Deposition and Optical Characterization of Sputter Deposited p-Type Delafossite CuGaO2 Thin Films Using Cu2O and Ga2O3 Targets
Abstract
1. Introduction
2. Experimental
2.1. Deposition of CuGaO2 Thin Films
2.2. Film Characterization
3. Results and Discussion
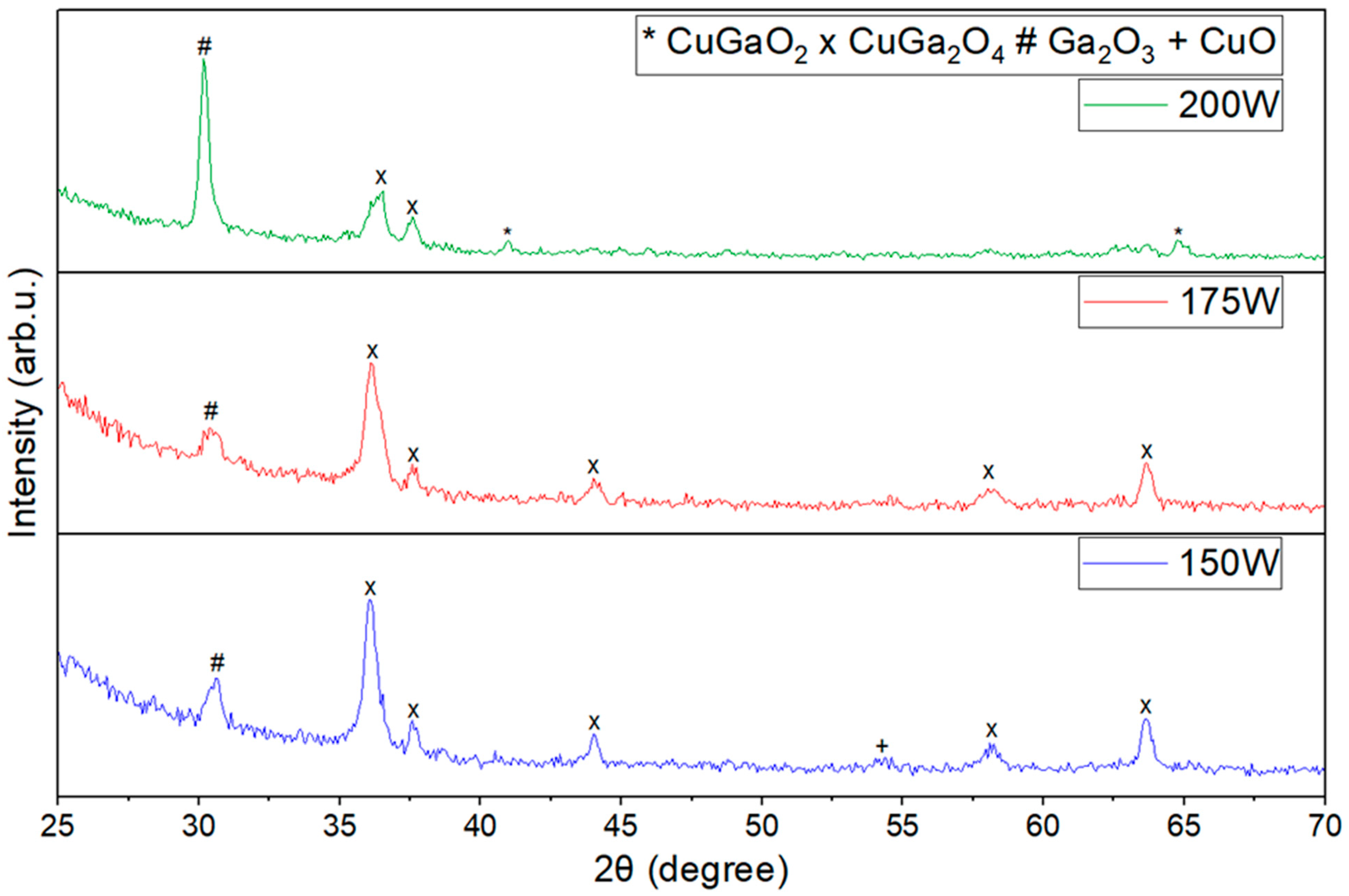
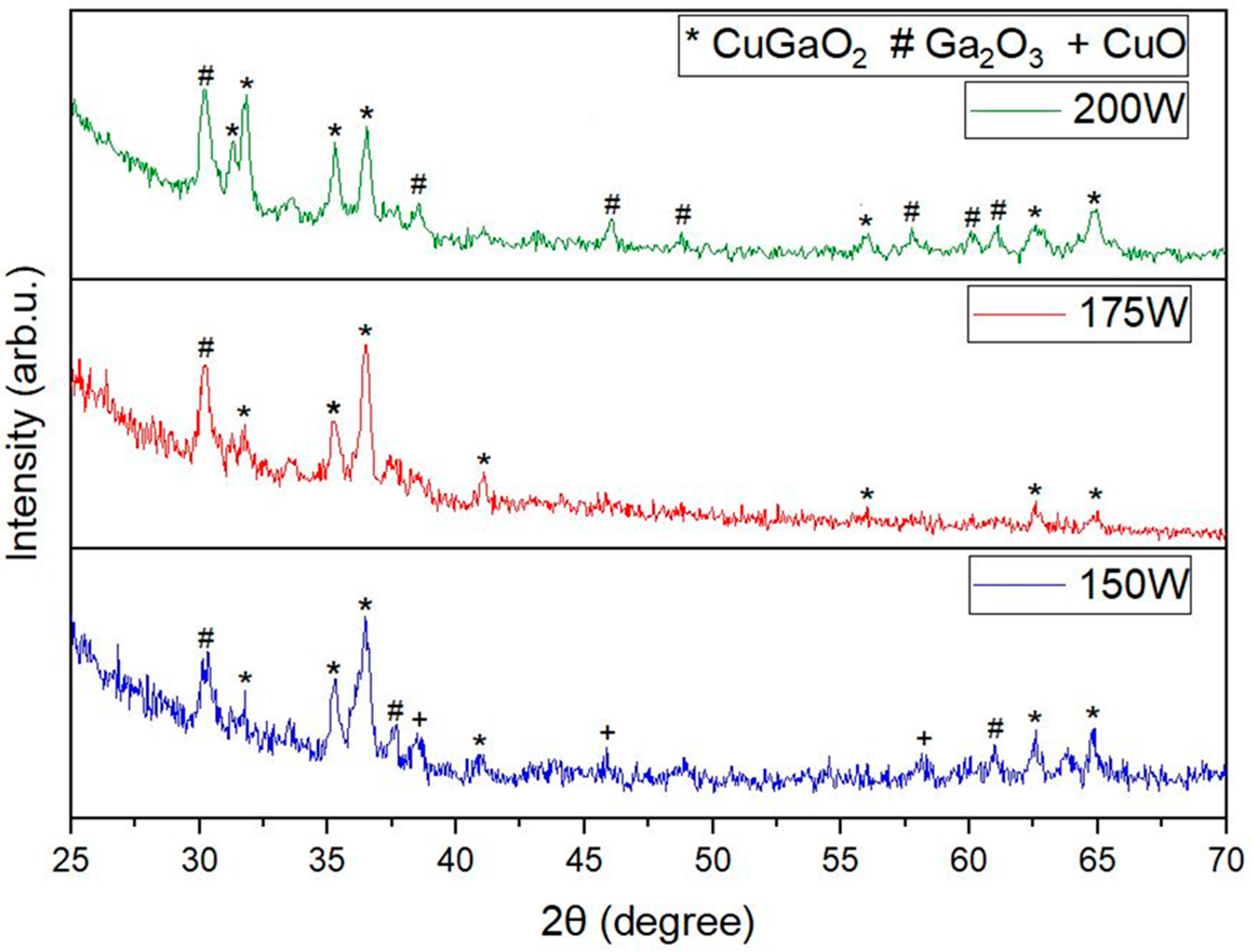
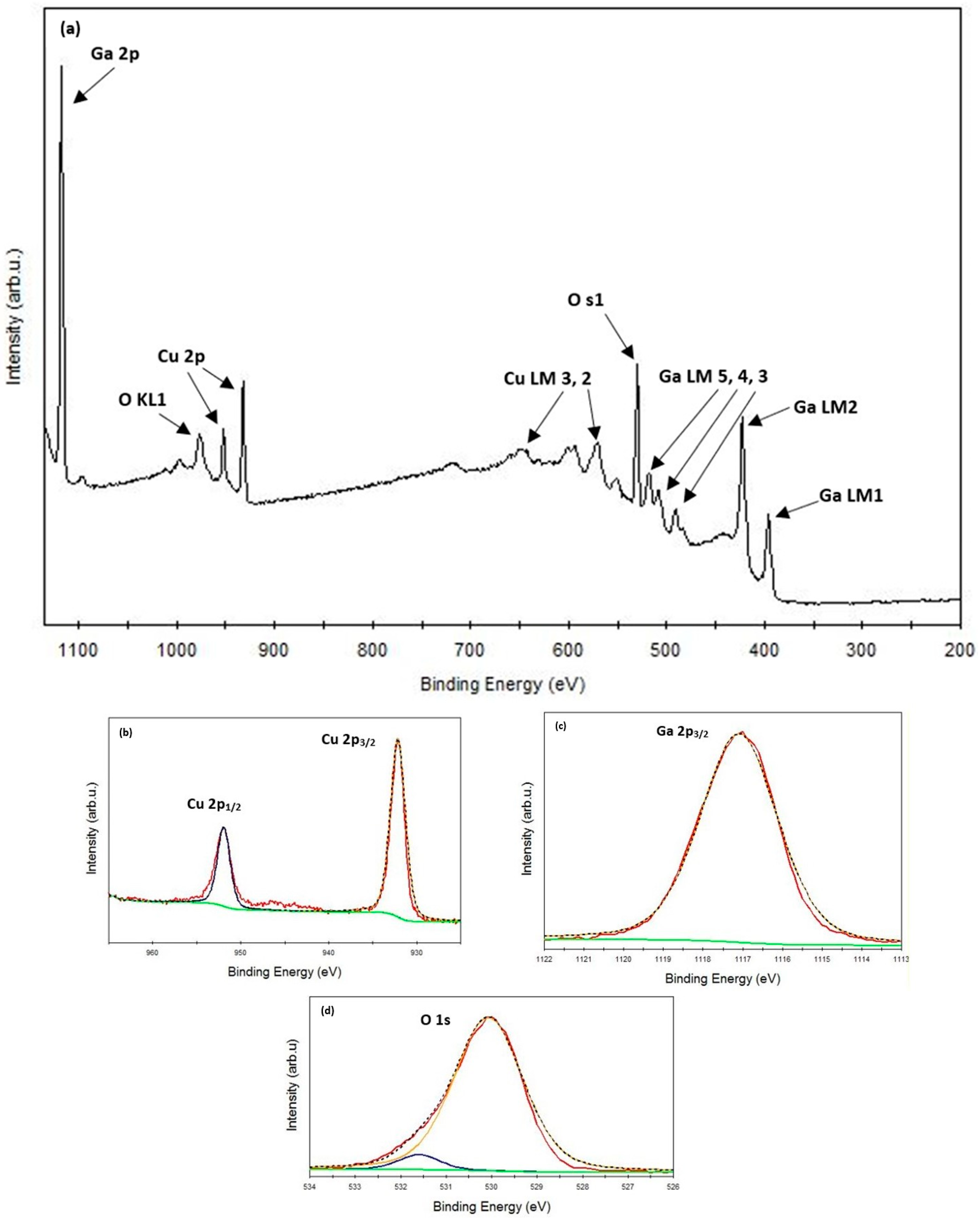
3.1. XRD and XPS Analysis
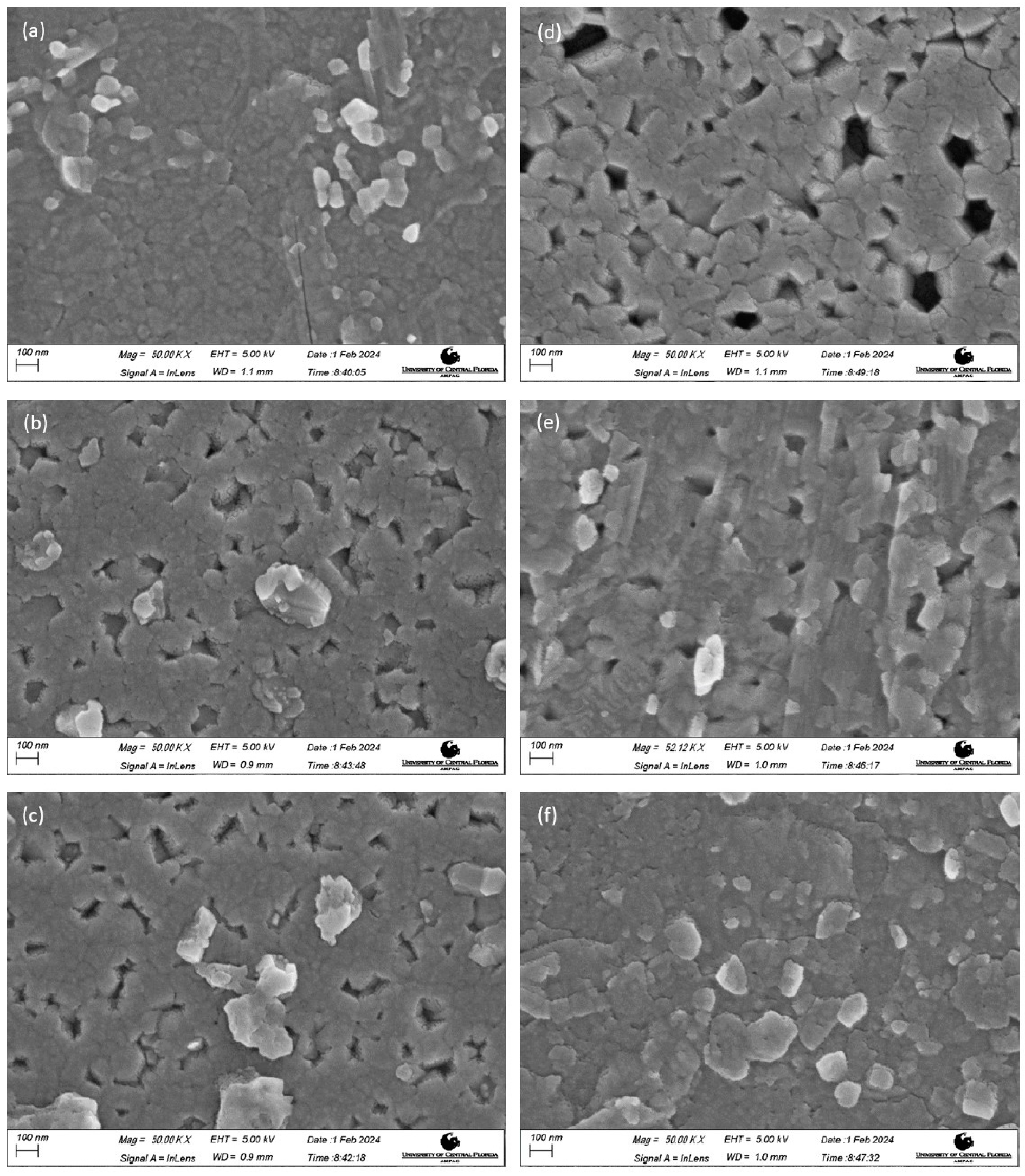
3.2. Morphology Studies
3.3. Optical Studies
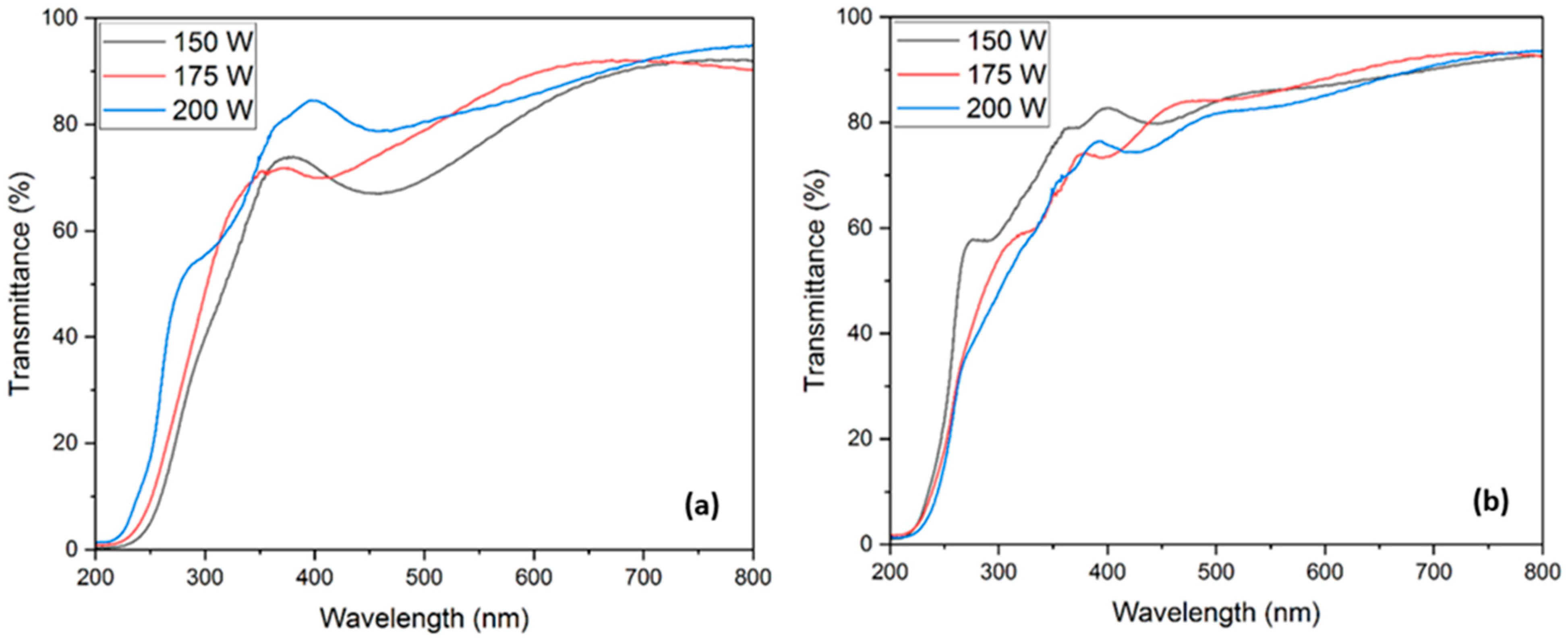
3.3.1. Optical Transmission
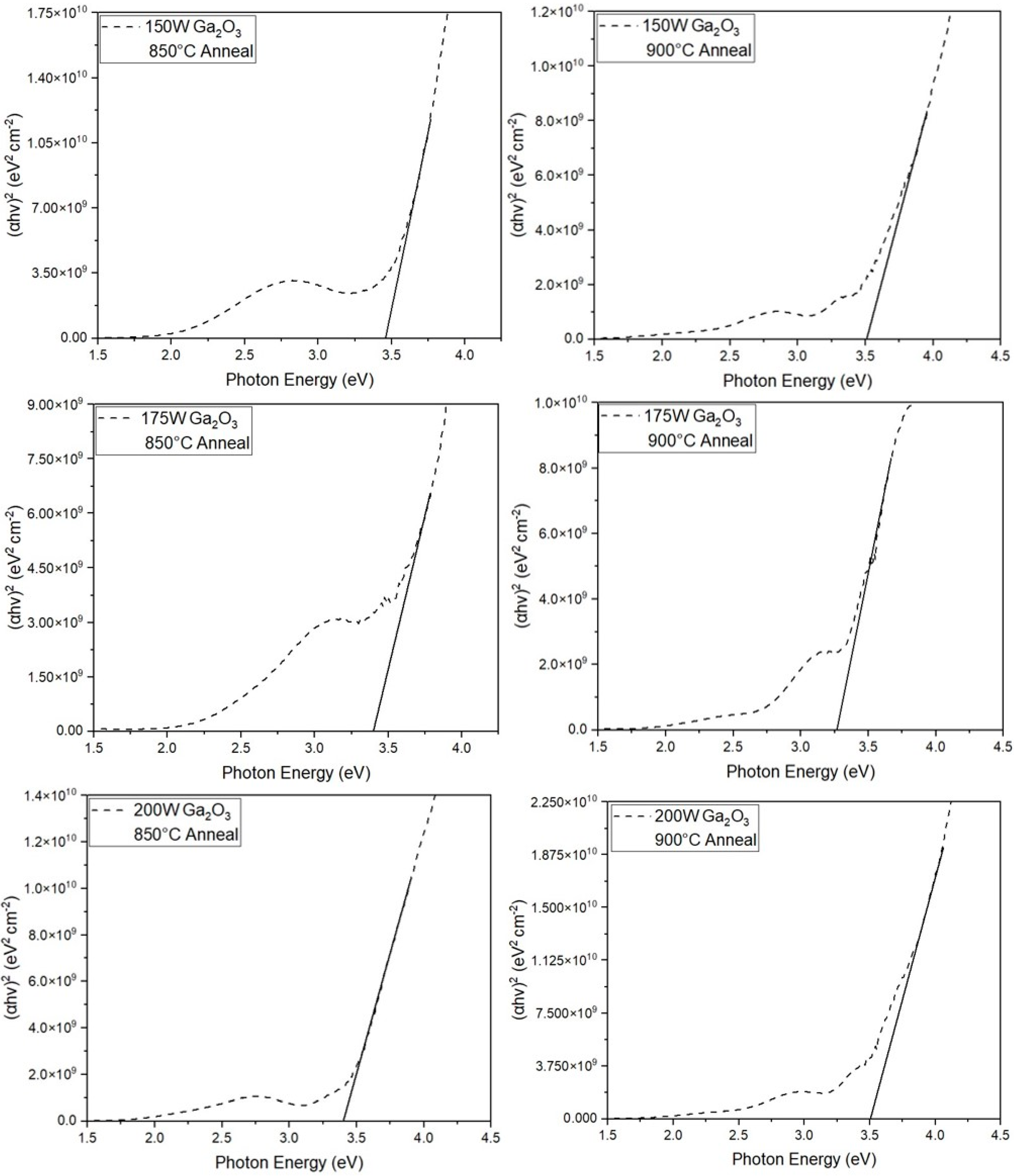
3.3.2. Optical Bandgap
3.3.3. Refractive Index Studies of CuGaO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, L.; Wei, R.H.; Tang, X.W.; Lu, W.J.; Zhu, X.B.; Sun, Y.P. Design strategy for p-type transparent conducting oxides. J. Appl. Phys. 2020, 128, 140902. [Google Scholar] [CrossRef]
- Stadler, A. Transparent Conducting Oxides-An Up-to-Date Overview. Materials 2012, 5, 661–683. [Google Scholar] [CrossRef]
- Lan, J.-H.; Kanicki, J.; Catalano, A.; Keane, J.; Boer, W.d.; Gu, T. Patterning of transparent conducting oxide thin films by wet etching for a-Si:H TFT-LCDs. J. Electron. Mater. 1996, 25, 1806–1817. [Google Scholar] [CrossRef]
- Saikumar, A.K.; Nehate, S.D.; Sundaram, K.B. Review—RF Sputtered Films of Ga2O3. ECS J. Solid State Sci. Technol. 2019, 8, Q3064. [Google Scholar] [CrossRef]
- Nehate, S.; Saikumar, A.K.; Sundaram, K.B. A review of recent developments in aluminum gallium oxide thin films and devices. Crit. Rev. Solid State Mater. Sci. 2022, 47, 538–569. [Google Scholar] [CrossRef]
