Effect of Dentin Irrigants on Push-Out Bond Strength in Resin Cementation Protocols for Fiber Posts in Endodontically Treated Teeth: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cementation and Polymerization Protocols
- -
- Distilled water (Dw) (Groups: 1, 2, 3) as control.
- -
- CanalPro™ EDTA 17% (EDTA) (Coltène/Whaledent AG, Altstätten, Switzerland) (Groups: 4, 5, 6).
- -
- NaOCl 5% (NaOCl) (Panreac Química SLU, Barcelona, Spain) (Groups: 7, 8, 9).
- -
- Chlorhexidine digluconate 2% in distilled water (CHX) (Groups: 10, 11, 12).
- −
- Etch-rinse adhesive (Groups: 1, 4, 7, 10): 37% orthophosphoric acid (Oa) (Ivoclar Vivadent AG, Schaan, Liechtenstein) was applied in the post space for 15 s, rinsing with distilled water (15 s) using a Canal Pro syringe with Slotted-End Tips, 27 ga. (Coltène/Whaledent AG, Altstätten, Switzerland), aspiration of the water in post space with a suction tip (Roeko surgitip-endo 0.35 mm, Coltène/Whaledent AG, Altstätten, Switzerland), and drying using standardized absorbent paper points #60 (Coltène/Whaledent GmbH, Langenau, Germany). The corresponding irrigant was applied and removed as previously explained. Subsequently, the adhesive system was applied: Parabond® Primer A and Primer B (PAB) (Coltène/Whaledent AG, Altstätten, Switzerland) was actively applied to the dentin surface of the post space for 30 s. A disposable mixing well (Dentsply DeTrey GmbH, Konstanz, Germany) and a microapplicator (Root Canal Applicator Tip, Dentsply DeTrey GmbH, Konstanz, Germany) were used. After the application time, excess adhesive was removed with standardized absorbent paper points #60 (Coltène/Whaledent GmbH, Langenau, Germany), and the area was air-dried using the syringe for two seconds. The combination of paper points and air was effective in removing residual water or solvent from the post space [34]. Cementation: The resin cement ParaCore® (PC) (Coltène/Whaledent AG, Altstätten, Switzerland) was directly applied to the post space using its own dispensing and mixing syringe. The applicator tip was placed at the base to fill the entire receiving space of the post. As the cement was extruded, the tip was withdrawn until it exited the post space.
- −
- Two-step self-etch adhesive (Groups: 2, 5, 8, 11): Application of the irrigant was performed as described in groups 1, 4, 7, and 10 followed by the etchant conditioning. It was performed by actively applying ParaBond® Non-Rinse Conditioner (Coltène/Whaledent AG, Altstätten, Switzerland) (PNR) for 30 s using a microapplicator (Root Canal Applicator Tip, Dentsply DeTrey GmbH, Konstanz, Germany). After the application time, excess was removed with standardized absorbent paper points #60 (Coltène/Whaledent GmbH, Langenau, Germany), and the area was air-dried using the syringe for two seconds. Subsequently, the adhesive system was applied: Parabond® Primer A and Primer B (PAB) (Coltène/Whaledent AG, Altstätten, Switzerland) was applied as described in groups 1, 4, 7, and 10. Cementation: ParaCore® resin cement (PC) (Coltène/Whaledent AG, Altstätten, Switzerland) was applied directly to the post space as described in groups 1, 4, 7, and 10.
- −
- Universal adhesive (Groups: 3, 6, 9, 12): Application of the corresponding irrigant was performed as described in groups 1, 4, 7, and 10 followed by the self-etch adhesive ClearfilTM Universal Bond (CUB) (Kuraray Noritake Dental Inc., Okayama, Japan). It was actively applied to the dentin surface of the post space for 10 s using a microapplicator (Root Canal Applicator Tip, Dentsply DeTrey GmbH, Konstanz, Germany). Excess adhesive and solvent were removed with standardized absorbent paper points #60 (Coltène/Whaledent GmbH, Langenau, Germany), and the area was air-dried using the syringe for five seconds. Photopolymerization of the universal dentin adhesive was performed for five seconds at 1600 mW/cm2 using the LED unit S.P.E.C. 3 (Coltène/Whaledent GmbH, Langenau, Germany). Cementation: The resin cement ClearfilTM DC Core Plus (CCP) (Kuraray Noritake Dental Inc., Okayama, Japan) was directly applied to the post space using its own dispensing and mixing syringe. The applicator tip was placed at the base to fill the entire receiving space of the post. As the cement was extruded, the tip was withdrawn until it exited the post space.
