Effect of Copper Surface Roughness on the High-Temperature Structural Stability of Single-Layer-Graphene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterizations
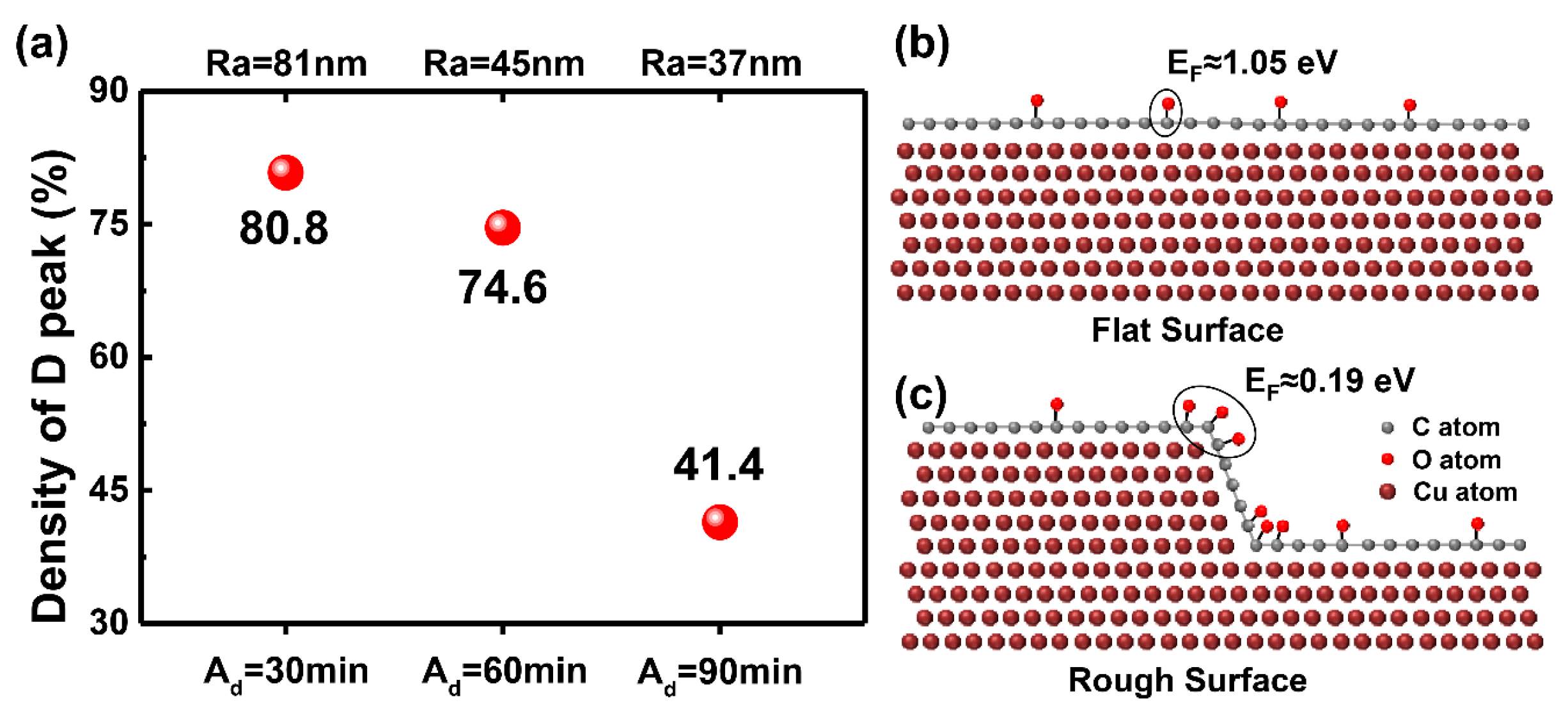
2.2. Density Functional Theory (DFT) Calculations
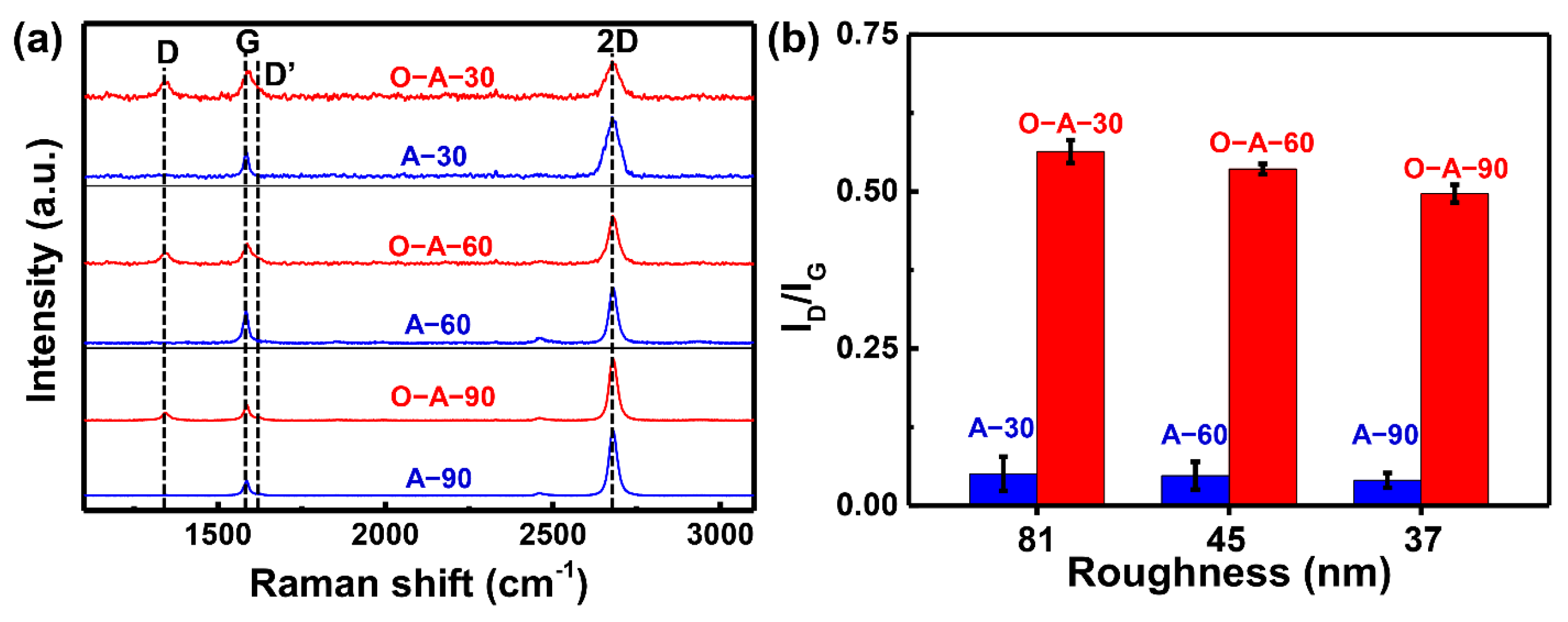
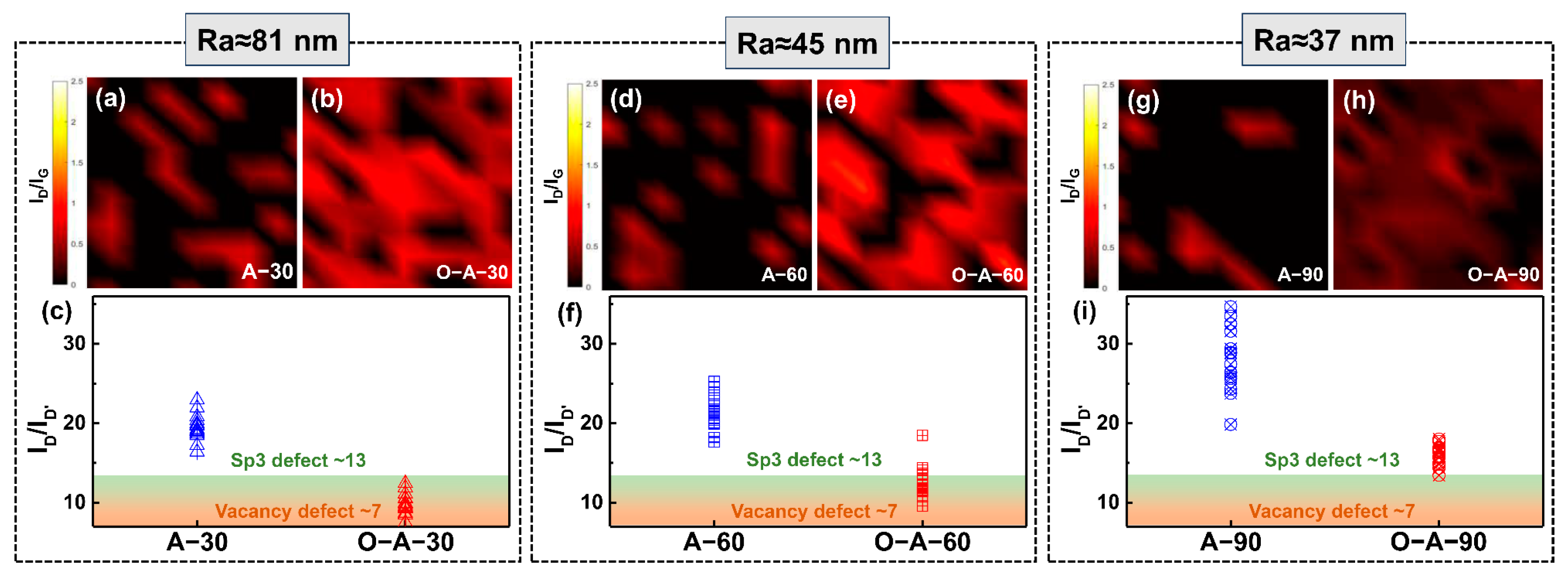
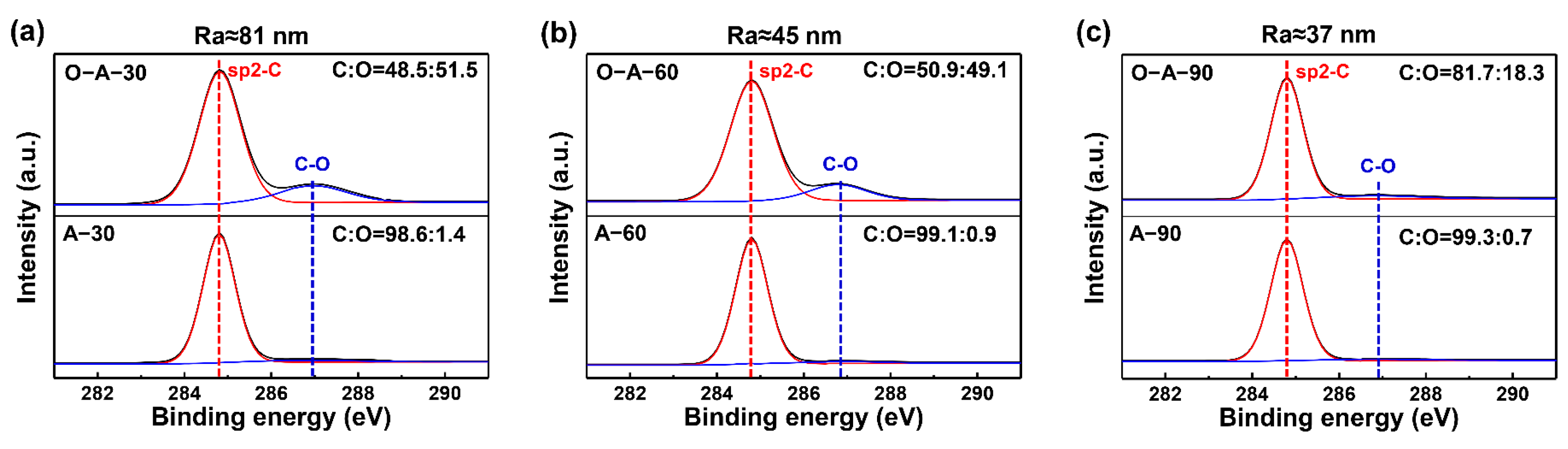
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, M.; Im, J.; Shin, M.; Min, Y.; Park, J.; Cho, H.; Park, S.; Shim, M.; Jeon, S.; Chung, D.; et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol. 2012, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Lee, S.; Lee, M.Y.; Lee, G.; Park, Y.; Kim, H.; Lee, Y.H.; Kim, M.; Ma, K.Y.; et al. Ultralow-k amorphous boron nitride film for copper interconnect capping layer. IEEE Trans. Electron. Dev. 2023, 5, 2588–2593. [Google Scholar] [CrossRef]
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh strength and high electrical conductivity in copper. Science 2004, 304, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Barranco, V.; Onofre, E.; Escudero, M.; García-Alonso, M.C. Characterization of roughness and pitting corrosion of surfaces modified by blasting and thermal oxidation. Surf. Coat. Technol. 2010, 204, 3783–3793. [Google Scholar] [CrossRef]
- Huang, C.; Weng, W.; Liao, C.; Tu, K.N. Suppression of interdiffusion-induced voiding in oxidation of copper nanowires with twin-modified surface. Nat. Commun. 2018, 9, 304. [Google Scholar] [CrossRef]
- Tomotoshi, D.; Kawasaki, H. Surface and interface designs in copper-based conductive inks for printed/flexible electronics. Nanomaterials 2020, 10, 1689. [Google Scholar] [CrossRef]
- Grag, M.; Grewal, H.S.; Sharma, R.K.; Arora, H.S. Improving the high temperature oxidation resistance of high entropy alloy by surface modification. Corros. Rev. 2023, 43, 39–56. [Google Scholar] [CrossRef]
