Spinel-Based ZnAl2O4: 0.5%Cr3+ Red Phosphor Ceramics for WLED
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Synthesis
2.2. Characterization
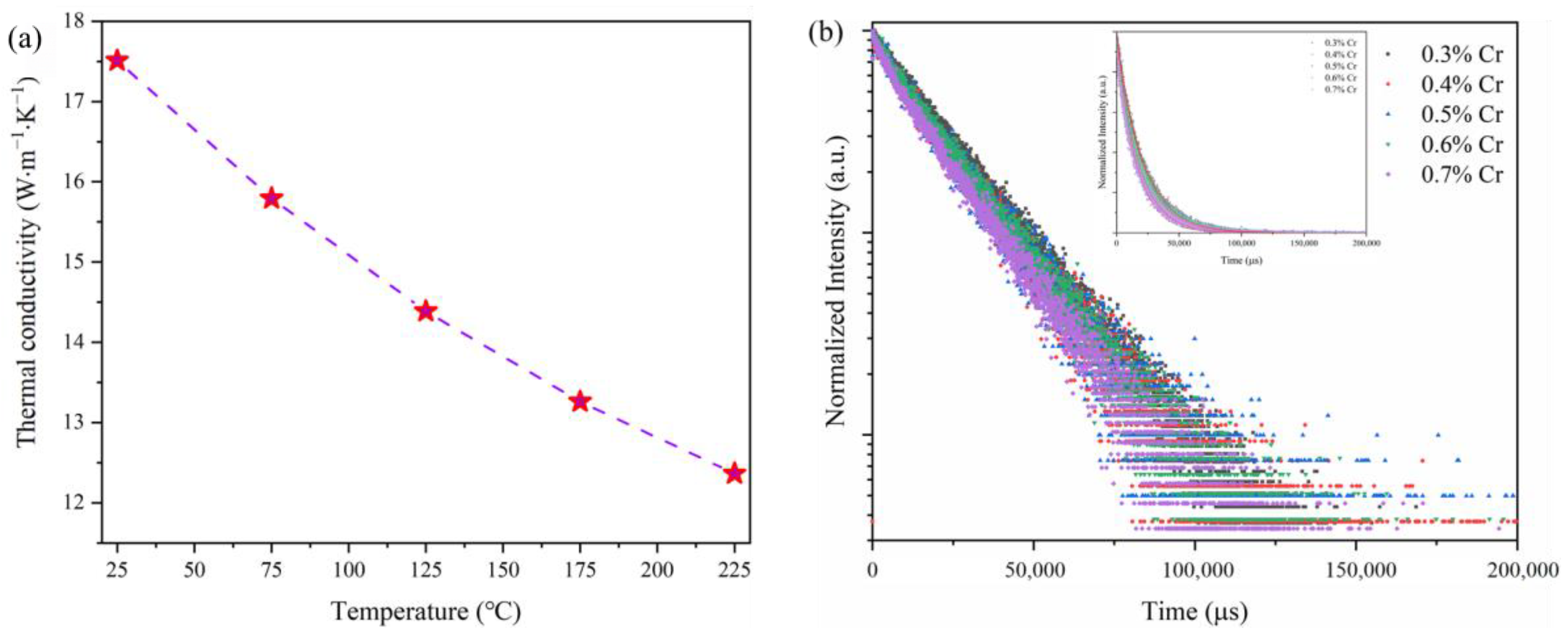
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Tang, Y.; Yin, X.; Qiang, M.; Yao, X.; Zhang, D. ZnAl2O4: Mn2+ transparent phosphor ceramic with narrow-band green emission by spark plasma sintering. J. Lumin. 2024, 265, 120198. [Google Scholar] [CrossRef]
- Markovskyi, A.; Gorbenko, V.; Zorenko, T.; Witkiewicz-Lukaszek, S.; Sidletskiy, O.; Fedorov, A.; Zorenko, Y. Development of Three-Layered Composite Color Converters for White LEDs Based on the Epitaxial Structures of YAG:Ce, TbAG:Ce and LuAG:Ce Garnets. Materials 2023, 16, 1848. [Google Scholar] [CrossRef]
- Bachmann, V.; Ronda, C.; Meijerink, A. Temperature quenching of yellow Ce3+ luminescence in YAG: Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- Pust, P.; Schmidt, P.J.; Schnick, W. A revolution in lighting. Nat. Mater. 2015, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Mangum, B.D.; Landes, T.S.; Theobald, B.R.; Kurtin, J.N. Exploring the bounds of narrow-band quantum dot downconverted LEDs. Photonics Res. 2017, 5, A13–A22. [Google Scholar] [CrossRef]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Golkovski, M.G.; Zhilgildinov, Z.S.; Popov, A.I.; Zhunusbekov, A.M.; Polisadova, E.; Tulegenova, A.; Mussakhanov, D.A.; Alpyssova, G.; et al. Electron Beam-Assisted Synthesis of YAG:Ce Ceramics. Materials 2023, 16, 4102. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Wu, Z.; Yang, T.; Zhao, H.; Wang, J.; Huang, H.; Liu, Y.; Kang, Z. Highly stable and bright blue light-emitting diodes based on carbon dots with a chemically inert surface. Nanoscale Adv. 2021, 3, 6949–6955. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.; Tulegenova, A.; Kaneva, E.; Mussakhanov, D.; Gritsenko, B. Express Synthesis of YAG:Ce Ceramics in the High-Energy Electrons Flow Field. Materials 2023, 16, 1057. [Google Scholar] [CrossRef]
- Nsresh, V.; Lee, N.J. KGaP2O7: Mn4+ deep red emitting phosphor: Synthesis, structure, concentration and temperature dependent photoluminescence characteristics. J. Lumin. 2019, 214, 116565. [Google Scholar] [CrossRef]
- Sivakumar, V.; Lakshmanan, A.; Kalpana, S.; Rani, R.S.; Kumar, R.S.; Jose, M. Low-temperature synthesis of Zn2SiO4: Mn green photoluminescence phosphor. J. Lumin. 2012, 132, 1917–1920. [Google Scholar] [CrossRef]
- Tolhurst, T.M.; Branu, C.; Schnick, W.; Moewes, A. Comprehensive Band Gap and Electronic Structure Investigations of the Prominent Phosphors M2Si5N8: Eu2+ (M = Ca, Sr, Ba) Determined Using Soft X-ray. Spectrosc. Density Funct. Theory 2021, 125, 25799–25806. [Google Scholar]
- Zhong, H.; Wang, J.W.; Dong, B.B.; Li, L.; Yang, M.Y.; Yu, J.L.; Xin, X.; Agathopoulos, S. A simple way to prepare a hydrophobic Sr[LiAl3N4]: Eu2+ phosphor with improved moisture resistance. Mater. Res. Bull. 2018, 105, 260–264. [Google Scholar] [CrossRef]
- Nanai, Y.; Ishida, R.; Urabe, Y.; Nishimura, S.; Fuchi, S. Octave-spanning broad luminescence of Cr3+, Cr4+ -codoped Mg2SiO4 phosphor for ultra-wideband near-infrared LEDs. Jpn. J. Appl. Phys. 2019, 58, SFFD02. [Google Scholar] [CrossRef]
