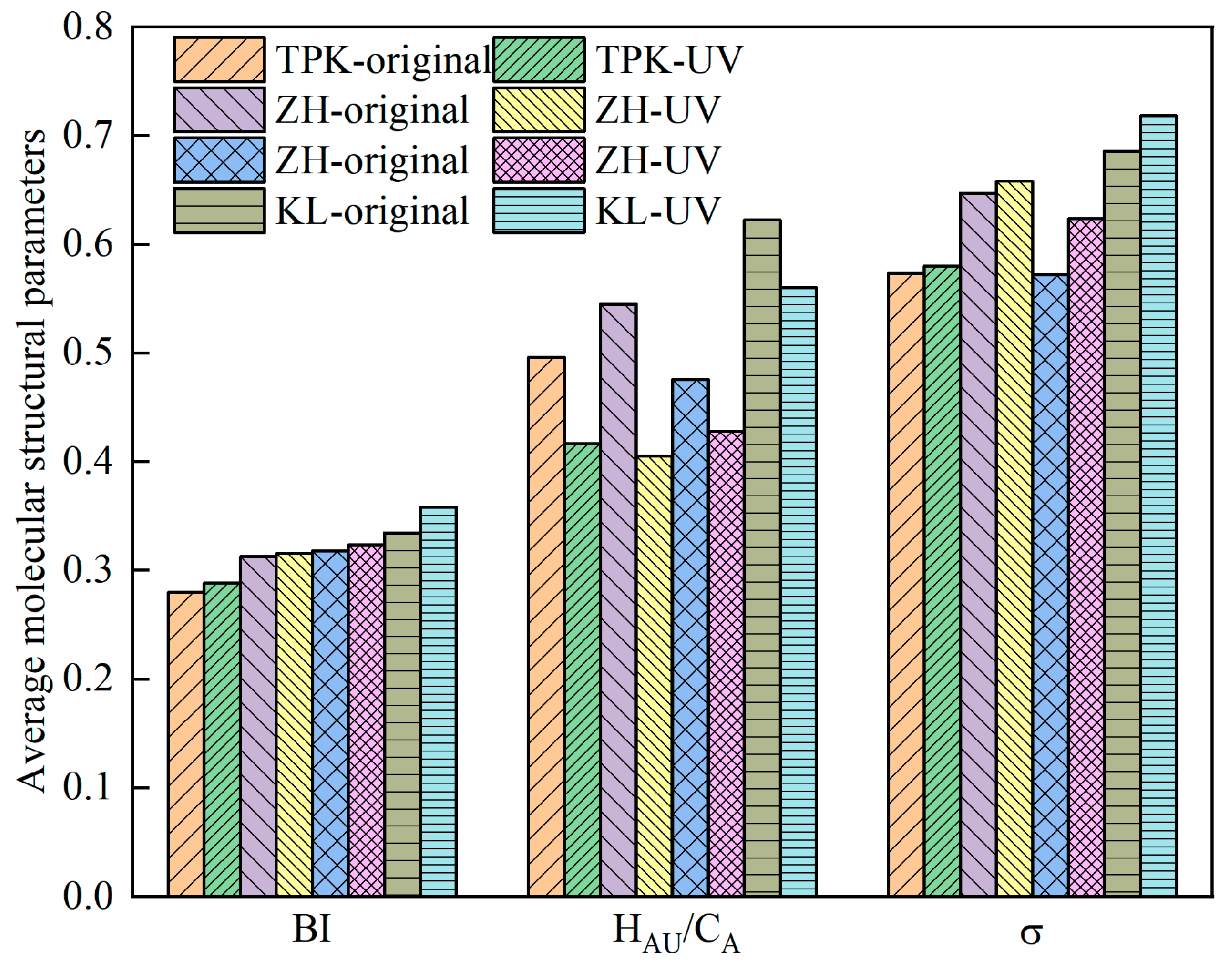
- Molecular structure parameters
3.4. Molecular Structure Analysis of Rheological Properties and Chemical Composition
4. Discussions
5. Conclusions
- (1)
- After UV aging, the anti-deformation, anti-high-temperature deformation, and elastic recovery capacity of asphalt are enhanced, while the flow performance is reduced. It can be observed that the internal elastic components of asphalt increase and transform into gel after aging;
- (2)
- Under the influence of ultraviolet light, the carbon and sulfur molecules within the asphalt will combine with oxygen elements to form stable chemical functional groups and increase the molecular weight of the asphalt. This indicates that the essence of the ultraviolet aging of asphalt is oxidation;
- (3)
- Ultraviolet aging leads to a reduction in HA, for which the decrease in KL was 55.88%, suggesting that hydrogen atoms in the benzene ring of asphalt are substituted and isomerized. This demonstrates that the dehydrogenation of asphalt takes place during the aging process;
- (4)
- After 16 days of aging, the aromatic carbon of TPK, DH, ZH, and KL increased by 34.23%, 49.74%, 28.91%, and 12.63%, respectively. This shows that the aromatic structure of asphalt is changed and the molecular stability is enhanced after ultraviolet aging;
- (5)
- The correlation analysis conducted herein shows that the correlation coefficients between sulfoxide group, carbonyl group, and molecular structure indexes are between 0.7–0.98, indicating that oxidation is the essence of UV aging and that the effect of ultraviolet light is mainly excitation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Zeng, X.; Wang, J.; Luo, S.; Meng, Y.; Gao, L.; Wang, X. Aging performance of high viscosity modified asphalt under complex Heat-light-water coupled conditions. Constr. Build. Mater. 2022, 325, 126314. [Google Scholar] [CrossRef]
- Yang, B.; Li, H.; Sun, Y.; Zhang, H.; Liu, J.; Yang, J.; Zhang, X. Chemo-rheological, mechanical, morphology evolution and environmental impact of aged asphalt binder coupling thermal oxidation, ultraviolet radiation, and water. J. Clean. Prod. 2023, 388, 135866. [Google Scholar] [CrossRef]
- Li, H.L.; Tong, P.P.; Zhang, X.J.; Lin, X.X.; Li, B. Influence of ultraviolet and oxygen coupling aging on rheological properties and functional group Index of warm mix asphalt binder. Materials 2020, 13, 4216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wu, S.P.; Liu, Q.T.; Xie, J.; Li, H.; Dai, Y.; Li, C.; Nie, S.; Song, W. Aging effects of ultraviolet lights with the same dominant wavelength and different wavelength ranges on a hydrocarbon-based polymer (asphalt). Polym. Test. 2019, 75, 64–75. [Google Scholar] [CrossRef]