- Laska, N.; Swadźba, R.; Nellessen, P.; Helle, O.; Anton, R. Oxidation behavior of Ti2AlC MAX phase-based coating on a γ-TiAl alloy TiAl48-2-2 produced by DC magnetron sputtering. Surf. Coat. Technol. 2024, 480, 130601. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.I.; Lamichhane, B.; Kim, Y.; Lee, Y.; Cho, C.R.; Cheon, M.; Kim, J.C.; Jeong, H.Y.; Ha, T.; et al. Flat-surface-assisted and self-regulated oxidation resistance of Cu(111). Nature 2022, 603, 434–438. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, B.; Wu, B.; Wang, Y.; Tian, X.; Yang, L.; Oguzie, E.E.; Ma, X. Enhanced corrosion resistance by engineering crystallography on metals. Nat. Commun. 2022, 13, 726. [Google Scholar] [CrossRef]
- Byrne, C.; Brady, A.; Walsh, L.; Mccoy, A.P.; Bogan, J.; McGlynn, E.; Rajani, K.V.; Hughes, G. Chemical and electrical characterisation of the segregation of Al from a CuAl alloy (90%:10% wt) with thermal anneal. Thin Solid Films 2016, 599, 59–63. [Google Scholar] [CrossRef]
- An, B.W.; Gwak, E.J.; Kim, K.; Kim, Y.C.; Jang, J.; Kim, J.Y.; Park, J.U. Stretchable, Transparent electrodes as wearable heaters using nanotrough networks of metallic glasses with superior mechanical properties and thermal stability. Nano Lett. 2016, 16, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Pérez, A.F.; Rodríguez-Vargas, B.R.; Calliari, I.; Pezzato, L. Corrosion resistance of GMAW duplex stainless steels welds. Materials 2023, 16, 1847. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.S.; Paiva, J.M.; Arif, A.F.M.; Amorim, F.L.; Torres, R.D.; Veldhuis, S.C. The effect of laser micro-scale textured tools on the tool-chip interface performance and surface integrity during austenitic stainless-steel turning. Appl. Surf. Sci. 2020, 510, 145455. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, C.; Peng, Z.; Yan, X.; Sun, Y.; Yu, Q.; Yu, B.; Cai, M.; Zhou, F. Corrosion and wear resistant polyp-xylene composite coating on AZ31 magnesium alloy prepared by micro-arc oxidation and vapor deposition. Prog. Organ. Coat. 2024, 186, 108016. [Google Scholar] [CrossRef]
- Saharkhiz, R.; Valefi, Z.; Mirjani, M.; Abdollahi, A.; Taghi-Ramezani, S. Effect of hydrogen and argon shrouding gas flow rate on high-temperature oxidation behavior of NiCrAlY coating by solid shielding shrouded plasma spray (SSPS). Surf. Coat. Technol. 2020, 394, 125818. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Coelho, L.B.; Dangreau, L.; Lanzutti, A.; Fedrizzi, L.; Olivier, M. Self-healing plasma electrolytic oxidation (PEO) coating developed by an assembly of corrosion inhibitive layer and sol-gel sealing on AA2024. Corros. Sci. 2023, 222, 111424. [Google Scholar] [CrossRef]
- Pezzato, L.; Settimi, A.G.; Fanchin, D.; Moschin, E.; Moro, I.; Dabalà, M. Effect of Cu Addition on the Corrosion and Antifouling Properties of PEO Coated Zinc-Aluminized Steel. Materials 2022, 15, 7895. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Sulyubayeva, L.; Maulet, M.; Sagdoldina, Z.; Buitkenov, D.; Issova, A. Investigation of high-temperature oxidation of homogeneous and gradient ni-cr-al coatings obtained by detonation spraying. Coatings 2024, 14, 11. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Gazenbiller, E.; Yasakau, K.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Wieland, D.F.; Zheludkevich, M.L. Corrosion inhibition of decylphosphonate on bare and PEO-coated Mg alloy. Corros. Sci. 2024, 226, 111651. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Ghadami, S. Microstructural characteristics and oxidation behavior of the modified MCrAlX coatings: A critical review. Vacuum 2021, 185, 109980. [Google Scholar] [CrossRef]
