1. Introduction
Zinc oxide varistor is a kind of polycrystalline semiconductor ceramic with a complex, multi-component oxide composition. The main component is zinc oxide, accounting for more than 90% (molar percentage), with a small amount of Bi
2O
3, Sb
2O
3, MnO
2, Cr
2O
3, SiO
2, Co
2O
3 and other auxiliary components [
1,
2,
3,
4]. In the 1970s, ZnO-Bi
2O
3 ceramics were invented by Matsuoka et al. ZnO varistors are widely used as the core components of high-voltage surge arresters and low-voltage surge protection devices because of their excellent nonlinear electric field-current density relationship and their ability to absorb energy surges [
1,
3]. In the Bi
2O
3-ZnO varistor, Bi
2O
3 is the main reason for the nonlinear characteristics of the varistor [
5]. Bi
2O
3 has a low melting point [
6], and during the sintering cooling process. Bi
2O
3 [
7,
8] can form a liquid phase, make the dopant more uniformly dispersed, and promote the material exchange between grains and grain boundaries, so that more cations of unsaturated excess metal oxides, such as Co
3+ and Mn
4+, can obtain electrons and transform from the high valence state to the stable low valence state. Oxides such as Co
2O
3 and MnO
2 [
4,
9,
10] have host-accepted properties, and their reaction at grain boundaries increases the surface density of states (
Ns) of ZnO grains, which leads to a significant increase in the height of the grain boundary layer potential barrier, and thus improves the voltage gradient (E
1mA) of the sample. At the same time, symmetric double Schottky barriers (
) are generated at the grain boundaries, and the higher the
are the larger the nonlinear coefficients (α). The oxygen desorption mechanism [
11,
12,
13] is used to explain the merits of Bi-rich phases during high temperature sintering. The adsorbed oxygen [
14] can introduce interface acceptor states and capture negative charge carriers, thus increasing the double Schottky barrier (
) in the grain boundary. This is because the interface acceptor state and the donor concentration together determine the height of
. However, the low melting point during the high-temperature sintering can also leads to its volatiliztion, and some pore can be formed inside the varistor, which affects its electrical properties [
14]. According to the theory of charge compensation mechanism [
15], oxygen vacancies, the main transportation channel of adsorbed oxygen, will be consumed in the solid solution process between additives with Bi
2O
3, which cause
to weaken. Accordingly, BiSbO
4 can be pre-synthesized using the materials Bi
2O
3 and Sb
2O
3 instead of the equal amounts of Bi
2O
3 and Sb
2O
3 in the original formulation. The depletion of oxygen vacancies at grain boundaries during sintering can be avoided increasing the concentration of adsorbed oxygen, and the densification and electrical properties of varistor can be improved [
16]. With the increasing application of high-voltage transmission, high-voltage transmission systems are increasingly pursuing smaller and lighter surge arresters, which can effectively improve the uneven potential distribution along the ZnO varistor columns within the arrester [
17]. At the same time, this puts higher requirements on the voltage gradient of the piezoresistors. It has been shown that decreasing the sintering temperature and holding time improves the voltage gradient but leads to a decrease in energy absorption capacity and nonlinearity [
18,
19]. Increasing the voltage gradient by sacrificing other electrical properties is not worth. The increasing the voltage gradient is equivalent to reducing the grain size, and it may be more effective to dope with additives that can inhibit grain growth and obtain a high voltage gradient of the varistors.
Therefore, it is particularly important to improve voltage gradients and nonlinear coefficients while taking into account other electrical characteristics. Al
3+ doping leads to lower grain resistance and a smaller residual voltage [
20,
21]. The smaller the grain resistance, the smaller the residual voltage ratio, thus improving the protection performance of the arrester [
22]. Yet, it leads to a significant increase in leakage current, shortening the service life of the arrester, and the voltage gradient is somewhat affected [
20,
23]. Some studies have shown that the doping of In
3+ can effectively suppress the leakage current, improve the nonlinear coefficient, and significantly reduce the residual voltage ratio, but the doping of In
3+ does not significantly improve the voltage gradient [
9,
22,
24]. Also, it has been shown that BiSbO
4 promotes grain growth, which is detrimental to obtaining ZnO varistors with high voltage gradients.The addition of rare-earth oxides [
25,
26] can effectively improve the grain boundary characteristics and thus enhance the electrical performance of ZnO pre-synthesizing ceramic materials. The rare earth element Y
3+ forms a bismuth-rich second phase containing Y
3+ in the sintered composition, which is mainly distributed at the junctions of the three grains and plays the role of “pinning” [
27,
28], similar to the inhibition effect of spinel on the relative grains, reducing the grain size and significantly improving the potential gradient. It also increases the number of grain boundaries, enlarges the number of Schottky barriers, and improves the nonlinear coefficients to some extent [
27,
29,
30]. This precisely compensates for the disadvantages of ln
3+ doping, which only slightly increases the voltage gradient of ZnO varistors, and BiSbO
4 doping, which promotes grain growth.
In order to prepare and obtain varistors with a high voltage gradient, a low residual voltage ratio, and a low leakage current. Therefore, experiments with different doping concentrations of In3+ were carried out to investigate the effect of combined doping of the three on the microstructure and electrical performance of the oxide varistor at given Al3+ and Y3+ doping concentrations and pre-synthesizing BiSbO4 in lieu of doping.
