Research on the Properties of Steel Slag with Different Preparation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.2.1. Density Test
2.2.2. Volume Expansion Test
2.2.3. XRD, SEM, and TG Test
3. Results and Discussion
3.1. Density and Water Absorption
3.2. Volume Expansion
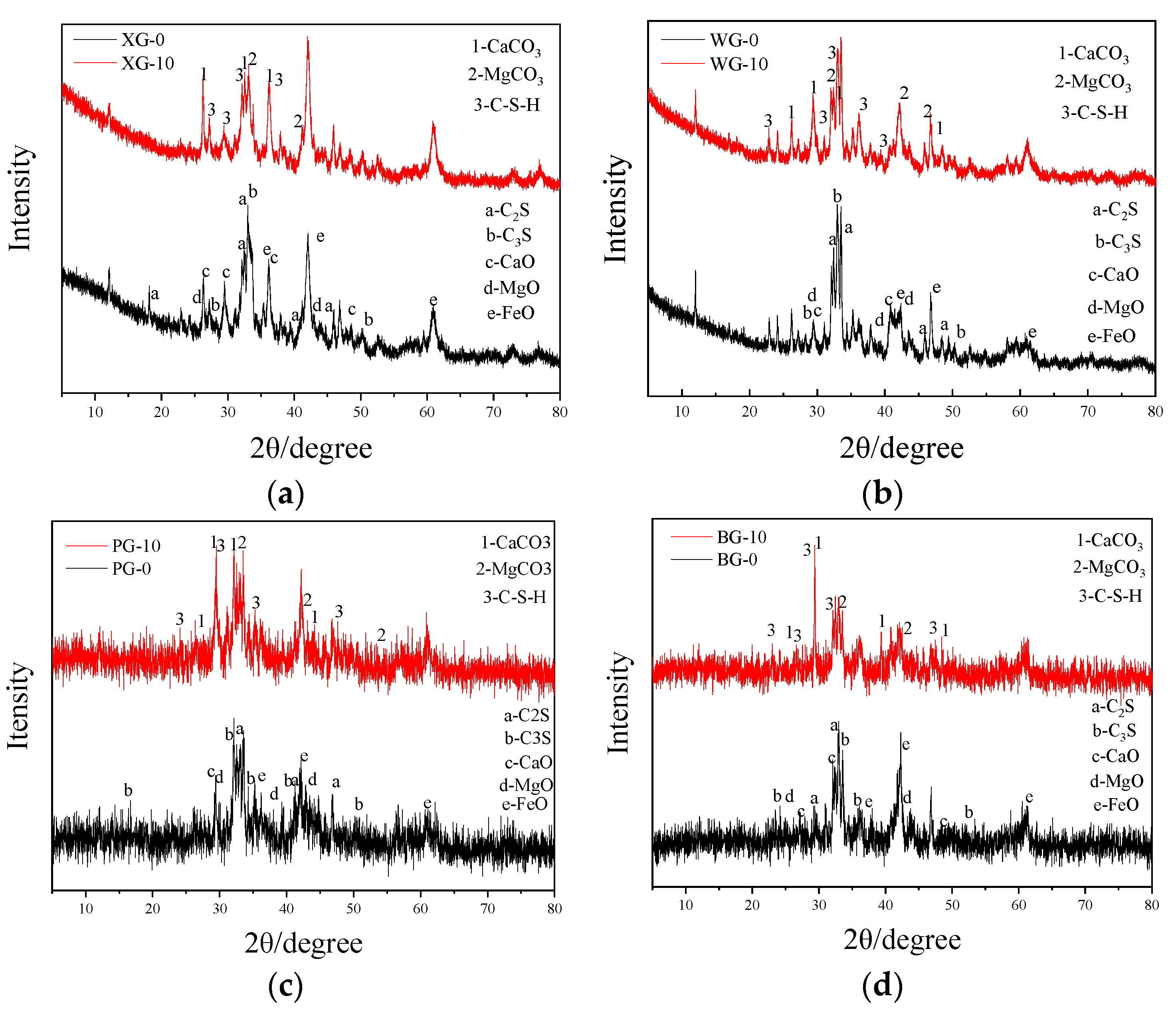
3.3. Mineral Changes
3.4. Simple Quantitative Analysis
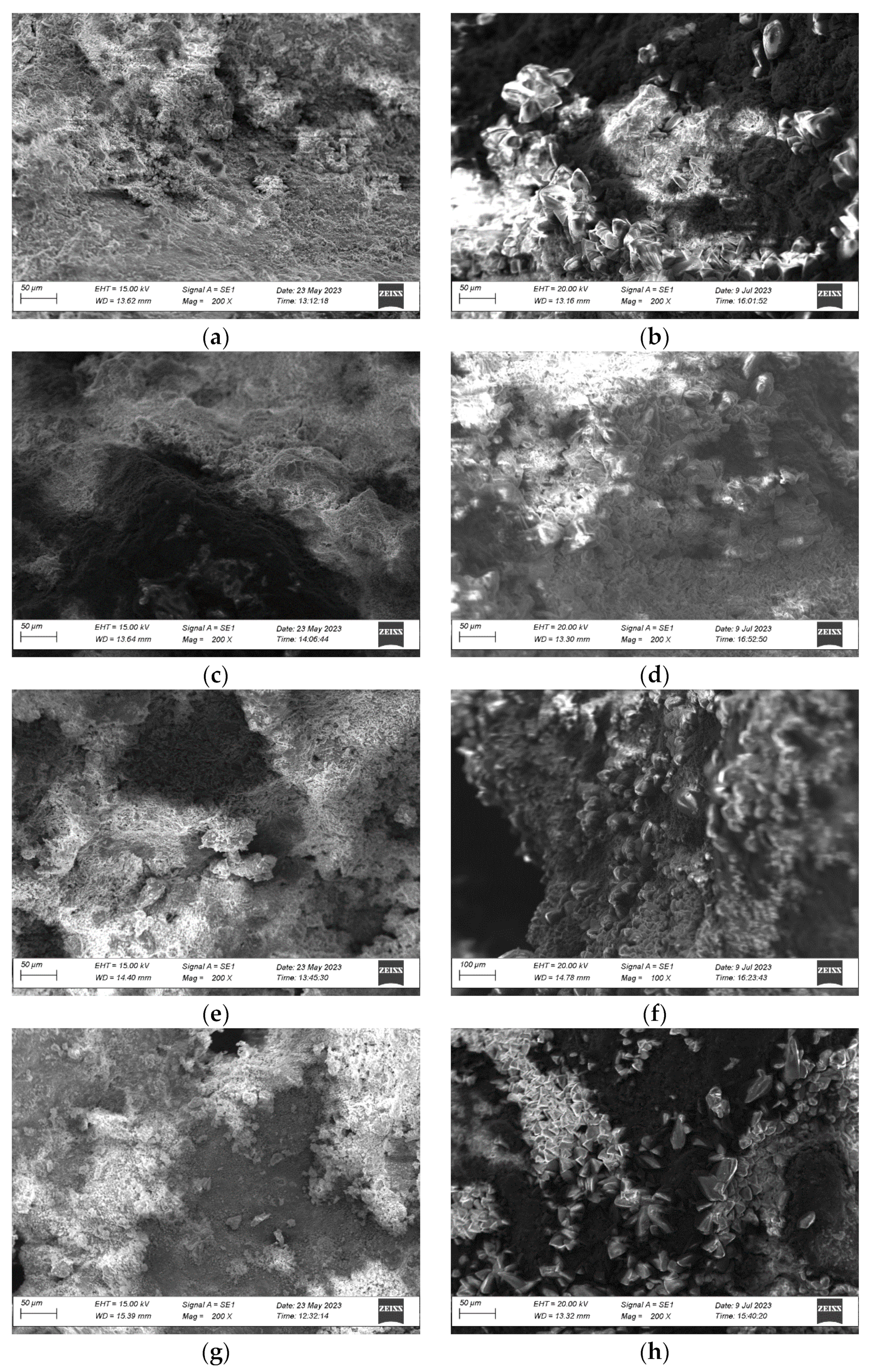
3.5. Microstructure Changes
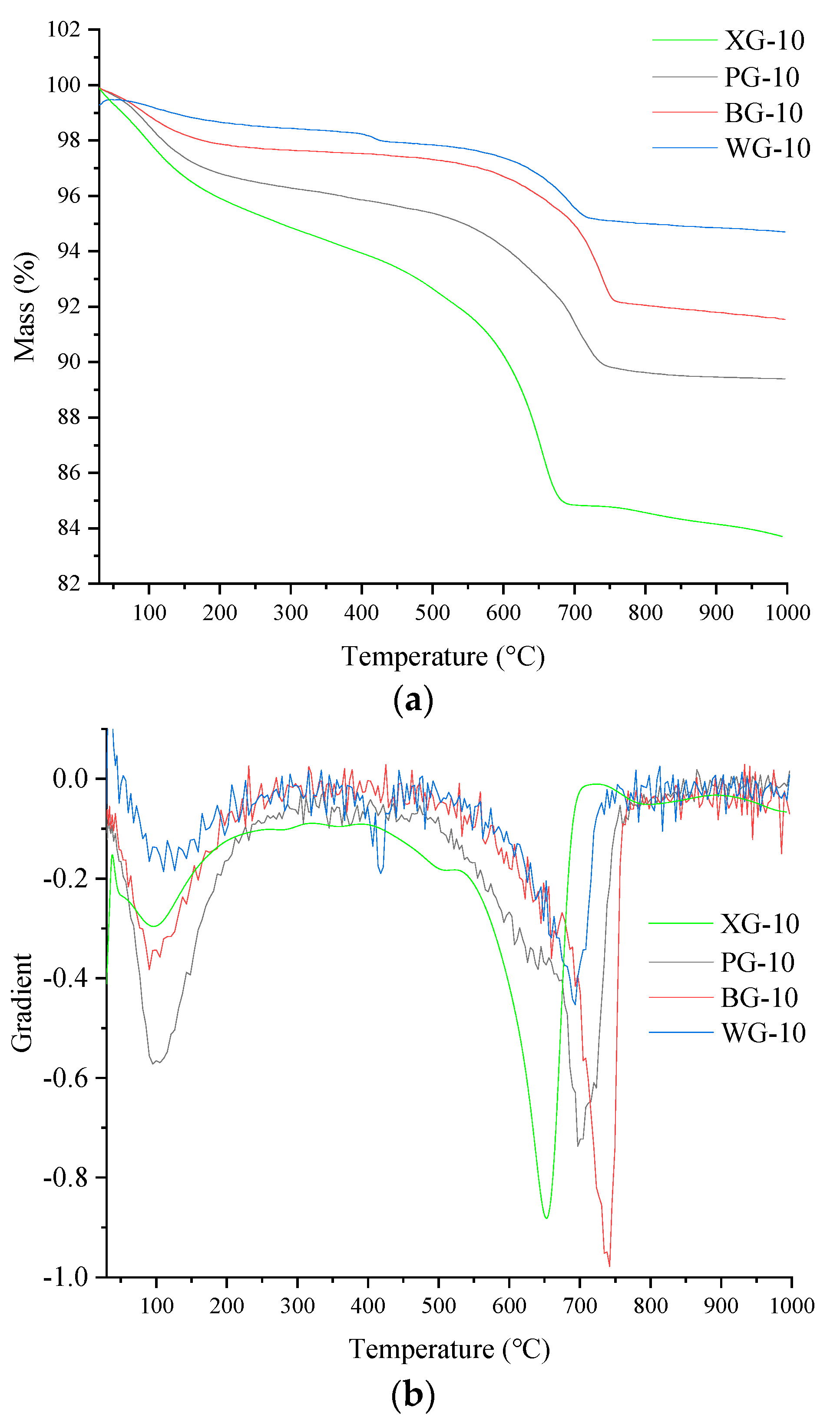
3.6. Thermogravimetric Analysis
4. Conclusions
- (1)
- The steel slag produced by the drum method has the highest density and lower expansion rate than the other three steel slags. It was also found that although BG has the highest content of free calcium oxide, the higher density of steel slag particles helps to resist the volume expansion caused by water contact.
- (2)
- The composition elements of steel slag produced by different processes are very similar, with the primary active materials being C2S, C3S, CaO, and MgO. After the expansion test, the main chemical products of steel slag are CaCO3, MgCO3, and C-S-H. C-S-H is generated by hydrolysis and a combination of C2S and C3S, and the content of CaO and MgO mainly influences the amount generated. This means a higher degree of alkalinity can promote the generation of C-S-H. Most C-S-H is generated on the surface of steel slag, forming clusters of mineral crystals.
- (3)
- The thermal stability of the two types of steel slag treated by the hot smothering method is relatively lower than that of steel slag treated by the hot splashing and drum methods. Additionally, it was found that the storage time may be related to the thermal stability of steel slag particles.
- (4)
- This research considers the density, volume stability, and thermal stability of the four types of steel slag. XG, PG, and BG are recommended as alternative coarse aggregates for road and building construction. Meanwhile, WG is unsuitable as a building material due to its high expansion, and further treatment should be carried out to reduce its expansion before testing. Among them, BG steel slag has the best comprehensive performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaopeng, W.; Peide, C.; Jun, X.; Quantao, L.; Ling, P. Expansive Inhibition Method of Steel Slag Aggregate and Volume Stability of Mixture: A Review. China J. Highw. Transp. 2021, 34, 166–179. [Google Scholar] [CrossRef]