- Hejrani, E.; Sebold, D.; Nowak, W.J.; Mauer, G.; Naumenko, D.; Vaßen, R.; Quadakkers, W.J. Isothermal and cyclic oxidation behavior of free standing MCrAlY coatings manufactured by high-velocity atmospheric plasma spraying. Surf. Coat. Technol. 2017, 313, 191–201. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Faĺko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Xiong, D.; Yang, L.; Li, S.; Xie, Y.; Guo, Q.; Li, Z.; Adams, H.; Gu, J.; Fan, T.; et al. Ultrahigh electrical conductivity of graphene embedded in metals. Adv. Funct. Mater. 2019, 29, 1806792. [Google Scholar] [CrossRef]
- Kashani, H.; Kim, C.; Rudolf, C.; Perkins, F.K.; Cleveland, E.R.; Kang, W. An axially continuous graphene-copper wire for high-power transmission: Thermoelectrical characterization and mechanisms. Adv. Mater. 2021, 33, 2104208. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Chugh, S.; Chen, Z. Enhanced electrical and thermal conduction in graphene-encapsulated copper nanowires. Nano Lett. 2015, 15, 2024–2030. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X. Layer dependence of graphene for oxidation resistance of Cu surface. Chin. J. Chem. Phys. 2017, 30, 193–199. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Li, B.; Zhu, C.; Huang, M.; Qiu, L.; Wang, M.; Jin, S.; Kim, M.; Ding, F.; et al. The Wet-Oxidation of a Cu(111) foil coated by Single Crystal Graphene. Adv. Mater. 2021, 33, 2102697. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yan, C.; Lu, J.; Zhang, L.; Cai, J. The effect of copper substrate’s roughness on graphene growth process via PECVD. Mater. Res. Express 2018, 5, 045604. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Zhang, Y.; Chen, Z.; Jin, Z.; Liu, X.; Hu, L.; Yu, G. Effect of Cu substrate roughness on growth of graphene domains at atmospheric pressure. Mater. Lett. 2014, 131, 138–140. [Google Scholar] [CrossRef]
- Wofford, J.M.; Oliveira, M.H., Jr.; Schumann, T.; Jenichen, B.; Ramsteiner, M.; Jahn, U.; Fölsch, S.; Lopes, J.M.J.; Riechert, H. Molecular beam epitaxy of graphene on ultra-smooth nickel: Growth mode and substrate interactions. New J. Phys. 2014, 16, 093055. [Google Scholar] [CrossRef]
- Procházka, P.; Mach, J.; Bischoff, D.; Lišková, Z.; Dvořák, P.; Vaňatka, M.; Simonet, P.; Varlet, A.; Hemzal, D.; Petrenec, M.; et al. Ultrasmooth metallic foils for growth of high quality graphene by chemical vapor deposition. Nanotechnology 2014, 25, 185601. [Google Scholar] [CrossRef]
- Hendriksen, B.L.M.; Ackermann, M.D.; Rijn, R.; Stoltz, D.; Popa, I.; Balmes, O.; Resta, A.; Wermeille, D.; Felici, R.; Ferrer, S.; et al. The role of steps in surface catalysis and reaction oscillations. Nat. Chem. 2010, 2, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Maurice, V.; Marcus, P. Progress in corrosion science at atomic and nanometric scales. Prog. Mater. Sci. 2018, 95, 132–171. [Google Scholar] [CrossRef]
- Takahashi, J.; Ueyama, T.; Kamei, K.; Kato, H.; Homma, Y. Experimental and theoretical studies on the surface morphology variation of a Ni substrate by graphene growth. J. Appl. Phys. 2021, 129, 024302. [Google Scholar] [CrossRef]
- Rakić, I.Š.; Kralj, M.; Jolie, W.; Lazić, P.; Sun, W.; Avila, J.; Asensio, M.; Craes, F.; Trontl, V.M.; Busse, C.; et al. Step-induced faceting and related electronic effects for graphene on Ir(332). Carbon 2016, 110, 267–277. [Google Scholar] [CrossRef]
- Lee, U.; Han, Y.; Lee, S.; Kim, S.; Lee, Y.H.; Kim, U.J.; Son, H. Time evolution studies on strain and doping of graphene grown on a copper substrate using Raman spectroscopy. ACS Nano 2020, 14, 919–926. [Google Scholar] [CrossRef]
- Arjmand, F.; Zhang, Y.; Zhao, Z.; Guan, K. Graphene oxide as an anti-corrosion coating on carbon steel: Effect of surface structure and wettability of steel. Corros. Eng. Sci. Technol. 2023, 58, 384–398. [Google Scholar] [CrossRef]
- Song, J.; Yao, S.; Li, Q.; Ni, J.; Yan, Z.; Yang, K.; Liu, G.; Liu, Y.; Wang, J. Reorientation Mechanisms of Graphene Coated Copper {001} Surfaces. Metals 2023, 13, 910. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Q.; Yao, S.; Yang, K.; Ma, H.; Ni, J.; Zhong, B.; Liu, Y.; Wang, J.; Fan, T. Achieving atomically flat copper surface: Formation of mono-atomic steps and associated strain energy mechanisms. Acta Mater. 2024, 263, 119414. [Google Scholar] [CrossRef]
- Yi, D.; Luo, D.; Wang, Z.; Dong, J.; Zhang, X.; Willinger, M.; Ruoff, R.S.; Ding, F. What drives metal-surface step bunching in graphene chemical vapor deposition? Phys. Rev. Lett. 2018, 120, 246101. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Davey, W.P. Precision Measurements of the Lattice Constants of Twelve Common Metals. Phys. Rev. 1925, 25, 753. [Google Scholar] [CrossRef]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1999, 59, 12301. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.; Tang, G.; Geng, W. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, P.; Lazzeri, M.; Mauri, F. Theory of double-resonant Raman spectra in graphene: Intensity and line shape of defect-induced and two-phonon bands. Phys. Rev. B 2011, 84, 035433. [Google Scholar] [CrossRef]
- Young, K.T.; Phillips, S.S.; Coley, J.T.T.; Perini, C.J.; Hitchcock, D.A.; Serkiz, S.M.; Vogel, E.M. The impact of defect density, grain size, and Cu orientation on thermal oxidation of graphene-coated Cu. Appl. Surf. Sci. 2019, 478, 959–968. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cheng, Y.; Zhang, L.; Zhang, K.; Huo, F.; Zhang, X.; Zhang, S. An effective polysulfides bridgebuilder to enable long-life lithium-sulfur flow batteries. Nano Energy 2018, 51, 113–121. [Google Scholar] [CrossRef]
- Ni, G.; Zheng, Y.; Bae, S.; Kim, H.R.; Pachoud, A.; Kim, Y.S.; Tan, C.; Im, D.; Ahn, J.; Hong, B.H.; et al. Quasi-Periodic nanoripples in graphene grown by chemical vapor deposition and its impact on charge transport. ACS Nano 2012, 6, 1158–1164. [Google Scholar] [CrossRef]

| Treatment Conditions | Samples | ||
|---|---|---|---|
| Synthesis of SLG on Cu | SLG/Cu | ||
| Re-annealing at 600 °C | A-30 (30 min) | A-60 (60 min) | A-90 (90 min) |
| 500 °C oxidation for 60 s | O-A-30 | O-A-60 | O-A-90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Zhong, B.; Guo, C.; Ni, J.; Yang, K.; Hu, S.; Gong, Z.; Liu, Y.; Song, J.; Fan, T. Effect of Copper Surface Roughness on the High-Temperature Structural Stability of Single-Layer-Graphene. Materials 2024, 17, 1648. https://doi.org/10.3390/ma17071648
Yao S, Zhong B, Guo C, Ni J, Yang K, Hu S, Gong Z, Liu Y, Song J, Fan T. Effect of Copper Surface Roughness on the High-Temperature Structural Stability of Single-Layer-Graphene. Materials. 2024; 17(7):1648. https://doi.org/10.3390/ma17071648
Chicago/Turabian StyleYao, Songsong, Boan Zhong, Chongxiao Guo, Jiamiao Ni, Kunming Yang, Siqi Hu, Zheng Gong, Yue Liu, Jian Song, and Tongxiang Fan. 2024. "Effect of Copper Surface Roughness on the High-Temperature Structural Stability of Single-Layer-Graphene" Materials 17, no. 7: 1648. https://doi.org/10.3390/ma17071648
APA StyleYao, S., Zhong, B., Guo, C., Ni, J., Yang, K., Hu, S., Gong, Z., Liu, Y., Song, J., & Fan, T. (2024). Effect of Copper Surface Roughness on the High-Temperature Structural Stability of Single-Layer-Graphene. Materials, 17(7), 1648. https://doi.org/10.3390/ma17071648










