Synthesis and Surface Strengthening Modification of Silica Aerogel from Fly Ash
Abstract
1. Introduction
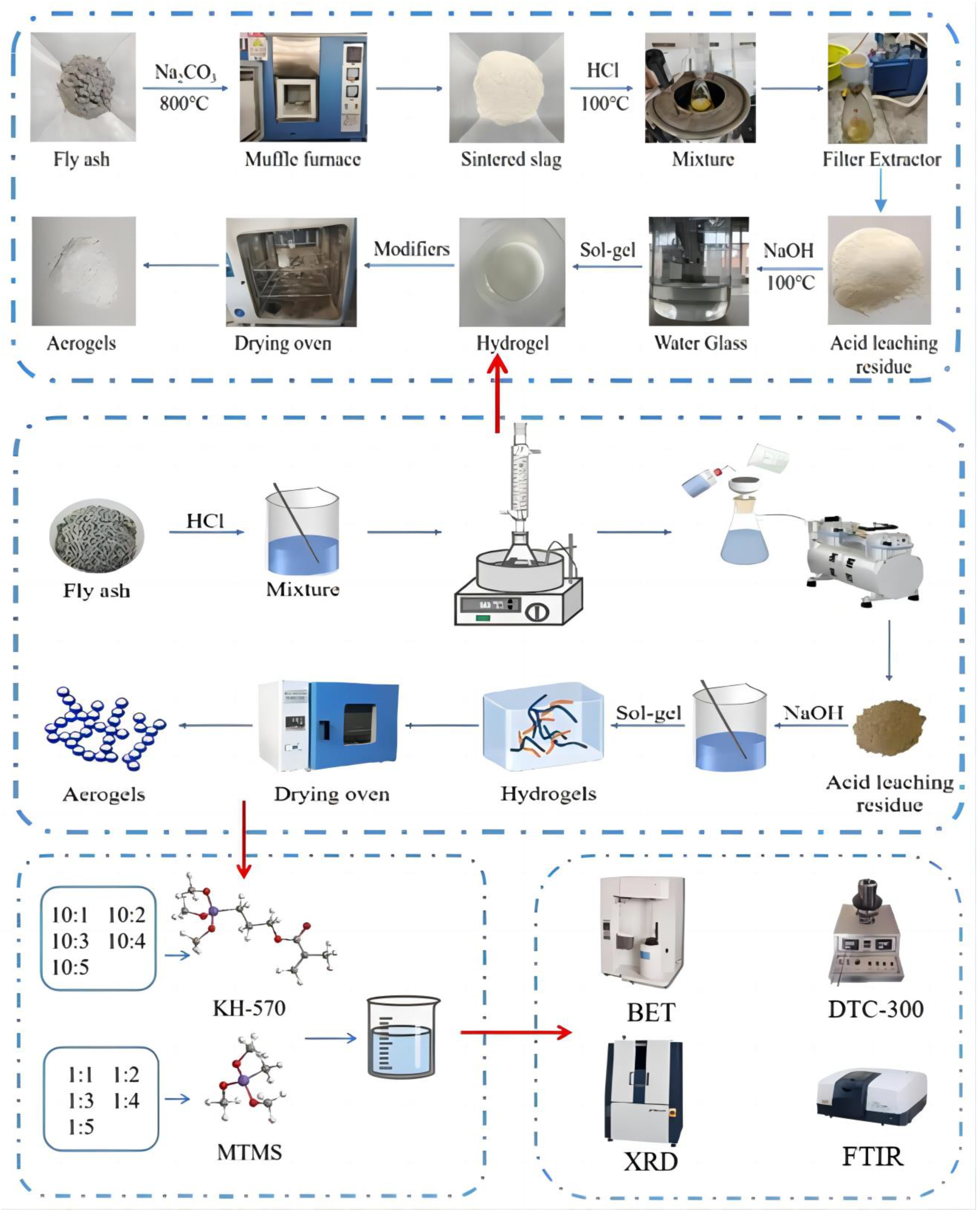
2. Experiment
2.1. Materials
2.2. Characterization Methods
2.2.1. XRD
2.2.2. BET
2.2.3. FTIR
2.2.4. Contact Angle
2.2.5. Thermal Conductivity
2.3. Preparation of Silica Aerogel Modified Silica Aerogel
2.3.1. Extraction of SiO2 through Acid Dissolution and Alkali Leaching from Fly Ash
- Acid Dissolution: Pre-treated fly ash is mixed with hydrochloric acid solutions of varying mass fractions (10 wt%, 15 wt%, 20 wt%, 25 wt%, and 30 wt%) in specific ratios (1:3, 1:4, 1:5, 1:6, and 1:7). The mixture is stirred in a magnetic stirrer at a designated temperature (80 °C, 90 °C, 100 °C, 110 °C, and 120 °C) for a specified duration (1 h, 2 h, 3 h, 4 h, and 5 h). After completion, the reaction mixture is filtered and repeatedly washed with distilled water until neutral. The residue is then dried at 105 °C for 3–5 h in a vacuum drying oven.
- Alkali Leaching: The filtered residue is mixed with sodium hydroxide solutions of various mass fractions (10 wt%, 15 wt%, and 20 wt%) in a 1:5 ratio. The mixture is stirred in a magnetic stirrer at a certain temperature (80 °C, 90 °C, and 100 °C) for a specific duration (1 h, 1.5 h, and 2 h). After completion, the mixture is filtered and distilled water is added for washing until neutral. The filtrate is collected and stored in a beaker, representing the NaSiO3 solution.
2.3.2. Preparation of Strengthened and Modified Silica Aerogel
- Preparation of wet gel and surface enhancement modification: The sodium silicate solution obtained from fly ash is poured into an ion exchange column containing strongly acidic cation exchange resin, resulting in a silicon acid solution with a pH of 2–3. Modified liquid is prepared by mixing MTMS and KH-570 with anhydrous ethanol in a 1:4 ratio. The silicon acid solution is mixed with the modified liquid in a certain proportion and stirred for 1 h. After stirring, the pH of the mixed solution is adjusted to 5–6 using 1 mol/L ammonia solution. The sealed gel is left to age, observed by tilting the beaker at a 45° angle to check for gel formation.
- Aging: deionized water is added to the beaker containing the wet gel, sealed, and placed in a 50 °C water bath for aging for 48 h.
- Solvent replacement: the aged wet gel is soaked in anhydrous ethanol and placed in a 50 °C water bath for solvent replacement for 24 h.
- Post-treatment modification: the wet gel, after solvent replacement, is soaked in a mixture of n-hexane, HMDSO, and ethanol (with HMDSO to wet gel volume ratio of 1:1) at 50 °C for surface modification for 24 h.
- Solvent replacement: the modified gel is soaked in n-hexane and placed in a 50 °C water bath for solvent replacement for 12 h.
- Ambient pressure drying: the gel, after solvent replacement, is placed in a vacuum drying oven and dried at 60 °C, 80 °C, 120 °C, and 180 °C for 2 h each, resulting in strengthened and modified SiO2 aerogels.