- Kamada, Y.; Hayasaka, R.; Uchida, K.; Suzuki, T.; Takei, T.; Kitaura, M.; Kominami, H.; Hara, K.; Matsushima, Y. Deep Red Photoluminescence from Cr3+ in Fluorine-Doped Lithium Aluminate Host Material. Materials 2024, 17, 338. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bessière, A.; Basavaraju, N.; Priolkar, K.R.; Binet, L.; Viana, B.; Gourier, D. Interplay between chromium content and lattice disorder on persistent luminescence of ZnGa2O4: Cr3+ for in vivo imaging. J. Lumin. 2014, 155, 251–256. [Google Scholar] [CrossRef]
- Huyen, N.; Tu, N.; Tung, D.; Trung, D.; Anh, D.; Duc, T.; Nga, T.; Huy, P. Photoluminescent properties of red-emitting phosphor BaMgAl10O17: Cr3+ for plant growth LEDs. Opt. Mater. 2020, 108, 110207. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mater. 2022, 15, 128062. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Bradley, R.K.; Fan, J.; Fan, X. Effects of humidity and phosphor on silicone/phosphor composite in white light-emitting diode package. J. Mater. Sci. Mater. Electron. 2019, 30, 20471–20478. [Google Scholar] [CrossRef]
- Dai, J.; Cao, M.; Kou, H.; Pan, Y.; Guo, J.; Li, J. Fabrication and properties of transparent Tb: YAG fluorescent ceramics with different doping concentrations. Ceram. Int. 2016, 42, 13812–13818. [Google Scholar] [CrossRef]
- Shakhno, A.; Markovskyi, A.; Zorenko, T.; Witkiewicz-Łukaszek, S.; Vlasyuk, Y.; Osvet, A.; Elia, J.; Brabec, C.J.; Batentschuk, M.; Zorenko, Y. Micropowder Ca2YMgScSi3O12:Ce Silicate Garnet as an Efficient Light Converter for White LEDs. Materials 2022, 15, 