2. Materials and Methods
Co-doped ZnO varistors were prepared from the following compositions: (93.9-X) mol% ZnO, 1 mol% Co
2O
3, 0.8 mol% MnO
2, 1.2 mol% SiO
2, 2 mol% BiSbO
4, 0.2 mol% Al(NO
3)
3·9H
2O, 0.9 mol% Y(NO
3)
3·6H
2O, X mol% In (NO
3)
3 (X = 0, 0.25, 0.5, 0.75, 1.00). All of the above experimental materials were analytically pure (Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China). Bi
2O
3 and Sb
2O
3 were accurately weighed in a molar ratio of 1:1, ball-milled (SFM-1 planetary ball mill, Hefei Kejing Materials Technology Co., Ltd., Hefei, China) for 12 h, dried, heat-treated at 800 °C for 2 h, and naturally cooled to 500 °C for 2 h of annealing treatment to obtain BiSbO
4. The main raw materials, zinc oxide, pre-synthesizing BiSbO
4, and its additives were then accurately weighed in molar proportions into the ball-milling jars and then mixed with the appropriate proportion of deionized water and grinding media (zirconia beads). The powder, grinding media, and surfactant (ammonium polyacrylate) were mixed with a suitable proportion of deionized water and then ball milled in a planetary ball mill with a combination of forward and reverse rotation at a speed of 450 r/min for 12 h. The slurry was removed and dried in a blast oven at 110 °C for 12 h. The dried powder was crushed, and a 5 wt% solution of polyvinyl alcohol (PVA) binder was added at a powder-to-PVA mass ratio of 1:9. The powder was ground in an onyx grinding bowl and passed through an 80 mesh stainless steel sieve for granulation to obtain the starting powder. 1 g of powder is weighed and pressed uniaxially at 10 Mpa for 90 s to form discs of 15 mm diameter and 1 mm thickness. Sintering was carried out in an air environment by placing the pressed discs in a muffle furnace (KSL-1700X, Hefei Kejing Materials Technology Co., Ltd., Hefei, China) and holding them at 400 °C for 4 h to release the binder (PVA), then increasing the temperature to 1100 °C and holding for 3 h. It is important to note that the temperature interval from 800 °C to 1000 °C during warming is the joint stage of densification of the varistors, while the temperature interval from 950 °C to 800 °C during cooling is critical for the nonlinear coefficient. In these two specific temperature intervals, the rate of temperature rise or fall should be slowed down to 2 °C/min and 1 °C/min, respectively. After the temperature was reduced to room temperature, the upper and lower surfaces of the ZnO varistors were sanded and polished, ultrasonically cleaned and dried, and then silver paste was applied to the upper and lower surfaces of the discs. 500 °C holding time of 60 min was used for curing to form the ohmic contacts as test electrodes. The flow chart for the preparation of ZnO varistors is shown in
Figure 1.
In addition, the microstructure of the cross-section of the sintered samples was observed using an electron microscope (SEM, JSM-6380, Japan Electronics Co., Ltd., Tokyo, Japan). The average grain size (d) was determined by the formula of the linear intercept method: d = 1.56 L⁄MN, where L is the length of a randomly delineated measurement line on the SEM image, M is the magnification of the SEM image, and N is the number of ZnO varistor boundaries covered by the delineated measurement line. X-ray diffraction (XRD) maps of the samples were obtained using CuKa radiation (XRD-7000X, Shimadzu Corporation, Kyoto, Japan) to determine the grain boundary structure and phase composition of the piezoresistors. The samples were scanned under CuKa radiation at a wavelength of 0.1540 nm at a scanning rate of 5° per minute over a diffraction angle range of 10° to 80°.The electric field-current density (E-J) relationship curves in the pre-breakdown and breakdown zones were measured using a source meter (Keithley 2410, Keithley Instruments, Inc., Cleveland, OH, USA) to calculate the electrical characteristics of the piezoresistors. For a given current density of 1.0 mA/cm−2, the voltage drop across the piezoresistor is the breakdown voltage (U1mA). The relationship between the voltage gradient (E1mA) and U1mA: E1mA = U1mA/D (D is the thickness of the varistor). The leakage current density (IL) was tested at 0.75E1mA electric field strength. The nonlinear coefficient was calculated as: , where V2 and V1 are the electric fields at current densities of 1.0 mA/cm2 and 0.1 mA/cm2, respectively. The capacitance-voltage (C-V) characteristics were measured using a broadband dielectric device (Novocontrol Concept 80, Novocontrol Technologies, Montabaur, Germany) at a frequency of 1 kHz. The residual voltage ratio (Kp) was calculated as KP = Un/E1mA, where Un is the residual voltage at a pulse current density of 63.7 A/cm2. A pulse generator (BS1005 8/20 µs) was used to generate a pulse current with a waveform of 8/20 µs. Every eight samples constitute a group, and the data in the text are their mean values to eliminate the effect of chance error on the reliability of the data.