2.4. The Influence of Acid Leaching Conditions on the Desilication Rate of Fly Ash
3. Results and Discussion
3.1. Effect of Acid Leaching Conditions on the Desilication Rate of Fly Ash
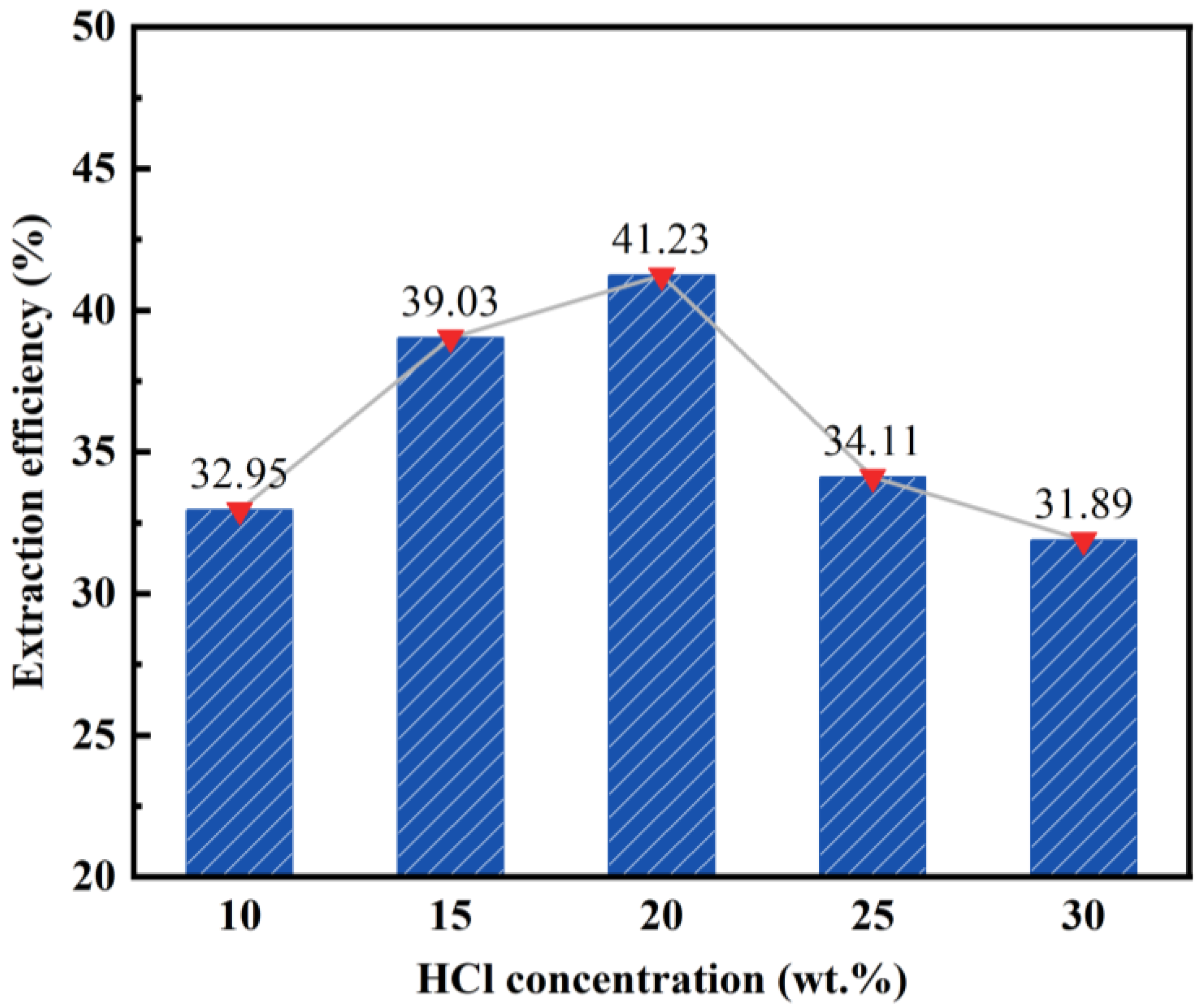
3.1.1. Effect of Hydrochloric Acid Concentration on the Desilication Rate of Fly Ash
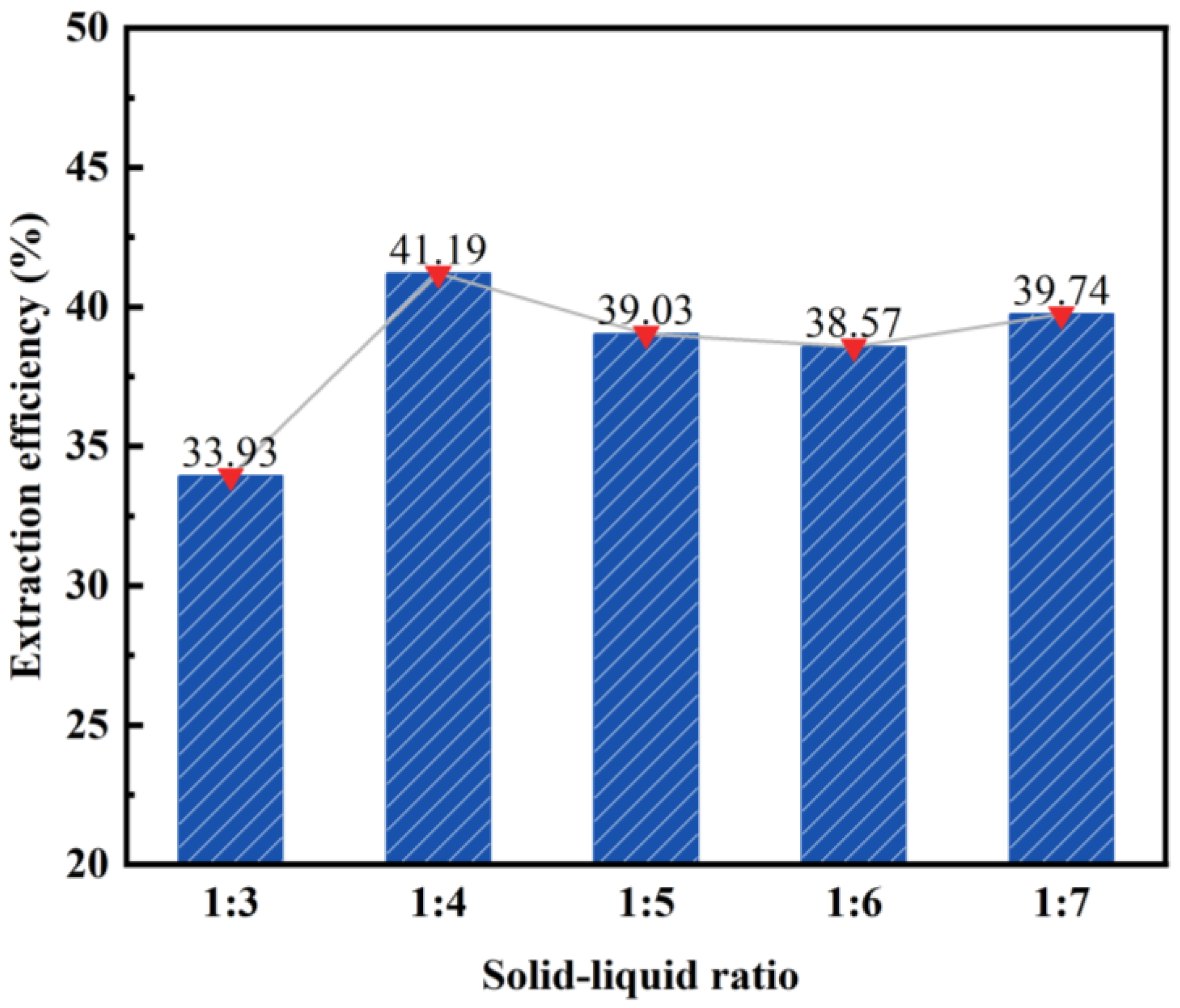
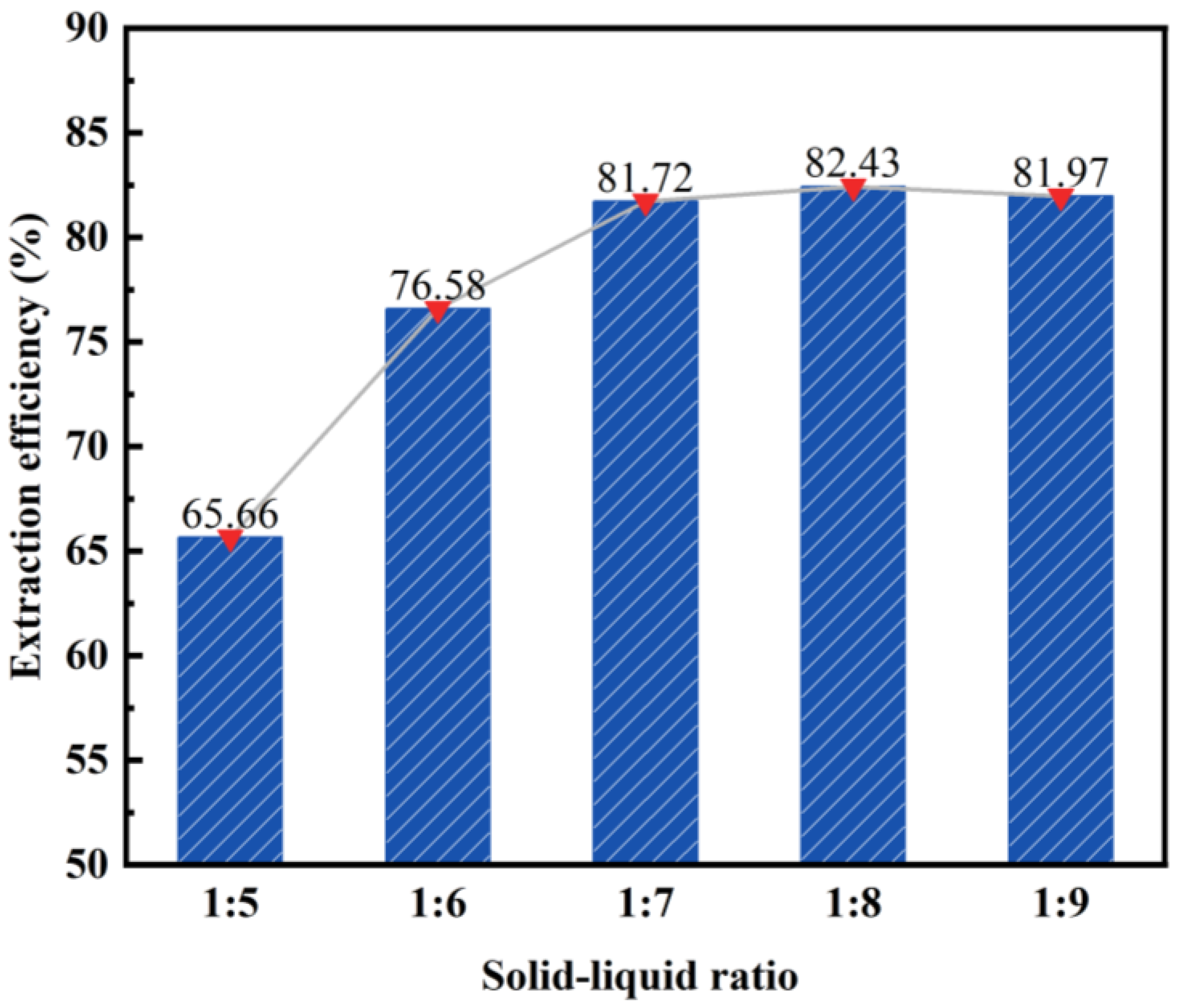
