Highly Sensitive Temperature Sensors Resulting from the Luminescent Behavior of Sm3+-Doped Ba2MgMoO6 High-Symmetry Double-Perovskite Molybdate Phosphors
Abstract
1. Introduction
1.1. Experimental
Samples Preparation
1.2. Research Techniques
2. Results and Discussion
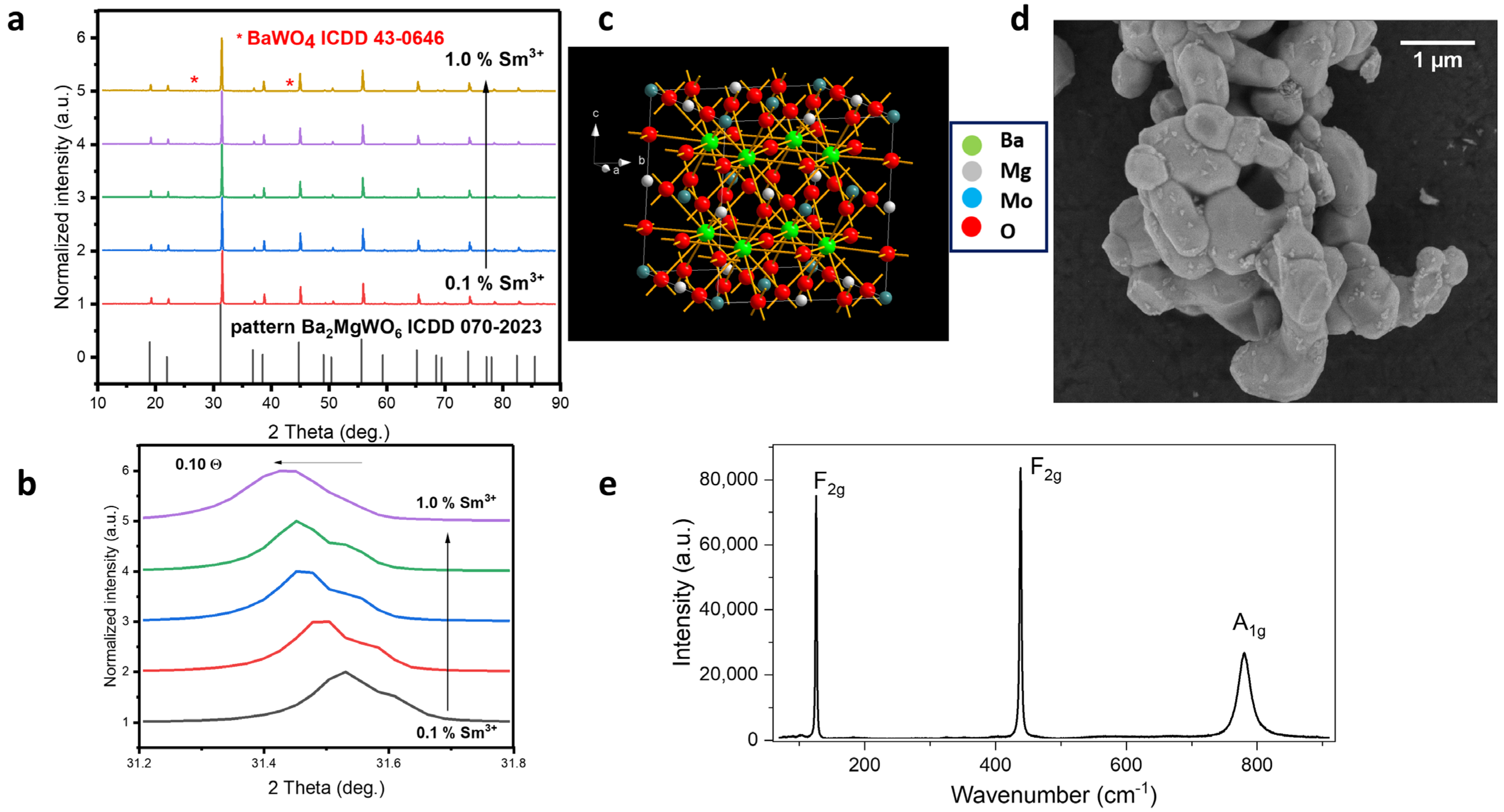
2.1. Structural Properties
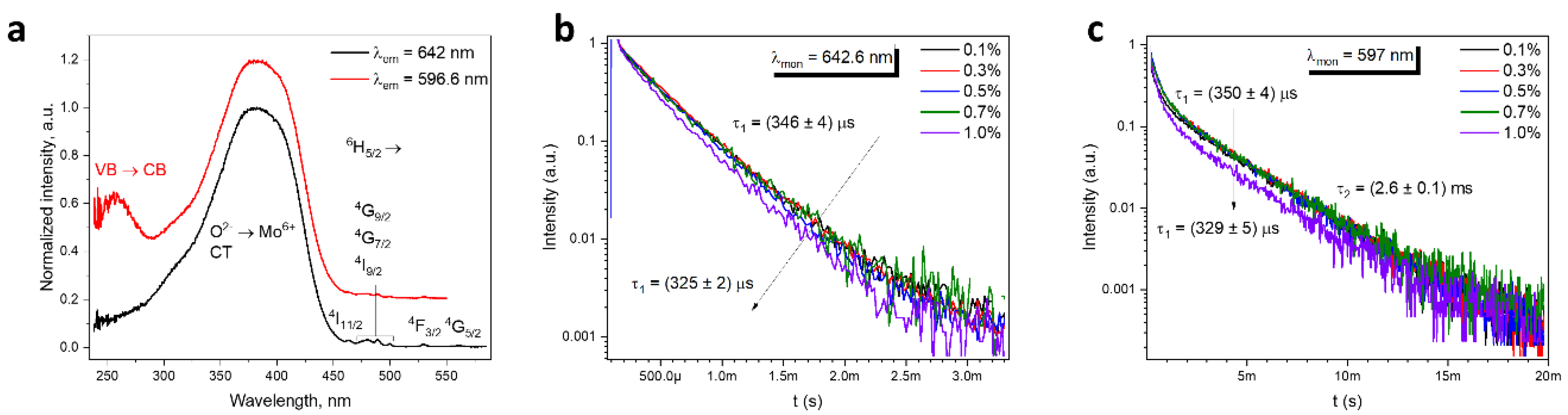
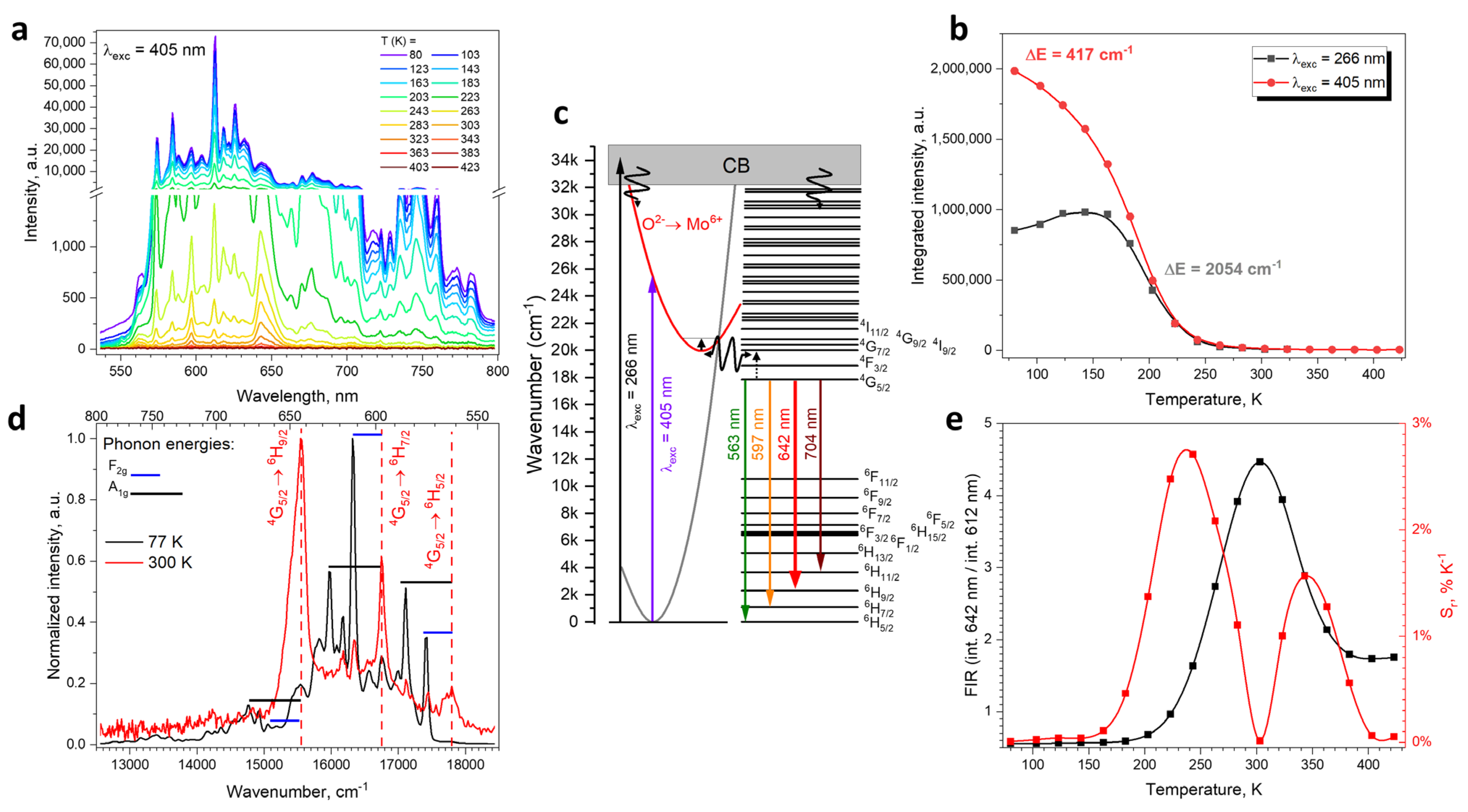
2.2. Spectroscopic Properties
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shkerin, S.N.; Uporov, S.A.; Yudin, V.N.; Solodovnikov, S.F.; Zolotova, E.S.; Tolkacheva, A.S. Paramagnetic properties of triple molybdates CsNa5M3(MoO4)6 (M = Ni, Co, Mn). Russ. J. Phys. Chem. A 2016, 90, 2292–2296. [Google Scholar] [CrossRef]
- Soltys, E.V.; Urazov, K.; Kharlamova, T.S.; Vodyankina, O.V. Redox and Catalytic Properties of Copper Molybdates with Various Composition. Kinet. Catal. 2018, 59, 58–69. [Google Scholar] [CrossRef]
- Soficha, D.; Tushinova, Y.L.; Shendrik, R.; Bazarov, B.G.; Dorzhieva, S.G.; Chimitova, O.D.; Bazarova, J.G. Optical spectroscopy of molybdates with composition Ln2Zr3(MoO4)9 (Ln: Eu, Tb). Opt. Mater. 2018, 81, 71–77. [Google Scholar] [CrossRef]
- Sofich, D.O.; Dorzhieva, S.G.; Chimitova, O.D.; Bazarov, B.G.; Tushinova, Y.L.; Bazarova, Z.G.; Shendrik, R.Y. Hypersensitive 5D0-7F2 Transition of Trivalent Europium in Double Molybdates. Bull. Russ. Acad. 2019, 83, 321–323. [Google Scholar] [CrossRef]
- Nagamani Naidu, B.; Koteswara Rao, K.; Murali Krishna, M.P.S. Sol-gel synthesis, crystal structure and luminescence properties of Eu3+ doped double perovskite molybdates NaCaBi1−xEuxMoO6 (x = 0–0.27). Mater. Res. Express. 2019, 6, 116210. [Google Scholar] [CrossRef]
- Takeda, T.; Ito, M.; Kikkawa, S. Preparation of magneto-resistive Sr2FeMoO6 through molybdic acid gelation. J. Alloys Compd. 2008, 449, 93–95. [Google Scholar] [CrossRef]