- Alkofahi, N.; Khedaywi, T. Evaluation the Effect of Asphalt Film Thickness on Stripping Resistance. Int. J. Appl. Eng. Res. 2019, 14, 560–570. Available online: https://www.researchgate.net/publication/331877065 (accessed on 17 January 2025).
- Abouelsaad, A.; White, G. The Combined Effect of Ultraviolet Irradiation and Temperature on Hot Mix Asphalt Mixture Aging. Sustainability 2022, 14, 5942. [Google Scholar] [CrossRef]
- Liu, H.B.; Zhang, Z.Q.; Xie, J.Q.; Gui, Z.; Li, N.; Xu, Y. Analysis of OMMT strengthened UV aging-resistance of Sasobit/SBS modified asphalt: Its preparation, characterization, and mechanism. J. Clean. Prod. 2021, 315, 128139. [Google Scholar] [CrossRef]
- Yang, J.; Muhammad, Y.; Yang, C.L.; Liu, Y.; Su, Z.; Wei, Y.; Li, J. Preparation of TiO2/PS-rGO incorporated SBS modified asphalt with enhanced resistance against ultraviolet aging. Constr. Build. Mater. 2021, 276, 121461. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Li, D.Q.; Zhang, H.L.; Wang, Z.; Li, J. Synergistic effect of surface modified nano-zinc oxide and organic vermiculite on long-term ultraviolet and thermal-oxidative aging resistance of different asphalt binders. Constr. Build. Mater. 2023, 409, 133832. [Google Scholar] [CrossRef]
- Chen, S.C.; Liu, Q.T.; Wu, H.J.; Yang, C.; Gong, X.; Wu, S.; Li, Y. Simultaneous enhancement of the thermal oxygen and ultraviolet aging resistance of asphalt by incorporating antioxidant intercalated layered double hydroxide. Constr. Build. Mater. 2022, 349, 128743. [Google Scholar] [CrossRef]
- Lyu, L.; Pei, J.Z.; Hu, D.L.; Fini, E.H. The durability of rubberized asphalt binders containing waste cooking oil under thermal and ultraviolet aging. Constr. Build. Mater. 2021, 299, 124282. [Google Scholar] [CrossRef]
- Lu, P.Z.; Ma, Y.H.; Ye, K.; Huang, S. Analysis of the high-temperature performance of polymer-modified asphalts through molecular dynamics simulations and experiments. Constr. Build. Mater. 2022, 350, 128903. [Google Scholar] [CrossRef]
- Li, H.H.; Yu, J.Y.; Wu, S.P.; Pang, L.; Li, Y.; Wu, Y. Property of anti-ultraviolet aging of LDHs modified asphalt. J. Wuhan Univ. Technol. (Mater. Sci.) 2018, 33, 634–638. [Google Scholar] [CrossRef]
- Eltwati, A.; Mohamed, A.; Hainin, M.R.; Jusli, E.; Enieb, M. Rejuvenation of aged asphalt binders by waste engine oil and SBS blend: Physical, chemical, and rheological properties of binders and mechanical evaluations of mixtures. Constr. Build. Mater. 2022, 346, 128441. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feng, J.L.; Yang, F.; Wu, S.; Liu, Q.; Bai, T.; Liu, Z.; Li, C.; Gu, D.; Chen, A.; et al. Gradient aging behaviors of asphalt aged by ultraviolet lights with various intensities. Constr. Build. Mater. 2021, 295, 123618. [Google Scholar] [CrossRef]