- Chiu, T.-W.; Yang, Y.-C.; Yeh, A.-C.; Wang, Y.-P.; Feng, Y.-W. Antibacterial property of CuCrO2 thin films prepared by RF magnetron sputtering deposition. Vacuum 2013, 87, 174–177. [Google Scholar] [CrossRef]
- Jabed, A.; Rahman, Z.U.; Khan, M.M.; Haider, W.; Shabib, I. Combinatorial development and in vitro characterization of the quaternary Zr–Ti–X–Y (X–Y = Cu–Ag/Co–Ni) metallic glass for prospective bioimplants. Adv. Eng. Mater. 2019, 21, 1900726. [Google Scholar] [CrossRef]
- Saikumar, A.K.; Sundaresh, S.; Sundaram, K.B. Preparation and characterization of p-type copper gallium oxide (CuGaO2) thin films by dual sputtering using Cu and Ga2O3 targets. ECS J. Solid State Sci. Technol. 2022, 11, 065010. [Google Scholar] [CrossRef]
- Fallah, H.R.; Ghasemi, M.; Hassanzadeh, A.; Steki, H. The effect of annealing on structural, electrical and optical properties of nanostructured ITO films prepared by e-beam evaporation. Mater. Res. Bull. 2007, 42, 487–496. [Google Scholar] [CrossRef]
- George, J.; Menon, C.S. Electrical and optical properties of electron beam evaporated ITO thin films. Surf. Coat. Technol. 2000, 132, 45–48. [Google Scholar] [CrossRef]
- Dixon, S.C.; Sathasivam, S.; Williamson, B.A.D.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. Transparent conducting n-type ZnO:Sc—Synthesis, optoelectronic properties and theoretical insight. J. Mater. Chem. C 2017, 5, 7585–7597. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Shan, F.; Yu, Y.s. Band gap energy of pure and Al-doped ZnO thin films. J. Eur. Ceram. Soc. 2004, 24, 1869–1872. [Google Scholar] [CrossRef]
- Maldonado, F.; Stashans, A. Al-doped ZnO: Electronic, electrical and structural properties. J. Phys. Chem. Solids 2010, 71, 784–787. [Google Scholar] [CrossRef]