- Roque, A.J.; Rodrigues, G.M.; da Silva, P.F. Re-cycling of construction and demolition waste and steel slag: Characterization of the durability. J. Mater. Cycles Waste Manag. 2020, 22, 1699–1711. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Tozsin, G.; Oztas, T. Utilization of Steel Slag as a Soil Amendment and Mineral Fertilizer in Agriculture: A Review. J. Agric. Sci. Tarim Bilim. Derg. 2023, 29, 906–913. [Google Scholar] [CrossRef]
- O’Connor, J.; Nguyen, T.B.T.; Honeyands, T.; Monaghan, B.; O’Dea, D.; Rinklebe, J.; Vinu, A.; Hoang, S.A.; Singh, G.; Kirkham, M.B.; et al. Production, characterisation, utilisation, and beneficial soil application of steel slag: A review. J. Hazard. Mater. 2021, 419, 126478. [Google Scholar] [CrossRef]
- Mal, U.; Adhikari, K.; Tripathi, A. Steel plant slag dumps: A potential source of groundwater contamination. J. Earth Syst. Sci. 2022, 131, 45. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, H.; Zhu, X.; Yao, D.; Peng, X.; Fan, X. Unified Characterization of Rubber Asphalt Mixture Strength under Different Stress Loading Paths. J. Mater. Civ. Eng. 2024, 36, 04023498. [Google Scholar] [CrossRef]
- Baalamurugan, J.; Kumar, V.G.; Padmapriya, R.; Raja, V.K.B. Recent applications of steel slag in construction industry. Environ. Dev. Sustain. 2024, 26, 2865–2896. [Google Scholar] [CrossRef]
- Masilamani, A.; Ramalingam, M.; Kathirvel, P.; Murali, G.; Vatin, N.I. Mechanical, Physico-Chemical and Morphological Characterization of Energy Optimised Furnace (EOF) Steel Slag as Coarse Aggregate in Concrete. Materials 2022, 15, 3079. [Google Scholar] [CrossRef] [PubMed]
- Edgar, M.; Hamdan, N.; Morales, D.; Boyer, T.H. Phosphorus removal by steel slag from tile drainage water: Lab and field evaluations. Chemosphere 2022, 307, 135850. [Google Scholar] [CrossRef] [PubMed]
- Angst, U.M. Steel corrosion in concrete-Achilles’ heel for sustainable concrete? Cem. Concr. Res. 2023, 172, 107239. [Google Scholar] [CrossRef]
- Stefanini, L.; Ghorbani, S.; De Schutter, G.; Matthys, S.; Walkley, B.; Provis, J.L. Evaluation of copper slag and stainless steel slag as replacements for blast furnace slag in binary and ternary alkali-activated cements. J. Mater. Sci. 2023, 58, 12537–12558. [Google Scholar] [CrossRef]