- Celauro, C.; Teresi, R.; Dintcheva, N.T. Effect of short-term and UV irradiation aging on the behavior of SBS-Modified bitumen. Sustainability 2022, 14, 6915. [Google Scholar] [CrossRef]
- Zhang, D.M.; Zheng, Y.M.; Yuan, G.C.; Guo, H.; Zhou, Q.; Qian, G.; Liang, B. Comparative analysis of rheological and microscopic performance of SBS modified asphalt based on field aging and laboratory aging. Fuel 2023, 352, 128933. [Google Scholar] [CrossRef]
- Hou, X.D.; Xiao, F.P.; Wang, J.A.; Amirkhanian, S. Identification of asphalt aging characterization by spectrophotometry technique. Fuel 2018, 226, 230–239. [Google Scholar] [CrossRef]
- Rajib, A.; Saadeh, S.; Katawal, P.; Mobasher, B.; Fini, E.H. Enhancing Biomass Value Chain by Utilizing Biochar as A Free Radical Scavenger to Delay Ultraviolet Aging of Bituminous Composites Used in Outdoor Construction. Resour. Conserv. Recycl. 2020, 168, 105302. [Google Scholar] [CrossRef]
- Yu, H.N.; Bai, X.P.; Qian, G.P.; Wei, H.; Gong, X.; Jin, J.; Li, Z. Impact of Ultraviolet Radiation on the Aging Properties of SBS-Modified Asphalt Binders. Polymers 2019, 11, 1111. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, Z.Q.; Xue, J.S.; Lei, J.; Liu, Y.; Wang, Y.; Fang, Y. Variation and mechanism of asphalt-aggregate interface features under ultraviolet aging based on meso- and micro-observations. Constr. Build. Mater. 2023, 400, 132830. [Google Scholar] [CrossRef]
- Xiao, M.M.; Fan, L. Ultraviolet aging mechanism of asphalt molecular based on microscopic simulation. Constr. Build. Mater. 2022, 319, 126157. [Google Scholar] [CrossRef]
- Research Institute of Highway Science Ministry of Transport. Highway Asphalt Pavement Construction Technical Code: JTG F40-2017; People’s Communications Press: Beijing, China, 2017. [Google Scholar]
- Research Institute of Highway Science Ministry of Transport. Test Regulation of Asphalt and Asphalt Mixture in Highway Engineering: JTG E20-2011; People’s Communications Press: Beijing, China, 2011. [Google Scholar]
- Liu, H.B.; Zhang, Z.Q.; Tian, Z.G.; Li, N.; Li, H.; Wang, P. Optimization of the evaluation indexes for asphalt UV aging behaviors based on multi-scale characterization methods: From the evolution of their physical-chemical properties and microscope morphology. Constr. Build. Mater. 2022, 347, 128532. [Google Scholar] [CrossRef]
- Zheng, W.X.; Xu, W.Y.; Xie, S.R. Study on Ultraviolet Aging Mechanism of Carbon Nanotubes/SBS Composite-Modified Asphalt in Two-Dimensional Infrared Correlation Spectroscopy. Materials 2021, 14, 5672. [Google Scholar] [CrossRef]
- GB/T 21863-2008; Gel Permeation Chromatography (GPC) Using Tetrahydrofuran as Eluent. Standardization Administration of China, Standards Press of China: Beijing, China, 2008.
