Theoretical Prediction and Experimental Synthesis of Zr3AC2 (A = Cd, Sb) Phases
Abstract
1. Introduction
2. Experimental Procedures
2.1. First-Principles Calculation
2.2. Synthesis of Zr3CdC2 and Zr3SbC2 Phases
2.3. Composition and Microstructure Characterization
3. Results and Discussion
3.1. Stability Calculation of Zr3CdC2 and Zr3SbC2 Phases
3.2. Theoretical Mechanical Properties of Zr3CdC2 and Zr3SbC2 Phases
3.3. Synthesis and Microstructure Characterization of Zr3CdC2 and Zr3SbC2 Phases
4. Conclusions
- It was confirmed that the Zr3CdC2 and Zr3SbC2 phases belonged to the space group of P63/mmc with the hexagonal crystal structure. The calculated crystal parameters of Zr3CdC2 were a = 3.319 Å and c = 20.393 Å, and those of Zr3SbC2 were a = 3.367 Å and c = 19.413 Å. The calculated formation energies of Zr3CdC2 and Zr3SbC2 were −1.104 and −1.306 eV/atom, respectively, which confirm the thermal stability of the two phases. The absence of imaginary frequencies in the acoustic branches of the phonon band structure and the calculation results using the Born–Huang criterion confirmed the thermodynamic and mechanical stability of the two MAX phases. Additionally, the calculated Young’s moduli of Zr3CdC2 and Zr3SbC2 were 162.846 GPa and 164.265 GPa, respectively. The theoretical Vickers hardnesses of the Zr3CdC2 (5.448 GPa) and Zr3SbC2 (4.309 GPa) phases were low due to the weaker covalent bonds, exhibiting excellent potential machinability.
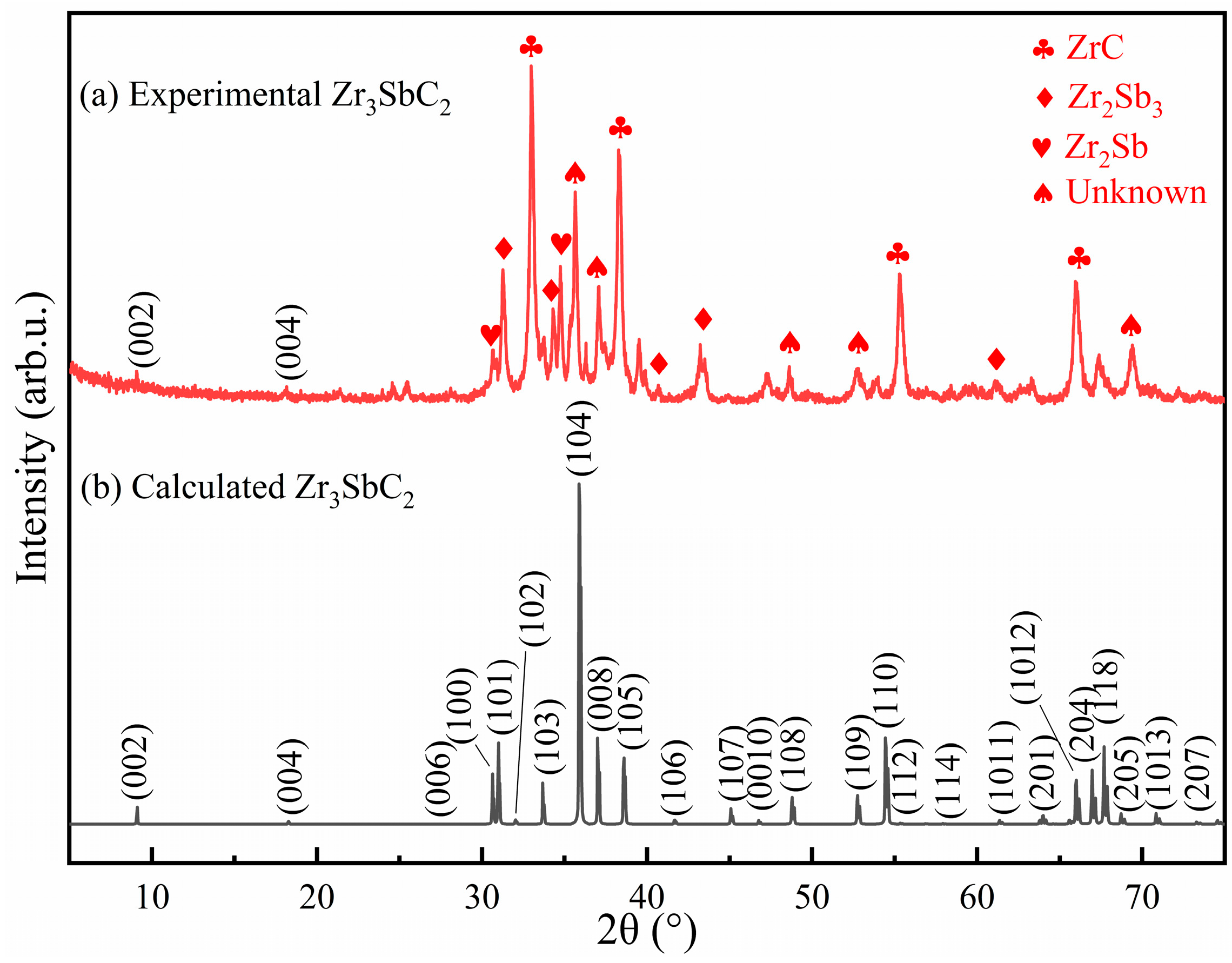
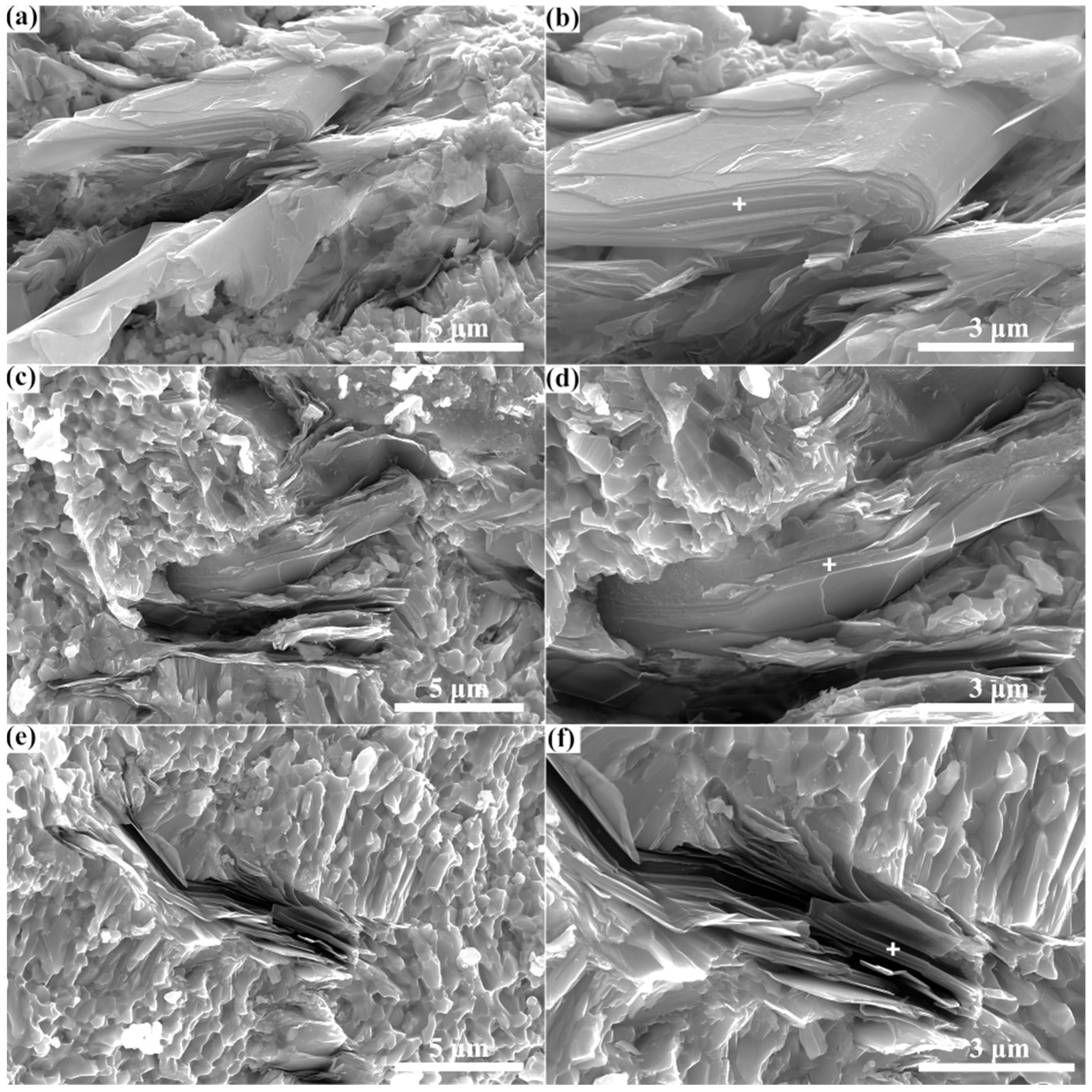