- Baalamurugan, J.; Kumar, V.G.; Prasad, B.; Padmapriya, R.; Karthick, V.; Govindaraju, K. Recycling of induction furnace steel slag in concrete for marine environmental applications towards ocean acidification studies. Int. J. Environ. Sci. Technol. 2022, 19, 5039–5048. [Google Scholar] [CrossRef]
- Zaibo, L.; Tusheng, H.; Xuguang, Z.; Sanyin, Z. Cementitious Activity Evaluation of Steel Slag by EDTA-NaOH Solution Extraction Method. Mater. Sci. Forum 2017, 893, 389–394. [Google Scholar]
- Ramezani, A.; Modaresi, S.; Dashti, P.; GivKashi, M.R.; Moodi, F.; Ramezanianpour, A.A. Effects of Different Types of Fibers on Fresh and Hardened Properties of Cement and Geopolymer-Based 3D Printed Mixtures: A Review. Buildings 2023, 13, 945. [Google Scholar] [CrossRef]
- Neubert, L.; Kovtun, O.; Kreschel, T.; Volkova, O. Phosphorus Partition Between Liquid Crude Steel and High-Basicity Basic Oxygen Furnace Slags Containing V2O5. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2023, 54, 1524–1531. [Google Scholar] [CrossRef]
- Gencel, O.; Karadag, O.; Oren, O.H.; Bilir, T. Steel slag and its applications in cement and concrete technology: A review. Constr. Build. Mater. 2021, 283, 122783. [Google Scholar] [CrossRef]
- Al-Saedi, M.; Sabbar, A.S. Treatment of Expansive Soils with Slag: A Review Study. Soil Mech. Found. Eng. 2024, 60, 574–580. [Google Scholar] [CrossRef]
- Devarangadi, M.; Shankar, M.U. Correlation studies on geotechnical properties of various industrial byproducts generated from thermal power plants, iron and steel industries as liners in a landfill-a detailed review. J. Clean. Prod. 2020, 261, 121207. [Google Scholar] [CrossRef]
- Yuan, S.; Xiao, H.; Wang, R.; Li, Y.; Gao, P. Improved iron recovery from low-grade iron ore by efficient suspension magnetization roasting and magnetic separation. Miner. Eng. 2022, 186, 107761. [Google Scholar] [CrossRef]
- Schneider, R.; Wiesinger, V.; Gelder, S.; Reiter, G. Effect of the Slag Composition on the Process Behavior, Energy Consumption, and Nonmetallic Inclusions during Electroslag Remelting. Steel Res. Int. 2023, 94, 2200483. [Google Scholar] [CrossRef]
- Chowdhury, S.R. Recycled Smelter Slags for In Situ and Ex Situ Water and Wastewater Treatment-Current Knowledge and Opportunities. Processes 2023, 11, 783. [Google Scholar] [CrossRef]