- Kong, L.Y.; Xi, H. Research review on ultraviolet aging and anti-aging materials of road asphalt. Mater. Rep. 2024, 38, 48–60. Available online: https://kns.cnki.net/kcms/detail//50.1078.TB.20230217.1731.009.html (accessed on 17 January 2025).
- Xi, H.; Kong, L.Y. Molecular Composition Changes of UV-aged asphalt and Its structure-Activity Relationship with rheological Properties and Chemical composition. Mater. Rev. 2025, 39, 86–94. [Google Scholar]
- Leite, L.F.M.; Aragão, F.T.S.; Macedo, T.F.; Osmari, P.H.; Cravo, M.C.C.; Nascimento, L.A.H.; Dantas, L.N. Effects of Chemical and Microstructural Constituents on the Healing Characteristics of Asphalt Binders; Springer: Cham, Switzerland, 2022; Volume 27. [Google Scholar] [CrossRef]
- Liu, M.X. Study on Multi-Scale Aging Factors of Matrix Asphalt Under Simulated Ultraviolet Environment. Master’s Thesis, Chongqing Jiaotong University, Chongqing, China, 2022. [Google Scholar]
- Szerb, E.I.; Nicotera, I.; Teltayev, B.; Vaiana, R.; Rossi, C.O. Highly stable surfactant-crumb rubber-modified bitumen: NMR and rheological investigation. Road Mater. Pavement Des. 2018, 19, 1192–1202. [Google Scholar] [CrossRef]
- Gui, X.; Long, X.; Zhan, W. Performance Prediction Method of Bridge Asphalt Pavement Material Based on Grey Theory. In Proceedings of the 2020 IEEE International Conference on Industrial Application of Artificial Intelligence (IAAI), Harbin, China, 25–27 December 2020; pp. 362–366. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Z.Q.; Yang, J.H.; Li, X. Performance characterization of biorecycled asphalt and gray correlation analysis between its components and macroproperties. J. Mater. Civ. Eng. 2022, 34, 04021490. [Google Scholar] [CrossRef]
- Zhao, W.F.; Li, X.J.; Feng, Z.G. Infrared spectrum analysis of aged asphalt regenerated by recycled engine oil bottom. Math. Probl. Eng. 2022, 2224094. [Google Scholar] [CrossRef]




| Asphalt Types | Penetration (25 °C, 0.1 mm) | Softening Point/°C | Elongation (15 °C)/cm |
|---|---|---|---|
| TRK | 75.0 | 47.5 | 83.2 |
| DH | 70.0 | 48.1 | 75.3 |
| ZH | 65.0 | 49.4 | 62.4 |
| KL | 67.0 | 48.7 | 70.1 |
| Technical requirement | 60.0~80.0 | ≥43.0 | ≥40.0 |
| Test method | T0604 | T0606 | T0605 |
| Atom | Area | Chemical Shift (δ)/ppm | Attribution of Atom |
|---|---|---|---|
| Hydrogen atom | HA | 6.0~9.0 | Hydrogen is directly linked to the aromatic carbon |
| Hα | 2.0~4.0 | Hydrogen attached to the α carbon of the aromatic ring | |
| Hβ | 1.0~2.0 | Hydrogen on the β carbon of the aromatic ring and hydrogen on the CH2 and CH groups beyond β | |