3.1.2. Effect of Solid–Liquid Ratio on the Desilication Rate of Fly Ash
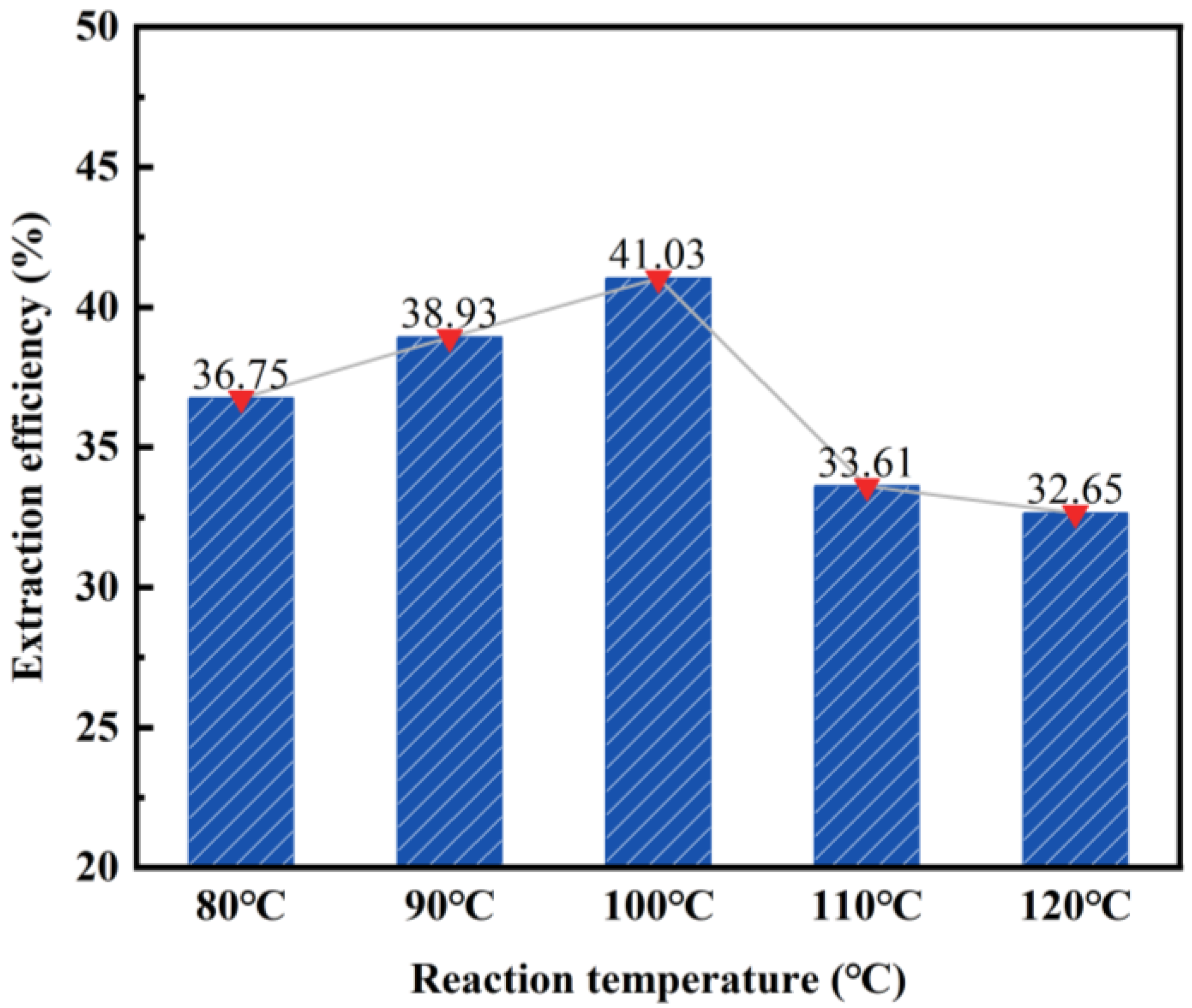
3.1.3. Effect of Reaction Temperature on the Desilication Rate of Fly Ash
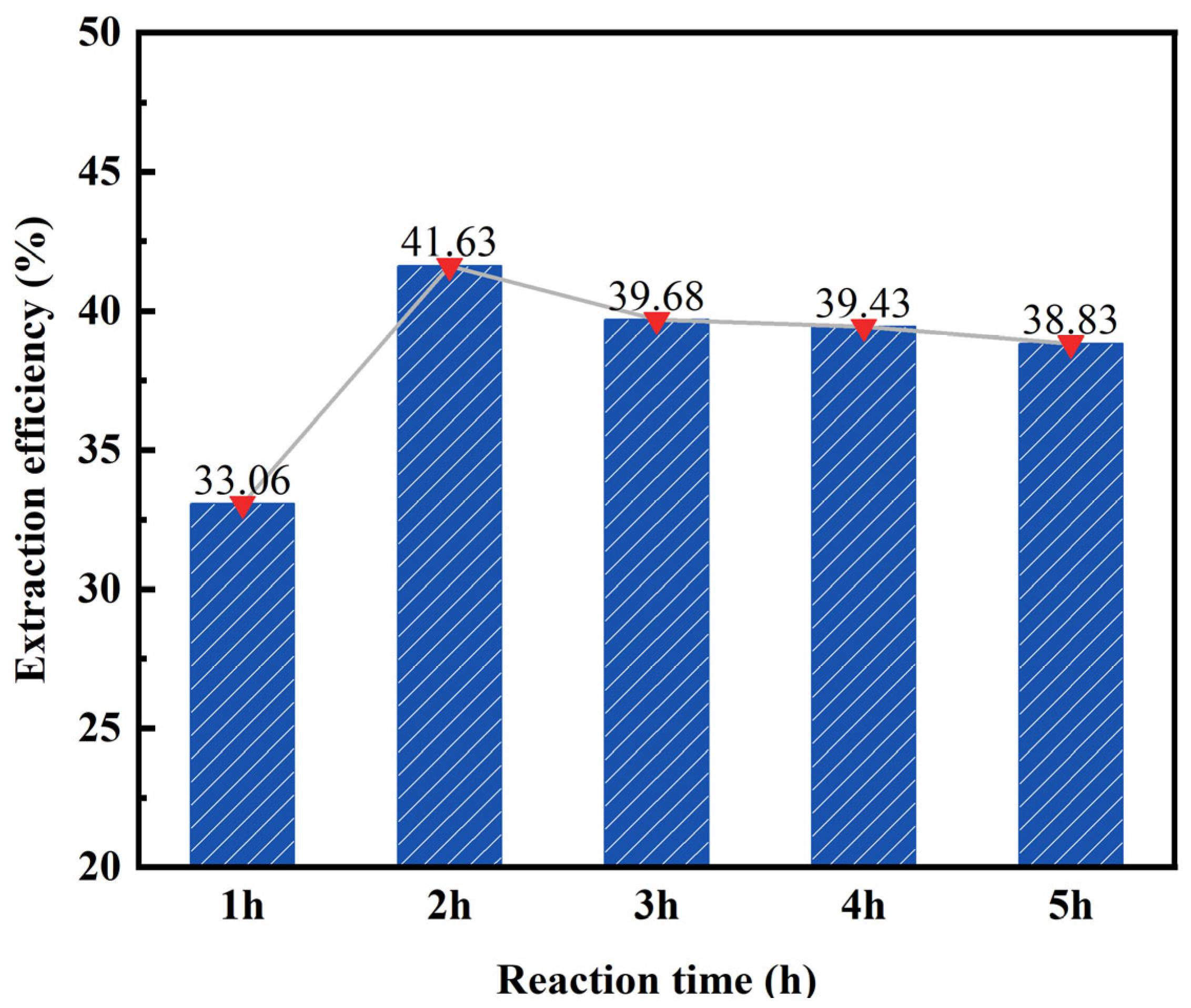
3.1.4. Effect of Reaction Time on the Desilication Rate of Fly Ash
3.1.5. Interactive Effects of Different Reaction Conditions on the Desilication Rate of Fly Ash
