A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers
Abstract
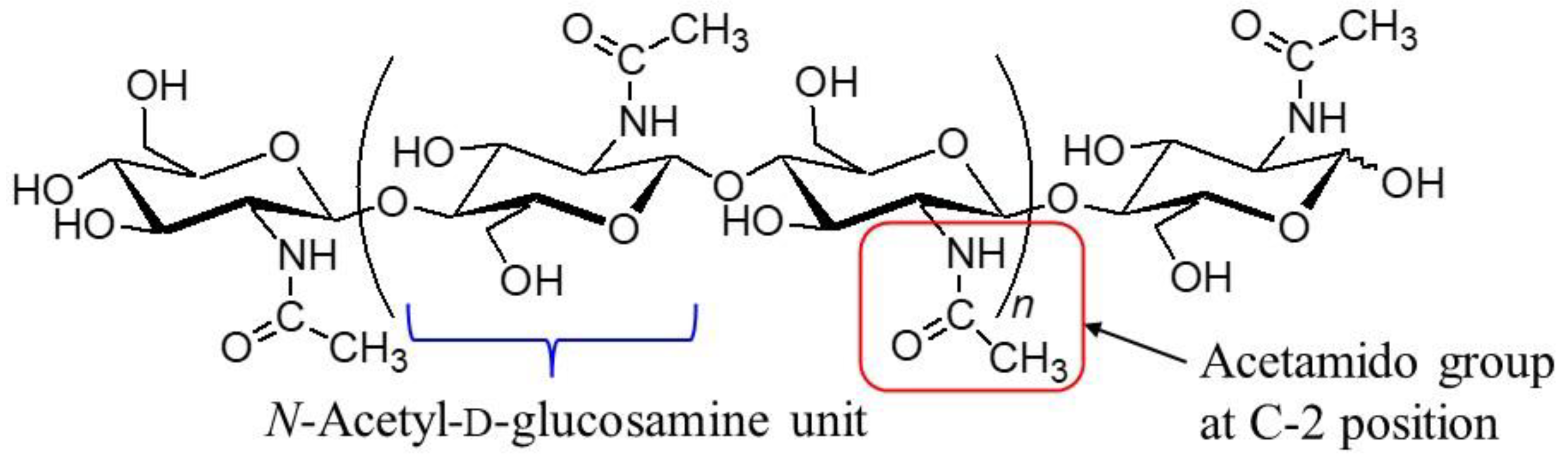
1. Introduction
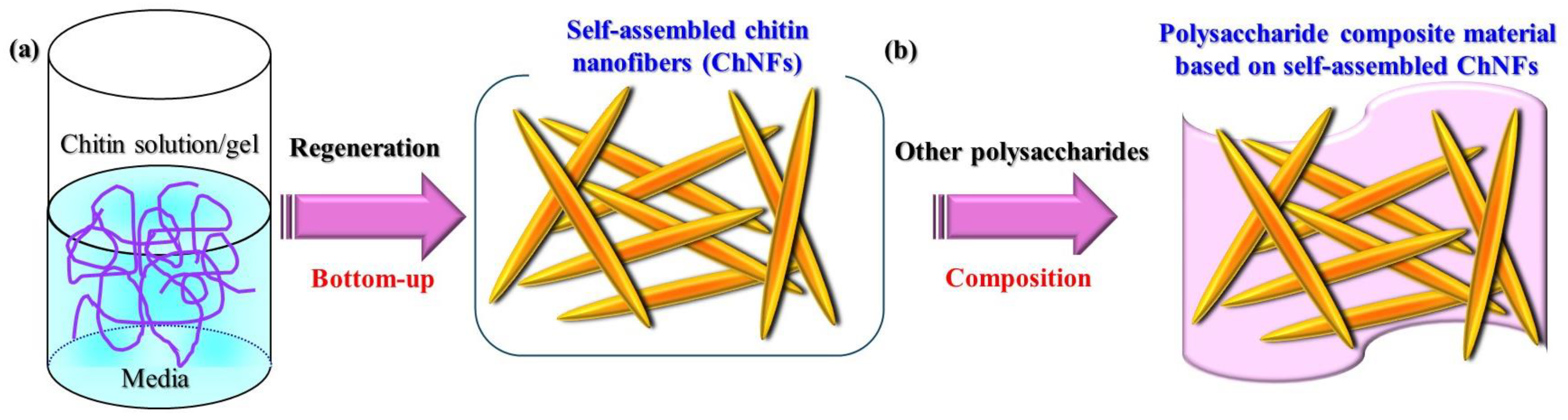
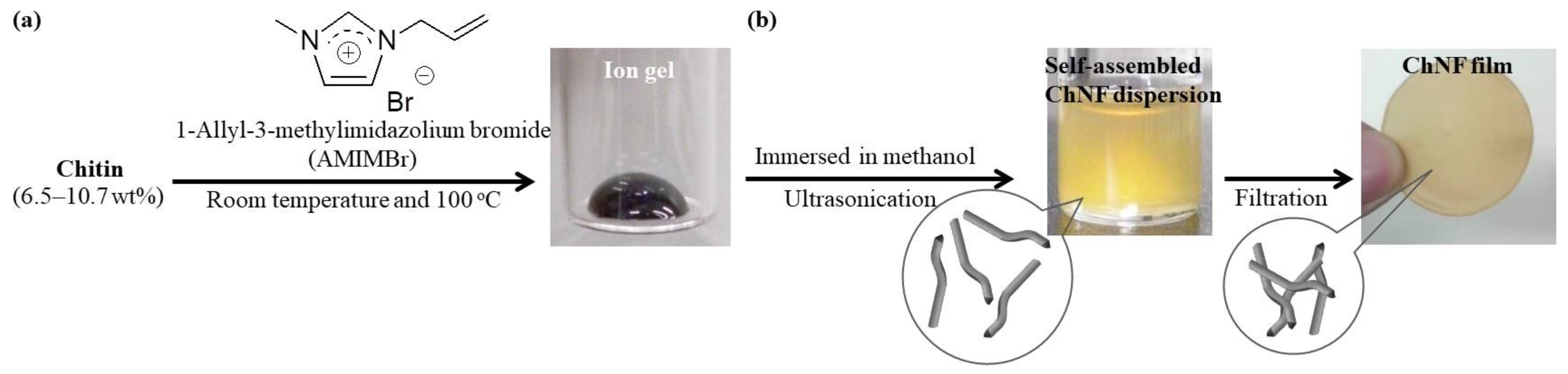
2. Preparation of Self-Assembled ChNFs from Ion Gels and Their Further Scaling Down into Thinner Fibrils
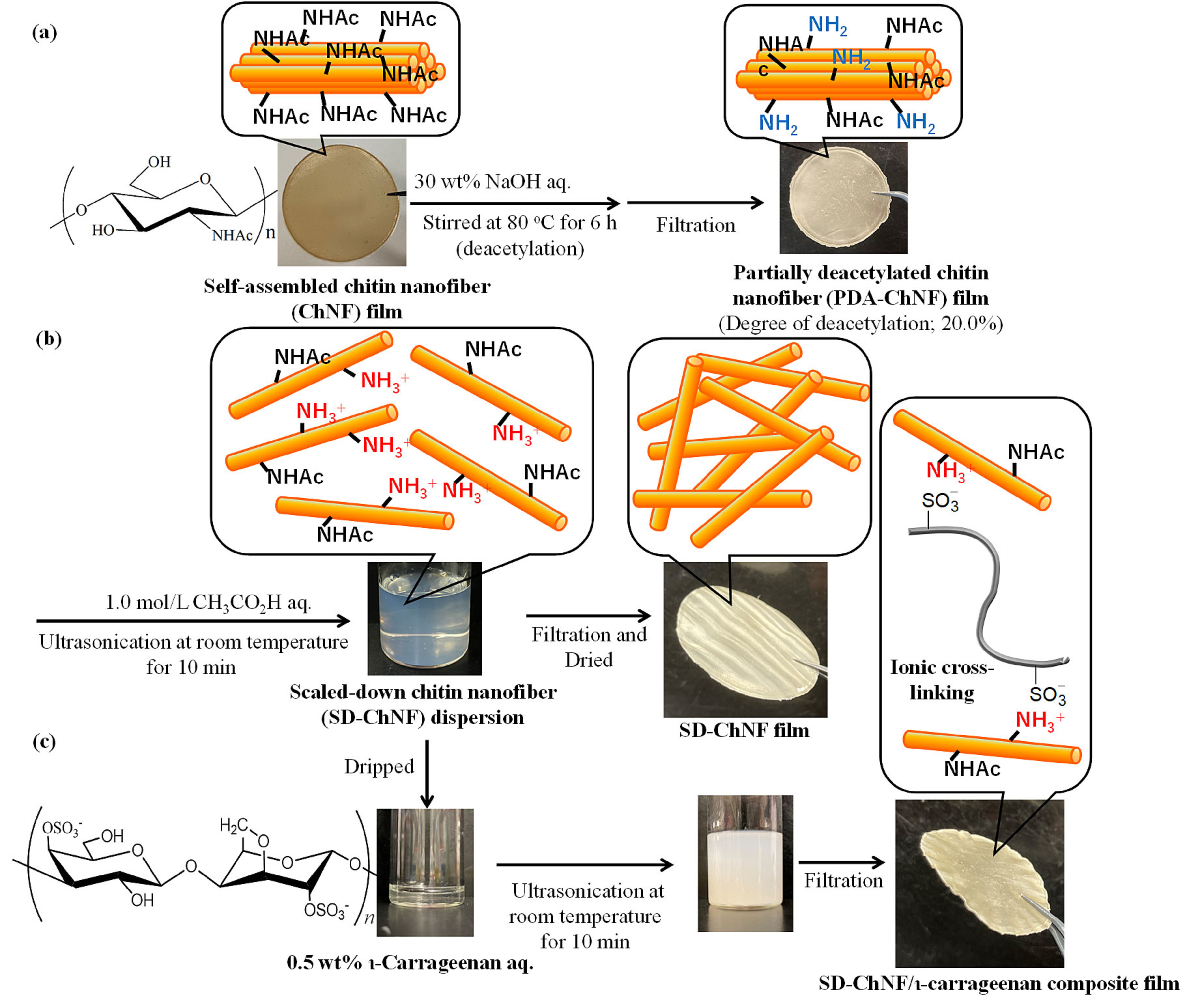
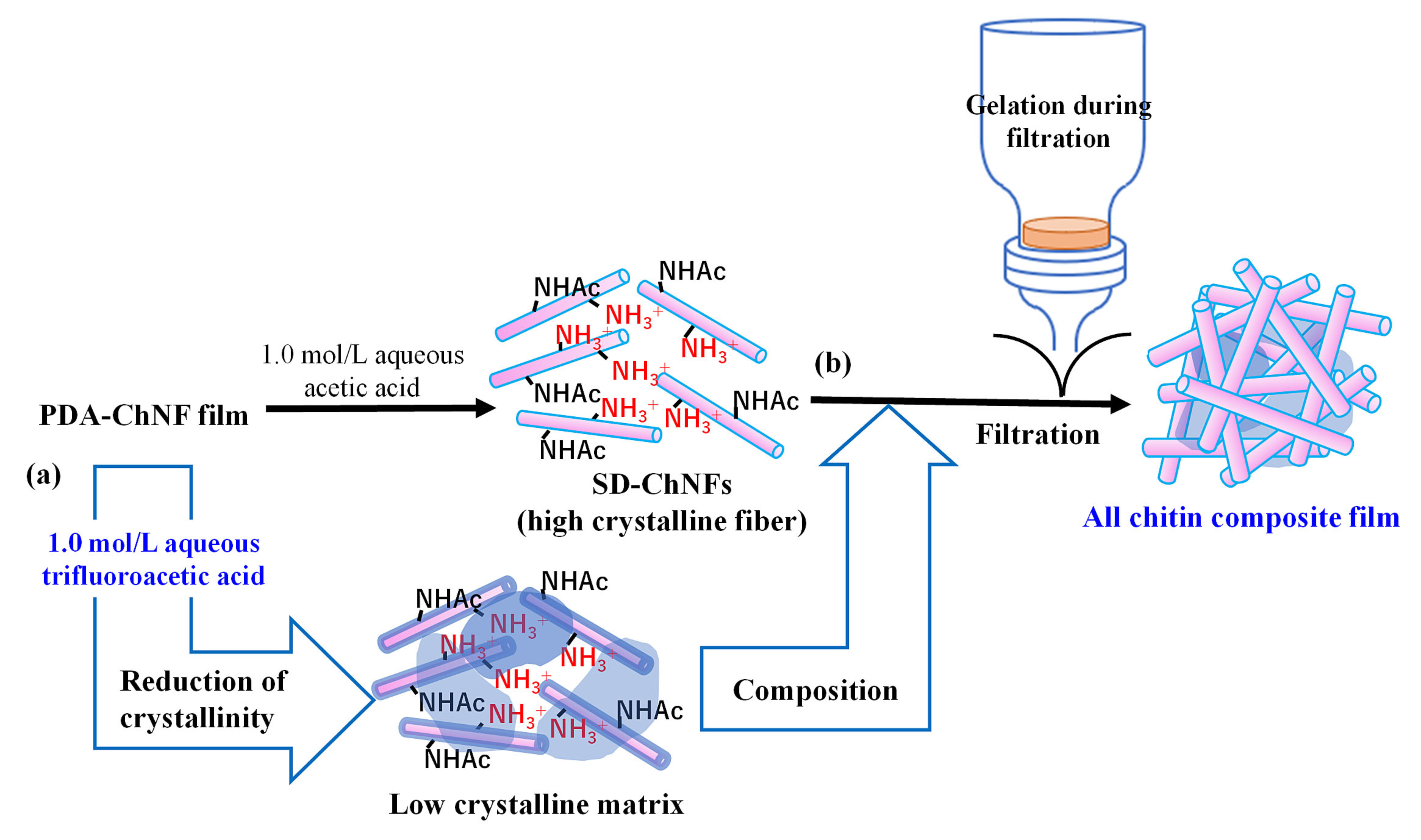
3. Composition of Self-Assembled ChNFs and SD-ChNFs with Other Chitin Components
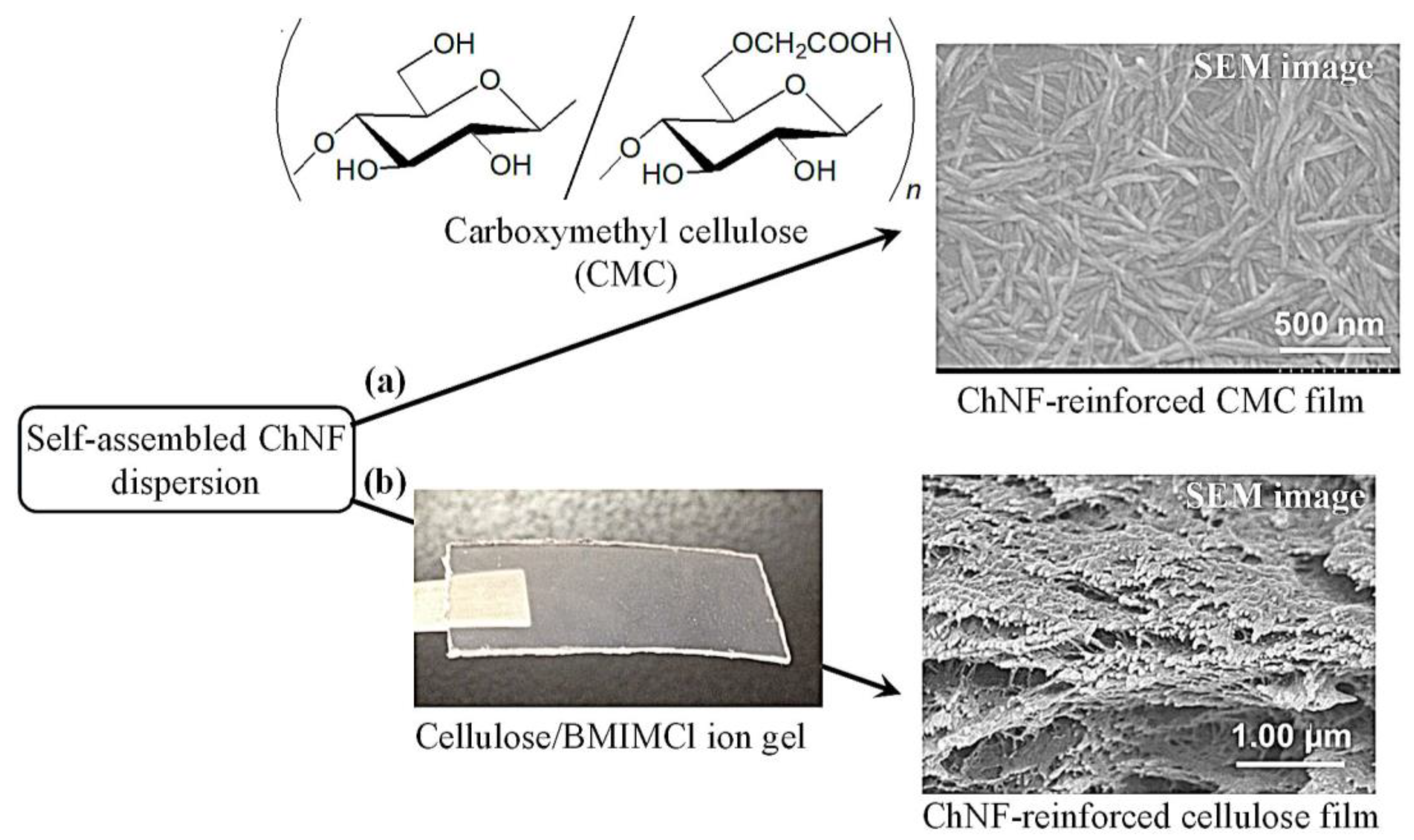
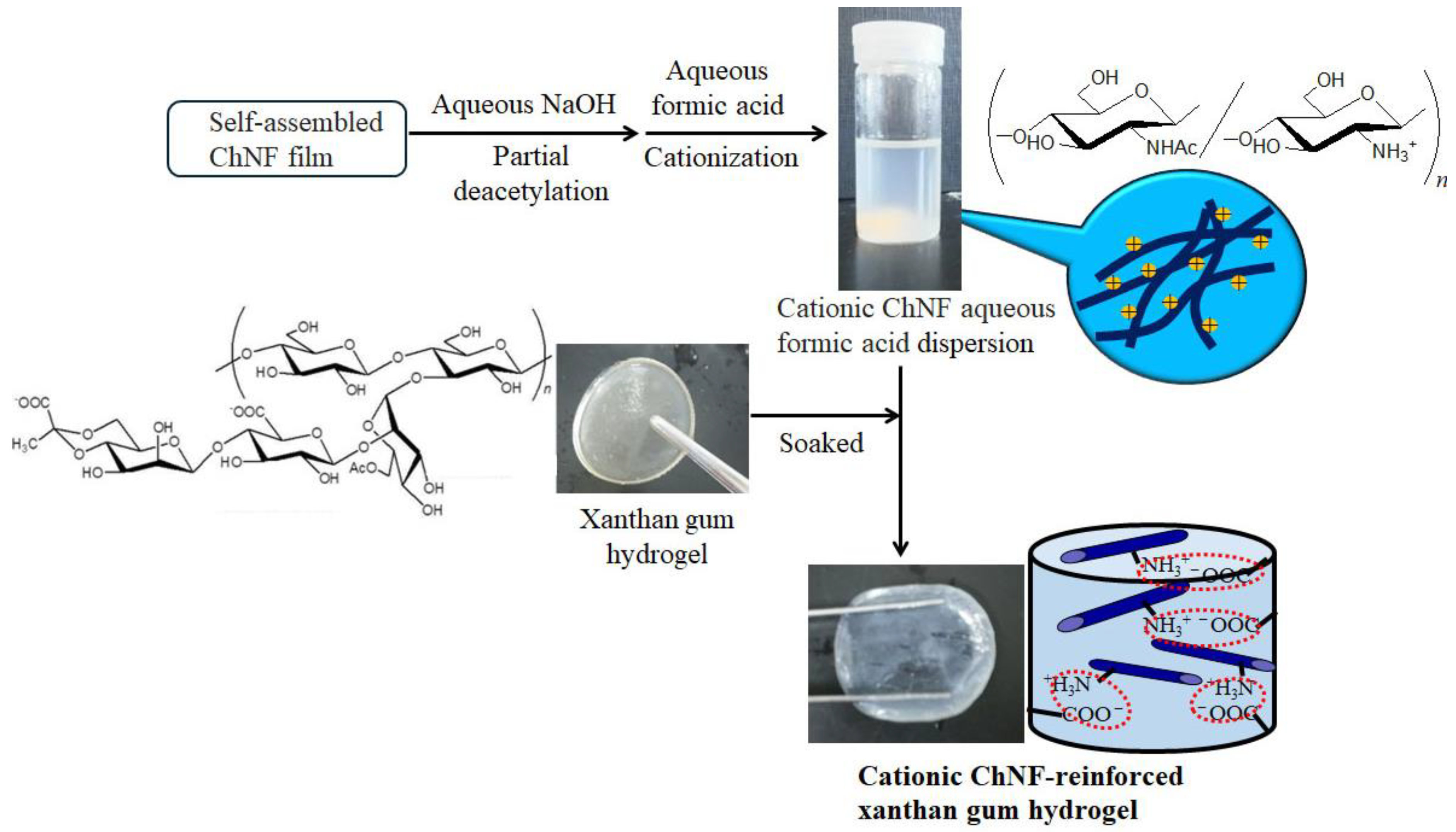
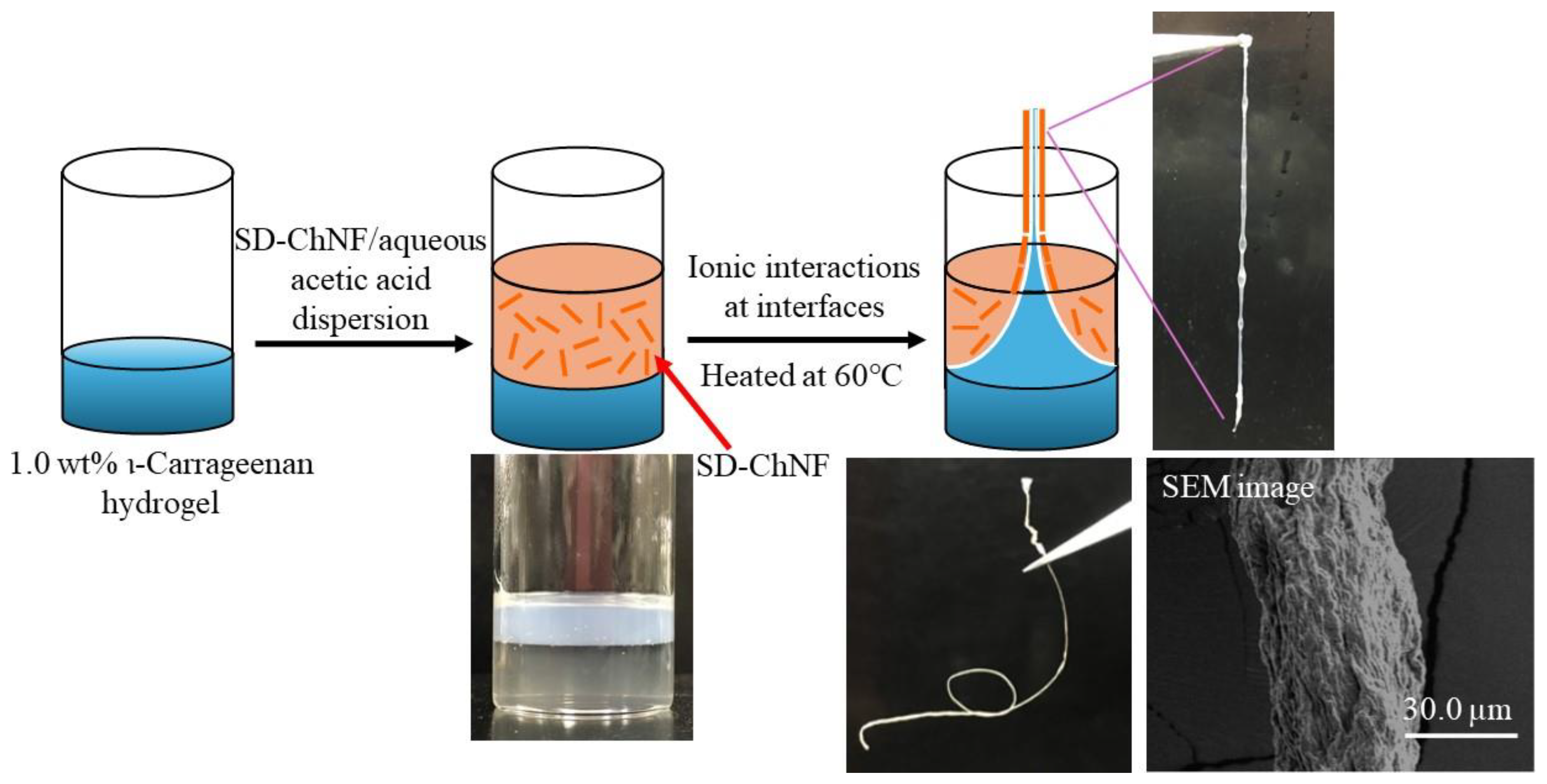
4. Composition of Self-Assembled ChNFs and SD-ChNFs with Other Polysaccharide Components
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daramola, M.O.; Ayeni, A.O. (Eds.) Valorization of Biomass to Value-Added Commodities: Current Trends, Challenges, and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Rinaudo, M. Materials based on chitin and chitosan. In Bio-Based Plastics: Materials and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 63–87. [Google Scholar]
- Li, B.; Mu, X. Recent progress in the utilization of chitin/chitosan for chemicals and materials. In Fuels, Chemicals and Materials from the Oceans and Aquatic Sources; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 151–187. [Google Scholar]