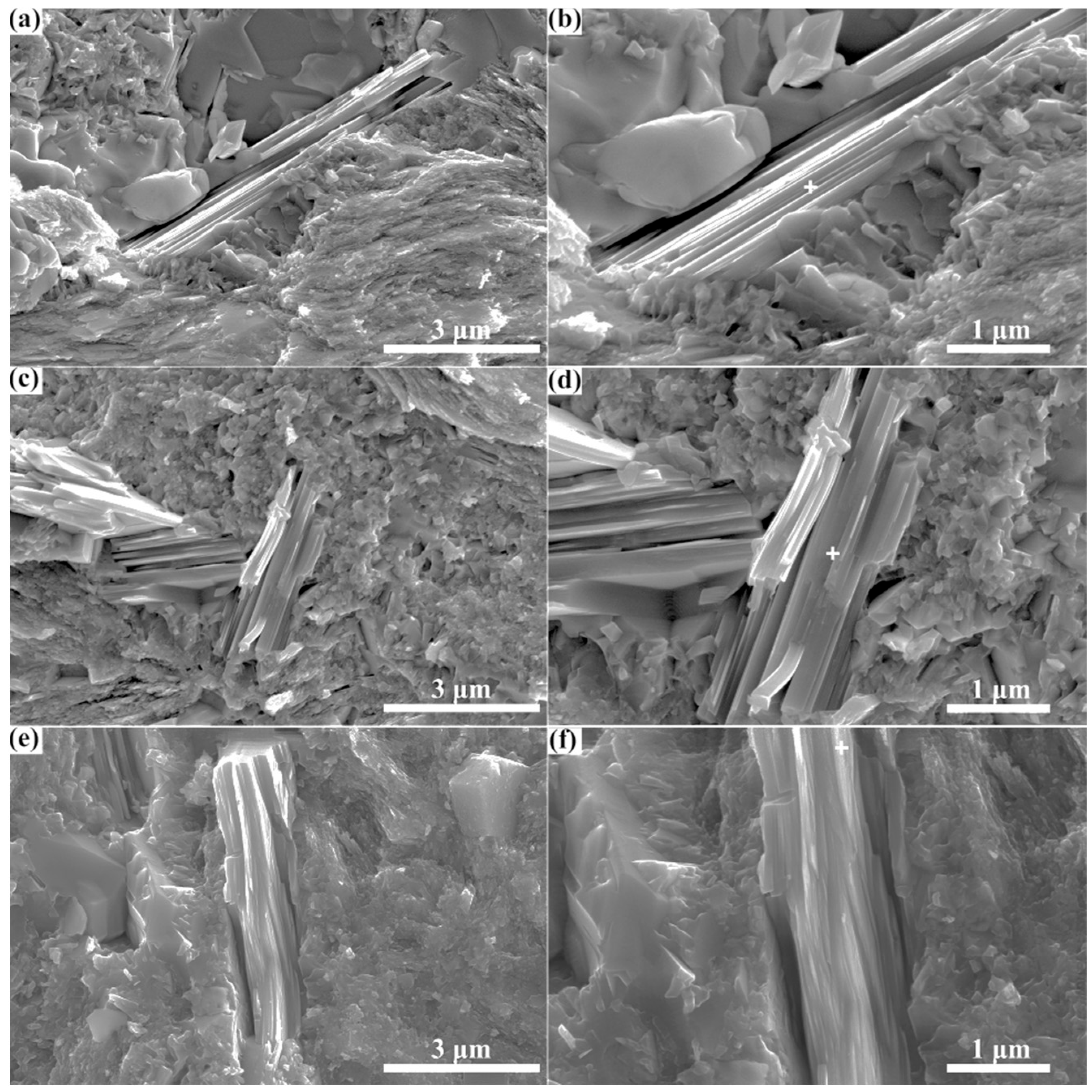
- Through spark plasma sintering, composites containing the Zr3CdC2 and Zr3SbC2 phases could be synthesized at temperatures of 850 °C and 1300 °C, respectively. The optimal molar ratio of Zr:Cd/Sb:C was determined as 3:1.5:1.5. Based on the SEM micrographs, the nanolayered characters of the Zr3CdC2 and Zr3SbC2 grains were determined. The average element ratios were Zr:Cd = 3:0.976 for Zr3CdC2 grains and Zr:Sb = 3:1.012 for Zr3SbC2 grains, respectively, very close to the ratio of 3:1.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barsoum, M.W.; El-Raghy, T. Synthesis and Characterization of a Remarkable Ceramic: Ti3SiC2. J. Am. Ceram. Soc. 1996, 79, 1953–1956. [Google Scholar] [CrossRef]
- Nowotny, V. Strukturchemie einiger Verbindungen der Übergangsmetalle mit den elementen C, Si, Ge, Sn. Prog. Solid State Chem. 1971, 5, 27–70. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Radovic, M. Elastic and Mechanical Properties of the MAX Phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, B.; Luo, J.; Zhou, Y.; San, X.; Bao, Y.; Chu, L.; Feng, Q.; Grasso, S.; Hu, C. Synthesis of new lead-containing MAX phases of Zr3PbC2 and Hf3PbC2. J. Am. Ceram. Soc. 2023, 106, 6390–6397. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T.; Ali, M. Processing and characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5. Metall. Mater. Trans. A 2000, 31, 1857–1865. [Google Scholar] [CrossRef]