3.1.6. Optimization of Acid Leaching Conditions
3.2. Surface Strengthening Modification of Silica Aerogel
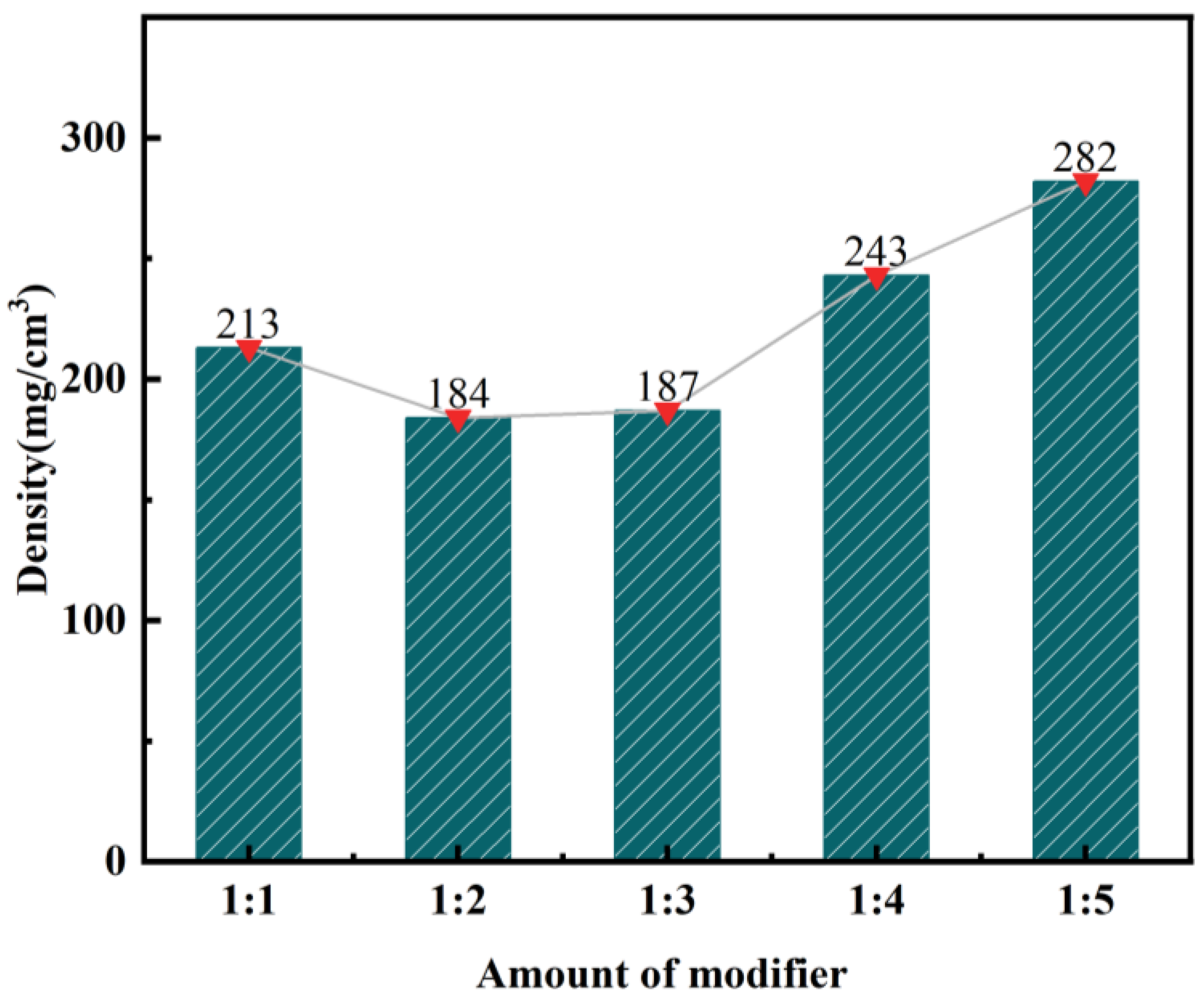
Effect of Modifier on the Density of Silica Aerogel
3.3. Analysis of Surface Enhancement and Modification Mechanism
3.3.1. KH-570 Modification Mechanism
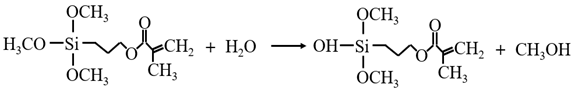
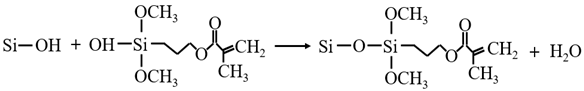
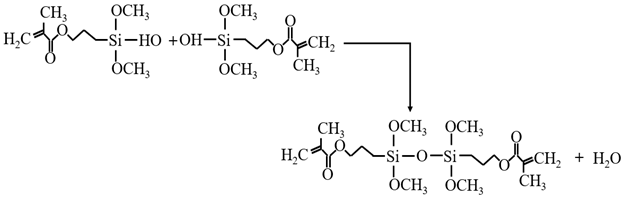