2.2. Push-Out Test
3. Results
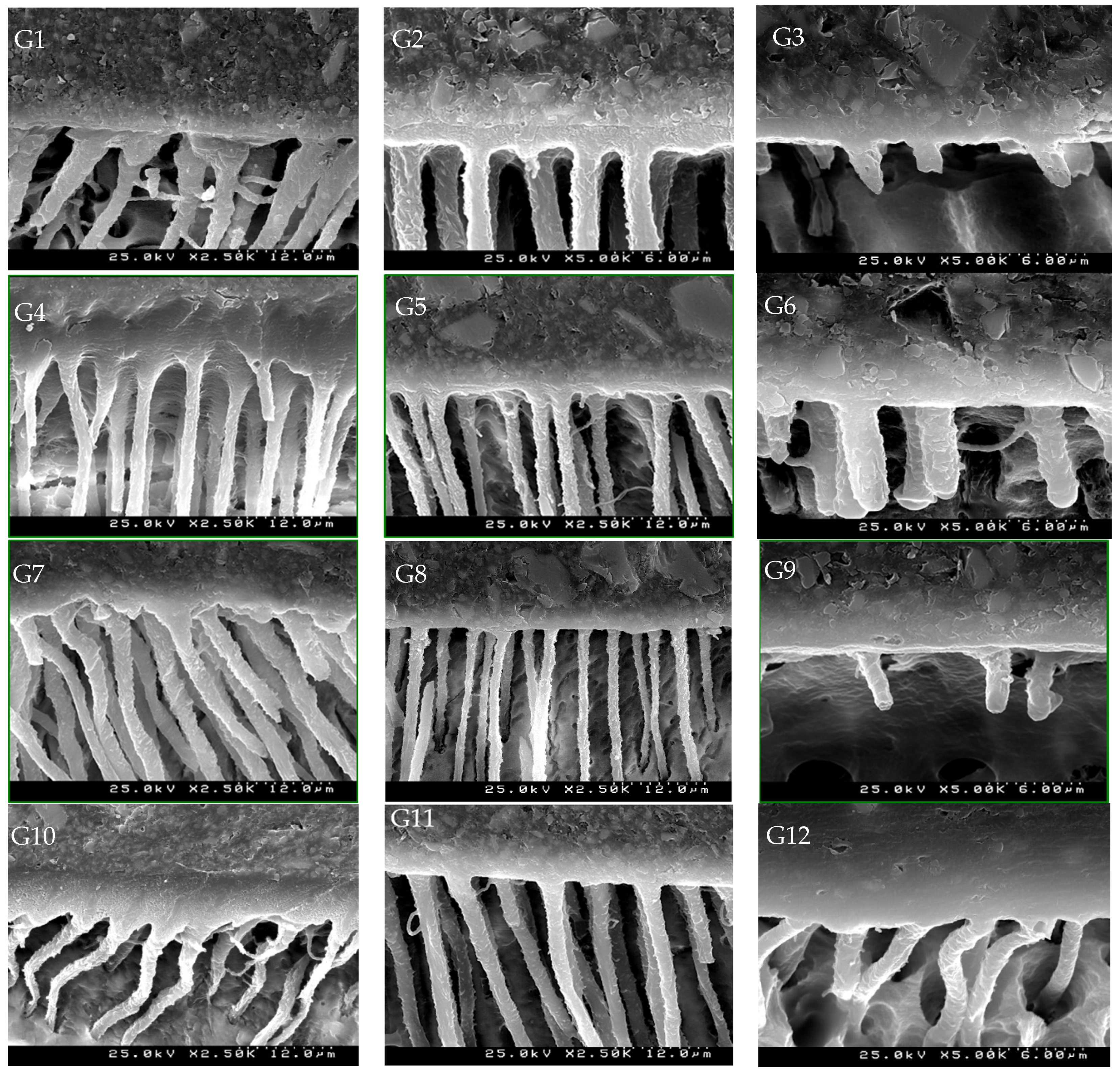
SEM Images
4. Discussion
5. Study Limitations
- −
- Limited scope regarding newer materials: this study focuses on specific irrigants and adhesive systems.
- −
- Short-term analysis: this study assesses bond strength shortly after cementation without considering long-term durability.
- −
- Lack of diversity in tooth selection: using only single-rooted, unrestored human teeth may limit the applicability of the results to a broader range of clinical scenarios.
- −
- Potential for bias in sample preparation: the methodology relies on manual processes, such as the application of adhesives and the preparation of teeth, which could introduce variability affecting this study’s outcomes.
6. Conclusions
- −
- 5% NaOCl for one minute significantly reduces adhesive strength compared to the other irrigants.
- −
- 5% NaOCl for one minute significantly reduces the adhesive strength of ClearfilTM Universal Bond.
- −
- There were no significant differences in adhesion between the three adhesive procedures studied.
- −
- There were no significant differences in adhesion between the irrigants: distilled water, EDTA 17%, and chlorhexidine digluconate 2% groups.