3. Results and Discussion
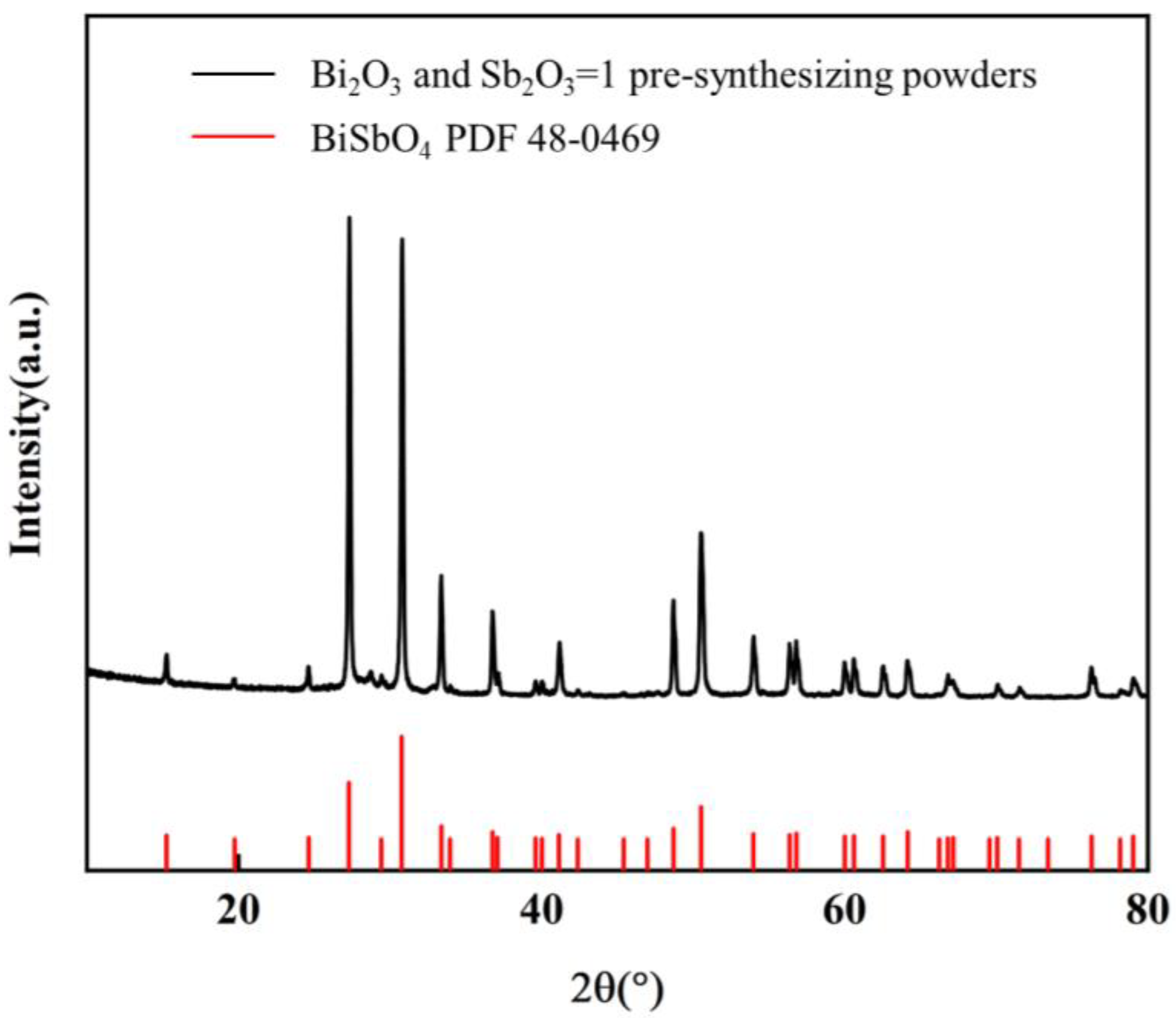
Figure 2 shows the XRD patterns of the complexes of Bi
2O
3 and Sb
2O
3 mixed in a 1:1 ratio and sintered at 800 °C for 2 h. The diffraction angles of the three strongest peaks correspond to about 26°, 30°, and 50°, and all the diffraction peaks coincide with the BiSbO
4 standard card (JCPDS card No. 48-0469). Bi
2O
3 and Sb
2O
3 diffraction peaks were not detected, and there were no secondary phases. This indicates that Bi
2O
3 and Sb
2O
3 reacted completely at 800 °C without residual impurities, and the sintered complex is a high-purity BiSbO
4. The chemical reaction equation is:
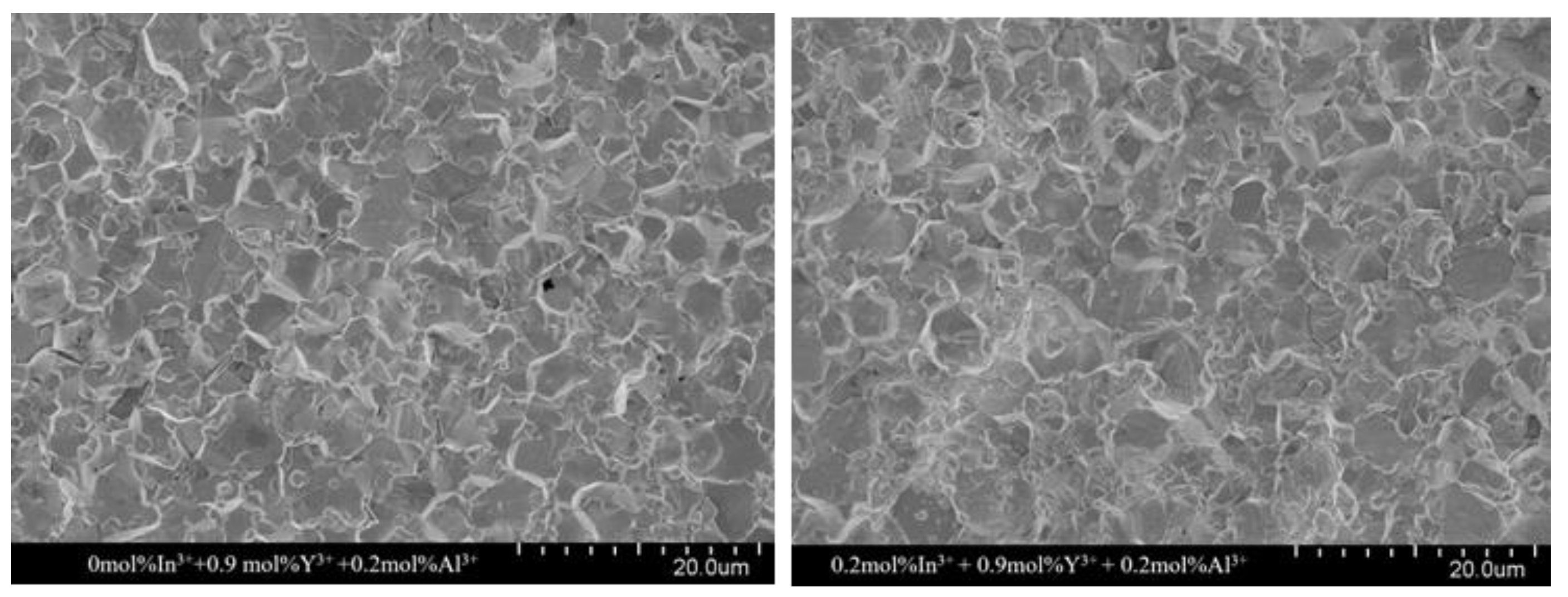
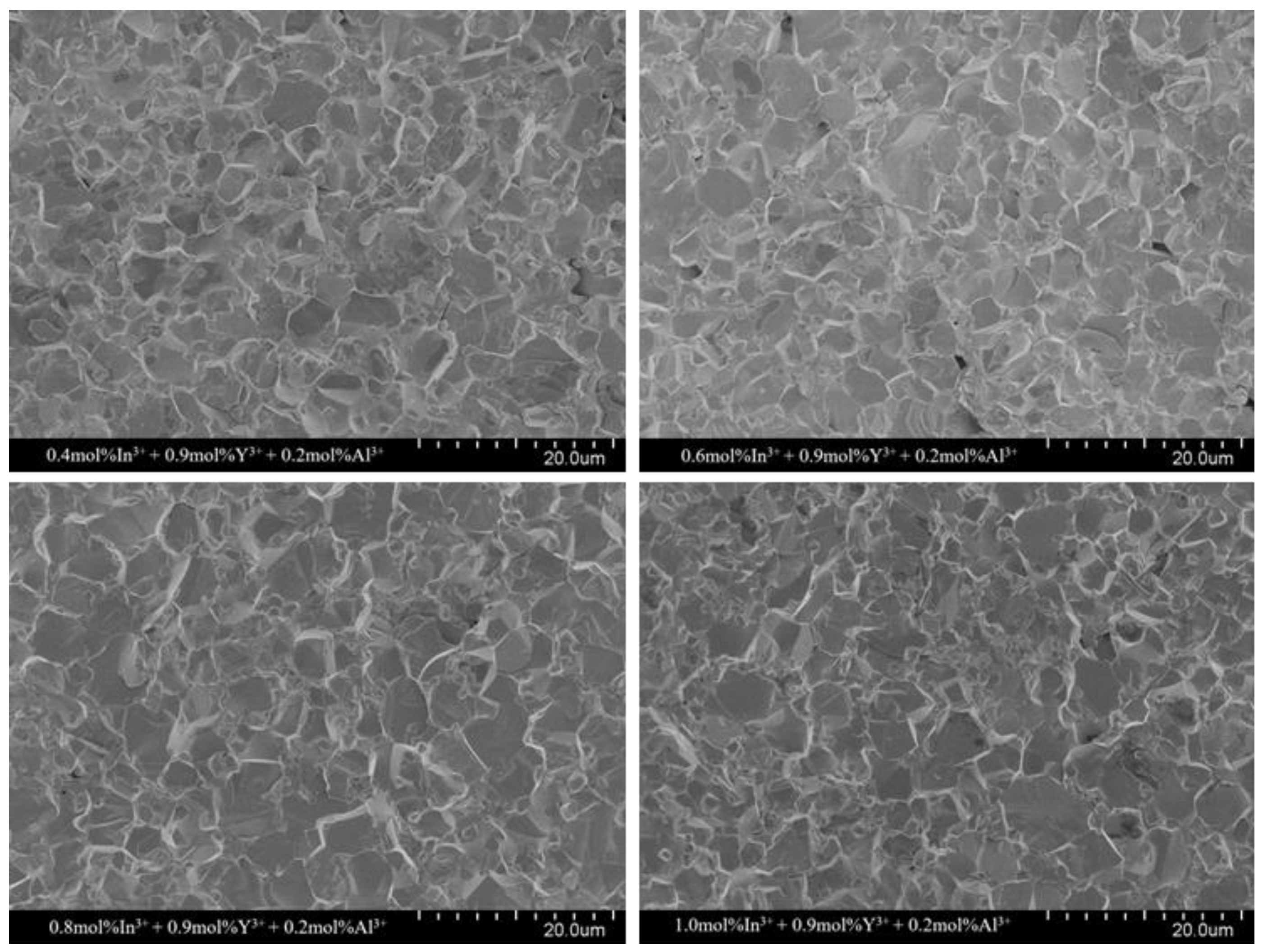
The microstructure of the sintered ceramics with different In
3+ doping molar ratios is shown in
Figure 3. The average grain size (d) was calculated by the above intercept method and is listed in
Table 1. The grain size decreases gradually from 6.31 µm to 4.46 µm with an increasing In
3+ doping ratio. This can be explained by the fact that some doped In
3+ form small particles at the grain edges, which act similar to the “pinning” effect of spinel and inhibit grain growth. This is consistent with the effect of added In
3+ on grain size reported by others [
22,
24]. It was also observed that at a doping concentration of 0.6 mol% of In
3+ ions, the homogeneity of the grains was better, which was favorable for the electrical properties of the ZnO resistor sheet [
31].