3942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, B.; Tu, B.; Wang, H.; Wang, W.; Fu, Z. Characterization in activators’ distribution and photoluminescence properties of Ce3+ doped MgAlON transparent fluorescent ceramic. J. Eur. Ceram. Soc. 2016, 36, 2801–2805. [Google Scholar] [CrossRef]
- Su, L.; Miao, L.; Miao, J.; Zheng, Z.; Yang, B.; Xia, R.; Chen, P.; Qian, J. Synthesis and optical property of zinc aluminate spinel cryogels. J. Asian Ceram. Soc. 2016, 4, 185–190. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Ramachandran, S.; Subramani, A.; Thamilselvan, A.; Venkatesan, S.; Sundararajan, M.; Dash, C.S. Impact of Mg2+ Ion on the Structural, Morphological, Optical, Vibrational, and Magnetic Behavior of Mg: ZnAl2O4 Spinel. J. Supercond. Nov. Magn. 2019, 33, 1199–1206. [Google Scholar] [CrossRef]
- Da Silva, A.A.; de Souza Gonçalves, A.; Davolos, M.R. Characterization of nanosized ZnAl2O4 spinel synthesized by the sol–gel method. J. Sol.-Gel Sci. Technol. 2009, 49, 101. [Google Scholar] [CrossRef]
- Basiri, A.; Nassajpour-Esfahani, A.H.; Haftbaradaran-Esfahani, M.R.; Alhaji, A.; Shafyei, A. Optimization of spray freeze drying parameters for spark plasma sintering of transparent MgAl2O4 spinel. Ceram. Int. 2022, 48, 10751–10761. [Google Scholar] [CrossRef]
- Khaidukov, N.; Pirri, A.; Brekhovskikh, M.; Toci, G.; Vannini, M.; Patrizi, B.; Makhov, V. Time-and Temperature-Dependent Luminescence of Manganese Ions in Ceramic Magnesium Aluminum Spinels. Materials 2021, 14, 420. [Google Scholar] [CrossRef]