- −
- There were no significant differences in adhesion among the coronal, middle, and apical regions of the post space.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, R.S.; Robbins, J.W. Post Placement and Restoration of Endodontically Treated Teeth: A Literature Review. J. Endod. 2004, 30, 289–301. [Google Scholar] [CrossRef]
- Hayashi, M.; Takahashi, Y.; Hirai, M.; Iwami, Y.; Imazato, S.; Ebisu, S. Effect of Endodontic Irrigation on Bonding of Resin Cement to Radicular Dentin. Eur. J. Oral Sci. 2005, 113, 70–76. [Google Scholar] [CrossRef]
- Salameh, Z.; Sorrentino, R.; Papacchini, F.; Ounsi, H.F.; Tashkandi, E.; Goracci, C.; Ferrari, M. Fracture Resistance and Failure Patterns of Endodontically Treated Mandibular Molars Restored Using Resin Composite with or without Translucent Glass Fiber Posts. J. Endod. 2006, 32, 752–755. [Google Scholar] [CrossRef]
- Barros, A.P.O.; de Melo Alencar, C.; Zambon, M.; de Andrade, M.F.; Fernández, E.; Kuga, M.C. Etch-and-Rinse versus Self-Etch Strategy of a Universal Adhesive in Different Application Methods at the Bonding Interface of Fiber Post Cementation. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. Al 2023, 35, 1249–1256. [Google Scholar] [CrossRef]
- Ferrari, M.; Mannocci, F.; Vichi, A.; Cagidiaco, M.C.; Mjör, I.A. Bonding to Root Canal: Structural Characteristics of the Substrate. Am. J. Dent. 2000, 13, 255–260. [Google Scholar]
- Varela, S.G.; Rábade, L.B.; Lombardero, P.R.; Sixto, J.M.L.; Bahillo, J.D.G.; Park, S.A. In Vitro Study of Endodontic Post Cementation Protocols That Use Resin Cements. J. Prosthet. Dent. 2003, 89, 146–153. [Google Scholar] [CrossRef]
- Geerts, S.; Bolette, A.; Seidel, L.; Guéders, A. An In Vitro Evaluation of Leakage of Two Etch and Rinse and Two Self-Etch Adhesives after Thermocycling. Int. J. Dent. 2012, 2012, 852841. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H.C. Immediate Bonding Properties of Universal Adhesives to Dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef]
- Mortazavi, V.; Khademi, A.; Khosravi, K.; Fathi, M.; Ebrahimi-Chaharom, M.; Shahnaseri, S.; Khalighinejad, N.; Badrian, H. Effect of MTAD on the Shear Bond Strength of Self-Etch Adhesives to Dentin. Dent. Res. J. 2012, 9, 24–30. [Google Scholar] [CrossRef]
- Brackett, W.W.; Tay, F.R.; Brackett, M.G.; Dib, A.; Sword, R.J.; Pashley, D.H. The Effect of Chlorhexidine on Dentin Hybrid Layers In Vivo. Oper. Dent. 2007, 32, 107–111. [Google Scholar] [CrossRef]
- Dionysopoulos, D. Effect of Digluconate Chlorhexidine on Bond Strength between Dental Adhesive Systems and Dentin: A Systematic Review. J. Conserv. Dent. JCD 2016, 19, 11–16. [Google Scholar] [CrossRef]
- Oh, S.; Jung, H.-S.; Kim, H.-J.; Jang, J.-H.; Kim, D.-S.; Choi, K.-K.; Kim, S.-Y. Effect of Zinc on the Collagen Degradation in Acid-Etched Dentin. J. Dent. Sci. 2018, 13, 97–102. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental Adhesion Review: Aging and Stability of the Bonded Interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Al-Ammar, A.; Drummond, J.L.; Bedran-Russo, A.K. The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 419–424. [Google Scholar] [CrossRef]
- Gilberto, H.H. Adhesión En Odontología Conservadora, 2nd ed.; Ripano Editorial Médica: Madrid, Spain, 2010. [Google Scholar]
- Mao, H.; Chen, Y.; Yip, K.H.-K.; Smales, R.J. Effect of Three Radicular Dentine Treatments and Two Luting Cements on the Regional Bond Strength of Quartz Fibre Posts. Clin. Oral Investig. 2011, 15, 869–878. [Google Scholar] [CrossRef]
- Akman, M.; Eldeniz, A.U.; Ince, S.; Guneser, M.B. Push-out Bond Strength of a New Post System after Various Post Space Treatments. Dent. Mater. J. 2016, 35, 876–880. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Comba, A.; Cadenaro, M.; Radovic, I.; Sebold, M.; Turco, G.; Breschi, L.; Mazzoni, A. The Effect of Carbodiimide on Push-out Bond Strength of Fiber Posts and Endogenous Enzymatic Activity. BMC Oral Health 2023, 23, 399. [Google Scholar] [CrossRef]
- Osorio, R.; Erhardt, M.C.G.; Pimenta, L.a.F.; Osorio, E.; Toledano, M. EDTA Treatment Improves Resin-Dentin Bonds’ Resistance to Degradation. J. Dent. Res. 2005, 84, 736–740. [Google Scholar] [CrossRef]