- Phelps, J.R.; Saikumar, A.K.; Abdolvand, R.; Sundaram, K.B. Comparison of RF and High Impulse Magnetron Sputtered Gallium-Doped Zinc Oxide Thin Films. Coatings 2023, 13, 71. [Google Scholar] [CrossRef]
- Shantheyanda, B.P.; Sundaram, K.B.; Shiradkar, N.S. Studies on the effect of hydrogen doping during deposition of Al: ZnO films using RF magnetron sputtering. Mater. Sci. Eng. B 2012, 177, 1777–1782. [Google Scholar] [CrossRef]
- Sundaresh, S.; Bharath, A.H.; Sundaram, K.B. Synthesis and Characterization of Radio-Frequency-Sputtered Delafossite Copper Indium Oxide (CuInO2) Thin Films. ECS J. Solid State Sci. Technol. 2023, 12, 043010. [Google Scholar] [CrossRef]
- Sundaresh, S.; Bharath, A.H.; Sundaram, K.B. Effect of Annealing Temperature on Radio Frequency Sputtered p-Type Delafossite Copper Chromium Oxide (CuCrO2) Thin Films and Investigation of Diode Characteristics Forming Transparent pn-Heterojunction. Coatings 2023, 13, 263. [Google Scholar] [CrossRef]
- Sundaresh, S.; Bharath, A.H.; Sundaram, K.B. Effect of Cu2O Sputtering Power Variation on the Characteristics of Radio Frequency Sputtered p-Type Delafossite CuCrO2 Thin Films. Coatings 2023, 13, 395. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Hautier, G.; Miglio, A.; Ceder, G.; Rignanese, G.-M.; Gonze, X. Identification and design principles of low hole effective mass p-type transparent conducting oxides. Nat. Commun. 2013, 4, 2292. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Chattopadhyay, K.K. Recent developments in the emerging field of crystalline p-type transparent conducting oxide thin films. Prog. Cryst. Growth Charact. Mater. 2005, 50, 52–105. [Google Scholar] [CrossRef]