- Kurniati, E.O.; Pederson, F.; Kim, H.J. Application of steel slags, ferronickel slags, and copper mining waste as construction materials: A review. Resour. Conserv. Recycl. 2023, 198, 107175. [Google Scholar] [CrossRef]
- Deus, A.C.F.; Büll, L.T.; Guppy, C.N.; Santos, S.D.M.C.; Moreira, L.L.Q. Effects of lime and steel slag application on soil fertility and soybean yield under a no till-system. Soil Tillage Res. 2020, 196, 104422. [Google Scholar] [CrossRef]
- Singh, S.K.; Rekha, P.; Surya, M. Utilization of Linz-Donawitz slag from steel industry for waste minimization. J. Mater. Cycles Waste Manag. 2020, 22, 611–627. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Hajiaghamemar, M.; Mostofinejad, D.; Bahmani, H. A high-strength concrete resistant to elevated temperatures using steel slag aggregates. Struct. Concr. 2023, 24, 3162–3177. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
- Alex, T.C.; Mucsi, G.; Venugopalan, T.; Kumar, S. BOF Steel Slag: Critical Assessment and Integrated Approach for Utilization. J. Sustain. Metall. 2021, 7, 1407–1424. [Google Scholar] [CrossRef]
- Pathak, S.; Choudhary, R.; Kumar, A. Effect of Long-Term Binder Draindown on Performance of Open Graded Asphalt Friction Courses with BOF Steel Slag Aggregates. J. Mater. Civ. Eng. 2022, 34, 04021383. [Google Scholar] [CrossRef]
- Ghorbani, S.; Sun, Y.B.; Mohan, M.K.; Matthys, S. Effect of copper and stainless steel slags on fresh, mechanical and pore structure properties of alkali activated ground granulated blast furnace slag. Case Stud. Constr. Mater. 2023, 18, e01981. [Google Scholar] [CrossRef]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. Sustainable solutions for road pavements: A multi-scale characterization of warm mix asphalts containing steel slags. J. Clean. Prod. 2017, 166, 835–843. [Google Scholar] [CrossRef]
- Tozsin, G.; Yonar, F.; Yucel, O.; Dikbas, A. Utilization possibilities of steel slag as backfill material in coastal structures. Sci. Rep. 2023, 13, 4318. [Google Scholar] [CrossRef] [PubMed]
- Meshram, S.; Raut, S.P.; Ansari, K.; Madurwar, M.; Daniyal, M.; Khan, M.A.; Katare, V.; Khan, A.H.; Khan, N.A.; Hasan, M.A. Waste slags as sustainable construction materials: A compressive review on physico mechanical properties. J. Mater. Res. Technol. 2023, 23, 5821–5845. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.; Salleh, M.; Ming, L.Y.; Li, L.Y.; Sandu, A.V.; Vizureanu, P.; Nemes, O.; Mahdi, S.N. Recent Developments in Steelmaking Industry and Potential Alkali Activated Based Steel Waste: A Comprehensive Review. Materials 2022, 15, 1948. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. Return of results to the families of children in genomic sequencing: Tallying risks and benefits. Genet. Med. 2013, 15, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, S.; Hou, H.; Zha, J. Experimental investigation of basic oxygen furnace slag used as aggregate in asphalt mixture. J. Hazard. Mater. 2006, 138, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Varma, S. A review on utilization of steel slag in hot mix asphalt. Int. J. Pavement Res. Technol. 2020, 14, 232–242. [Google Scholar] [CrossRef]
- Rajesh, A.A.; Senthilkumar, S.; Samson, S. Optimal proportional combinations of rubber crumbs and steel slag for enhanced concrete split tensile strength. Mater. Rio De Jan. 2023, 28, e2023020. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Li, D.S. Application of Steel Slag as an Aggregate in Concrete Production: A Review. Materials 2023, 16, 5841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ling, T.-C.; Shi, C.; Pan, S.-Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Loureiro, C.D.A.; Moura, C.F.N.; Rodrigues, M.; Martinho, F.C.G.; Silva, H.; Oliveira, J.R.M. Steel Slag and Recycled Concrete Aggregates: Replacing Quarries to Supply Sustainable Materials for the Asphalt Paving Industry. Sustainability 2022, 14, 5022. [Google Scholar] [CrossRef]
- Liu, J.Z.; Xu, J.; Liu, Q.; Wang, S.Y.; Yu, B. Steel Slag for Roadway Construction: A Review of Material Characteristics and Application Mechanisms. J. Mater. Civ. Eng. 2022, 34, 03122001. [Google Scholar] [CrossRef]
- Nugmanova, A.; Shon, C.S.; Kim, J.R.; Rossi, C.O. Characterizing Chronologically Aged Basic Oxygen Furnace Slags as Aggregates and Their Use in Asphalt Concrete Mix as Filler. Appl. Sci. 2023, 13, 10126. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Li, S. Research and Application of Drum Method Stainless Steel Slag Treatment Technology. Baosteel Technol. 2020, 73–77. Available online: https://kns.cnki.net/kcms2/article/abstract?v=1fhXvtqifPLIRZfH5frPT9ewWMGkVTVocbaRZQHn5UMlDUW6v_5au5Pa3uaUa_Ncd7FxfLHmoLesMPaJh4bRsNq65Uv76SewK8XcqVCcEEN8tS7kEF60TQt6dCABqCR9JsQJUNlyiQNqeskM2jehAA==&uniplatform=NZKPT&language=CHS (accessed on 15 January 2024).