- Olszowski, S.; Mak, P.; Olszowska, E.; Marcinkiewicz, J. Collagen Type II Modification by Hypochlorite. Acta Biochim. Pol. 2003, 50, 471–479. [Google Scholar] [CrossRef]
- Kasraei, S.; Azarsina, M.; Khamverdi, Z. Effect of Ethylene Diamine Tetra Acetic Acid and Sodium Hypochlorite Solution Conditioning on Microtensile Bond Strength of One-Step Self-Etch Adhesives. J. Conserv. Dent. JCD 2013, 16, 243–246. [Google Scholar] [CrossRef]
- Kim, J.; Uchiyama, T.; Carrilho, M.; Agee, K.A.; Mazzoni, A.; Breschi, L.; Carvalho, R.M.; Tjäderhane, L.; Looney, S.; Wimmer, C.; et al. Chlorhexidine Binding to Mineralized versus Demineralized Dentin Powder. Dent. Mater. 2010, 26, 771–778. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.S.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing Dentin Bond Durability: Control of Collagen Degradation by Matrix Metalloproteinases and Cysteine Cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Ou, Q.; Hu, Y.; Yao, S.; Wang, Y.; Lin, X. Effect of Matrix Metalloproteinase 8 Inhibitor on Resin-Dentin Bonds. Dent. Mater. 2018, 34, 756–763. [Google Scholar] [CrossRef]
- Tarazona, J.A.F.; Rivera, Z.J.T. Influence of the Application of Chlorhexidine at 2% in the Adhesive Protocol on the Tensile Bond Strength of Glass Fiber Post. Int. J. Dent. Res. 2018, 3, 55–58. [Google Scholar] [CrossRef]
- Bitter, K.; Gläser, C.; Neumann, K.; Blunck, U.; Frankenberger, R. Analysis of Resin-Dentin Interface Morphology and Bond Strength Evaluation of Core Materials for One Stage Post-Endodontic Restorations. PLoS ONE 2014, 9, e86294. [Google Scholar] [CrossRef]
- Alaghemand, H.; Mirzae, M.; Ahmadi, E.; Saidi, A. Effect of Different Post-Space Pretreatments on Fiber Post Bonding to Root Dentine. Dent. Res. J. 2013, 10, 545–552. [Google Scholar]
- Mendoza, D.B.; Eakle, W.S. Retention of Posts Cemented with Various Dentinal Bonding Cements. J. Prosthet. Dent. 1994, 72, 591–594. [Google Scholar] [CrossRef]
- Machado, M.B.M.; Alves Morgan, L.F.D.S.; Gomes, G.M.; Vasconcellos, W.A.; Cardoso, F.P.; Albuquerque, R.d.C. Effects of Immediate and Delayed Intraradicular Preparation on Bond Strength of Fiber Posts. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2015, 26, 244–247. [Google Scholar] [CrossRef]
- Ertas, H.; Ok, E.; Uysal, B.; Arslan, H. Effects of Different Irrigating Solutions and Disinfection Methods on Push-out Bond Strengths of Fiber Posts. Acta Odontol. Scand. 2014, 72, 783–787. [Google Scholar] [CrossRef]
- Mohsen, C.A. Evaluation of Push-out Bond Strength of Surface Treatments of Two Esthetic Posts. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2012, 23, 596–602. [Google Scholar] [CrossRef]
- Shori, D.; Pandey, S.; Kubde, R.; Rathod, Y.; Atara, R.; Rathi, S. To Evaluate and Compare the Effect of Different Post Surface Treatments on the Tensile Bond Strength between Fiber Posts and Composite Resin. J. Int. Oral Health JIOH 2013, 5, 27–32. [Google Scholar]
- Prasansuttiporn, T.; Nakajima, M.; Foxton, R.M.; Tagami, J. Scrubbing Effect of Self-Etching Adhesives on Bond Strength to NaOCl-Treated Dentin. J. Adhes. Dent. 2012, 14, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Thitthaweerat, S.; Nakajima, M.; Foxton, R.M.; Tagami, J. Effect of Solvent Evaporation Strategies on Regional Bond Strength of One-Step Self-Etch Adhesives to Root Canal Dentine. Int. Endod. J. 2013, 46, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Hamouda, I.M.; Ghazy, M.H.; Abo-Madina, M.M. Immediate and Delayed Micro-Tensile Bond Strength of Different Luting Resin Cements to Different Regional Dentin. J. Biomed. Res. 2013, 27, 151–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadam, A.; Pujar, M.; Patil, C. Evaluation of Push-out Bond Strength of Two Fiber-Reinforced Composite Posts Systems Using Two Luting Cements In Vitro. J. Conserv. Dent. JCD 2013, 16, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.S.; Swartz, M.L.; Moore, B.K.; Rhodes, B.F. Influence of Selected Variables on Adhesion Testing. Dent. Mater. 