- Afre, R.; Sharma, N.; Sharon, M.; Sharon, M. Transparent Conducting Oxide Films for Various Applications: A Review. Rev. Adv. Mater. Sci. 2019, 53, 79–89. [Google Scholar] [CrossRef]
- Zhang, K.; Xi, K.; Blamire, M.; Egdell, R. P-type transparent conducting oxides. J. Phys. Condens. Matter Inst. Phys. J. 2016, 28, 383002. [Google Scholar] [CrossRef]
- Moreira, M.; Afonso, J.; Crepelliere, J.; Lenoble, D.; Lunca-Popa, P. A review on the p-type transparent Cu–Cr–O delafossite materials. J. Mater. Sci. 2022, 57, 3114–3142. [Google Scholar] [CrossRef]
- Iozzi, M.F.; Vajeeston, P.; Vidya, R.; Ravindran, P.; Fjellvåg, H. Structural and electronic properties of transparent conducting delafossite: A comparison between the AgBO2 and CuBO2 families (B = Al, Ga, In and Sc, Y). RSC Adv. 2015, 5, 1366–1377. [Google Scholar] [CrossRef]
- Saikumar, A.K.; Sundaresh, S.; Nehate, S.D.; Sundaram, K.B. Preparation and Characterization of Radio Frequency Sputtered Delafossite p-type Copper Gallium Oxide (p-CuGaO2) Thin Films. ECS J. Solid State Sci. Technol. 2022, 11, 023005. [Google Scholar] [CrossRef]
- Omata, T.; Nagatani, H.; Suzuki, I.; Kita, M.; Yanagi, H.; Ohashi, N. Wurtzite CuGaO2: A New Direct and Narrow Band Gap Oxide Semiconductor Applicable as a Solar Cell Absorber. J. Am. Chem. Soc. 2014, 136, 3378–3381. [Google Scholar] [CrossRef]
- Han, M.; Huang, T.; Zhang, J.; Li, Y.; Hu, Z. Structural, optical and electrical properties of delafossite CuGaO2 films grown by sol-gel method. In Proceedings of the Eighth International Conference on Thin Film Physics and Applications, Shanghai, China, 20–23 September 2013. [Google Scholar]