- Tshabalala, K.; Cho, S.-H.; Park, J.-K.; Pitale, S.S.; Nagpure, I.; Kroon, R.; Swart, H.; Ntwaeaborwa, O. Luminescent properties and X-ray photoelectron spectroscopy study of ZnAl2O4: Ce3+,Tb3+ phosphor. J. Alloys Compd. 2011, 509, 10115–10120. [Google Scholar] [CrossRef]
- Strachowski, T.; Grzanka, E.; Mizeracki, J.; Chlanda, A.; Baran, M.; Małek, M.; Niedziałek, M. Microwave-Assisted Hydrothermal Synthesis of Zinc-Aluminum Spinel ZnAl2O4. Materials 2021, 15, 245. [Google Scholar] [CrossRef]
- Menon, S.; Dhabekar, B.; Raja, E.A.; More, S.; Rao, T.G.; Kher, R. TSL, OSL and ESR studies in ZnAl2O4: Tb phosphor. J. Lumin. 2008, 128, 1673–1678. [Google Scholar] [CrossRef]
- Kim, I.-W.; Kaur, S.; Yadav, A.; Rao, A.; Saravanakumar, S.; Rao, J.; Singh, V. Structural, luminescence and EPR properties of deep red emitting MgY2Al4SiO12: Cr3+ garnet phosphor. J. Lumin. 2020, 220, 116975. [Google Scholar] [CrossRef]
- Brik, M.; Srivastava, A.; Popov, A. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Qiang, M.; Yin, X.; Chao, J.; Hu, Q.; Lin, H.; Hong, R.; Zheng, L.; Zhang, D. Effect of Ga3+ substitution on the photoluminescence properties of ZnAl2O4 red phosphor. Opt. Mater. 2023, 143, 114262. [Google Scholar] [CrossRef]
- Tran, M.; Trung, D.; Tu, N.; Anh, D.; Thu, L.; Du, N.; Quang, N.; Huyen, N.; Kien, N.; Viet, D.; et al. Single-phase far-red-emitting ZnAl2O4: Cr3+ phosphor for application in plant growth LEDs. J. Alloys Compd. 2021, 5, 161077. [Google Scholar] [CrossRef]
- Du, P.; Tang, J.; Li, W.; Luo, L. Exploiting the diverse photoluminescence behaviors of NaLuF4: xEu3+ nanoparticles and g-C3N4 to realize versatile applications in white light-emitting diode and optical thermometer. Chem. Eng. J. 2021, 406, 127165. [Google Scholar] [CrossRef]