1992, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Grandini, S.; Bossù, M.; Bertelli, E.; Ferrari, M. Laboratory Assessment of the Retentive Potential of Adhesive Posts: A Review. J. Dent. 2007, 35, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Tavares, A.U.; Fabianelli, A.; Monticelli, F.; Raffaelli, O.; Cardoso, P.C.; Tay, F.; Ferrari, M. The Adhesion between Fiber Posts and Root Canal Walls: Comparison between Microtensile and Push-out Bond Strength Measurements. Eur. J. Oral Sci. 2004, 112, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Cekic-Nagas, I.; Ergun, G.; Nagas, E.; Tezvergil, A.; Vallittu, P.K.; Lassila, L.V.J. Comparison between Regional Micropush-out and Microtensile Bond Strength of Resin Composite to Dentin. Acta Odontol. Scand. 2008, 66, 73–81. [Google Scholar] [CrossRef]
- Albaladejo, A. Método de Preparación Del Espécimen Para Evaluar La Micromorfología de La Interfase Adhesiva Resina-Dentina Con Un Microscopio Electrónico de Barrido. Av. En Odontoestomatol. 2007, 23, 197–206. [Google Scholar]
- Tay, F.R.; Pashley, D.H. Monoblocks in Root Canals: A Hypothetical or a Tangible Goal. J. Endod. 2007, 33, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Carvalho, C.A.; Goracci, C.; Antoniolli, F.; Mazzoni, A.; Mazzotti, G.; Cadenaro, M.; Breschi, L. Influence of Luting Material Filler Content on Post Cementation. J. Dent. Res. 2009, 88, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Marchesi, G.; Cadenaro, M.; Mazzotti, G.; Di Lenarda, R.; Ferrari, M.; Breschi, L. Push-out Stress for Fibre Posts Luted Using Different Adhesive Strategies. Eur. J. Oral Sci. 2009, 117, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zicari, F.; Couthino, E.; De Munck, J.; Poitevin, A.; Scotti, R.; Naert, I.; Van Meerbeek, B. Bonding Effectiveness and Sealing Ability of Fiber-Post Bonding. Dent. Mater. 2008, 24, 967–977. [Google Scholar] [CrossRef]
- Radovic, I.; Mazzitelli, C.; Chieffi, N.; Ferrari, M. Evaluation of the Adhesion of Fiber Posts Cemented Using Different Adhesive Approaches. Eur. J. Oral Sci. 2008, 116, 557–563. [Google Scholar] [CrossRef]
- Perdigão, J.; Gomes, G.; Augusto, V. The Effect of Dowel Space on the Bond Strengths of Fiber Posts. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2007, 16, 154–164. [Google Scholar] [CrossRef]
- Sterzenbach, G.; Karajouli, G.; Naumann, M.; Peroz, I.; Bitter, K. Fiber Post Placement with Core Build-up Materials or Resin Cements-an Evaluation of Different Adhesive Approaches. Acta Odontol. Scand. 2012, 70, 368–376. [Google Scholar] [CrossRef]
- Boschian Pest, L.; Cavalli, G.; Bertani, P.; Gagliani, M. Adhesive Post-Endodontic Restorations with Fiber Posts: Push-out Tests and SEM Observations. Dent. Mater. 2002, 18, 596–602. [Google Scholar] [CrossRef]
- Matochek, M.H.M.; Tomaz, P.L.S.; Oliveira, T.d.S.; Polassi, M.R.; Alonso, R.C.B.; Scremin, F.M.; Sauro, S.; Marcucci, M.C.; D’Alpino, P.H.P. Influence of a Propolis-Based Irrigant Solution on Gap Formation and Bond Strength of Posts Bonded to Root Canal Dentin Using Different Resin Cements. Dent. Mater. J. 2020, 39, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Inokoshi, S.; Braem, M.; Lambrechts, P.; Vanherle, G. Morphological Aspects of the Resin-Dentin Interdiffusion Zone with Different Dentin Adhesive Systems. J. Dent. Res. 1992, 71, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Nadler, A.-M.-O.; da Silva, E.-J.; Lins-Filho, P.-C.; Dias, M.-F.; Guimarães, R.-P.; da Silva, C.-H.-V.; Silva, S.-D.S.; Gomes, A.-S.-L. Influence of Different Adhesion Strategies on Glass Fiber Post Retention. J. Clin. Exp. Dent. 2023, 15, e649–e657. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore Memorial Lecture. Adhesion to Enamel and Dentin: Current Status and Future Challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative Study on Adhesive Performance of Functional Monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Peumans, M.; Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Three-Year Clinical Effectiveness of a Two-Step Self-Etch Adhesive in Cervical Lesions. Eur. J. Oral Sci. 2005, 113, 512–518. [Google Scholar] [CrossRef]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical Interaction of Phosphoric Acid Ester with Hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef]