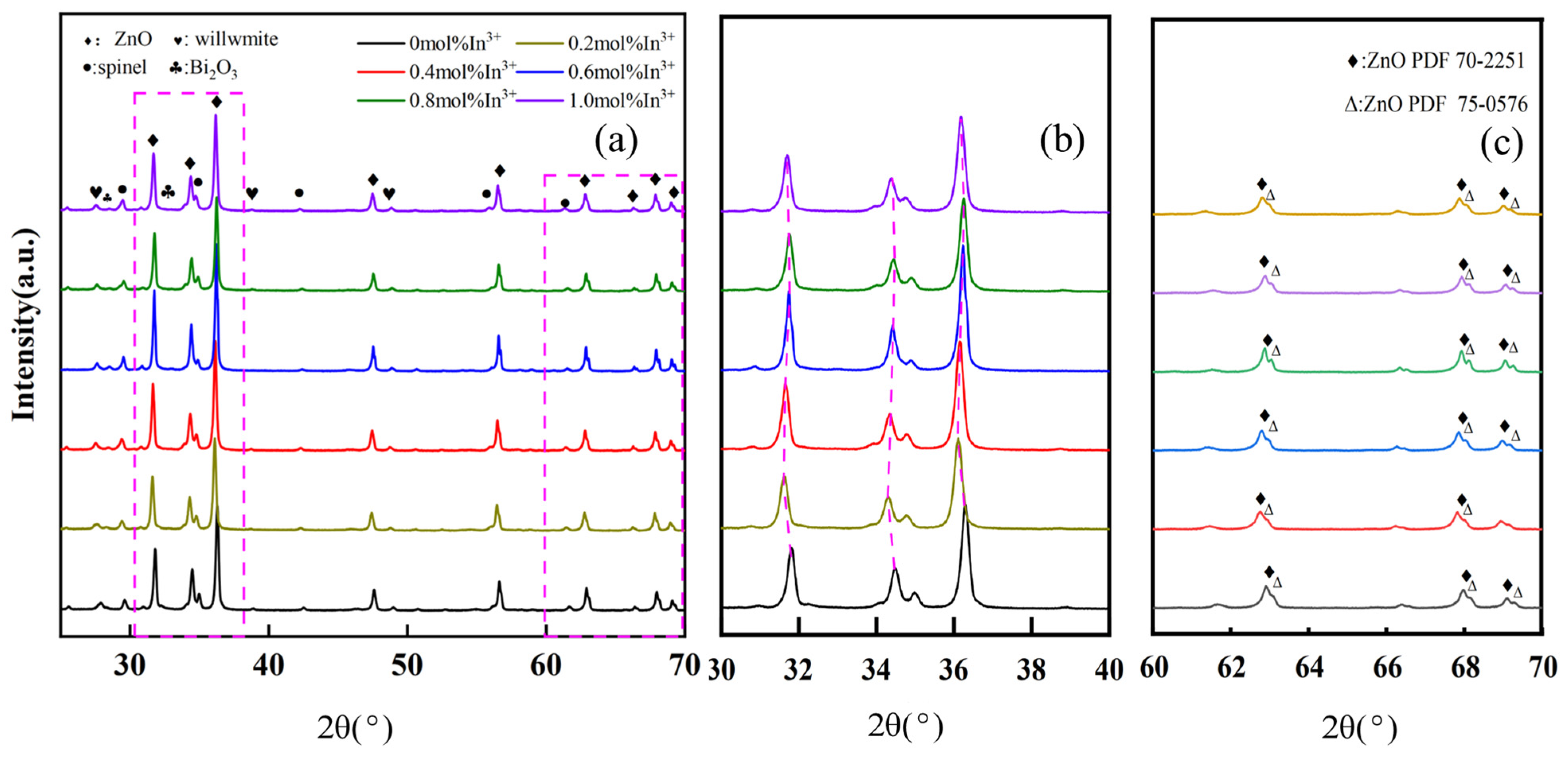
Figure 4 shows the XRD patterns of ZnO varistor ceramics sintered at 1100 °C for 3 h with different amounts of In
3+ doping, which are predominantly hexagonal-structured ZnO (PDF 70-2251 and PDF 75-0576), and a few amounts of second phases, including spinel, willemite, and Bi-rich phases. The presence of the Bi-rich liquid phase is mainly to provide a place for the dissolution and reaction of other additives to promote the sintering of ceramics and make the reaction more complete. From
Figure 4a, it is clearly observed that the diffraction intensity of the strongest peak (diffraction angle of about 38°) and the second strongest peak (diffraction angle of about 32°) is maximum at 0.6 mol% doping concentration of In
3+ as compared to other doping concentrations. This indicates that the crystallinity of ZnO grains is higher when the doping concentration of In
3+ is 0.6 mol%.
Notably, it can be observed from
Figure 4b that the diffraction angles of the three strongest peaks slightly tend to move to lower diffraction angles with the increase of the In
3+ doping ratio, and an inflection point occurs at the In
3+ doping concentration of 0.6%. At 0.6% to 1.0%, the diffraction angles of the three strongest peaks are almost unchanged. The ZnO lattice parameter a = 3.1258 Å and c = 5.2213 Å is used when there is no doping of In
3+. As the concentration of In
3+ increases, In
3+ enters the ZnO lattice and replaces the defects in the position of Zn
2+, which results in an increase in the lattice parameter. The radius of In
3+ is larger than that of Zn
2+, and there is a limit to the solid solution reaction inside the grains. When the doping concentration reaches 0.6 mol%, the lattice parameter tends to be stabilized at a = 3.3591 Å and c = 5.6318 Å. In the doping concentration of In
3+ from 0.6 mol% to 1.0 mol%, the lattice parameter of ZnO is basically unchanged. This slight decrease in lattice parameter values. This is due to the presence of small stresses in the ZnO structure, which leads to the enlargement of the single cell of the ZnO lattice [
32]. This can also be explained by the fact that at low doping concentrations [
33], In
3+ is rapidly and mostly solidified into the ZnO lattice, but the solidification is limited by the fact that the ionic radius of In
3+ (ion radius of 8.0 × 10
−11 m) is larger than that of Zn
2+ (ion radius of 7.4 × 10
−11 m), and thus the diffraction angles of the three strongest peaks are almost the same at doping ratios of 0.6%, 0.8%, and 1.0%. In the doping concentration of In
3+ from 0.6 mol% to 1.0 mol%, the lattice parameter of ZnO is basically unchanged. This is due to the presence of small stresses in the ZnO structure, which leads to the enlargement of the single cell of the ZnO lattice. This can also be explained by the fact that at low doping concentrations, In
3+ is rapidly and mostly solidified into the ZnO lattice, but the solidification is limited by the fact that the ionic radius of In
3+ (ion radius of 8.0 × 10
−11 m) is larger than that of Zn
2+ (ion radius of 7.4 × 10
−11 m), and thus the diffraction angles of the three strongest peaks are almost the same at doping ratios of 0.6%, 0.8%, and 1.0%. At the same time, this also confirms the theory that “larger ion doping will move the XRD diffraction peaks to a lower diffraction angle, while smaller radius ion doping will move the corresponding diffraction peaks to a higher diffraction angle” [
32,
34]. This peak shift indirectly reflects the solid solution of In
3+ ions in ZnO grains. In
Figure 4c, the detachment of the ZnO diffraction peaks with lower intensities is observed in the diffraction interval with diffraction angles from 60° to 70°, which again suggests that the doping of In
3+ distorts the ZnO lattice. There are two main reasons for the absence of diffraction peaks of the corresponding substances of other ions in the XRD spectra: it is related to the ionic radius, and the additive ions with smaller ionic radius are easy to be doped into the ZnO crystal lattice; and some of the additive ions are supposed to be due to the lower concentration of doping, and so the diffraction peaks of the corresponding ions do not appear.