- Zheng, T.; Luo, L.; Du, P.; Lis, S.; Rodríguez-Mendoza, U.R.; Lavín, V.; Martín, I.R.; Runowski, M. Pressure-triggered enormous redshift and enhanced emission in Ca2Gd8Si6O26: Ce3+ phosphors: Ultrasensitive, thermally-stable and ultrafast response pressure monitoring-ScienceDirect. Chem. Eng. J. 2022, 443, 136414. [Google Scholar] [CrossRef]
- Yin, X.; Qiang, M.; Lin, H.; Zhang, D.; Hong, R.; Han, Z. Effect of Ga3+ ion doping on emission thermal stability and efficiency of MgAl2O4: Cr3+ phosphor. J. Am. Ceram. Soc. 2023, 106, 7069–7077. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Ezerskyte, E.; Minderyte, A.; Stanionyte, S.; Juskenas, R.; Sakirzanovas, S.; Katelnikovas, A. Optical Properties of Red-Emitting Rb2Bi(PO4)(MoO4): Eu3+ Powders and Ceramics with High Quantum Efficiency for White LEDs. Materials 2019, 12, 3275. [Google Scholar] [CrossRef]
- Gutowski, M. Antisymmetric exchange interactions in Cr3+-Cr3+ pairs in the inverse spinel LiGa5O8. Phys. Rev. B 1978, 18, 5984–5989. [Google Scholar] [CrossRef]
- Qiang, M.; Yin, X.; Lin, H.; Hong, R.; Zhang, D.; Zhang, Z.; Zhou, S.; Chen, J.; Tian, Y.; Zheng, G.; et al. ZnAl2O4: Cr3+ translucent ceramic phosphor with thermally stable far-red luminescence. Opt. Mater. 2022, 133, 112887. [Google Scholar] [CrossRef]
- Yu, D.; Zhou, Y.; Ma, C.; Melman, J.H.; Baroudi, K.M.; LaCapra, M.; Riman, R.E. Non-Rare-Earth Na3AlF6: Cr3+ Phosphors for Far-Red Light Emitting Diodes. ACS Appl. Electron. Mater. 2019, 1, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron–Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- Torchia, G.; Martinez-Matos, O.; Khaidukov, N.; Tocho, J. Phonon coupling of Cr3+ ions in Cs2NaAlF6 crystals. Solid State Commun. 2004, 130, 159–163. [Google Scholar] [CrossRef]