- Nagakane, K.; Yoshida, Y.; Hirata, I.; Fukuda, R.; Nakayama, Y.; Shirai, K.; Ogawa, T.; Suzuki, K.; Van Meerbeek, B.; Okazaki, M. Analysis of Chemical Interaction of 4-MET with Hydroxyapatite Using XPS. Dent. Mater. J. 2006, 25, 645–649. [Google Scholar] [CrossRef]
- Ernst, C.-P. Options for Dentin Bonding. J. Esthet. Restor. Dent. 2006, 18, 61–67. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between Bond-Strength Tests and Clinical Outcomes. Dent. Mater. 2010, 26, e100-121. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the Art of Self-Etch Adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Koshiro, K.; Yoshida, Y.; De Munck, J.; Nagakane, K.; Suzuki, K.; Sano, H.; Van Meerbeek, B. Hydrolytic Stability of Self-Etch Adhesives Bonded to Dentin. J. Dent. Res. 2005, 84, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Manuja, N.; Tyagi, S.P.; Singh, U.P. In Vitro Bonding Effectiveness of Self-Etch Adhesives with Different Application Techniques: A Microleakage and Scanning Electron Microscopic Study. J. Conserv. Dent. JCD 2011, 14, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Volpato, C.A.M. Current Perspectives on Dental Adhesion: (3) Adhesion to Intraradicular Dentin: Concepts and Applications. Jpn. Dent. Sci. Rev. 2020, 56, 216–223. [Google Scholar] [CrossRef]
- Pashley, D.H.; Ciucchi, B.; Sano, H.; Carvalho, R.M.; Russell, C.M. Bond Strength versus Dentine Structure: A Modelling Approach. Arch. Oral Biol. 1995, 40, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Eick, J.D.; Gwinnett, A.J.; Pashley, D.H.; Robinson, S.J. Current Concepts on Adhesion to Dentin. Crit. Rev. Oral Biol. Med. 1997, 8, 306–335. [Google Scholar] [CrossRef] [PubMed]
- Chersoni, S.; Suppa, P.; Breschi, L.; Ferrari, M.; Tay, F.R.; Pashley, D.H.; Prati, C. Water Movement in the Hybrid Layer after Different Dentin Treatments. Dent. Mater. 2004, 20, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, A.; Ramos, J.; Messias, A.; Marques, F.; Caramelo, F.; Mata, A. Microtensile Bond Strength and Micromorphology of Bur-Cut Enamel Using Five Adhesive Systems. J. Adhes. Dent. 2015, 17, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Dhem, A.; Goret-Nicaise, M.; Braem, M.; Lambrechts, P.; VanHerle, G. Comparative SEM and TEM Examination of the Ultrastructure of the Resin-Dentin Interdiffusion Zone. J. Dent. Res. 1993, 72, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, A.J.; Tay, F.R.; Pang, K.M.; Wei, S.H. Quantitative Contribution of the Collagen Network in Dentin Hybridization. Am. J. Dent. 1996, 9, 140–144. [Google Scholar]
- Nakabayashi, N.; Saimi, Y. Bonding to Intact Dentin. J. Dent. Res. 1996, 75, 1706–1715. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Conn, L.J.; Duke, E.S.; Eick, J.D.; Robinson, S.J.; Guerrero, D. Correlative Transmission Electron Microscopy Examination of Nondemineralized and Demineralized Resin-Dentin Interfaces Formed by Two Dentin Adhesive Systems. J. Dent. Res. 1996, 75, 879–888. [Google Scholar] [CrossRef]
- Foxton, R.M.; Nakajima, M.; Tagami, J.; Miura, H. Adhesion to Root Canal Dentine Using One and Two-Step Adhesives with Dual-Cure Composite Core Materials. J. Oral Rehabil. 2005, 32, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.S.; Silva-Sousa, Y.T.C.; Sousa-Neto, M.D. Bond Strength of Fiber Posts to Weakened Roots after Resin Restoration with Different Light-Curing Times. J. Endod. 2009, 35, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C.; de Melo, R.M.; Chaves, C.; Galhano, G.A.P.; Bottino, M.A.; Balducci, I. The Adhesive System and Root Canal Region Do Not Influence the Degree of Conversion of Dual Resin Cement. J. Appl. Oral Sci. Rev. FOB 2010, 18, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Serafino, C.; Gallina, G.; Cumbo, E.; Ferrari, M. Surface Debris of Canal Walls after Post Space Preparation in Endodontically Treated Teeth: A Scanning Electron Microscopic Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yu, H.; Lin, Q.; Taira, Y.; Cheng, H. Effects of Different Root Canal Obturation Techniques on the Bond Strength of Fiber Post to Intraradicular Dentine. Chin. J. Dent. Res. 2019, 22, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Troesch, S.; Wataha, J.C.; Krejci, I.; Meyer, J.M.; Pashley, D.H. Microtensile Bond Strength between Adhesive Cements and Root Canal Dentin. Dent. Mater. 2003, 19, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Vichi, A.; Grandini, S.; Geppi, S. Influence of Microbrush on Efficacy of Bonding into Root Canals. Am. J. Dent. 2002, 15, 227–231. [Google Scholar]