3.3.2. MTMS Modification Mechanism
3.4. Effects of Surface Enhancement Modification on the Structure and Properties of Silica Aerogel
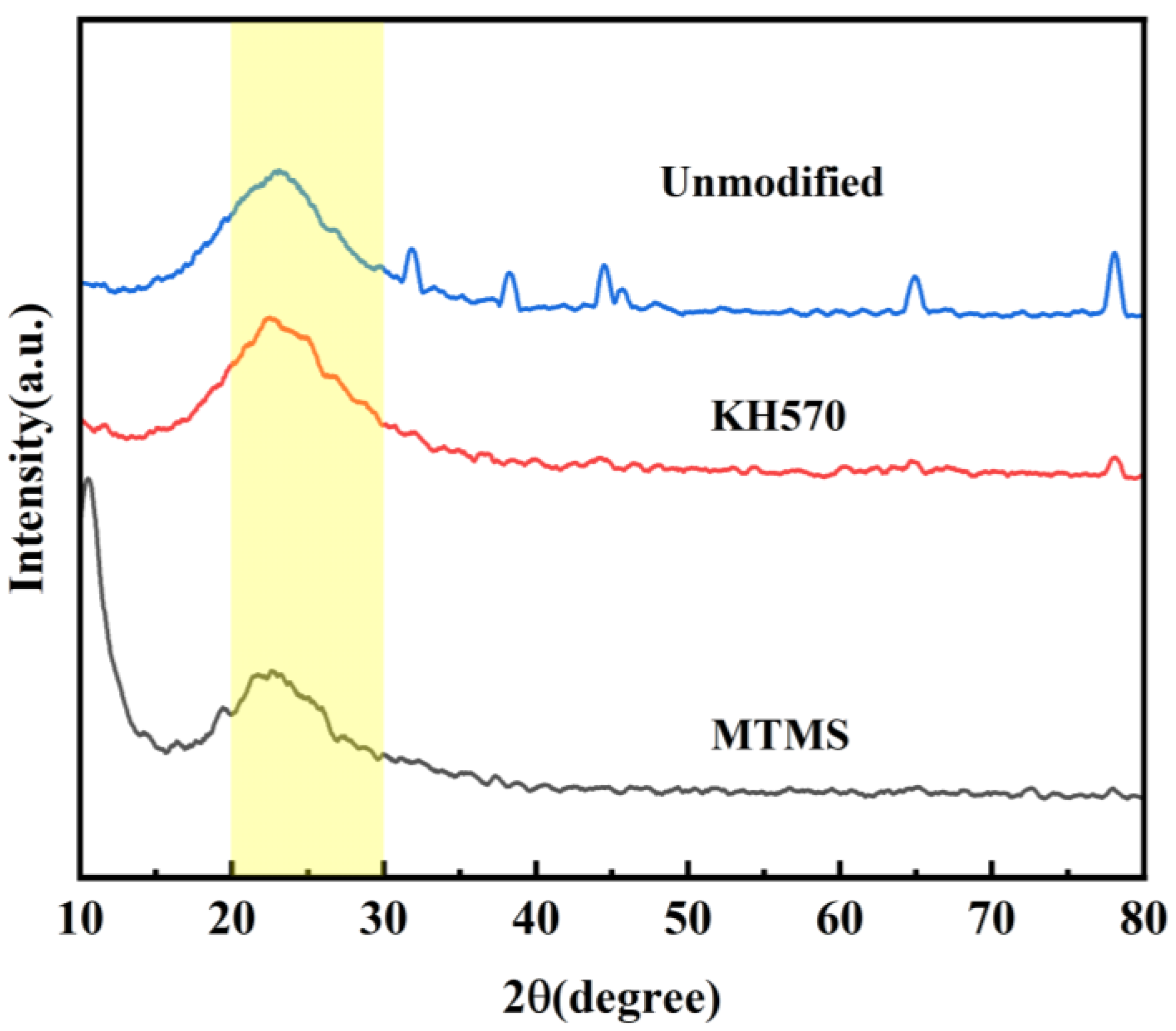
3.4.1. Phase Analysis
3.4.2. Physical Property Analysis
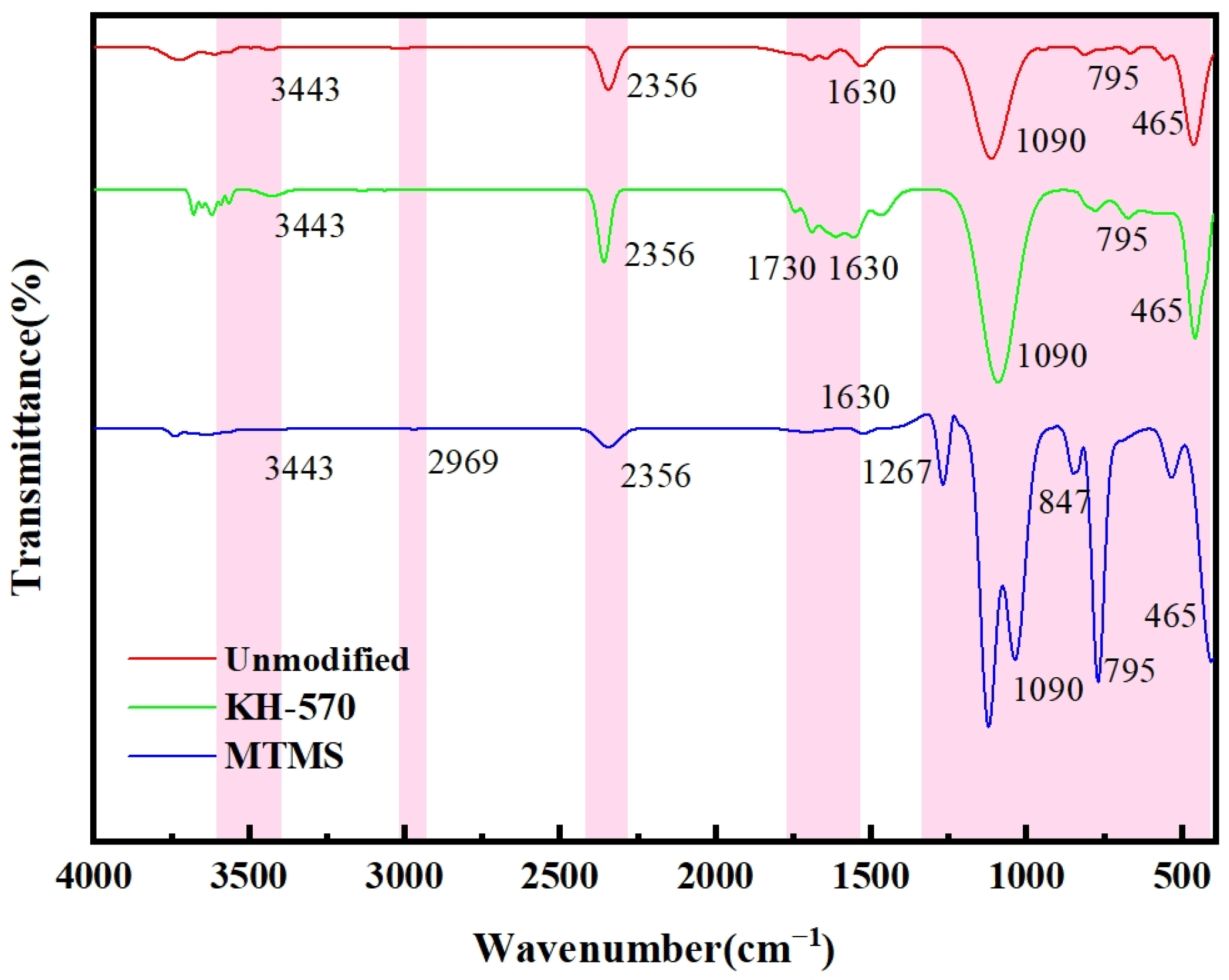
3.4.3. Chemical Property Analysis
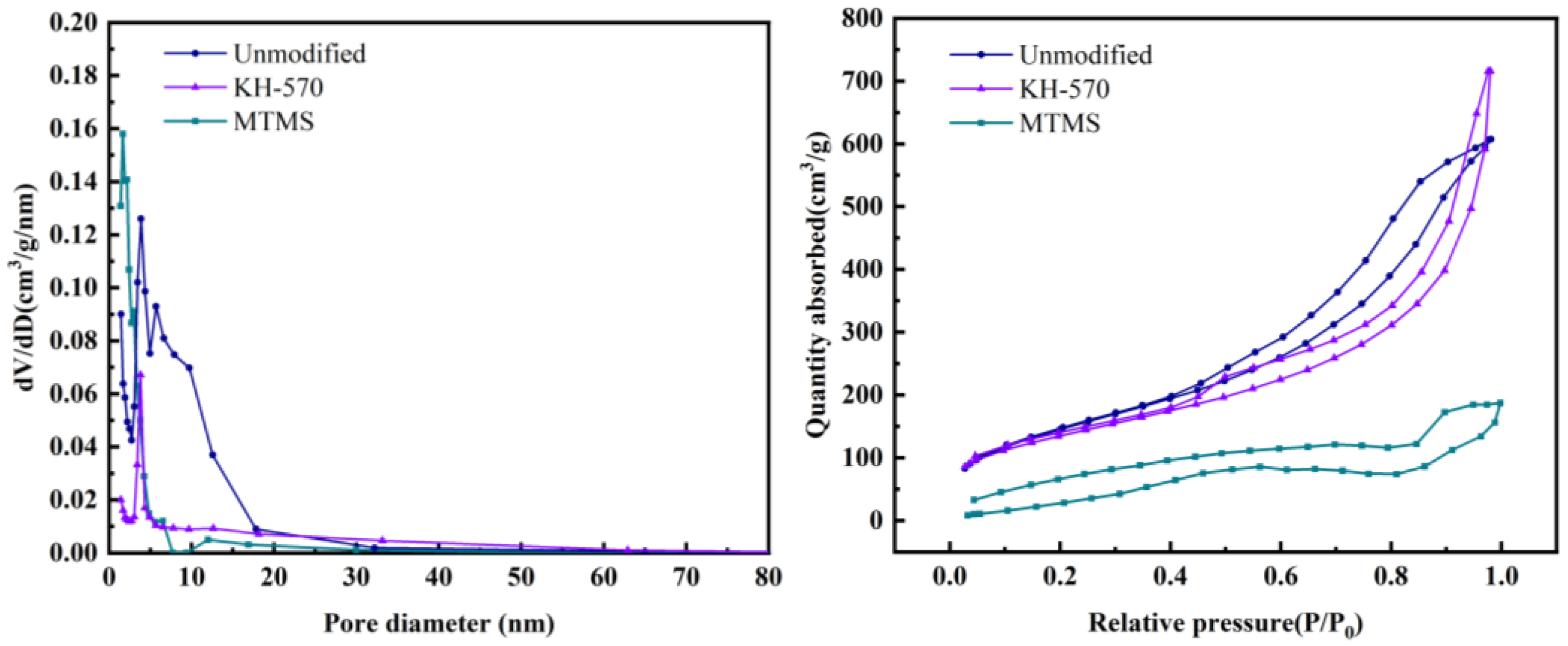
3.4.4. Analysis of Pore Structure and Specific Surface Area
3.4.5. Hydrophobicity Analysis
4. Conclusions
- The impact of different acid leaching conditions on the desilication rate of fly ash can be ranked as follows: hydrochloric acid concentration > solid–liquid ratio > reaction temperature > reaction time. The hydrochloric acid concentration and solid–liquid ratio were found to have the most significant effects on the desilication rate.