- Dilbas, H. Optimizing the Treatment of Recycled Aggregate (>4 mm), Artificial Intelligence and Analytical Approaches. Materials 2023, 16, 2994. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, Y.; Zhang, Z.; Ma, Z.; Gao, F.; Xue, Q.; Xu, Z. Effect of acid-activation on CaO existential state and reactive properties of hot-splashed steel slag in cement-based materials. Struct. Concr. 2022, 23, 3819–3833. [Google Scholar] [CrossRef]
- Teo, P.T.; Zakaria, S.K.; Salleh, S.Z.; Taib, M.A.A.; Sharif, N.M.; Abu Seman, A.; Mohamed, J.J.; Yusoff, M.; Yusoff, A.H.; Mohamad, M.; et al. Assessment of Electric Arc Furnace (EAF) Steel Slag Waste’s Recycling Options into Value Added Green Products: A Review. Metals 2020, 10, 1347. [Google Scholar] [CrossRef]
- JTG E42-2005; Test Code for Aggregate in Highway Engineering. Ministry of Communications Highway Science Research Institute: Beijing, China, 2005.
- GB/T 24765-2009; Steel Slag for Wearing Asphalt Pave. China Iron and Steel Association: Beijing, China, 2010.
- Fu, Q.; Xue, G.; Xu, S.; Li, J.J.; Dong, W. Mechanical performance, microstructure, and damage model of concrete containing steel slag aggregate. Struct. Concr. 2023, 24, 2189–2207. [Google Scholar] [CrossRef]
- Hussain, A.; Hussaini, S.K.K. Use of steel slag as railway ballast: A review. Transp. Geotech. 2022, 35, 100779. [Google Scholar] [CrossRef]
- Hassan, K.E.; Attia, M.I.E.; Reid, M.; Al-Kuwari, M.B.S. Performance of steel slag aggregate in asphalt mixtures in a hot desert climate. Case Stud. Constr. Mater. 2021, 14, e00534. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, Q. Steel Slag Treatment and Resource Utilization; Metallurgical Industry Press: Beijing, China, 2015. [Google Scholar]
- Kang, H.; Lee, Y.; Lee, J.; Moon, J. Importance of amorphous content, surface energy, and preferred orientation on the accurate quantification of cement minerals in clinkers. J. Build. Eng. 2023, 66, 105887. [Google Scholar] [CrossRef]