- Rath, M.K.; Lee, K.-T. Characterization of novel Ba2LnMoO6 (Ln = Pr and Nd) double perovskite as the anode material for hydrocarbon-fueled solid oxide fuel cells. J. Alloys Compd. 2018, 737, 152–159. [Google Scholar] [CrossRef]
- Chan, W.-L.; Liu, Z.; Lu, S.; Tanner, P.A.; Wong, K.-L. The reported anomalous emission intensity of the 5D0 → 7F4 transition of Eu3+ in a molybdate double perovskite. J. Mater. Chem. C 2015, 3, 860–963. [Google Scholar] [CrossRef]
- Bondzior, B.; Stefańska, D.; Vu, T.H.Q.; Dereń, P.J. Optimization of Eu3+-to-Host Emission Ratio in Double-Perovskite Molybdenites for Highly Sensitive Temperature Sensors. J. Phys. Chem. C 2022, 126, 13247–13255. [Google Scholar] [CrossRef]
- Krishnan, R.; Kroon, R.E.; Swart, H.C. Charge transfer characteristics and luminescence properties of Eu3+ activated Ba2YMoO6 and BaY2(MoO4)4 phosphors. Mater. Res. Bull. 2022, 145, 111554. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Y.; Chen, L.; Wang, X.; Ju, G.; Fan, Y. A novel Ba2MgMoO6:Eu3+ orange-red phosphor: Photoluminescence properties and mechanism of charge and energy transfer. J. Mater. Res. 2013, 28, 3130–3136. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, B.; Shao, C.; Zhen, F.; Wei, S.; Bu, W.; Yao, Q.; Jiang, Z.; Chen, H. Preparation, band-structure and luminescence of double perovskite Ba2MgMoO6:Eu3+ orange-red phosphor for white LEDs. Ceram. Int. 2018, 44, 17305–17312. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, Y.; Chen, S.; Luo, J.; Shu, S.; Gao, J.; Deng, B.; Yu, R. Luminescence investigation of red-emitting Sr2MgMoO6:Eu3+ phosphor for visualization of latent fingerprint. J. Colloid Interface Sci. 2021, 583, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Huang, T.; Nie, J.; Zhang, L.; Chen, Y.; Li, L.; Lan, B.; Wang, J. Energy transfer and tunable–color luminescence properties of a single-phase CaSrNb2O7:Sm3+; Bi3+. J. Mol. Struct. 2024, 1297, 136962. [Google Scholar] [CrossRef]
- Cao, R.; Wei, J.; Chen, T.; Lan, B.; Li, L.; Liu, R.; Luo, Z.; Wang, J. Synthesis, adjustable-color emission and energy transfer of Ba2MgSi2O7: Sm3+, Bi3+ phosphor. J. Mol. Struct. 2023, 1274, 134404. [Google Scholar] [CrossRef]