- Fattahi, M.; Zarezadeh Mehrizi, M. Formation mechanism for synthesis of Ti3SnC2 MAX phase. Mater. Today Commun. 2020, 25, 101623. [Google Scholar] [CrossRef]
- Hossein-Zadeh, M.; Mirzaee, O.; Mohammadian-Semnani, H. An investigation into the microstructure and mechanical properties of V4AlC3 MAX phase prepared by spark plasma sintering. Ceram. Int. 2019, 45, 7446–7457. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, B.; Luo, J.; Zhou, Y.; San, X.; Bao, Y.; Feng, Q.; Grasso, S.; Hu, C. Synthesis of new rare earth containing ternary laminar Sc2PbC ceramic. J. Eur. Ceram. Soc. 2023, 43, 1735–1739. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; San, X.; Li, W.; Bao, Y.; Feng, Q.; Grasso, S.; Hu, C. Zr2SeB and Hf2SeB: Two new MAB phase compounds with the Cr2AlC-type MAX phase (211 phase) crystal structures. J. Adv. Ceram. 2022, 11, 1764–1776. [Google Scholar] [CrossRef]
- Cuskelly, D.T.; Richards, E.R.; Kisi, E.H.; Keast, V.J. Ti3GaC2 and Ti3InC2: First bulk synthesis, DFT stability calculations and structural systematics. J. Solid State Chem. 2015, 230, 418–425. [Google Scholar] [CrossRef]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. On the chemical diversity of the MAX phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Hu, C. Extension of MAX phases from ternary carbides and nitrides (X = C and N) to ternary borides (X = B, C, and N): A general guideline. Int. J. Appl. Ceram. Technol. 2023, 20, 803–822. [Google Scholar] [CrossRef]