- The optimal conditions for acid leaching were determined as follows: a hydrochloric acid concentration of 20 wt%, a solid–liquid ratio of 1:4, a reaction time of 2 h, and a reaction temperature of 100 °C.
- After the calcination and activation of fly ash using sodium carbonate, the highest desilication rate of 81.72% was achieved with an acid-leaching solid-to-liquid ratio of 1:7.
- Silica aerogel was prepared by ambient pressure drying using a sodium silicate solution obtained from fly ash as a silicon source. KH-570 and MTMS were used as strengthening modifiers. The lowest density of silica aerogel, 183 mg·cm−3, was obtained with a volume ratio of silica solution to KH-570 of 10:1. Likewise, the lowest density of silica aerogel, 184 mg·cm−3, was achieved with a volume ratio of silicic acid solution to MTMS of 1:2. And after characterizing by XRD, FTIR, and BET, the KH-570-enhanced aerogel exhibited the best overall performance.
- The optimal performance parameters of silica aerogel were characterized by XRD, FTIR, and BET, resulting in thermal conductivity, a specific surface area, pore volume, average pore size, and contact angle values of 0.0421 W·(m·K)−1, 487.9 m2·g−1, 1.107 cm3·g−1, 9.075 nm, and 123° respectively.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Hamidi, A.; Nazari, P.; Shakibania, S.; Rashchi, F. Microwave irradiation for the recovery enhancement of fly ash components: Thermodynamic and kinetic aspects. Chem. Eng. Process. Process. Intensif. 2023, 191, 109472. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud Univ. Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pakshirajan, K.; Pugazhenthi, G. Process intensification through waste fly ash conversion and application as ceramic membranes: A review. Sci. Total. Environ. 2021, 808, 151968. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, Z.B.; Çam, T. Performance of eco-friendly fly ash-based geopolymer mortars with stone-cutting waste. Mater. Chem. Phys. 2023, 307, 128112. [Google Scholar] [CrossRef]
- Ferrante, F.; Bertini, M.; Ferlito, C.; Lisuzzo, L.; Lazzara, G.; Duca, D. A computational and experimental investigation of halloysite silicic surface modifications after alkaline treatment. Appl. Clay Sci. 2023, 232, 106813. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Fauzi, A.; Nuruddin, M.F.; Malkawi, A.B.; Abdullah, M.M.A.B. Study of Fly Ash Characterization as a Cementitious Material. Procedia Eng. 2016, 148, 487–493. [Google Scholar] [CrossRef]
- Ding, J.; Ma, S.; Shen, S.; Xie, Z.; Zheng, S.; Zhang, Y. Research and industrialization progress of recovering alumina from fly ash: A concise review. Waste Manag. 2017, 60, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Mathapati, M.; Amate, K.; Prasad, C.D.; Jayavardhana, M.; Raju, T.H. A review on fly ash utilization. Mater. Today Proc. 2022, 50, 1535–1540. [Google Scholar] [CrossRef]
- Hait, P.; Dhara, D.; Ghanta, I.; Biswas, C.; Basu, P. Simultaneous Utilization of Fly Ash and Waste Plastics for Making Bricks and Paver Blocks. J. Inst. Eng. India Ser. D 2024, 1–8. [Google Scholar] [CrossRef]
- Das, D.; Rout, P.K. A Review of Coal Fly Ash Utilization to Save the Environment. Water, Air, Soil Pollut. 2023, 234, 1–23. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Y.; Feng, J.; Feng, J.; Yue, C. Infrared-opacified Al2O3–SiO2 aerogel composites reinforced by SiC-coated mullite fibers for thermal insulations. Ceram. Int. 2015, 41, 437–442. [Google Scholar] [CrossRef]
- Bin Rashid, A.; Shishir, S.I.; Mahfuz, A.; Hossain, T.; Hoque, E. Silica Aerogel: Synthesis, Characterization, Applications, and Recent Advancements. Part. Part. Syst. Charact. 2023, 40, 202200186. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Pontinha, A.D.R.; Santos, P.; Durães, L. Aerogel Composites Produced from Silica and Recycled Rubber Sols for Thermal Insulation. Materials 2022, 15, 7897. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, X.; Zhang, M.; Guo, M. Synthesis of SiO2–Al2O3 composite aerogel from fly ash: A low-cost and facile approach. J. Sol-Gel Sci. Technol. 2019, 93, 281–290. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A review on silica aerogel-based materials for acoustic applications. J. Non-Crystalline Solids 2021, 562, 120770. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Woignier, T.; Primera, J.; Alaoui, A.; Etienne, P.; Despestis, F.; Calas-Etienne, S. Mechanical Properties and Brittle Behavior of Silica Aerogels. Gels 2015, 1, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Lamy-Mendes, A.; Pontinha, A.D.R.; Alves, P.; Santos, P.; Durães, L. Progress in silica aerogel-containing materials for buildings’ thermal insulation. Constr. Build. Mater. 2021, 286, 122815. [Google Scholar] [CrossRef]
- Hasan, M.A.; Sangashetty, R.; Esther, A.C.M.; Patil, S.B.; Sherikar, B.N.; Dey, A. Prospect of Thermal Insulation by Silica Aerogel: A Brief Review. J. Inst. Eng. India Ser. D 2017, 98, 297–304. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Kanamori, K.; Shimizu, T.; Zhu, Y.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Versatile Double-Cross-Linking Approach to Transparent, Machinable, Supercompressible, Highly Bendable Aerogel Thermal Superinsulators. Chem. Mater. 2018, 30, 2759–2770. [Google Scholar] [CrossRef]
- Xue, J.; Han, R.; Li, Y.; Zhang, J.; Liu, J.; Yang, Y. Advances in multiple reinforcement strategies and applications for silica aerogel. J. Mater. Sci. 2023, 58, 14255–14283. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; Zhong, Y.; Gao, J.; Jing, F.; Cui, S.; Shen, X. Comparative studies on the physicochemical properties of in-situ hydrophobic silica aerogels by ambient pressure drying method. J. Porous Mater. 2023, 30, 2043–2055. [Google Scholar] [CrossRef]