- Stefańska, D.; Bondzior, B.; Vu, T.H.Q.; Miniajluk-Gaweł, N.; Dereń, P.J. The Influence of Morphology and Eu3+ Concentration on Luminescence and Temperature Sensing Behavior of Ba2MgWO6 Double Perovskite as a Potential Optical Thermometer. J. Alloys Compd. 2020, 842, 155742. [Google Scholar] [CrossRef]
- Yan, L.; Xing, M.; Ma, Y.; Kang, L.; Fu, Y.; Pang, Q.; Xin, F.; Wang, H.; Luo, X. Promising lanthanide-doped double molybdates KYb(MoO4)2 phosphor for highly efficient upconversion luminescence and temperature sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 308, 123751. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, Y.; Fang, F.; Lin, H.; Li, Y.; Feng, G.; Xiong, Y.; Meng, F.; Cao, L.; Ren, H. Up-conversion phosphor Na2MoO4: Er3+/Yb3+ for the optical temperature sensing and anticounterfeiting. J. Lumin. 2024, 265, 120244. [Google Scholar] [CrossRef]
- Tian, X.; Xu, S.; Wen, J.; Zhu, L.; Ji, C.; Huang, Z.; Wang, X.; Luo, F.; Liu, X.; Lu, Y.; et al. Thermochromic Pr3+ doped CaMoO4 phosphor with diverse thermal responses for temperature sensing. Ceram. Int. 2023, 49, 27126–27137. [Google Scholar] [CrossRef]
- Armaroli, N.; Bolink, H.J. Photoluminescent Materials and Electroluminescent Devices. In Topics in Current Chemistry Collections; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Ye, S.; Wang, C.-H.; Jing, X.-P. Photoluminescence and Raman Spectra of Double-Perovskite Sr2Ca(Mo/W)O6 with A- and B-Site Substitutions of Eu3+. J. Electrochem. Soc. 2008, 155, 148. [Google Scholar] [CrossRef]
- Pásztorová, J.; Bi, W.H.; Gaal, R.; Krämer, K.; Živković, I.; Rønnow, H.M. Structure, heat capacity and Raman spectra of Ba2MgWO6 single crystals grown in BaCl2-MgCl2 flux. J. Solid State Chem. 2023, 326, 124184. [Google Scholar] [CrossRef]
- Fujioka, Y.; Frantti, J.; Kakihana, M. Raman Scattering Studies of the Ba2MnWO6 and Sr2MnWO6 Double Perovskites. J. Phys. Chem. B 2006, 110, 777–783. [Google Scholar] [CrossRef]
- Görller-Walrand, C.; Binnemans, K. Chapter 167 Spectral Intensities of f-f Transitions. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1998; Volume 25, pp. 101–264. [Google Scholar]
- Shih, H.-R.; Chang, Y.-S. Structure and Photoluminescence Properties of Sm3+ Ion-Doped YInGe2O7 Phosphor. Materials 2017, 10, 779. [Google Scholar] [CrossRef]
- Chen, P.; Hu, W.; Yang, D.; Zhu, J.; Zhang, J.; Wu, Y. Ba2ZnWO6:Sm3+ as promising orange-red emitting phosphors: Photoluminescence properties and energy transfer process. Phys. B Condens. 2018, 530, 127–132. [Google Scholar] [CrossRef]
- Herrmann, A.; Friedrich, D.; Zscheckel, T.; Rüssel, C. Luminescence properties of Sm3+ doped alkali/earth alkali orthoborates of the type XZBO3 with X = Li, Na, Cs and Z = Ca, Sr, Ba. J. Lumin. 2019, 214, 116550. [Google Scholar] [CrossRef]
- Wen, M.; Wu, H.; Su, X.; Lu, J.; Yang, Z.; Wu, X.; Pan, S. ACaBO3 (A = Cs, Rb): Two new cubic borates with isolated BO3 groups. Dalton Trans. 2017, 46, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, X.L.; Li, H.; He, M.; Dai, L.; Li, X.Z.; Xu, Y.P. Structure determination of a new compound LiCaBO3. J. Solid State Chem. 2004, 177, 1111–1116. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Zhang, Y.; Li, X.; Lian, H.; Lin, J. Host-Sensitized Luminescence Properties in CaNb2O6:Ln3+ (Ln3+ = Eu3+/Tb3+/Dy3+/Sm3+) Phosphors with Abundant Colors. Inorg. Chem. 2015, 54, 323–333. [Google Scholar] [CrossRef]
- Luo, W.; Li, R.; Chen, X. Host-Sensitized Luminescence of Nd3+ and Sm3+ Ions Incorporated in Anatase Titania Nanocrystals. J. Phys. Chem. C 2009, 113, 8772–8777. [Google Scholar] [CrossRef]
- Timoshenko, A.D.; Matvienko, O.O.; Doroshenko, A.G.; Parkhomenko, S.V.; Vorona, I.O.; Kryzhanovska, O.S.; Safronova, N.A.; Vovk, O.O.; Tolmachev, A.V.; Baumer, V.N.; et al. Highly-doped YAG:Sm3+ transparent ceramics: Effect of Sm3+ ions concentration. Ceram. Int. 2023, 49, 7524–7533. [Google Scholar] [CrossRef]
- Puchalska, M.; Zych, E. The effect of charge compensation through alkali metal co-doping on the luminescence behaviour of SrAl4O7:Sm3+ phosphor. J. Lumin. 2018, 197, 211–218. [Google Scholar] [CrossRef]
- Gupta, S.K.; Pathak, N.; Thulasidas, S.K.; Natarajan, V. Local site symmetry of Sm3+ in sol–gel derived α′-Sr2SiO4: Probed by emission and fluorescence lifetime spectroscopy. J. Lumin. 2016, 169, 669–673. [Google Scholar] [CrossRef]
- Solarz, P.; Dominika-Dzik, G.; Lisiecki, R.; Ryba-Romanowski, W.; Beregi, E.; Foldvari, I.; Lengyel, K. Spectroscopic properties of Sm3+ impurity in YAl3(BO3)4 single crystal. Opt. Mater. 2010, 32, 1446–1450. [Google Scholar] [CrossRef]
- Li, R.; Li, P.; Wei, G.; Li, J.; Shi, Y.; Wang, Y.; He, S.; Yang, Y.; Ding, W.; Wang, Z. Ultra-sensitive low-temperature thermometer regulated by the crystal field strength. Ceram. Int. 2023, 49, 7913–7919. [Google Scholar] [CrossRef]
- Cai, P.; Wang, X.; Seo, H.J. Excitation power dependent optical temperature behaviors in Mn4+ doped oxyfluoride Na2WO2F4. Phys. Chem. Chem. Phys. 2018, 20, 2028–2035. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Chapter 281—Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Yao, G.; Li, S.; Valiev, D.; Chen, M.; Stepanow, S.; Lu, Y.; Li, C.; Zhou, Y.; Su, Z.; Zeng, F. Luminescence behavior and temperature sensing properties of Sm3+-doped Cs4PbBr6 quantum dots encapsulated in borogermanate glass. J. Non-Cryst. Solids 2022, 582, 121462. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qiu, J.; Wang, L.-G.; Ye, L. Optical temperature sensing characteristics of Sm3+ doped YAG single crystal fiber based on luminescence emission. J. Alloys Compd. 2022, 890, 161844. [Google Scholar] [CrossRef]
- Singn, R.; Bedyyal, A.K.; Manhas, M.; Durani, F.; Swart, H.C.; Kumar, V. Effect of the synthesis route on luminescence dynamics and thermographic properties of Sm3+ doped Ba2Mg(PO4)2 phosphor. J. Alloys Compd. 2024, 973, 172911. [Google Scholar] [CrossRef]
- Lan, Q.; Meng, Q. Temperature sensing materials based on the FIR of doped ions and the matrix in CaWO4:Sm3+ phosphors. Sens. Actuator A-Phys. 2023, 363, 114708. [Google Scholar] [CrossRef]
- Dramićanin, M.D.; Antić, Ž.; Ćulubrk, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Self-referenced luminescence thermometry with Sm3+ doped TiO2 nanoparticles. Nanotechnology 2014, 25, 485501. [Google Scholar] [CrossRef]


| Level | Wavenumber |
|---|---|
| (cm−1) | |
| 4I11/2 | 21,575 |
| 4G9/2 | 20,812 |
| 4G7/2 | 20,429 |
| 4I9/2 | 20,008 |
| 4F3/2 | 18,879 |
| 4G5/2 | 17,857 |
| 6F11/2 | 10,520 |
| 6F9/2 | 9163 |
| 6F7/2 | 7999 |
| 6F5/2 | 7129 |
| 6F3/2 | 6634 |
| 6H15/2 | 6492 |
| 6F1/2 | 6380 |
| 6H13/2 | 5076 |
| 6H11/2 | 3663 |
| 6H9/2 | 2302 |
| 6H7/2 | 1095 |
| 6H5/2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miniajluk-Gaweł, N.; Bondzior, B.; Ptak, M.; Dereń, P.J. Highly Sensitive Temperature Sensors Resulting from the Luminescent Behavior of Sm3+-Doped Ba2MgMoO6 High-Symmetry Double-Perovskite Molybdate Phosphors. Materials 2024, 17, 1897. https://doi.org/10.3390/ma17081897
Miniajluk-Gaweł N, Bondzior B, Ptak M, Dereń PJ. Highly Sensitive Temperature Sensors Resulting from the Luminescent Behavior of Sm3+-Doped Ba2MgMoO6 High-Symmetry Double-Perovskite Molybdate Phosphors. Materials. 2024; 17(8):1897. https://doi.org/10.3390/ma17081897
Chicago/Turabian StyleMiniajluk-Gaweł, Natalia, Bartosz Bondzior, Maciej Ptak, and Przemysław Jacek Dereń. 2024. "Highly Sensitive Temperature Sensors Resulting from the Luminescent Behavior of Sm3+-Doped Ba2MgMoO6 High-Symmetry Double-Perovskite Molybdate Phosphors" Materials 17, no. 8: 1897. https://doi.org/10.3390/ma17081897
APA StyleMiniajluk-Gaweł, N., Bondzior, B., Ptak, M., & Dereń, P. J. (2024). Highly Sensitive Temperature Sensors Resulting from the Luminescent Behavior of Sm3+-Doped Ba2MgMoO6 High-Symmetry Double-Perovskite Molybdate Phosphors. Materials, 17(8), 1897. https://doi.org/10.3390/ma17081897