| Hγ | 0.5~1.0 | Hydrogen at the γ position of the aromatic ring and the CH3 group from γ away | |
| Carbon atom | Aromatic carbon | 150~170 | Aromatic carbon associated with an -OH or -OR |
| 130~150 | Aromatic carbon or Aromatic ring carbon associated with -R | ||
| 100~130 | Aromatic carbon associated with -H | ||
| Fatty carbon | 14.1 | CH3—(CH2)n— n ≥ 3 | |
| 19.7 | —CH2—CH (CH3)—CH2— | ||
| 22.7 | CH3—CH2—(CH2)n— n ≥ 2 | ||
| 29.7 | CH3—CH2—CH2—(CH2)n—CH2—CH2— | ||
| 32.0 | CH3—CH2—CH2—(CH2)n— n ≥ 2 |
| Asphalt Type | Aging Condition | 0.5~1 ppm | 1~2 ppm | 6~9 ppm | 7~8 ppm |
|---|---|---|---|---|---|
| TPK | original | 0.2039 | 0.2796 | 0.4960 | 0.5798 |
| UV | 0.2310 | 0.2880 | 0.4164 | 0.5732 | |
| ZH | original | 0.1634 | 0.3125 | 0.5445 | 0.6470 |
| UV | 0.2020 | 0.3155 | 0.4051 | 0.6579 | |
| DH | original | 0.1963 | 0.3233 | 0.4756 | 0.5718 |
| UV | 0.1955 | 0.3176 | 0.4275 | 0.6232 | |
| KL | original | 0.0811 | 0.3344 | 0.6222 | 0.6855 |
| UV | 0.1317 | 0.3582 | 0.5600 | 0.5180 |
| Asphalt Type | Aging Condition | CA | CN+P | fA |
|---|---|---|---|---|
| TPK | original | 12.2251 | 45.3986 | 0.2122 |
| UV | 16.4098 | 44.1438 | 0.2710 | |
| DH | original | 8.0504 | 51.8002 | 0.1345 |
| UV | 12.0555 | 47.2284 | 0.2034 | |
| ZH | original | 9.6214 | 43.6227 | 0.1807 |
| UV | 12.4025 | 40.2156 | 0.2357 | |
| KL | original | 12.8250 | 46.8221 | 0.2150 |
| UV | 14.4445 | 47.3007 | 0.2339 |
| Index | X01 | X02 | X03 | X04 | X05 |
|---|---|---|---|---|---|
| X1 | 0.78 | 0.62 | 0.55 | 0.70 | 0.71 |
| X2 | 0.58 | 0.70 | 0.70 | 0.77 | 0.76 |
| X3 | 0.57 | 0.74 | 0.66 | 0.82 | 0.76 |
| X4 | 0.57 | 0.61 | 0.59 | 0.90 | 0.82 |
| X5 | 0.56 | 0.63 | 0.60 | 0.93 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, H.; Kong, L.; Hu, S.; Zhu, S. Correlating the Effects of UV Aging on the Macro-Micro Behaviors of Asphalt with Its Molecular Mechanisms. Materials 2025, 18, 2165. https://doi.org/10.3390/ma18102165
Xi H, Kong L, Hu S, Zhu S. Correlating the Effects of UV Aging on the Macro-Micro Behaviors of Asphalt with Its Molecular Mechanisms. Materials. 2025; 18(10):2165. https://doi.org/10.3390/ma18102165
Chicago/Turabian StyleXi, Han, Lingyun Kong, Shixiong Hu, and Songxiang Zhu. 2025. "Correlating the Effects of UV Aging on the Macro-Micro Behaviors of Asphalt with Its Molecular Mechanisms" Materials 18, no. 10: 2165. https://doi.org/10.3390/ma18102165
APA StyleXi, H., Kong, L., Hu, S., & Zhu, S. (2025). Correlating the Effects of UV Aging on the Macro-Micro Behaviors of Asphalt with Its Molecular Mechanisms. Materials, 18(10), 2165. https://doi.org/10.3390/ma18102165