- Xu, Z.; Xiong, D.; Wang, H.; Zhang, W.; Zeng, X.; Ming, L.; Chen, W.; Xu, X.; Cui, J.; Wang, M.; et al. Remarkable Photocurrent of P-type Dye-sensitized Solar Cell Achieved by Size Controlled CuGaO2 Nanoplates. J. Mater. Chem. A 2014, 2, 2968–2976. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Synthesis, characterization and electrocatalytic properties of delafossite CuGaO2. J. Solid State Chem. 2016, 242, 77–85. [Google Scholar] [CrossRef]
- Nie, X.; Wei, S.-H.; Zhang, S. Bipolar Doping and Band-Gap Anomalies in Delafossite Transparent Conductive Oxides. Phys. Rev. Lett. 2002, 88, 066405. [Google Scholar] [CrossRef]
- Robertson, J.; Peacock, P.W.; Towler, M.; Needs, R. Electronic Structure of p-Type Conducting Transparent Oxides. Thin Solid Film. 2002, 411, 96–100. [Google Scholar] [CrossRef]
- Yu, M.; Draskovic, T.I.; Wu, Y. Understanding the crystallization mechanism of delafossite CuGaO2 for controlled hydrothermal synthesis of nanoparticles and nanoplates. Inorg. Chem. 2014, 53, 5845–5851. [Google Scholar] [CrossRef] [PubMed]
- Ehara, T. Preparation of CuGaO2 thin film by a sol-gel method using two kinds of metal source combination. J. Mater. Sci. Chem. Eng. 2018, 6, 68–78. [Google Scholar]
- Tsay, C.-Y.; Chen, C.-L. Improved electrical properties of p-type CuGaO2 semiconductor thin films through Mg and Zn doping. Ceram. Int. 2017, 43, 2563–2567. [Google Scholar] [CrossRef]
- Ueda, K.; Hase, T.; Yanagi, H.; Kawazoe, H.; Hosono, H.; Ohta, H.; Orita, M.; Hirano, M. Epitaxial growth of transparent p-type conducting CuGaO2 thin films on sapphire (001) substrates by pulsed laser deposition. J. Appl. Phys. 2001, 89, 1790–1793. [Google Scholar] [CrossRef]