- Lapauw, T.; Tunca, B.; Cabioc’h, T.; Lu, J.; Persson, P.O.; Lambrinou, K.; Vleugels, J. Synthesis of MAX phases in the Hf–Al–C System. Inorg. Chem. 2016, 55, 10922–10927. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-H.; Ma, X.; Du, S.; Zhang, R.-Q.; Tao, R.; Luo, J.-T.; Fu, C. Role of the A-element in the structural, mechanical, and electronic properties of Ti3AC2 MAX phases. Inorg. Chem. 2022, 61, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.G.; Birowska, M.; Bury, D.; Jakubczak, M.; Rosenkranz, A.; Jastrzębska, A.M. 2D MBenes: A novel member in the flatland. Adv. Mater. 2022, 34, 2108840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Liu, Y.; Li, S.; Wang, X.; Ying, G.; Xia, Q.; Zhang, P. From structural ceramics to 2D materials with multi-applications: A review on the development from MAX phases to MXenes. J. Adv. Ceram. 2021, 10, 1194–1242. [Google Scholar] [CrossRef]
- Fu, L.; Xia, W. MAX phases as nanolaminate materials: Chemical composition, microstructure, synthesis, properties, and applications. Adv. Eng. Mater. 2021, 23, 2001191. [Google Scholar] [CrossRef]
- Zhang, T.; Shuck, C.E.; Shevchuk, K.; Anayee, M.; Gogotsi, Y. Synthesis of three families of titanium carbonitride MXenes. J. Am. Chem. Soc. 2023, 145, 22374–22383. [Google Scholar] [CrossRef] [PubMed]
- Lapauw, T.; Tunca, B.; Cabioc’h, T.; Vleugels, J.; Lambrinou, K. Reactive spark plasma sintering of Ti3SnC2, Zr3SnC2 and Hf3SnC2 using Fe, Co or Ni additives. J. Eur. Ceram. Soc. 2017, 37, 4539–4545. [Google Scholar] [CrossRef]