- Maleki, G.; Milani, J.M. Functional properties of chitin and chitosan-based polymer materials. In Handbook of Chitin and Chitosan: Volume 3: Chitin- and Chitosan-Based Polymer Materials for Various Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–198. [Google Scholar]
- Halloub, A.; Marya, R.; Essabir, H.; Bouhfid, R.; El Kacem Qaiss, A. Chitin and chitosan derivatives to proffer new functional materials. In Polysaccharides: Advanced Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2023; pp. 140–154. [Google Scholar]
- Anusiya, G.; Jaiganesh, R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S. Chitin nanomaterials as multifunctional systems in advanced applications—Progress and challenges toward sustainability. Macromol. Mater. Eng. 2023, 308, 2300053. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef]
- You, J.; Li, M.; Ding, B.; Wu, X.; Li, C. Crab chitin-based 2D soft nanomaterials for fully biobased electric devices. Adv. Mater. 2017, 29, 1606895. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Tabuchi, R.; Ifuku, S.; Nagae, T.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Miyamura, S.; Hirayama, F.; et al. An oral absorbent, surface-deacetylated chitin nano-fiber ameliorates renal injury and oxidative stress in 5/6 nephrectomized rats. Carbohydr. Polym. 2017, 161, 21–25. [Google Scholar] [CrossRef]
- Koizumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Ochi, K.; Tsuka, T.; Imagawa, T.; Osaki, T.; Ito, N.; Okamoto, Y.; et al. Oral administration of surface-deacetylated chitin nanofibers and chitosan inhibit 5-fluorouracil-induced intestinal mucositis in mice. Int. J. Mol. Sci. 2017, 18, 279. [Google Scholar] [CrossRef]
- Satam, C.C.; Irvin, C.W.; Lang, A.W.; Jallorina, J.C.R.; Shofner, M.L.; Reynolds, J.R.; Meredith, J.C. Spray-coated multilayer cellulose nanocrystal—Chitin nanofiber films for barrier applications. ACS Sustain. Chem. Eng. 2018, 6, 10637–10644. [Google Scholar] [CrossRef]
- Mushi, N.E.; Nishino, T.; Berglund, L.A.; Zhou, Q. Strong and tough chitin film from α-chitin nanofibers prepared by high pressure homogenization and chitosan addition. ACS Sustain. Chem. Eng. 2019, 7, 1692–1697. [Google Scholar] [CrossRef]
- Naghdi, T.; Golmohammadi, H.; Yousefi, H.; Hosseinifard, M.; Kostiv, U.; Horák, D.; Merkoçi, A. Chitin nanofiber paper toward optical (bio)sensing applications. ACS Appl. Mater. Interfaces 2020, 12, 15538–15552. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Preparation and applications of chitin nanofibers/nanowhiskers. In Biopolymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2013; pp. 131–151. [Google Scholar]