- Saikumar, A.K.; Sundaresh, S.; Nehate, S.D.; Sundaram, K.B. Properties of RF Magnetron-Sputtered Copper Gallium Oxide (CuGa2O4) Thin Films. Coatings 2021, 11, 921. [Google Scholar] [CrossRef]
- Yu, R.-S.; Lee, Y.-C. Effects of annealing on the optical and electrical properties of sputter-deposited CuGaO2 thin films. Thin Solid Film. 2018, 646, 143–149. [Google Scholar] [CrossRef]
- Dong, L.; Jia, R.; Xin, B.; Zhang, Y. Effects of post-annealing temperature and oxygen concentration during sputtering on the structural and optical properties of β-Ga2O3 films. J. Vac. Sci. Technol. A 2016, 34, 060602. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Chen, C.-L. Growth and characterization of sol-gel derived CuGaO2 semiconductor thin films for UV photodetector application. J. Cryst. Growth 2017, 468, 662–665. [Google Scholar] [CrossRef]
- Zhao, Q.-M.; Zhao, Z.-Y.; Liu, Q.-L.; Yao, G.-Y.; Dong, X.-D. Delafossite CuGaO2 as promising visible-light-driven photocatalyst: Synthesize, properties, and performances. J. Phys. D Appl. Phys. 2020, 53, 135102. [Google Scholar] [CrossRef]
- Sundaram, K.; Bhagavat, G. High-temperature annealing effects on tin oxide films. J. Phys. D Appl. Phys. 1983, 16, 69. [Google Scholar] [CrossRef]
- Ramana, C.; Smith, R.; Hussain, O. Grain size effects on the optical characteristics of pulsed-laser deposited vanadium oxide thin films. Phys. Status Solidi (A) 2003, 199, R4–R6. [Google Scholar] [CrossRef]
- Shakti, N.; Gupta, P. Structural and optical properties of sol-gel prepared ZnO thin film. Appl. Phys. Res. 2010, 2, 19. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi (B) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Davis, E.; Mott, N. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Bakar, M.A.; Li, L.; Mohamad, K.; Sulaiman, S.; Salleh, S.; Alias, A. Effect of Annealing Temperature on CuGaO2 Thin Films Deposited by RF Sputtering Technique. J. Adv. Res. Appl. Mech 2015, 14, 12–17. [Google Scholar]