- Hadi, M.A.; Christopoulos, S.-R.G.; Naqib, S.H.; Chroneos, A.; Fitzpatrick, M.E.; Islam, A.K.M.A. Physical properties and defect processes of M3SnC2 (M = Ti, Zr, Hf) MAX phases: Effect of M-elements. J. Alloys Compd. 2018, 748, 804–813. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wen, B.; Zhou, Y.; Chu, L.; Feng, Q.; Hu, C. Determination of new α-312 MAX phases of Zr3InC2 and Hf3InC2. J. Eur. Ceram. Soc. 2023, 43, 7228–7233. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Die H-Phasen: Ti2CdC, Ti2GaC, Ti2GaN, Ti2InN, Zr2InN und Nb2GaC. Monatshefte Chem. 1964, 95, 178–179. [Google Scholar] [CrossRef]
- Bai, Y.; He, X.; Li, M.; Sun, Y.; Zhu, C.; Li, Y. Ab initio study of the bonding and elastic properties of Ti2CdC. Solid State Sci. 2010, 12, 144–147. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Hadi, M.A.; Nasir, M.T.; Naqib, S.H.; Islam, A.K.M.A. Structural, elastic and electronic properties of nitride Ti2 CdN phase in comparison with the carbide Ti2CdC phase from First-principles Study. J. Phys. Conf. Ser. 2021, 1718, 012019. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Islam, J.; Hossain, A.K.M.A. Investigation of physical and superconducting properties of newly synthesized CaPd2P2 and SrPd2P2. J. Alloys Compd. 2021, 868, 159199. [Google Scholar] [CrossRef]
- Fine, M.E.; Brown, L.D.; Marcus, H.L. Elastic constants versus melting temperature in metals. Scr. Met. 1984, 18, 951–956. [Google Scholar] [CrossRef]
- Born, M. On the stability of crystal lattices. I. Math. Proc. Camb. Philos. Soc. 1940, 36, 160–172. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, J.; Jiang, X. Correlation between hardness and elastic moduli of the covalent crystals. Comput. Mater. Sci. 2011, 50, 2287–2290. [Google Scholar] [CrossRef]