- Raabe, D.; Romano, P.; Sachs, C.; Fabritius, H.; Al-Sawalmih, A.; Yi, S.B.; Servos, G.; Hartwig, H.G. Microstructure and crystallographic texture of the chitin–protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus. Mater. Sci. Eng. A 2006, 421, 143–153. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Lin, A.Y.-M.; McKittrick, J.; Meyers, M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008, 4, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10-20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin nanofibers: Preparations, modifications, and applications. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2015; pp. 165–178. [Google Scholar]
- Ifuku, S.; Anraku, M.; Azuma, K. Preparation of chitin nanofiber and its derivatives from crab shell and their efficient biological properties. Adv. Polym. Sci. 2021, 287, 301–318. [Google Scholar]
- Tanaka, K.; Yamamoto, K.; Kadokawa, J. Facile nanofibrillation of chitin derivatives by gas bubbling and ultrasonic treatments in water. Carbohydr. Res. 2014, 398, 25–30. [Google Scholar] [CrossRef]
- Rolandi, M.; Rolandi, R. Self-assembled chitin nanofibers and applications. Adv. Colloid Interface Sci. 2014, 207, 216–222. [Google Scholar] [CrossRef]
- Kadokawa, J. Fabrication of nanostructured and microstructured chitin materials through gelation with suitable dispersion media. RSC Adv. 2015, 5, 12736–12746. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef]
- Ganesh, S.S.; Anushikaa, R.; Swetha Victoria, V.S.; Lavanya, K.; Shanmugavadivu, A.; Selvamurugan, N. Recent advancements in electrospun chitin and chitosan nanofibers for bone tissue engineering applications. J. Funct. Biomater. 2023, 14, 288. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Kadokawa, J. Application of ionic liquids for the functional materialization of chitin. Mater. Adv. 2022, 3, 3355–3364. [Google Scholar] [CrossRef]
- Kadokawa, J. Processing techniques of chitin-based gels, blends, and composites using ionic liquids. In Handbook of Chitin and Chitosan: Volume 2: Composites and Nanocomposites from Chitin and Chitosan, Manufacturing and Characterisations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 47–60. [Google Scholar]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids—Promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Khan, I.; Usmani, M.A.; Umapathi, R.; Al-Kindy, S.M.Z. Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 2019, 129, 750–777. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhu, J.; Wang, X.L.; Huang, Y.; Wang, Y.Z. Dissolution behavior of chitin in ionic liquids. J. Macromol. Sci. Phys. 2010, 49, 528–541. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Kozlecki, T.; Gorak, A. Review of the application of ionic liquids as solvents for chitin. J. Polym. Eng. 2012, 32, 67–69. [Google Scholar] [CrossRef]