- Ahmed, J.; Poltavets, V.V.; Prakash, J.; Alshehri, S.M.; Ahamad, T. Sol–gel synthesis, structural characterization and bifunctional catalytic activity of nanocrystalline delafossite CuGaO2 particles. J. Alloys Compd. 2016, 688, 1157–1161. [Google Scholar] [CrossRef]
- Onuma, T.; Fujioka, S.; Yamaguchi, T.; Higashiwaki, M.; Sasaki, K.; Masui, T.; Honda, T. Correlation between blue luminescence intensity and resistivity in β-Ga2O3 single crystals. Appl. Phys. Lett. 2013, 103, 041910. [Google Scholar] [CrossRef]
- Carreño, F.; Martínez-Antón, J.C.; Bernabeu, E. Optical interference method to obtain thickness and refractive indices of a uniaxial medium. Rev. Sci. Instrum. 1994, 65, 2489–2493. [Google Scholar] [CrossRef][Green Version]
- Wang, G.; Wu, H.; Wang, X.; Xu, Y.; Shi, L.; Zhang, H.; Jin, L.; Yin, J. Study on photoluminescence characteristics and whispering gallery mode resonance from Cr doped CuGaO2 nanoplates. Opt. Commun. 2020, 461, 125231. [Google Scholar] [CrossRef]
| Deposition Parameter | Specification |
|---|---|
| Base pressure | 5 × 10−7 Torr |
| Deposition pressure | 10 mTorr |
| Sputtering gas | Ar |
| Sputtering gas flow rate | 10 sccm |
| Cu2O power | 50 W |
| Ga2O3 power | 150 W, 175 W, and 200 W |
| Substrate temperature | Room temperature |
| Thickness of the deposited film | 200 nm |
| Annealing temperature | 850 °C and 900 °C |
| Annealing time | 5 h |
| Annealing gas and flow rate | N2 at 300 sccm |
| 850 °C | 900 °C | |
|---|---|---|