- Teter, D.M. Computational Alchemy: The Rational Design of New Superhard Materials. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 1998. [Google Scholar]
- Jiang, X.; Zhao, J.; Wu, A.; Bai, Y.; Jiang, X. Mechanical and electronic properties of B12-based ternary crystals of orthorhombic phase. J. Phys. Condens. Matter 2010, 22, 315503. [Google Scholar] [CrossRef] [PubMed]
- Miao, N.; Sa, B.; Zhou, J.; Sun, Z. Theoretical investigation on the transition-metal borides with Ta3B4-type structure: A class of hard and refractory materials. Comput. Mater. Sci. 2011, 50, 1559–1566. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Jamal, M.; Jalali Asadabadi, S.; Ahmad, I.; Rahnamaye Aliabad, H.A. Elastic constants of cubic crystals. Comput. Mater. Sci. 2014, 95, 592–599. [Google Scholar] [CrossRef]
- Shein, I.R.; Ivanovskii, A.L. Elastic properties of superconducting MAX phases from first-principles calculations: Elastic properties of superconducting MAX phases. Phys. Status Solidi B 2011, 248, 228–232. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Layered machinable and electrically conductive Ti2AlC and Ti3AlC2 ceramics: A review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Shuck, C.E.; Han, M.; Maleski, K.; Hantanasirisakul, K.; Kim, S.J.; Choi, J.; Reil, W.E.B.; Gogotsi, Y. Effect of Ti3AlC2 MAX phase on structure and properties of resultant Ti3C2Tx MXene. ACS Appl. Nano Mater. 2019, 2, 3368–3376. [Google Scholar] [CrossRef]