The nonlinear E-J curve of the zinc oxide varistor from the pre-breakdown region to the breakdown region is shown in
Figure 5A.
Figure 5B shows the E-J characteristics of the current density from 0 to 1.0 × 10
−3 A/cm
2. As shown in
Figure 5B, in the pre-breakdown region, the piezosensitive voltage increases rapidly with how the current density is added when the piezoresistor shows a high resistance state, while in the breakdown region, the growth rate of the piezosensitive voltage slows down or even remains unchanged [
19].
It can be observed from
Figure 5B that when the In
3+ doping concentration is 0 mol%, the increase in voltage in the corresponding E-J curve starts to change slowly at a current density of 4.0 × 10
−4 A/cm
2, and an inflection point appears in the curve trend. Compared with other doping concentrations, the inflection point occurs at the lowest current density of the varistor when the In
3+ doping concentration is 0.6 mol%, so that the nonlinear coefficient now reaches a maximum value of 62.2 when the In
3+ doping concentration is 0.6 mol%. This is because the more drastic the change in the inflection point between the pre-breakdown region and the breakdown region is, the greater the nonlinear coefficient will be, and this means that the faster the re-response time will be, the more effective the protection of the device will be [
35]. Based on the E-J curve and the above equations, the voltage gradient, leakage current density, and nonlinear coefficient of the ZnO varistor can be calculated, and the results are listed in
Table 1.
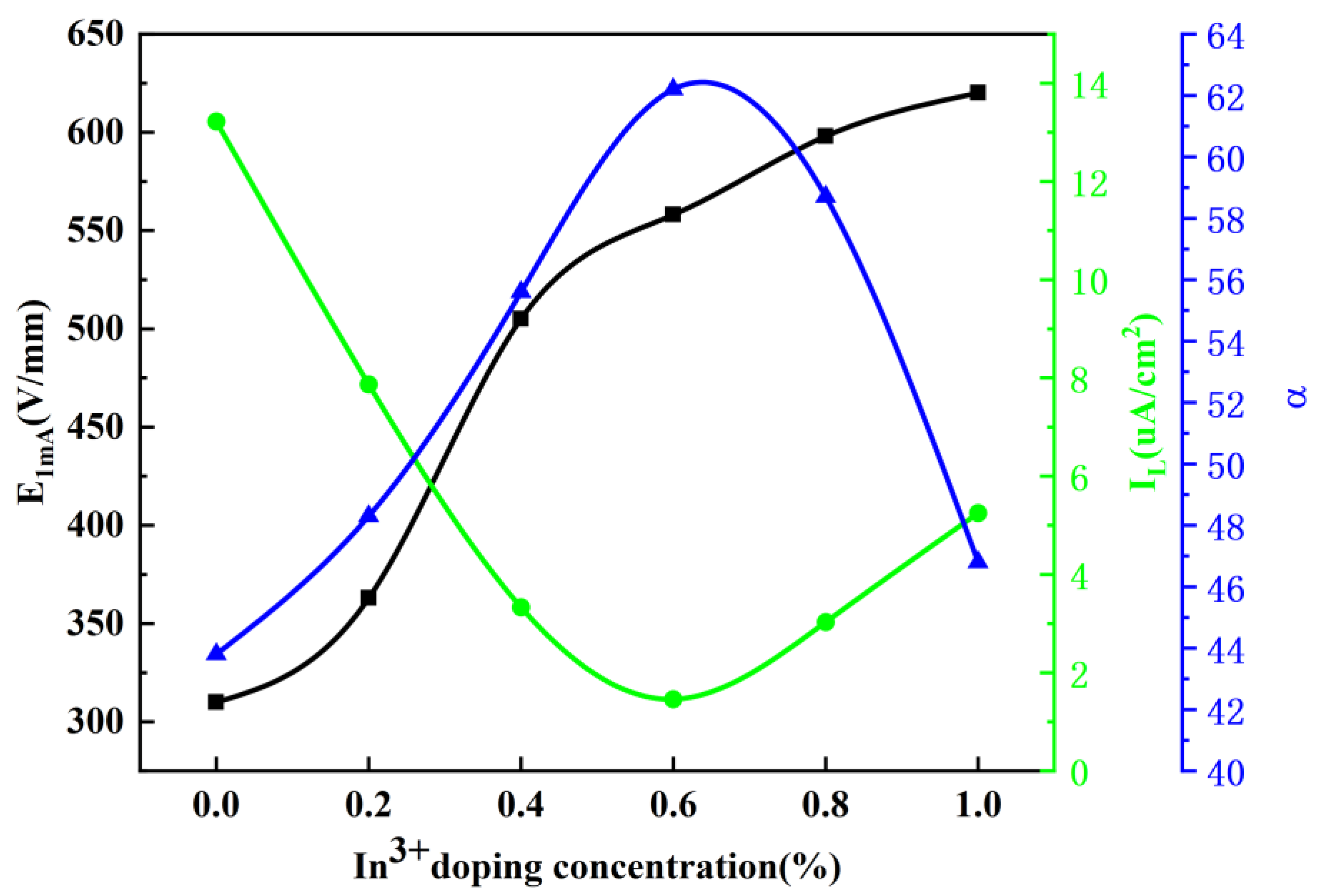
The effects of different In
3+ doping concentrations on the electrical breakdown strength, nonlinear coefficient, and leakage current of the materials are shown in
Figure 6. The results show that the breakdown voltage increases continuously from 310 V/mm to 620 V/mm with the increase in doping concentration. The increase in breakdown voltage age is partly attributed to the positive effect of Y
3+ doping. Y
3+ forms spinel at the grain boundaries during high temperature sintering, which inhibits the growth of the grains and thus reduces the average grain size. The increase in breakdown voltage is partly attributed to the positive effect of Y
3+ doping. Another reason is that since the ionic radius of In
3+ is slightly larger than that of Zn
2+, the solid solution of In
3+ in ZnO grains has a certain limit. It will not continue to solidify with the increase in In
3+ doping concentration, and the remaining In
3+ will remain at the grain boundaries to form a spinel phase, which exists in the triangular region where the grains intersect, restricting the growth of the grains and increasing the voltage gradient. When the doping concentration of In
3+ is increased from 0.0 mol% to 1.0 mol%, the leakage current (I
L) decreases rapidly from 13.22 µA/cm
2 to 1.46 µA/cm
2, and then rises slowly to 3.32 µA/cm
2. On the contrary, the nonlinear coefficient (α), which shows a tendency of increasing and then decreasing from 43.8 to 62.8, then decreasing rapidly to 46.8. I
L and α showed a negative correlation trend, which is consistent with their [
2] findings. The residual voltage ratio (K
p) value is in the range of 0 to 1.0 mol%. K
p values varied between 1.73 and 1.97, with a minimum value of 1.73 for the In
3+ doping concentration of 0.6 mol%. Thus, improving the protection performance of the arrester. Meanwhile, the different residual pressure ratios provide some evidence that the solid solution of In