- Kadokawa, J. Ionic liquid as useful media for dissolution, derivatization, and nanomaterial processing of chitin. Green Sustain. Chem. 2013, 3, 19–25. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Shamshina, J.L. Chitin in ionic liquids: Historical insights into the polymer’s dissolution and isolation. A review. Green Chem. 2019, 21, 3974–3993. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Tajiri, R.; Setoguchi, T.; Wakizono, S.; Yamamoto, K.; Kadokawa, J. Preparation of self-assembled chitin nanofibers by regeneration from ion gels using calcium halide · dihydrate/methanol solutions. J. Biobased Mater. Bioener. 2013, 7, 655–659. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kawano, A.; Yamamoto, K. Fabrication of semi-crystalline film by hexanoylation on self-assembled chitin nanofibers. ChemistrySelect 2019, 4, 797–801. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 118369. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamamoto, K.; Kadokawa, J. Preparation of cationic/anionic chitin nanofiber composite materials. J. Polym. Environ. 2018, 26, 3540–3549. [Google Scholar] [CrossRef]
- Krajenta, J.; Safandowska, M.; Pawlak, A.; Galeski, A. All-polymer composites—A new approach with the use of disentangled semi-crystalline polymers Part I. Disentangling and properties of disentangled polylactide. Polimery/Polymers 2020, 65, 165–173. [Google Scholar] [CrossRef]
- Krajenta, J.; Pawlak, A.; Galeski, A. All-polymer composites—A new approach with the use of disentangled semi-crystalline polymers Part II. Preparation of composites from partially disentangled polylactide. Polimery/Polymers 2020, 65, 261–267. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A critical review of all-cellulose composites. J. Mater. Sci. 2012, 47, 1171–1186. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Uusi-Tarkka, E.K.; Skrifvars, M.; Haapala, A. Fabricating sustainable all-cellulose composites. Appl. Sci. 2021, 11, 10069. [Google Scholar] [CrossRef]
- Egi, Y.; Kontani, A.; Kadokawa, J. Fabrication of all-chitin composite films. Int. J. Biol. Macromol. 2023, 253, 127512. [Google Scholar] [CrossRef]
- Hatanaka, D.; Yamamoto, K.; Kadokawa, J. Preparation of chitin nanofiber-reinforced carboxymethyl cellulose films. Int. J. Biol. Macromol. 2014, 69, 35–38. [Google Scholar] [CrossRef]