- Wozniak, J.; Petrus, M.; Cygan, T.; Adamczyk-Cieślak, B.; Moszczyńska, D.; Olszyna, A.R. Synthesis of Ti3SiC2 phases and consolidation of MAX/SiC composites—Microstructure and mechanical properties. Materials 2023, 16, 889. [Google Scholar] [CrossRef] [PubMed]
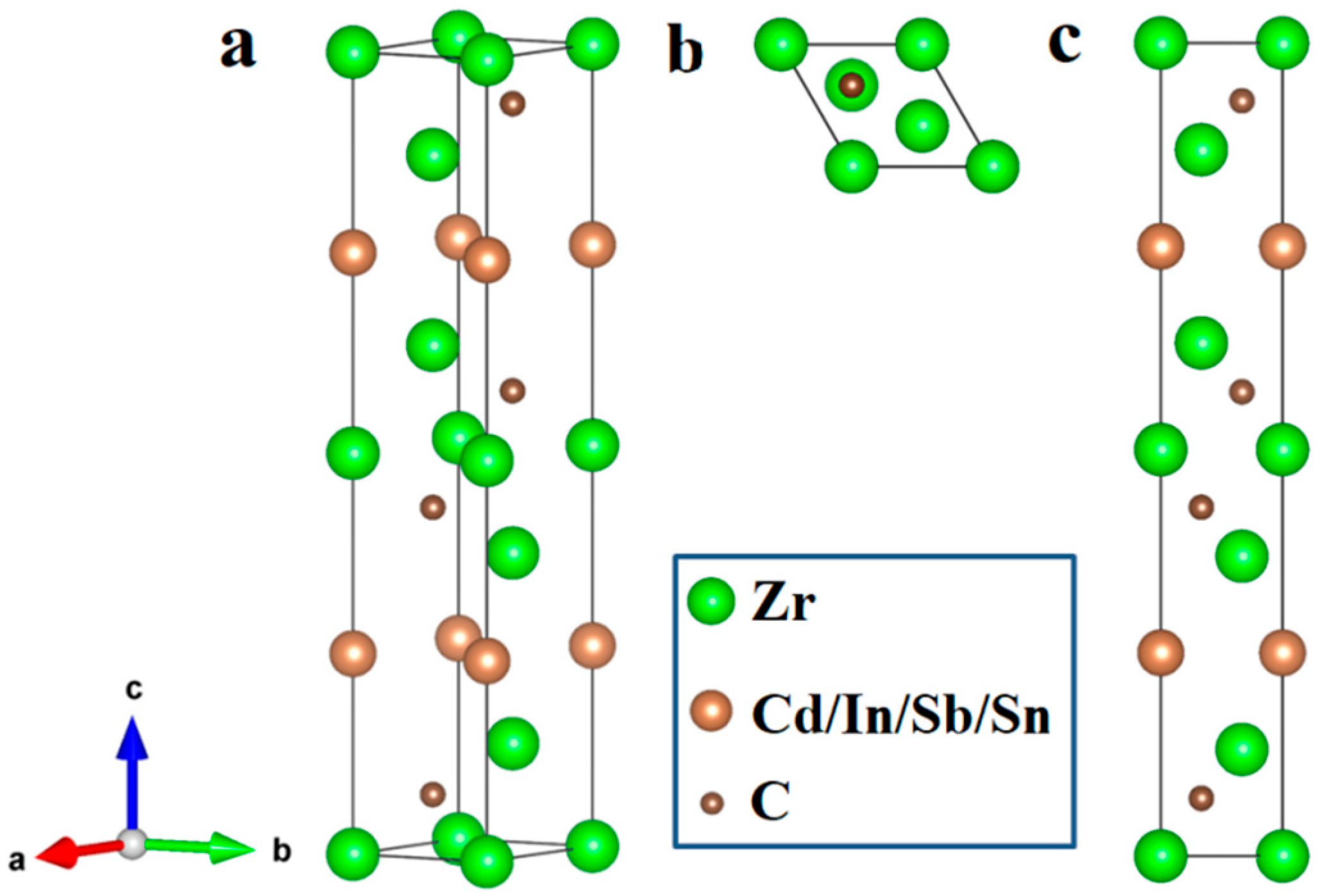
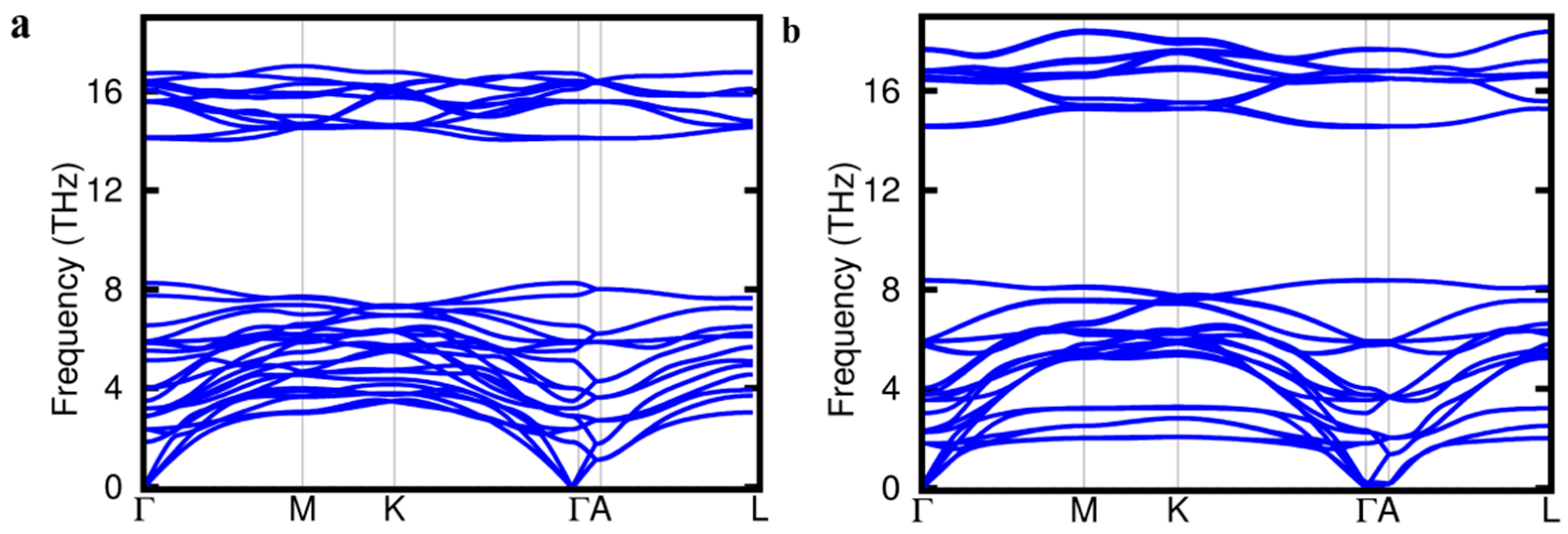
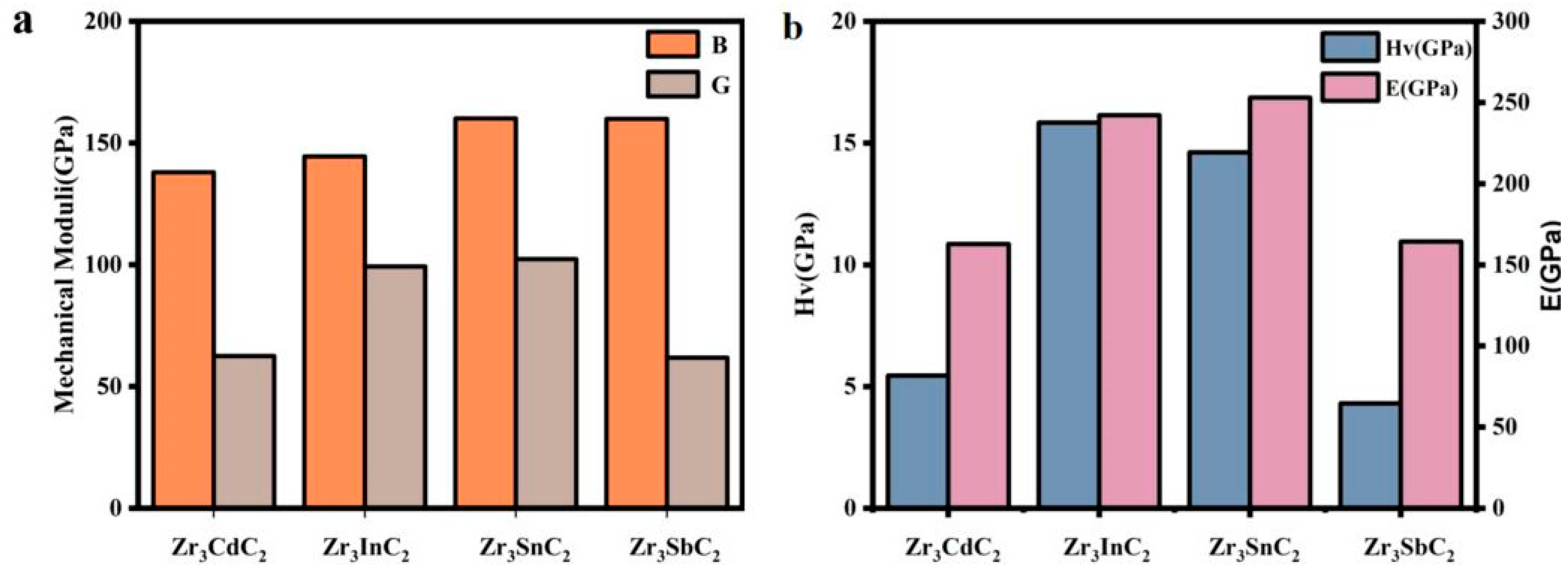
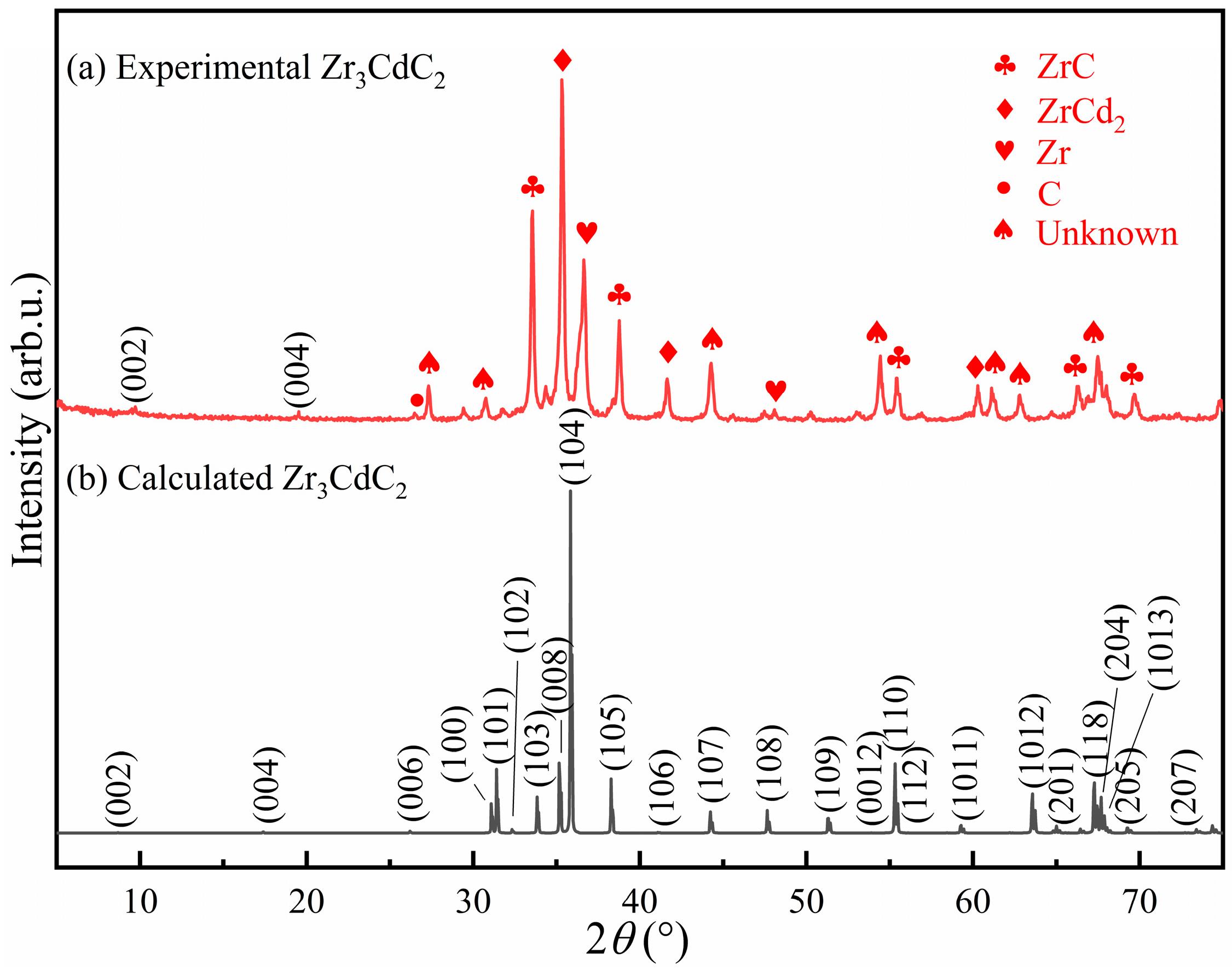
| Phase | a (Å) | c (Å) | ΔH (eV/atom) |
|---|---|---|---|
| Zr3CdC2 | 3.319 | 20.393 | −1.104 |
| Zr3InC2 | 3.337 3.352 [21] | 20.251 20.252 [21] | −1.220 |
| Zr3SnC2 | 3.348 3.359 [19] | 19.870 19.876 [19] | −1.282 |
| Zr3SbC2 | 3.367 | 19.413 | −1.306 |
| Phase | C11 (GPa) | C12 (GPa) | C33 (GPa) | C13 (GPa) | C44 (GPa) |
|---|---|---|---|---|---|
| Zr3CdC2 | 283.717 | 94.155 | 228.795 | 67.108 | 33.936 |
| Zr3InC2 | 310.372 | 81.969 | 248.679 | 68.846 | 84.965 |
| Zr3SnC2 | 286.005 | 109.654 | 290.242 | 89.750 | 118.166 |
| Zr3SbC2 | 239.549 | 108.369 | 252.822 | 126.183 | 62.190 |
| Phase | B (GPa) | G (GPa) | E (GPa) | B/G | HV (GPa) | Tm (K) | TD (K) |
|---|---|---|---|---|---|---|---|
| Zr3CdC2 | 137.942 | 62.477 | 162.846 | 2.208 | 5.448 | 1548.344 | 392.7 |
| Zr3InC2 | 144.437 | 95.251 | 242.262 | 1.455 | 15.842 | 1658.135 | 488.9 |
| Zr3SnC2 | 160.068 | 102.306 | 253.013 | 1.565 | 14.625 | 1647.378 | 493.7 |
| Zr3SbC2 | 159.913 | 61.810 | 164.265 | 2.587 | 4.309 | 1451.880 | 386.2 |