- Xiaogang, Z. Synthesis, Composition, Structure, and Morphology of Hydrated Calcium Silicate. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2010. [Google Scholar]
- Zhang, H. Preparation and Performance Study of Cement-Based Active Powder Concrete for 3D Printing; North University of China: Taiyuan, China, 2020. [Google Scholar] [CrossRef]
- Yao, D.; Yu, H.A.; Chen, X.; Yu, X.L.; Yao, J.L.; Zheng, X.G.; Zhang, C.; Gong, L.J. Effect of surface modification on properties of steel slag aggregate and mixture. Constr. Build. Mater. 2023, 402, 133058. [Google Scholar] [CrossRef]
- Kaya, G.G.; Yilmaz, E.; Deveci, H. Synthesis of sustainable silica xerogels/aerogels using inexpensive steel slag and bean pod ash: A comparison study. Adv. Powder Technol. 2020, 31, 926–936. [Google Scholar] [CrossRef]


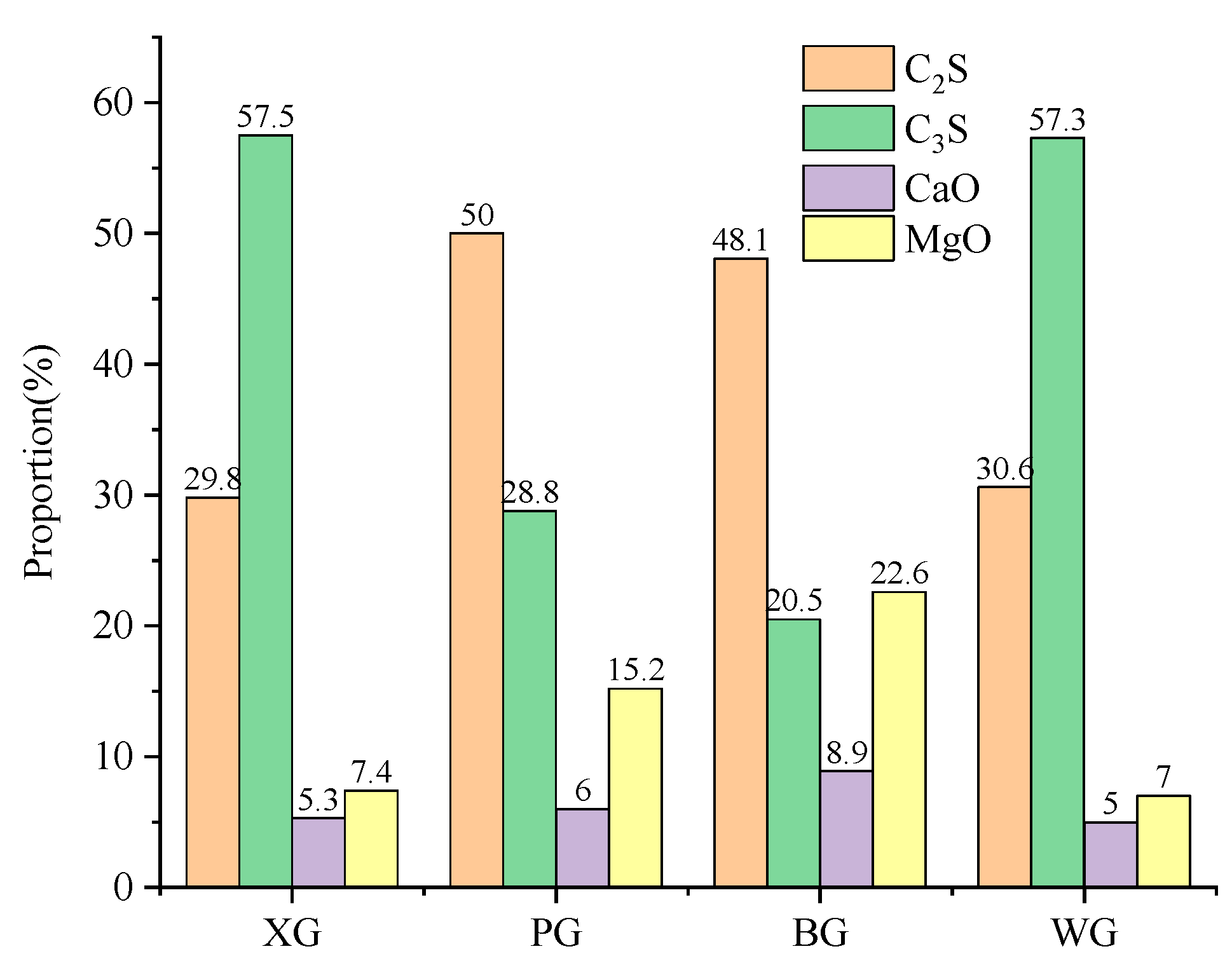
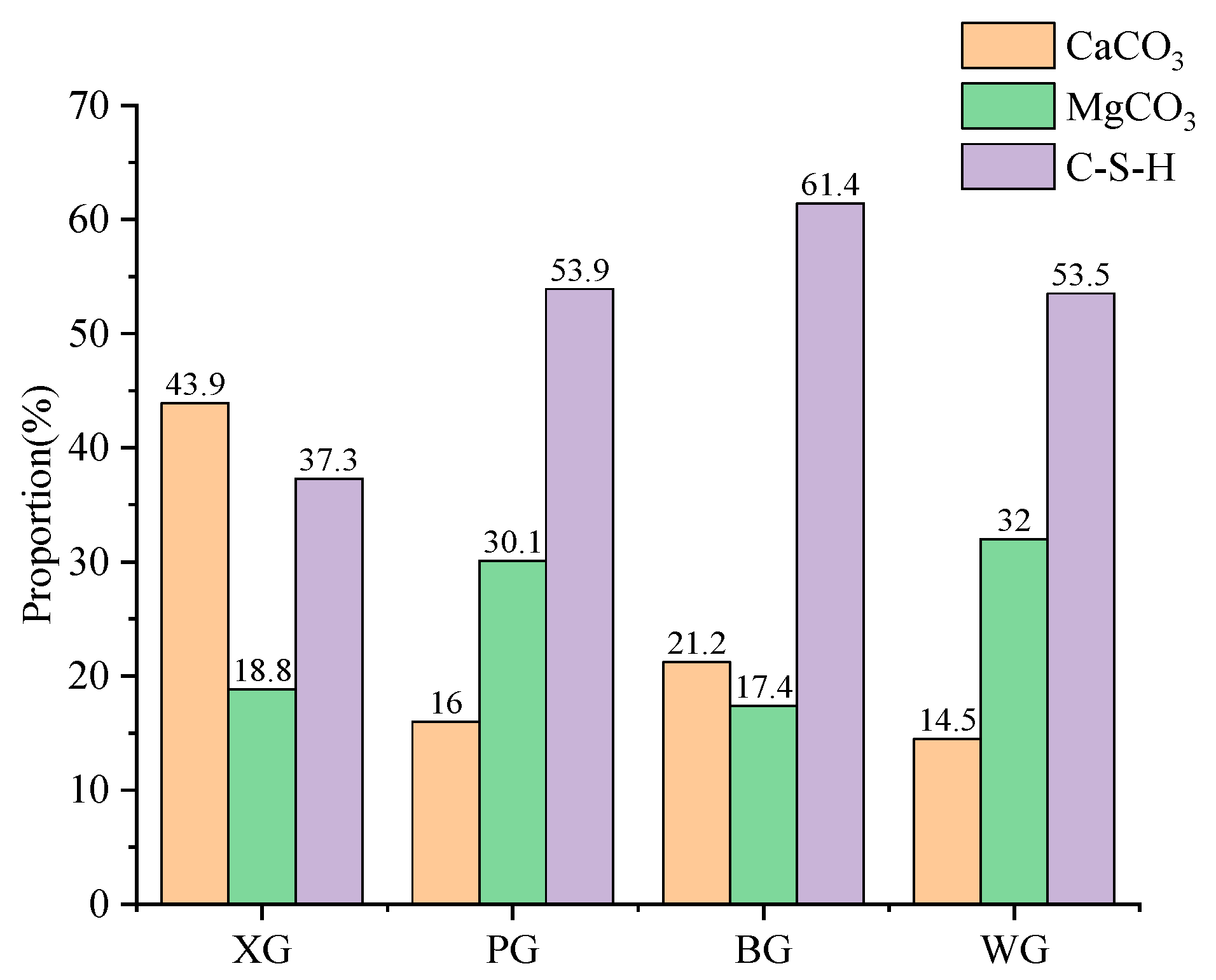

| Active Ingredients | Application | Advantages and Disadvantages |
|---|---|---|
| C2S, C3S, C4AF, and C3A | Replace natural aggregate in concrete | Good durability. Poor water stability |
| C3S and β-C2S | Steel slag powder as supplementary cementitious materials | Improve mechanical properties and durability. Potential expansion problem |
| CaCO3, C2S and C3S | Replace natural aggregate in asphalt mixture | Higher mechanical behavior of mixtures with steel slag aggregate. But require a higher asphalt content |
| CaO (%) | Fe2O3 (%) | SiO2 (%) | MgO (%) | MnO (%) | Al2O3 (%) | P2O5 (%) | TiO2 (%) | Cr2O3 (%) | SO3 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| BG | 40.80 | 33.51 | 13.59 | 4.39 | 2.91 | 1.99 | 0.99 | 0.75 | 0.39 | 0.23 |
| WG | 45.33 | 31.63 | 10.46 | 4.37 | 3.32 | 1.51 | 1.38 | 0.99 | 0.43 | 0.10 |
| PG | 46.13 | 29.85 | 11.66 | 4.08 | 3.65 | 1.39 | 1.33 | 0.93 | 0.49 | 0.18 |
| XG | 43.36 | 26.53 | 13.02 | 3.92 | 5.15 | 0.87 | 3.68 | 1.37 | 0.63 | 0.74 |
| Types | Parameter | 4.75–9.5 mm | 9.5–13.2 mm | 13.2–16 mm |
|---|---|---|---|---|
| XG | Density (g/cm3) | 3.498 | 3.393 | 3.377 |
| Apparent density (g/cm3) | 3.281 | 3.192 | 3.224 | |
| Water absorption (%) | 1.89% | 1.85% | 1.41% | |
| PG | Density (g/cm3) | 3.488 | 3.437 | 3.110 |
| Apparent density (g/cm3) | 3.321 | 3.333 | 2.865 | |
| Water absorption (%) | 1.45% | 0.90% | 2.75% | |
| BG | Density (g/cm3) | 3.579 | 3.628 | 3.628 |
| Apparent density (g/cm3) | 3.351 | 3.456 | 3.450 | |
| Water absorption (%) | 1.90% | 1.38% | 1.42% | |
| WG | Density (g/cm3) | 3.523 | 3.478 | 3.512 |
| Apparent density (g/cm3) | 3.327 | 3.343 | 3.387 | |
| Water absorption (%) | 1.67% | 1.16% | 1.05% |
| Temperature (°C) | XG (%) | PG (%) | BG (%) | WG (%) |
|---|---|---|---|---|
| 100–200 | 1.927 | 1.228 | 0.953 | 0.581 |
| 400–500 | 1.199 | 0.264 | −0.013 | 0.337 |
| 600–750 | 4.216 | 2.674 | 2.314 | 1.791 |
| Temperature (°C) | XG (%) | PG (%) | BG (%) | WG (%) |
|---|---|---|---|---|
| 100–200 | 2.035 | 1.736 | 1.015 | 0.589 |
| 400–500 | 1.289 | 0.498 | 0.223 | 0.398 |
| 600–750 | 5.467 | 4.336 | 4.268 | 2.273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, C.; Yu, H.; Qian, G.; Zheng, X.; Zhou, H.; Huang, L.; Zhang, F.; Zhong, Y. Research on the Properties of Steel Slag with Different Preparation Processes. Materials 2024, 17, 1555. https://doi.org/10.3390/ma17071555
Liu X, Zhang C, Yu H, Qian G, Zheng X, Zhou H, Huang L, Zhang F, Zhong Y. Research on the Properties of Steel Slag with Different Preparation Processes. Materials. 2024; 17(7):1555. https://doi.org/10.3390/ma17071555
Chicago/Turabian StyleLiu, Xingbei, Chao Zhang, Huanan Yu, Guoping Qian, Xiaoguang Zheng, Hongyu Zhou, Lizhang Huang, Feng Zhang, and Yixiong Zhong. 2024. "Research on the Properties of Steel Slag with Different Preparation Processes" Materials 17, no. 7: 1555. https://doi.org/10.3390/ma17071555
APA StyleLiu, X., Zhang, C., Yu, H., Qian, G., Zheng, X., Zhou, H., Huang, L., Zhang, F., & Zhong, Y. (2024). Research on the Properties of Steel Slag with Different Preparation Processes. Materials, 17(7), 1555. https://doi.org/10.3390/ma17071555