3+ into ZnO grains decreases the grain resistance. The voltage gradient and I
L are significantly improved, the α is slightly increased, and the residual voltage ratio is slightly improved compared to the results of Pengfei, M. et al. [
36].
Figure 7 shows the EDX analysis image of a typical sample. The scanning image confirms the presence of In
3+, Al
3+, and Y
3+ inside the grains and at the grain edges. During the high temperature sintering process, the dopant ions are solidly dissolved into the defects inside the grains and on the ZnO grain boundaries due to the presence of defects inside the grains and on the ZnO grain boundaries. In
Figure 7a, the white line shows the measurement path, which contains two grains and an intergranular layer.
Figure 7b shows the intensity of the distribution of In
3+, Al
3+, and Y
3+ on the detecting path. As can be seen in
Figure 7b, along the scanning path, the distribution of In
3+ ions inside the grains is slightly lower than that outside the grains. Some of them are solidly dissolved in the ZnO grains, while others are distributed along the grain boundaries. In
Figure 4b, the diffraction angles of the three strongest peaks of the main crystalline phase ZnO are shifted to the lower direction, which also confirms that part of In
3+ solidifies into the ZnO grains. The Bi element is volatilized in a small amount during the sintering process, and very little solid solution is found inside the grains, which is mainly distributed in the triangular area between the grains.
The distribution intensity of Y3+ at grain boundaries is significantly higher than that of Al3+ and In3+ ions, which can be attributed to two aspects: the higher Y3+ doping concentration than that of Al3+ and In3+ doping concentrations, and the large radius of Y3+. The distribution pattern of Al3+ ions is opposite to that of Y3+ ions because the relationship between the ionic radii of Y3+ and Zn2+ is: . It is easier for Al3+ to solidly dissolve into the ZnO grains. Replacing Zn2+ with Y3+ leads to huge lattice distortions, and for this to happen, more energy needs to be supplied from the outside. This is therefore very difficult to happen, so a large amount of Y3+ will accumulate at the grain boundaries.
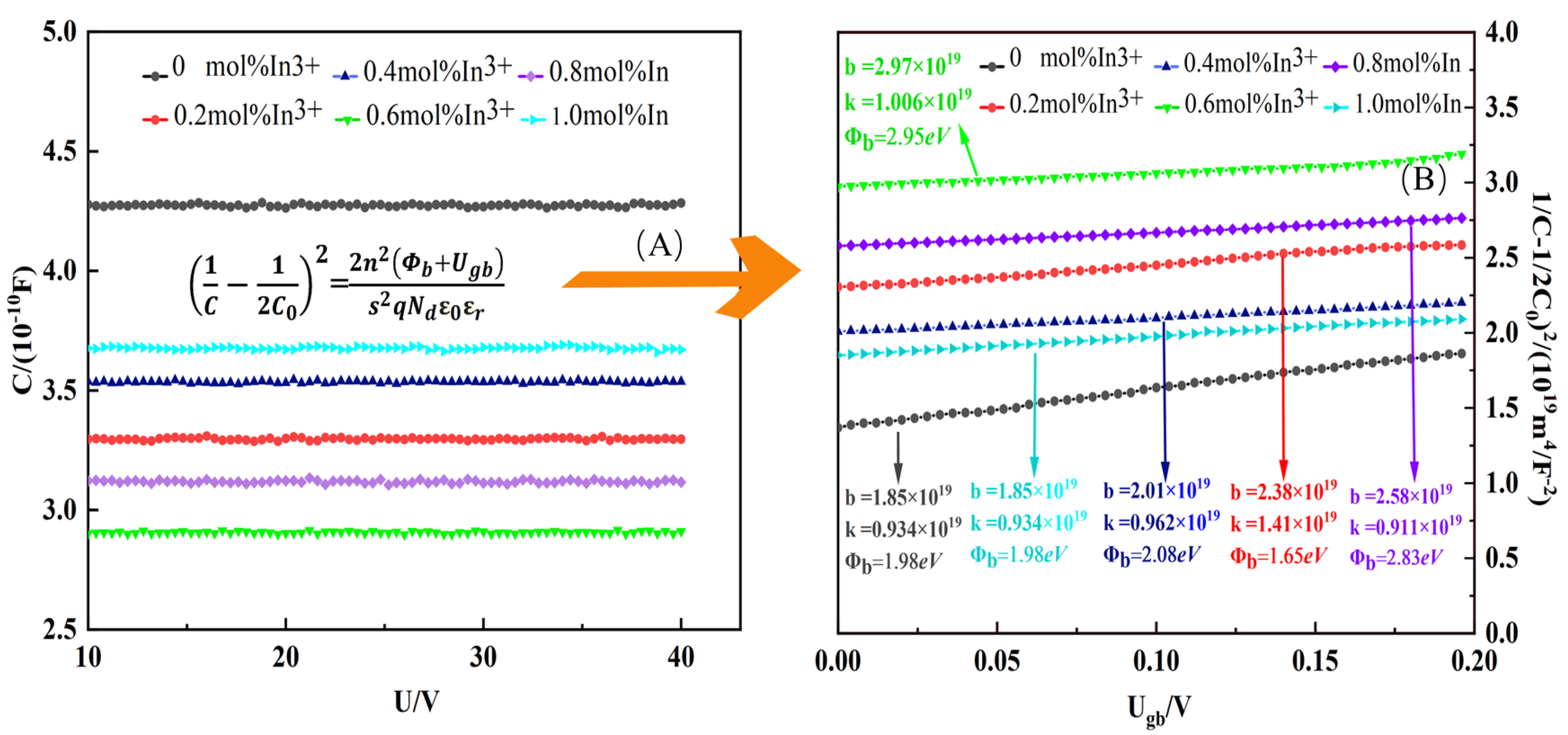
The nonlinear electrical properties are determined by the symmetric double Schottky barriers at the grain boundaries of ZnO. To further investigate the effect of different In
3+ doping concentrations on the nonlinear characteristics, the capacitance-voltage (C-V) curves of the ZnO varistor are plotted in
Figure 8A. It can be seen that the introduction of In
3+ ions has a first significant effect on the varistor capacitance (C), and the capacitance C value shows a decreasing and then increasing trend as the In
3+ doping ratio rises. Additionally, no obvious capacitance changes in every sample under the applied bias voltage (U). Based on the following normalized equation [
37]:
where
C and
C0 are the capacitance of the sample with
Ugb and zero bias voltage, respectively,
n is the number of grain boundaries between the electrodes, which can be calculated based on
(
h is the thickness of the sample),
Ugb is the bias voltage applied to each grain boundary,
s is the area of the electrodes,
q is the electronic charge,
ε0 is the vacuum permittivity, and
εr is the relative permittivity of the ZnO. Based on the normalization equation, the normalization curve is obtained, as shown in
Figure 8B. Donor density (
Nd) can be calculated from the slope (K) of the straight line and