| Phase | Zr1-C Length (Å) | Zr1-C Population | Zr2-C Length (Å) | Zr2-C Population | ΔQZr1 | ΔQZr2 | ΔQC |
|---|---|---|---|---|---|---|---|
| Zr3CdC2 | 2.377 | 0.85 | 2.242 | 1.24 | 1.591 | 1.135 | −1.692 |
| Zr3InC2 | 2.383 | 0.87 | 2.245 | 1.24 | 1.592 | 1.111 | −1.698 |
| Zr3SnC2 | 2.380 | 0.87 | 2.260 | 1.23 | 1.578 | 0.948 | −1.577 |
| Zr3SbC2 | 2.384 | 0.88 | 2.268 | 1.24 | 1.642 | 1.016 | −1.700 |
| Element | At.% in Figure 6b | At.% in Figure 6d | At.% in Figure 6f |
|---|---|---|---|
| Zr | 75.67 | 75.31 | 75.38 |
| Cd | 24.33 | 24.69 | 24.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Zhang, F.; Wen, B.; Zhang, Q.; Chu, L.; Zhou, Y.; Feng, Q.; Hu, C. Theoretical Prediction and Experimental Synthesis of Zr3AC2 (A = Cd, Sb) Phases. Materials 2024, 17, 1556. https://doi.org/10.3390/ma17071556
Luo J, Zhang F, Wen B, Zhang Q, Chu L, Zhou Y, Feng Q, Hu C. Theoretical Prediction and Experimental Synthesis of Zr3AC2 (A = Cd, Sb) Phases. Materials. 2024; 17(7):1556. https://doi.org/10.3390/ma17071556
Chicago/Turabian StyleLuo, Jia, Fengjuan Zhang, Bo Wen, Qiqiang Zhang, Longsheng Chu, Yanchun Zhou, Qingguo Feng, and Chunfeng Hu. 2024. "Theoretical Prediction and Experimental Synthesis of Zr3AC2 (A = Cd, Sb) Phases" Materials 17, no. 7: 1556. https://doi.org/10.3390/ma17071556
APA StyleLuo, J., Zhang, F., Wen, B., Zhang, Q., Chu, L., Zhou, Y., Feng, Q., & Hu, C. (2024). Theoretical Prediction and Experimental Synthesis of Zr3AC2 (A = Cd, Sb) Phases. Materials, 17(7), 1556. https://doi.org/10.3390/ma17071556