- Malysa, B.; Meijerink, A.; Jüstel, T. Temperature dependent luminescence Cr3+-doped GdAl3(BO3)4 and YAl3(BO3)4. J. Lumin. 2016, 171, 246–253. [Google Scholar] [CrossRef]
- Qiang, M.; Tang, Y.; Ye, S.; Lou, W.; Chao, J.; Lin, H.; Zhang, D. Novel YAGG: Cr garnet phosphor ceramic with broadband near infrared (NIR) emission and luminescence thermal stability for plant growth. Ceram. Int. 2024, 50, 4823–4830. [Google Scholar] [CrossRef]
- Gao, T.; Zhuang, W.; Liu, R.; Liu, Y.; Yan, C.; Tian, J.; Chen, G.; Chen, X.; Zheng, Y.; Wang, L. Site occupancy and enhanced luminescence of broadband NIR gallogermanate phosphors by energy transfer. J. Am. Ceram. Soc. 2020, 103, 202–213. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, H.; Zhang, D.; Shen, Y.; Li, Y.; Hong, R.; Tao, C.; Han, Z.; Chen, L.; Zhou, S. Deep-red emitting Mg2TiO4: Mn4+ phosphor ceramics for plant lighting. J. Adv. Ceram. 2021, 10, 10. [Google Scholar] [CrossRef]
- Xue, P.; Tian, L. A far-red phosphor LaSrZnNbO6: Mn4+ for plant growth lighting. Opt. Mater. 2021, 115, 111063. [Google Scholar] [CrossRef]
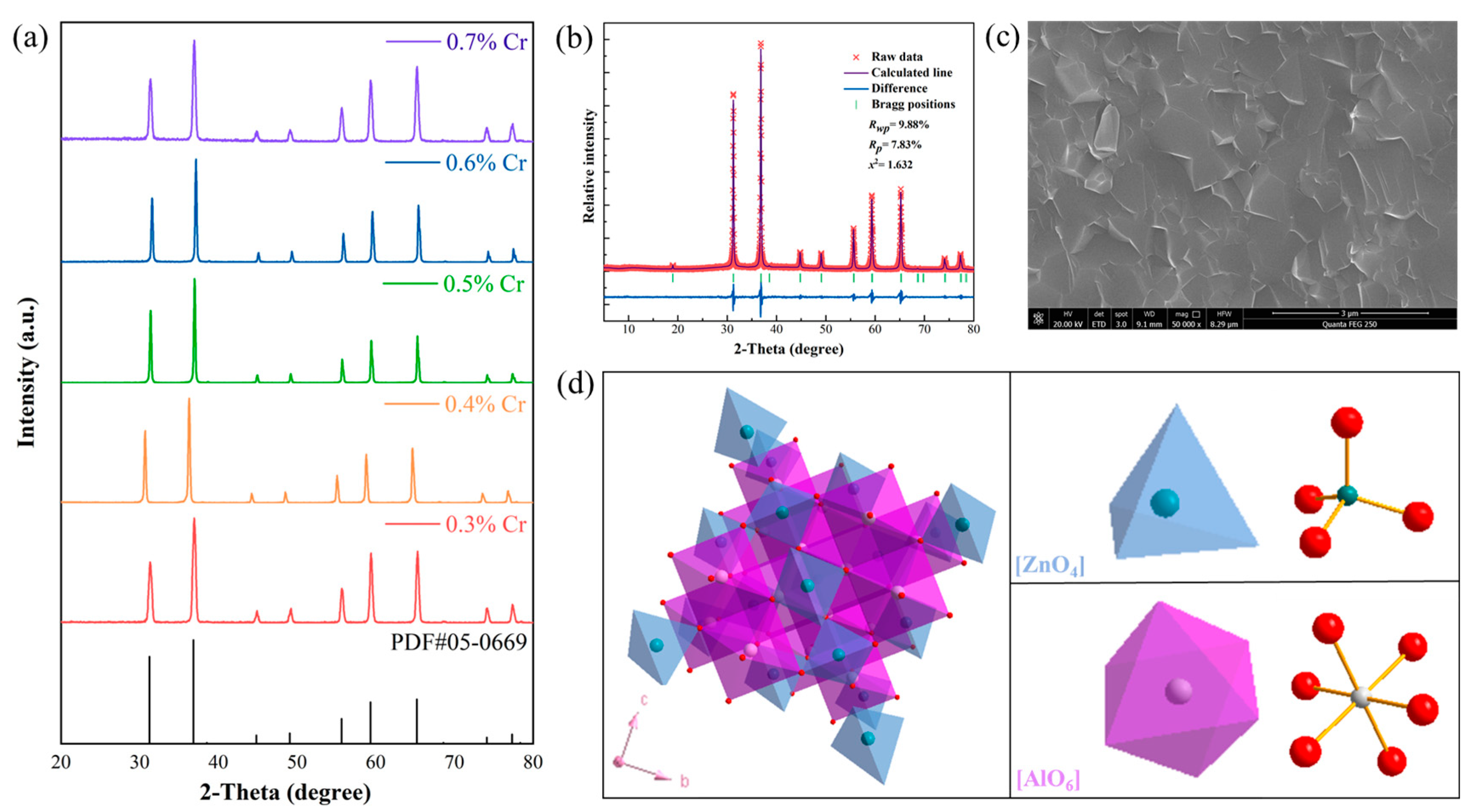
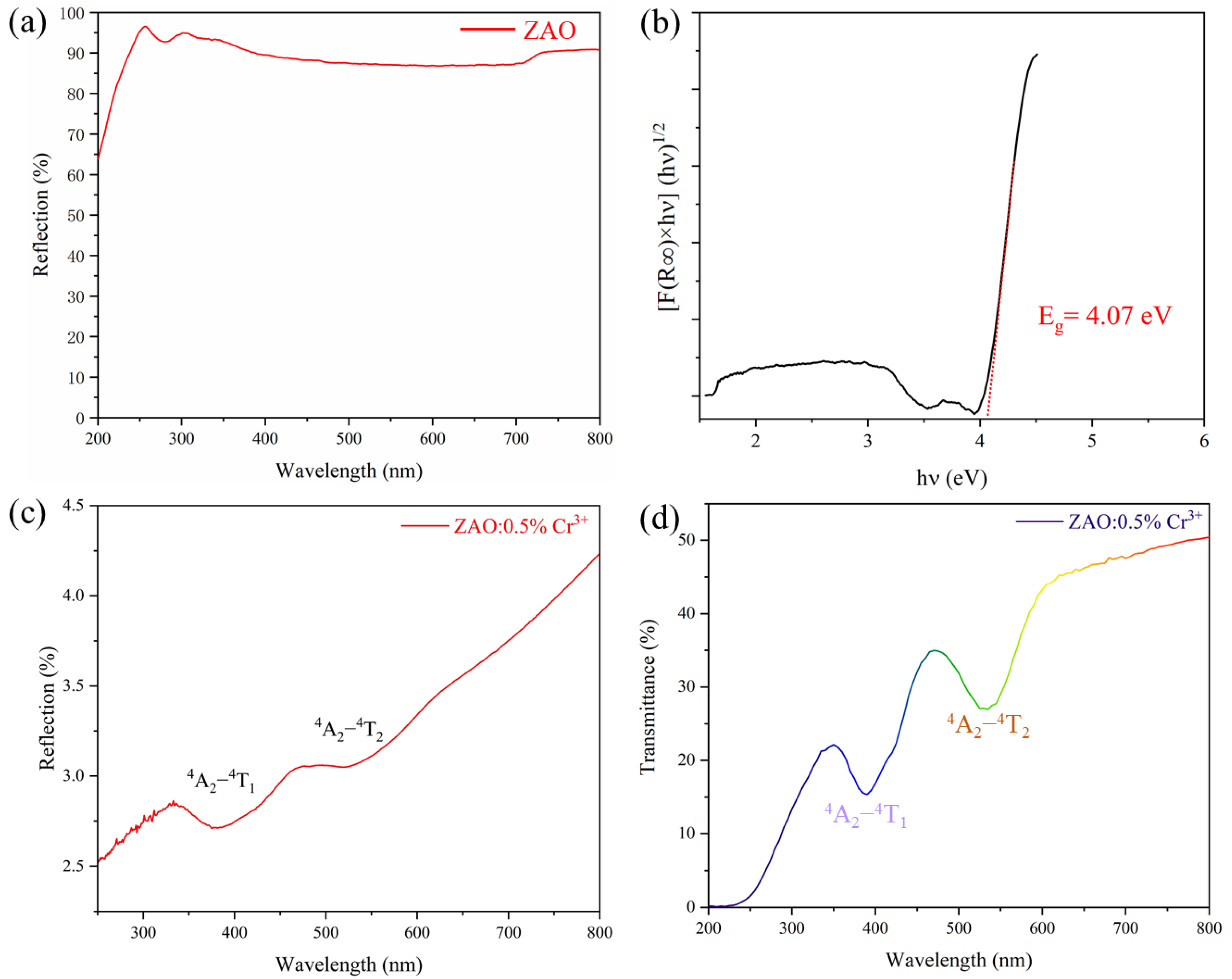

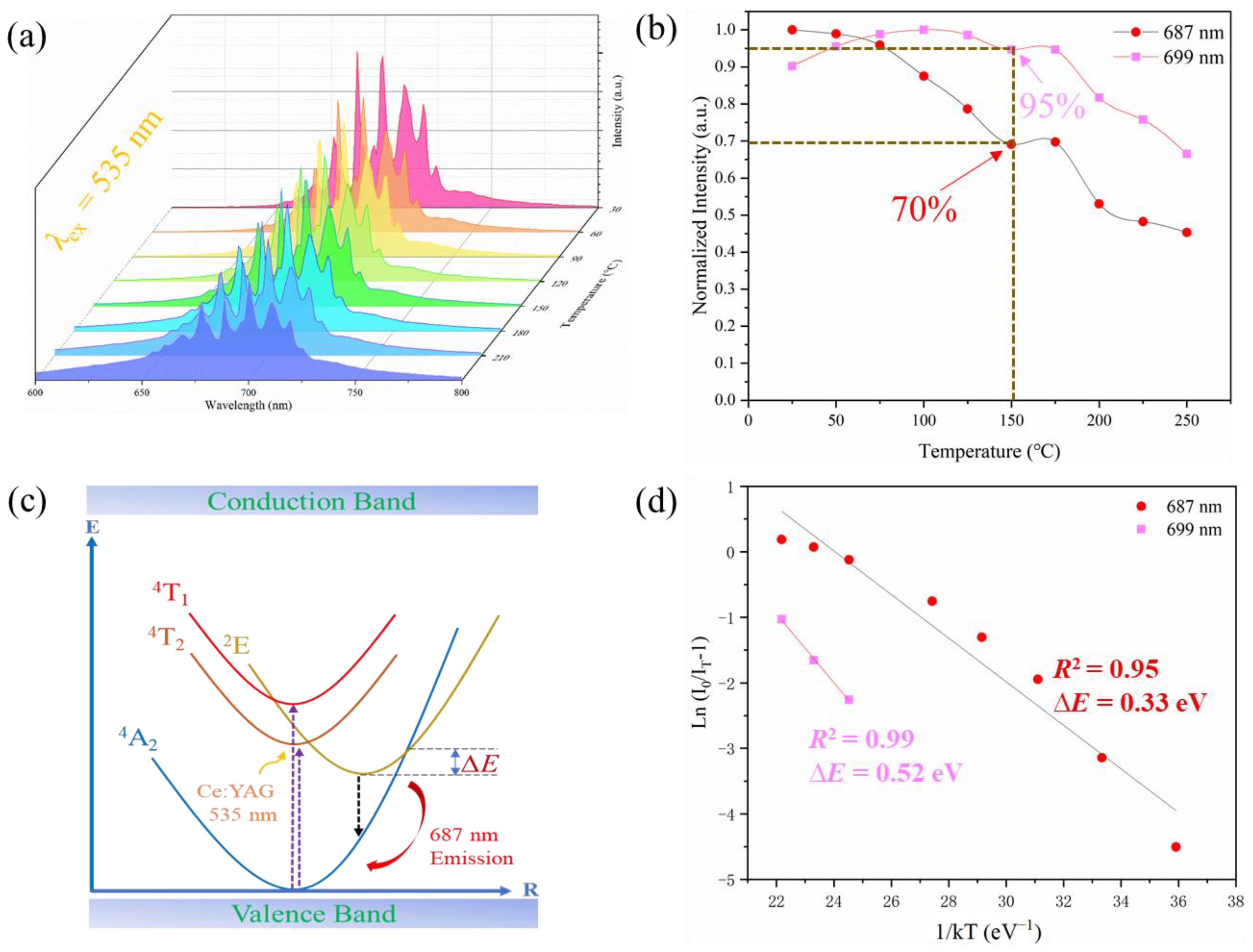
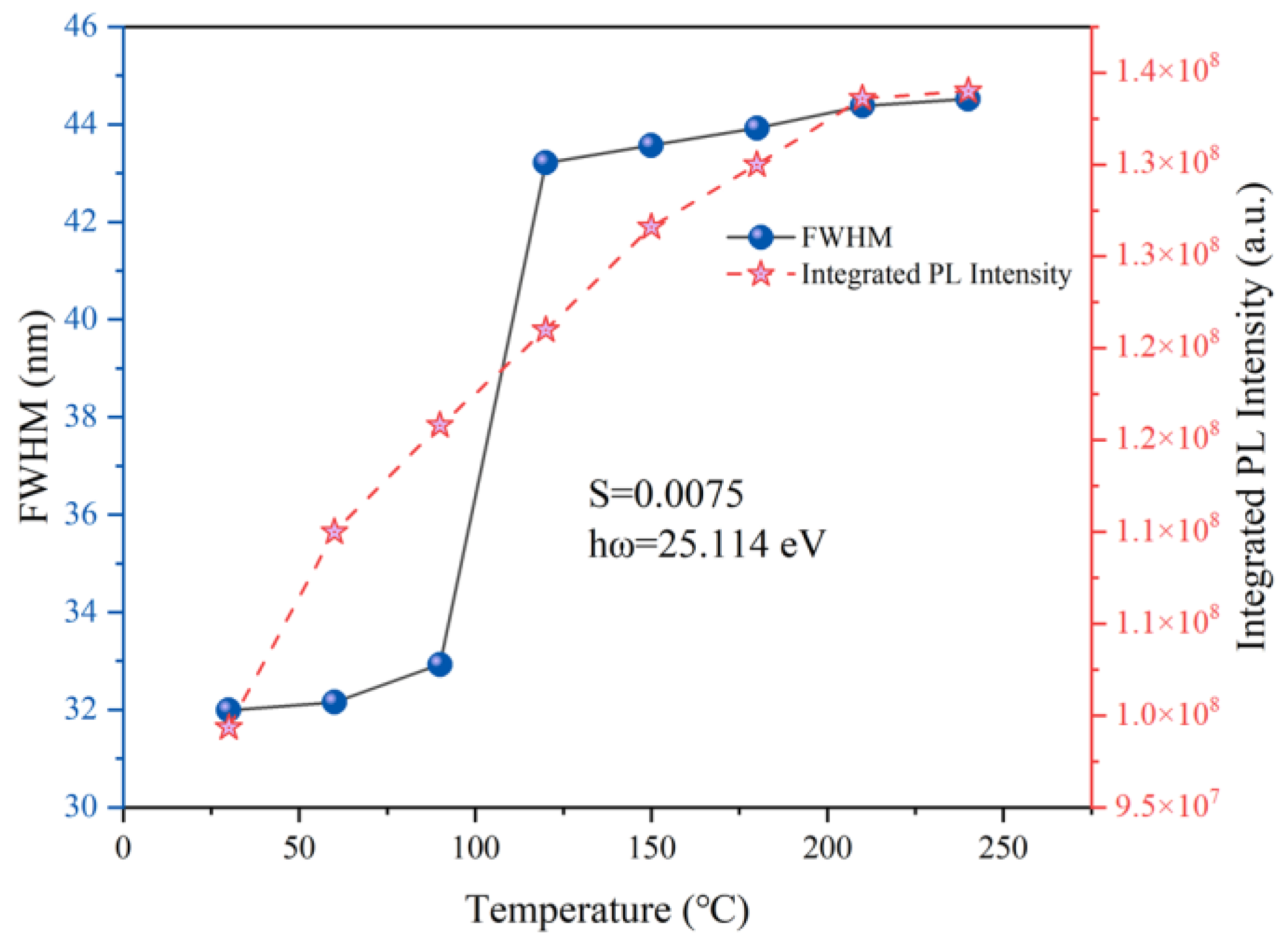

| Sample | a (Å) | b (Å) | c (Å) | V (Å3) | [Zn-O] (Å) | [Al/Cr-O] (Å) |
|---|---|---|---|---|---|---|
| ZAO | 8.0665 | 8.0665 | 8.0665 | 524.87 | 1.9525 | 1.9117 |