- Kadokawa, J.; Murakami, M.; Kaneko, Y. A facile preparation of gel materials from a solution of cellulose in ionic liquid. Carbohydr. Res. 2008, 343, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Endo, R.; Hatanaka, D.; Yamamoto, K. Preparation of chitin nanofiber-reinforced cellulose films through stepwise regenerations from individually prepared ion gels. J. Polym. Environ. 2015, 23, 348–355. [Google Scholar] [CrossRef]
- Kadokawa, J.; Noguchi, S.; Gotanda, T.; Kawano, A.; Yamamoto, K. Fabrication of cationized chitin nanofiber-reinforced xanthan gum hydrogels. Polym. Bull. 2020, 77, 4095–4103. [Google Scholar] [CrossRef]
- Izawa, H.; Kadokawa, J. Preparation and characterizations of functional ionic liquid-gel and hydrogel materials of xanthan gum. J. Mater. Chem. 2010, 20, 5235–5241. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadokawa, J.-i. A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers. Materials 2024, 17, 1898. https://doi.org/10.3390/ma17081898
Kadokawa J-i. A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers. Materials. 2024; 17(8):1898. https://doi.org/10.3390/ma17081898
Chicago/Turabian StyleKadokawa, Jun-ichi. 2024. "A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers" Materials 17, no. 8: 1898. https://doi.org/10.3390/ma17081898
APA StyleKadokawa, J.-i. (2024). A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers. Materials, 17(8), 1898. https://doi.org/10.3390/ma17081898