- Oskoee, S.S.; Bahari, M.; Kimyai, S.; Asgary, S.; Katebi, K. Push-out Bond Strength of Fiber Posts to Intraradicular Dentin Using Multimode Adhesive System. J. Endod. 2016, 42, 1794–1798. [Google Scholar] [CrossRef]
- do Amaral, R.C.; Stanislawczuk, R.; Zander-Grande, C.; Michel, M.D.; Reis, A.; Loguercio, A.D. Active Application Improves the Bonding Performance of Self-Etch Adhesives to Dentin. J. Dent. 2009, 37, 82–90. [Google Scholar] [CrossRef]
- Reis, A.; Pellizzaro, A.; Dal-Bianco, K.; Gones, O.M.; Patzlaff, R.; Loguercio, A.D. Impact of Adhesive Application to Wet and Dry Dentin on Long-Term Resin-Dentin Bond Strengths. Oper. Dent. 2007, 32, 380–387. [Google Scholar] [CrossRef]
- Elnaghy, A.M. Effect of QMix Irrigant on Bond Strength of Glass Fibre Posts to Root Dentine. Int. Endod. J. 2014, 47, 280–289. [Google Scholar] [CrossRef]
- Reyes-Carmona, J. Irrigation Protocols Effects on Radicular Dentin: Cleaning, Disinfection and Remaining Ultrastructure. ODOVTOS Int. J. Dent. Sc. 2023, 25, 14–21. [Google Scholar] [CrossRef]
- Khoroushi, M.; Kachuei, M. Pull-out Bond Strength of a Self-Adhesive Resin Cement to NaOCl-Treated Root Dentin: Effect of Antioxidizing Agents. Restor. Dent. Endod. 2014, 39, 95–103. [Google Scholar] [CrossRef]
- Kambara, K.; Nakajima, M.; Hosaka, K.; Takahashi, M.; Thanatvarakorn, O.; Ichinose, S.; Foxton, R.M.; Tagami, J. Effect of Smear Layer Treatment on Dentin Bond of Self-Adhesive Cements. Dent. Mater. J. 2012, 31, 980–987. [Google Scholar] [CrossRef]
- Taniguchi, G.; Nakajima, M.; Hosaka, K.; Iwamoto, N.; Ikeda, M.; Foxton, R.M.; Tagami, J. Improving the Effect of NaOCl Pretreatment on Bonding to Caries-Affected Dentin Using Self-Etch Adhesives. J. Dent. 2009, 37, 769–775. [Google Scholar] [CrossRef]
- Saber, S.-E.D.M.; El-Askary, F.S. The Outcome of Immediate or Delayed Application of a Single-Step Self-Etch Adhesive to Coronal Dentin Following the Application of Different Endodontic Irrigants. Eur. J. Dent. 2009, 3, 83–89. [Google Scholar] [CrossRef]
- Torii, Y.; Hikasa, R.; Iwate, S.; Oyama, F.; Itou, K.; Yoshiyama, M. Effect of EDTA Conditioning on Bond Strength to Bovine Dentin Promoted by Four Current Adhesives. Am. J. Dent. 2003, 16, 395–400. [Google Scholar]
- Rasimick, B.J.; Shah, R.P.; Musikant, B.L.; Deutsch, A.S. Effect of EDTA Conditioning upon the Retention of Fibre Posts Luted with Resin Cements. Int. Endod. J. 2008, 41, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Taşman, F.; Cehreli, Z.C.; Oğan, C.; Etikan, I. Surface Tension of Root Canal Irrigants. J. Endod. 2000, 26, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in Endodontics. Dent. Clin. N. Am. 2010, 54, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Denehy, G.E.; Swift, E.J. Effects of Chlorhexidine on Dentin Surfaces and Shear Bond Strengths. Am. J. Dent. 1994, 7, 81–84. [Google Scholar] [PubMed]
- el-Housseiny, A.A.; Jamjoum, H. The Effect of Caries Detector Dyes and a Cavity Cleansing Agent on Composite Resin Bonding to Enamel and Dentin. J. Clin. Pediatr. Dent. 2000, 25, 57–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Castro, F.L.A.; de Andrade, M.F.; Duarte Júnior, S.L.L.; Vaz, L.G.; Ahid, F.J.M. Effect of 2% Chlorhexidine on Microtensile Bond Strength of Composite to Dentin. J. Adhes. Dent. 2003, 5, 129–138. [Google Scholar]
- Say, E.C.; Nakajima, M.; Senawongse, P.; Soyman, M.; Ozer, F.; Tagami, J. Bonding to Sound vs Caries-Affected Dentin Using Photo- and Dual-Cure Adhesives. Oper. Dent. 2005, 30, 90–98. [Google Scholar] [PubMed]
- Hashem, A.A.R.; Ghoneim, A.G.; Lutfy, R.A.; Fouda, M.Y. The Effect of Different Irrigating Solutions on Bond Strength of Two Root Canal-Filling Systems. J. Endod. 2009, 35, 537–540. [Google Scholar] [CrossRef]
- Lindblad, R.M.; Lassila, L.V.J.; Salo, V.; Vallittu, P.K.; Tjäderhane, L. Effect of Chlorhexidine on Initial Adhesion of Fiber-Reinforced Post to Root Canal. J. Dent. 2010, 38, 796–801. [Google Scholar] [CrossRef]