- Koyuncu, D.D.E.; Okur, M. Investigation of dye removal ability and reusability of green and sustainable silica and carbon-silica hybrid aerogels prepared from paddy waste ash. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127370. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M. Silica aerogel; synthesis, properties and characterization. J. Am. Acad. Dermatol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Liu, Z.; Zang, C.; Zhang, S.; Zhang, Y.; Yuan, Z.; Li, H.; Jiang, J. Atmospheric drying preparation and microstructure characterization of fly ash aerogel thermal insulation material with superhydrophobic. Constr. Build. Mater. 2021, 303, 124425. [Google Scholar] [CrossRef]
- Akhter, F.; Soomro, S.A.; Inglezakis, V.J. Silica aerogels; a review of synthesis, applications and fabrication of hybrid composites. J. Porous Mater. 2021, 28, 1387–1400. [Google Scholar] [CrossRef]
- Yue, S.; Li, X.; Yu, H.; Tong, Z.; Liu, Z. Preparation of high-strength silica aerogels by two-step surface modification via ambient pressure drying. J. Porous Mater. 2021, 28, 651–659. [Google Scholar] [CrossRef]
- Çok, S.S.; Gizli, N. Microstructural properties and heat transfer characteristics of in-situ modified silica aerogels prepared with different organosilanes. Int. J. Heat Mass Transf. 2022, 188, 122618. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.-X.; Song, K.; Wang, Z.-Y. Cost-effective synthesis of silica aerogels from fly ash via ambient pressure drying. J. Non-Crystalline Solids 2010, 356, 2241–2246. [Google Scholar] [CrossRef]
- Wu, X.; Fan, M.; Mclaughlin, J.F.; Shen, X.; Tan, G. A novel low-cost method of silica aerogel fabrication using fly ash and trona ore with ambient pressure drying technique. Powder Technol. 2018, 323, 310–322. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Mai, N.T.; Reddy, V.R.M.; Jung, J.H.; Truong, N.T.N. Synthesis of silica aerogel particles and its application to thermal insulation paint. Korean J. Chem. Eng. 2020, 37, 1803–1809. [Google Scholar] [CrossRef]
- Shao, Z.; He, X.; Niu, Z.; Huang, T.; Cheng, X.; Zhang, Y. Ambient pressure dried shape-controllable sodium silicate based composite silica aerogel monoliths. Mater. Chem. Phys. 2015, 162, 346–353. [Google Scholar] [CrossRef]
- Torres, R.B.; Vareda, J.P.; Lamy-Mendes, A.; Durães, L. Effect of different silylation agents on the properties of ambient pressure dried and supercritically dried vinyl-modified silica aerogels. J. Supercrit. Fluids 2019, 147, 81–89. [Google Scholar] [CrossRef]
- Mahadik, S.; Pedraza, F.; Parale, V.; Park, H.-H. Organically modified silica aerogel with different functional silylating agents and effect on their physico-chemical properties. J. Non-Crystalline Solids 2016, 453, 164–171. [Google Scholar] [CrossRef]
- Pan, Y.; He, S.; Gong, L.; Cheng, X.; Li, C.; Li, Z.; Liu, Z.; Zhang, H. Low thermal-conductivity and high thermal stable silica aerogel based on MTMS/Water-glass co-precursor prepared by freeze drying. Mater. Des. 2017, 113, 246–253. [Google Scholar] [CrossRef]
- Chen, K.; Feng, Q.; Ma, D.; Huang, X. Hydroxyl modification of silica aerogel: An effective adsorbent for cationic and anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126331. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Zhai, J.; Yang, D. The effect of different alkaline catalysts on the formation of silica aerogels prepared by the sol–gel approach. J. Ceram. Soc. Jpn. 2020, 128, 395–403. [Google Scholar] [CrossRef]





| Component (wt%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | MnO | TiO2 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly ash | 50.04 | 33.52 | 6.60 | 4.16 | 0.33 | 0.02 | 1.33 | 0.94 | 0.075 | 1.48 | 1.52 |
| Level Number of Group | HCl/(wt%) | Solid–Liquid Ratio | Reaction Time/h | Reaction Temperature/°C |
|---|---|---|---|---|
| 1 | 15% | 1:3 | 2 h | 90 °C |
| 2 | 20% | 1:4 | 3 h | 100 °C |
| 3 | 25% | 1:5 | 4 h | 110 °C |
| Test Group Number | Factor | Desilication Rate/% | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 15% | 1:3 | 2 h | 90 °C | 34.99 |
| 2 | 15% | 1:4 | 3 h | 100 °C | 41.19 |
| 3 | 15% | 1:5 | 4 h | 110 °C | 41.5 |
| 4 | 20% | 1:3 | 3 h | 110 °C | 35.7 |
| 5 | 20% | 1:4 | 4 h | 90 °C | 39.88 |
| 6 | 20% | 1:5 | 2 h | 100 °C | 42.67 |
| 7 | 25% | 1:3 | 4 h | 100 °C | 32.58 |
| 8 | 25% | 1:4 | 2 h | 110 °C | 37.28 |
| 9 | 25% | 1:5 | 3 h | 90 °C | 33.19 |
| Level | A | B | C | D |
|---|---|---|---|---|
| 1 | 15% | 1:3 | 2 h | 90 °C |
| 2 | 20% | 1:4 | 3 h | 100 °C |
| 3 | 25% | 1:5 | 4 h | 110 °C |
| Extreme deviation | 5.07 | 5.03 | 1.62 | 2.79 |
| Serial Number | Silicon Source | Modified Liquid 1 | Modified Liquid 2 | ||
|---|---|---|---|---|---|
| Silicic Acid Solution/mL | KH-570/mL | EtOH/mL | MTMS/mL | EtOH/mL | |
| 1 | 10 | 5 | 20 | 10 | 40 |
| 2 | 10 | 4 | 16 | 20 | 80 |
| 3 | 10 | 3 | 12 | 30 | 120 |
| 4 | 10 | 2 | 8 | 40 | 160 |
| 5 | 10 | 1 | 4 | 50 | 200 |
| Sample | Specific Surface Area/(m2/g) | Pore Volume/(cm3/g) | Average Pore Diameter/(nm) |
|---|---|---|---|
| Unmodified | 538.7 | 0.991 | 7.358 |
| KH-570 | 487.9 | 1.107 | 9.075 |
| MTMS | 146.8 | 0.425 | 11.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, Q.; Zhao, H.; Song, R.; Chen, Y.; Liu, C.; Han, Z. Synthesis and Surface Strengthening Modification of Silica Aerogel from Fly Ash. Materials 2024, 17, 1614. https://doi.org/10.3390/ma17071614
Zhang L, Wang Q, Zhao H, Song R, Chen Y, Liu C, Han Z. Synthesis and Surface Strengthening Modification of Silica Aerogel from Fly Ash. Materials. 2024; 17(7):1614. https://doi.org/10.3390/ma17071614
Chicago/Turabian StyleZhang, Lei, Qi Wang, Haocheng Zhao, Ruikang Song, Ya Chen, Chunjiang Liu, and Zhikun Han. 2024. "Synthesis and Surface Strengthening Modification of Silica Aerogel from Fly Ash" Materials 17, no. 7: 1614. https://doi.org/10.3390/ma17071614
APA StyleZhang, L., Wang, Q., Zhao, H., Song, R., Chen, Y., Liu, C., & Han, Z. (2024). Synthesis and Surface Strengthening Modification of Silica Aerogel from Fly Ash. Materials, 17(7), 1614. https://doi.org/10.3390/ma17071614