| 150 W | CuGa2O4 + Ga2O3 + CuO | CuGaO2 + Ga2O3 + CuO |
| 175 W | CuGa2O4 + Ga2O3 | CuGaO2 + Ga2O3 |
| 200 W | CuGa2O4 + CuGaO2 + Ga2O3 | CuGaO2 + Ga2O3 |
| 2θ Angle | Index |
|---|---|
| 31.4° | (006) |
| 35.21° | (101) |
| 36.43° | (012) |
| 41.06° | (105) |
| 62.59° | (110) |
| 2θ Angle | Index |
|---|---|
| 38.69° | (111) |
| 45.09° | ) |
| 57.94° | (202) |
| 2θ Angle | Index |
|---|---|
| 30.22° | (400) |
| 38.55° | (401) |
| 46.05° | (202) |
| 48.98 | 01) |
| 57.8 | (511) |
| 59.97 | 01) |
| 2θ Angle | Index |
|---|---|
| 36.17° | (311) |
| 37.71° | (222) |
| 44.08° | (400) |
| 58.29° | (333) |
| 63.68° | (440) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharath, A.H.; Saikumar, A.K.; Sundaram, K.B. Deposition and Optical Characterization of Sputter Deposited p-Type Delafossite CuGaO2 Thin Films Using Cu2O and Ga2O3 Targets. Materials 2024, 17, 1609. https://doi.org/10.3390/ma17071609
Bharath AH, Saikumar AK, Sundaram KB. Deposition and Optical Characterization of Sputter Deposited p-Type Delafossite CuGaO2 Thin Films Using Cu2O and Ga2O3 Targets. Materials. 2024; 17(7):1609. https://doi.org/10.3390/ma17071609
Chicago/Turabian StyleBharath, Akash Hari, Ashwin Kumar Saikumar, and Kalpathy B. Sundaram. 2024. "Deposition and Optical Characterization of Sputter Deposited p-Type Delafossite CuGaO2 Thin Films Using Cu2O and Ga2O3 Targets" Materials 17, no. 7: 1609. https://doi.org/10.3390/ma17071609
APA StyleBharath, A. H., Saikumar, A. K., & Sundaram, K. B. (2024). Deposition and Optical Characterization of Sputter Deposited p-Type Delafossite CuGaO2 Thin Films Using Cu2O and Ga2O3 Targets. Materials, 17(7), 1609. https://doi.org/10.3390/ma17071609