- Erdemir, A.; Ari, H.; Güngüneş, H.; Belli, S. Effect of Medications for Root Canal Treatment on Bonding to Root Canal Dentin. J. Endod. 2004, 30, 113–116. [Google Scholar] [CrossRef]
- Santos, J.N.; Carrilho, M.R.d.O.; De Goes, M.F.; Zaia, A.A.; Gomes, B.P.F.d.A.; de Souza-Filho, F.J.; Ferraz, C.C.R. Effect of Chemical Irrigants on the Bond Strength of a Self-Etching Adhesive to Pulp Chamber Dentin. J. Endod. 2006, 32, 1088–1090. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Pinto, T.A.; Silva, L.M.; Araújo, D.F.G.; Martins, L.M.; Hannas, A.R.; Pedreira, A.P.R.V.; Francisconi, P.a.S.; Honório, H.M. Effect of 2% Chlorhexidine Digluconate on Bond Strength of a Glass-Fibre Post to Root Dentine. Int. Endod. J. 2013, 46, 847–854. [Google Scholar] [CrossRef]
- Haragushiku, G.A.; Back, E.D.E.E.; Tomazinho, P.H.; Baratto Filho, F.; Furuse, A.Y. Influence of Antimicrobial Solutions in the Decontamination and Adhesion of Glass-Fiber Posts to Root Canals. J. Appl. Oral Sci. Rev. FOB 2015, 23, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Sanabe, M.E.; Costa, C.A.; Hebling, J. Exposed Collagen in Aged Resin-Dentin Bonds Produced on Sound and Caries-Affected Dentin in the Presence of Chlorhexidine. J. Adhes. Dent. 2011, 13, 117–124. [Google Scholar] [CrossRef] [PubMed]


| Orthophosphoric Acid 37% PARABOND A+B PARACORE | Non-Rinse Conditioner PARABOND A+B PARACORE | CLEARFIL UNIVERSAL CLEARFIL DC CORE PLUS | |
|---|---|---|---|
| Distilled water (control) | G1 | G2 | G3 |
| EDTA 17% | G4 | G5 | G6 |
| NaOCl5% | G7 | G8 | G9 |
| CHX 2% | G10 | G11 | G12 |
| Etch-and-Rinse Adhesive Orthophosphoric Acid 37% Parabond A+B Paracore | Two Steps Self-Etch Adhesive ParaBond® Non-Rinse Conditioner Parabond A+B Paracore | Universal Adhesive Clearfil Universal Clearfil DC Core Plus | Total | |
|---|---|---|---|---|
| Distilled Water (control) | G1 = 16,760 aa ± 4.43 | G2 = 16,261 aa ± 5.88 | G3 = 17,067 abc ± 4.33 | 16,696 ac |
| EDTA 17% | G4 = 17,069 aa ± 5.03 | G5 = 14,951 aa ± 5.14 | G6 = 16,663 aac ± 4.84 | 16,228 ab |
| NaOCl 5% | G7 = 14,606 aa ± 3.81 | G8 = 14,095 aa ± 4.64 | G9 = 12,919 a ± 3.79 | 13,873 |
| CHX 2% | G10 = 17,304 aa ± 5.95 | G11= 16,479 aa ± 3.04 | G12 = 16,604 aab ± 4.58 | 16,796 bc |
| Total | 16,435 a | 15,447 a | 15,813 a | 15,898 a |
| Coronal | Media | Apical | |
|---|---|---|---|
| (MPa) | 16,198 | 16,333 | 15,163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Varela, S.; Ramos, J.C.; Ginzo-Villamayor, M.J.; Castelo-Baz, P.; Méndez-Díaz, R.; Anache-D’Abate, M.A.; Gancedo-Gancedo, T.; Ruíz-Piñón, M.; Mareque-Bueno, S.; Martín-Biedma, B.J. Effect of Dentin Irrigants on Push-Out Bond Strength in Resin Cementation Protocols for Fiber Posts in Endodontically Treated Teeth: An In Vitro Study. Materials 2024, 17, 1432. https://doi.org/10.3390/ma17061432
García-Varela S, Ramos JC, Ginzo-Villamayor MJ, Castelo-Baz P, Méndez-Díaz R, Anache-D’Abate MA, Gancedo-Gancedo T, Ruíz-Piñón M, Mareque-Bueno S, Martín-Biedma BJ. Effect of Dentin Irrigants on Push-Out Bond Strength in Resin Cementation Protocols for Fiber Posts in Endodontically Treated Teeth: An In Vitro Study. Materials. 2024; 17(6):1432. https://doi.org/10.3390/ma17061432
Chicago/Turabian StyleGarcía-Varela, Sandra, João Carlos Ramos, María José Ginzo-Villamayor, Pablo Castelo-Baz, Ramón Méndez-Díaz, Marcos Aníbal Anache-D’Abate, Tania Gancedo-Gancedo, Manuel Ruíz-Piñón, Soledad Mareque-Bueno, and Benjamín José Martín-Biedma. 2024. "Effect of Dentin Irrigants on Push-Out Bond Strength in Resin Cementation Protocols for Fiber Posts in Endodontically Treated Teeth: An In Vitro Study" Materials 17, no. 6: 1432. https://doi.org/10.3390/ma17061432
APA StyleGarcía-Varela, S., Ramos, J. C., Ginzo-Villamayor, M. J., Castelo-Baz, P., Méndez-Díaz, R., Anache-D’Abate, M. A., Gancedo-Gancedo, T., Ruíz-Piñón, M., Mareque-Bueno, S., & Martín-Biedma, B. J. (2024). Effect of Dentin Irrigants on Push-Out Bond Strength in Resin Cementation Protocols for Fiber Posts in Endodontically Treated Teeth: An In Vitro Study. Materials, 17(6), 1432. https://doi.org/10.3390/ma17061432