| ZAO:0.5%Cr3+ | 8.0839 | 8.0839 | 8.0839 | 528.28 | 1.9475 | 2.0161 |
| Concentration (%) | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 |
|---|---|---|---|---|---|
| Lifetime (ms) | 20.8 | 18.9 | 18.6 | 18.9 | 17.1 |
| Drive Current (mA) | Color Coordinates (x, y) | Luminous Efficacy (lm/W) | Luminous Flux (lm) | Color Rendering Index | R9 | Color Temperature (K) |
|---|---|---|---|---|---|---|
| * 50 | (0.3063, 0.3421) | 8.3 | 3.2 | 75.7 | −10 | 6735 |
| 50 | (0.3315, 0.3206) | 6.1 | 2.4 | 85 | 38 | 5543 |
| 100 | (0.3311, 0.3189) | 6.1 | 4.9 | 85.3 | 38.9 | 5558 |
| 300 | (0.3304, 0.3147) | 5.5 | 14.3 | 85.9 | 41.9 | 5599 |
| 500 | (0.3303, 0.3128) | 5.0 | 22.6 | 86.1 | 42.9 | 5606 |
| 800 | (0.329, 0.3085) | 4.4 | 33.6 | 86.9 | 51.1 | 5683 |
| 1000 | (0.3283, 0.3062) | 4.1 | 40.1 | 87.3 | 55.9 | 5730 |
| 1300 | (0.3275, 0.3026) | 3.9 | 50.3 | 87.8 | 61.3 | 5780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, W.; Xu, X.; Qiang, M.; Dun, A. Spinel-Based ZnAl2O4: 0.5%Cr3+ Red Phosphor Ceramics for WLED. Materials 2024, 17, 1610. https://doi.org/10.3390/ma17071610
Ji W, Xu X, Qiang M, Dun A. Spinel-Based ZnAl2O4: 0.5%Cr3+ Red Phosphor Ceramics for WLED. Materials. 2024; 17(7):1610. https://doi.org/10.3390/ma17071610
Chicago/Turabian StyleJi, Wenchao, Xueke Xu, Ming Qiang, and Aihuan Dun. 2024. "Spinel-Based ZnAl2O4: 0.5%Cr3+ Red Phosphor Ceramics for WLED" Materials 17, no. 7: 1610. https://doi.org/10.3390/ma17071610
APA StyleJi, W., Xu, X., Qiang, M., & Dun, A. (2024). Spinel-Based ZnAl2O4: 0.5%Cr3+ Red Phosphor Ceramics for WLED. Materials, 17(7), 1610. https://doi.org/10.3390/ma17071610