can be calculated from the intercept (b) of the curve. Interface state density (
Ns) can be calculated based on
. The detailed calculation relations are given below [
14,
37]:
By transforming Equations (4) and (5),
Ns,
Nd, and
are calculated as follows:
Parameters such as
Nd,
Ns, and
were calculated based on
Figure 8B and the above equation and are listed in
Table 1. The interfacial state density
Ns of the samples showed an increasing and then decreasing trend with the increase and decrease of In
3+ doping concentration, which appeared at the peak value of 2.75 × 10
16 m
−2 at 0.6 mol% In
3+ doping. This trend may be caused by the addition of more oxygen vacancies and zinc gaps by the substitution process at the ZnO grain boundaries.
Nd and
Ns show a similar variational tendency. According to
, it can be known that
is mainly affected by
Ns, and the trend of
tends to be the same as that of
Ns. As a result, the value of
increases significantly with In
3+ ions dopant and reaches up to eV when x = 0.6 mol%, and then decreases to 1.98 eV at x = 1.0 mol% in
Table 1.
The radius of the Y
3+ ion is larger than that of the Zn
2+ ion, and a small fraction of the Y
3+ ion is solidly dissolved into the ZnO grains. The ionic radius deviation A value of Y
3+ ions and Zn
2+ ions during high temperature sintering of ZnO varistors is 20.4% according to the solid solution ionic formula
< A [
38] (
r1 denotes ions with large ionic radius values,
r2 denotes ions with small ionic radius values, and A denotes ionic radius deviation). Some of the Y
3+ ions will act as donors into the ZnO lattice, affecting the donor density
Nd. Most of the Y
3+ ions form fine spinel particles at the edges of ZnO grains, creating a pinning effect that inhibits the growth of ZnO grains [
27], increases the number of grain boundaries inside the varistor, and increases the number of Schottky barriers. Significantly improved voltage gradients and nonlinear coefficients.
The radius of Al
3+ is smaller than that of Zn
2+, and the absolute value of the difference between the two is larger, so Al
3+ is easy to solid-solve into ZnO grains, generating a large number of electronic charges, lowering the grain resistance, and keeping the residual pressure ratio low [
32,
36,
39]. At the same time, Al
3+ doping induces a significant increase in the N
d and a slight increase in the surface state density [
21], which together lead to a decrease in
.
The In
3+ ionic radius is slightly larger than that of Zn
2+, and a part of some In
3+ ions are solidly dissolved into the grains, which acts in the same role as Al
3+, increasing the electrical conductivity of the ZnO grains, decreasing the grain resistance, and reducing the residual pressure ratio [
22]. A fraction of the In
3+ ions act as donors at grain boundaries, increasing the interface state density (
Ns) and enhancing the double Schottky barrier (
) [
2,
36]. Furthermore, the residual In
3+ ions form particles, similar to spinel, that inhibit the growth of ZnO grains. With the further increase of the In
3+ doping ratio, since the ionic radius of In
3+ is smaller than that of Y
3+, it will occupy the position of Y
3+ in solid solution, which would lead to an excess of Y
3+ in the grain boundaries. In addition, the liquid phase of Sb-Bi-O appears at high temperatures, which will make the grain spacing larger, leading to the reduction of the Schottky barrier height and the nonlinear coefficient. The grain defect equation for In
3+-, Al
3+-, and Y
3+-doped ZnO can be expressed as [
40,
41]: