The Influence of Corrosion Processes on the Degradation of Concrete Cover
Abstract
:1. Introduction
Significance and Novelty
2. Materials
3. Test Methods
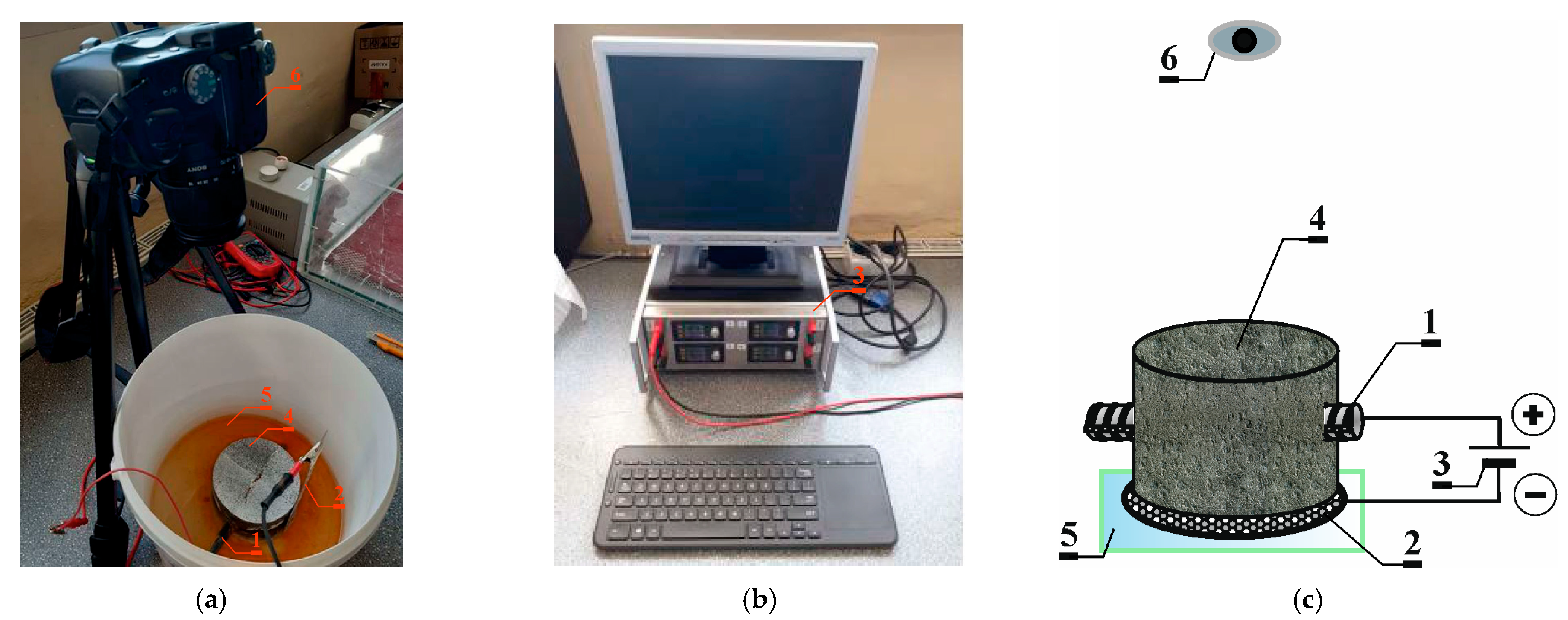
3.1. Accelerated Corrosion Process Method 1
3.1.1. Accelerated Process of Chloride Migration in Concrete
3.1.2. Accelerated Process of Corrosion
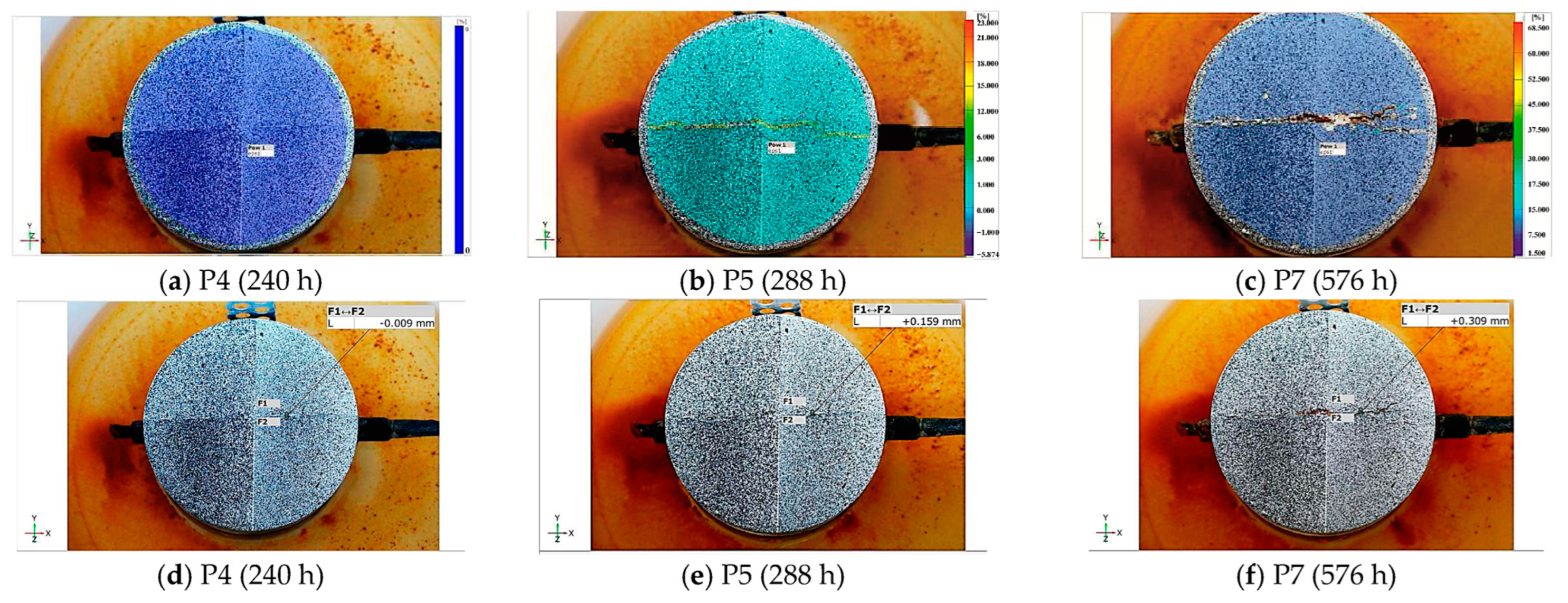
3.1.3. Measurement of the Crack Opening Width Using the Optical Method
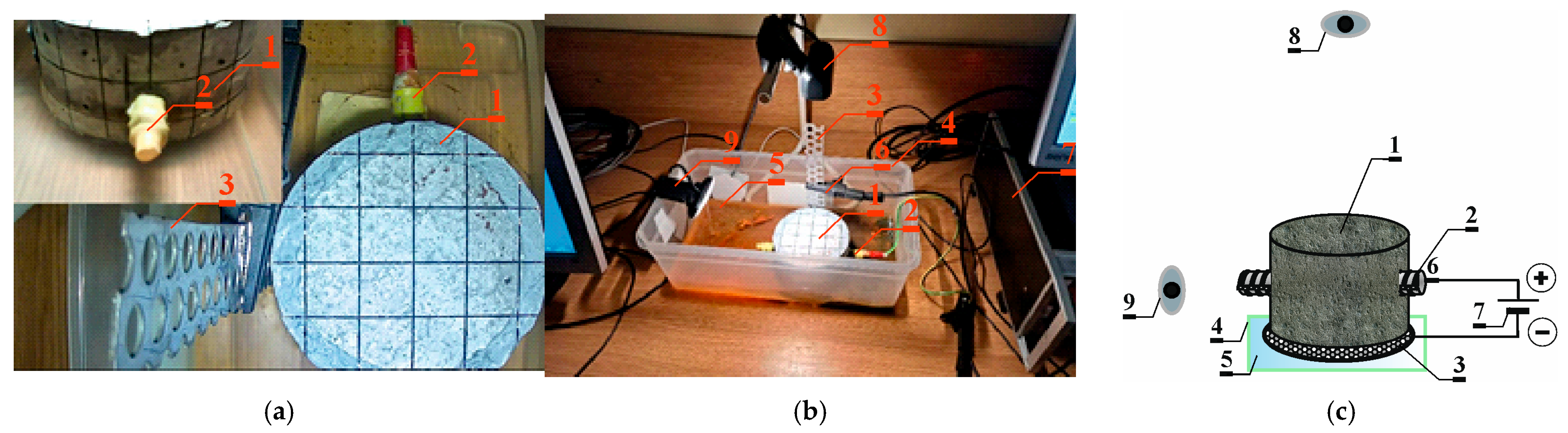
3.2. Accelerated Corrosion Process Method 2
4. Results and Discussion
4.1. Results of the Accelerated Corrosion Process—Method 1
4.1.1. Result of the Accelerated Process of Chloride Migration in Concrete
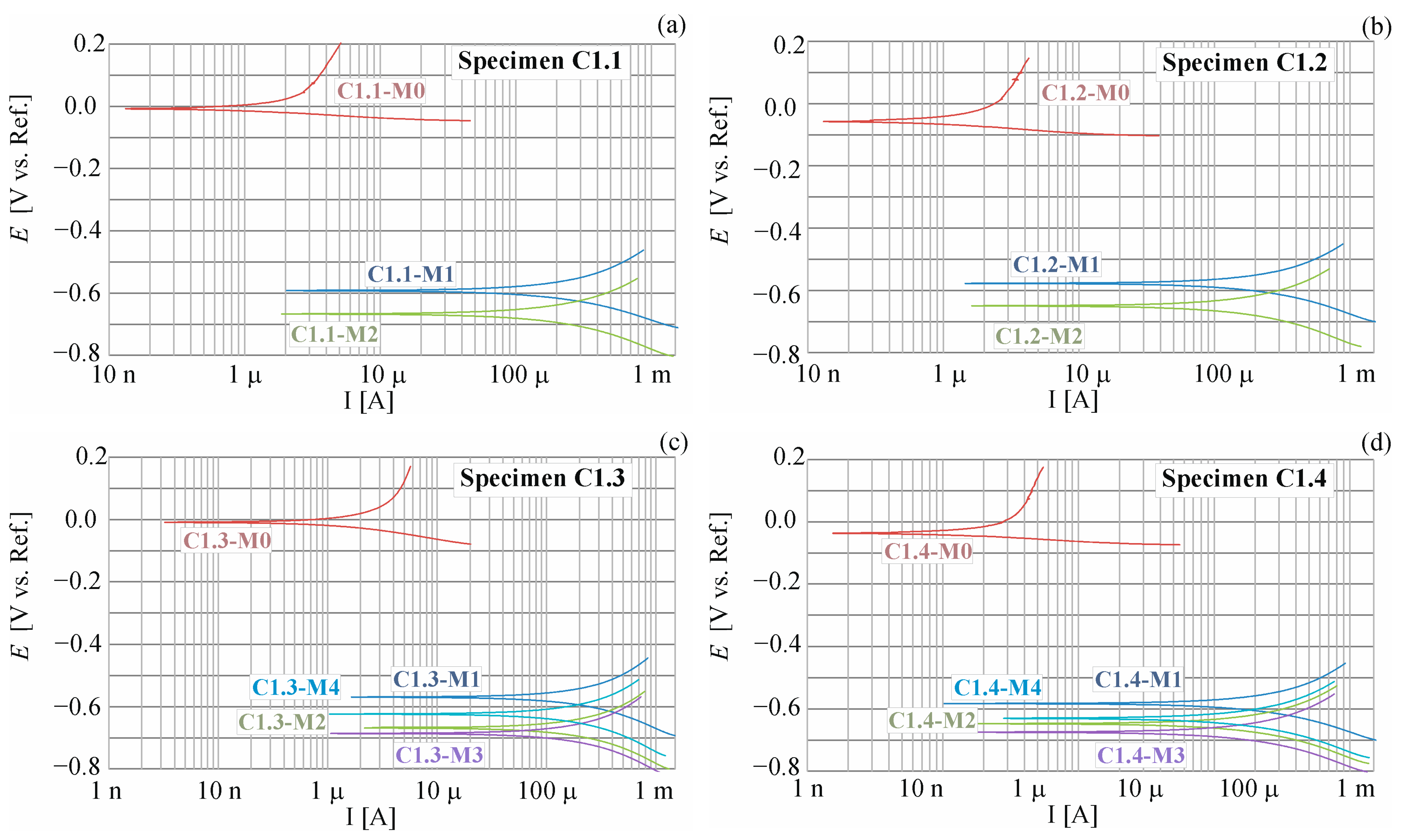
4.1.2. Result of the Accelerated Process of Corrosion
5. Conclusions
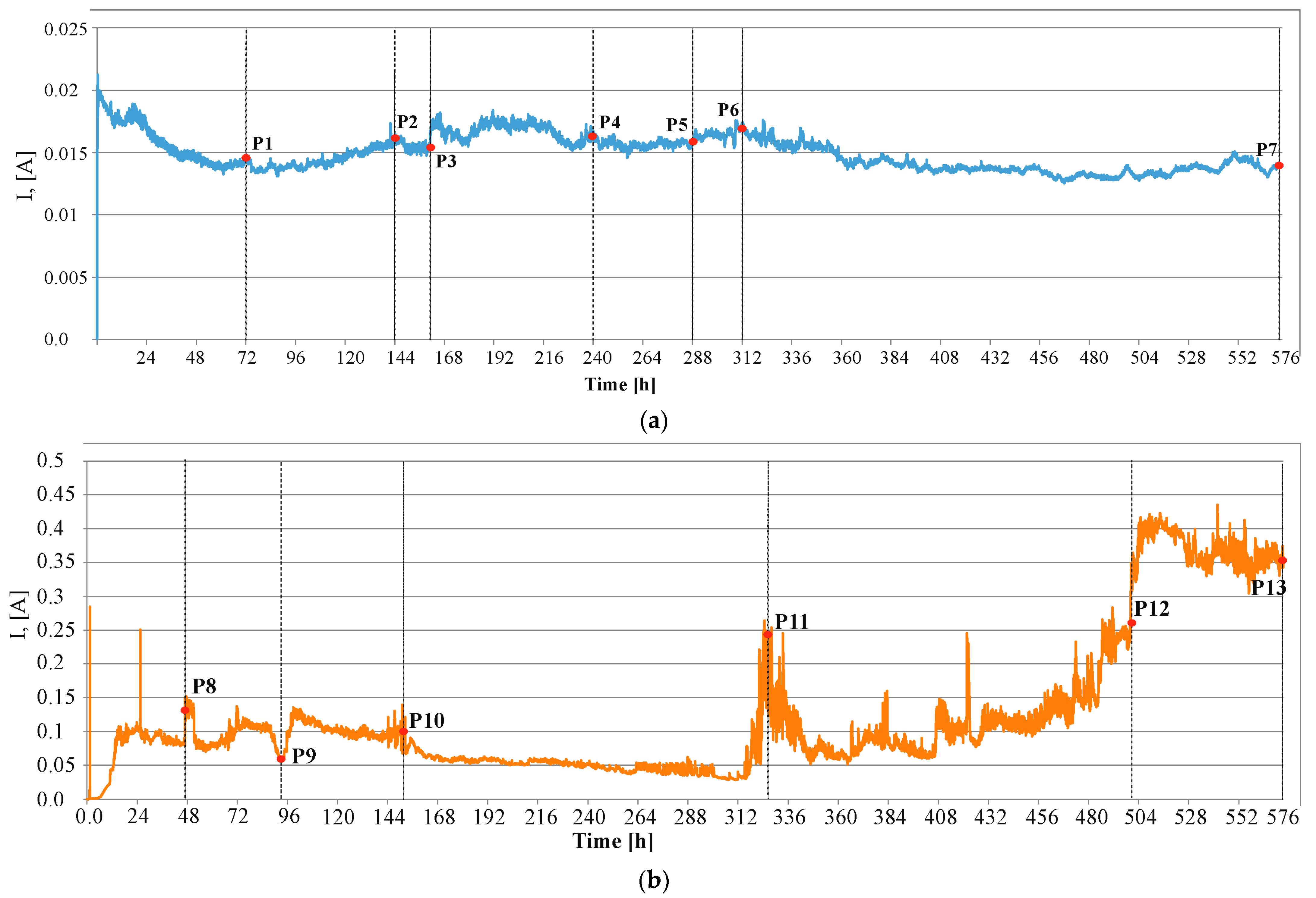
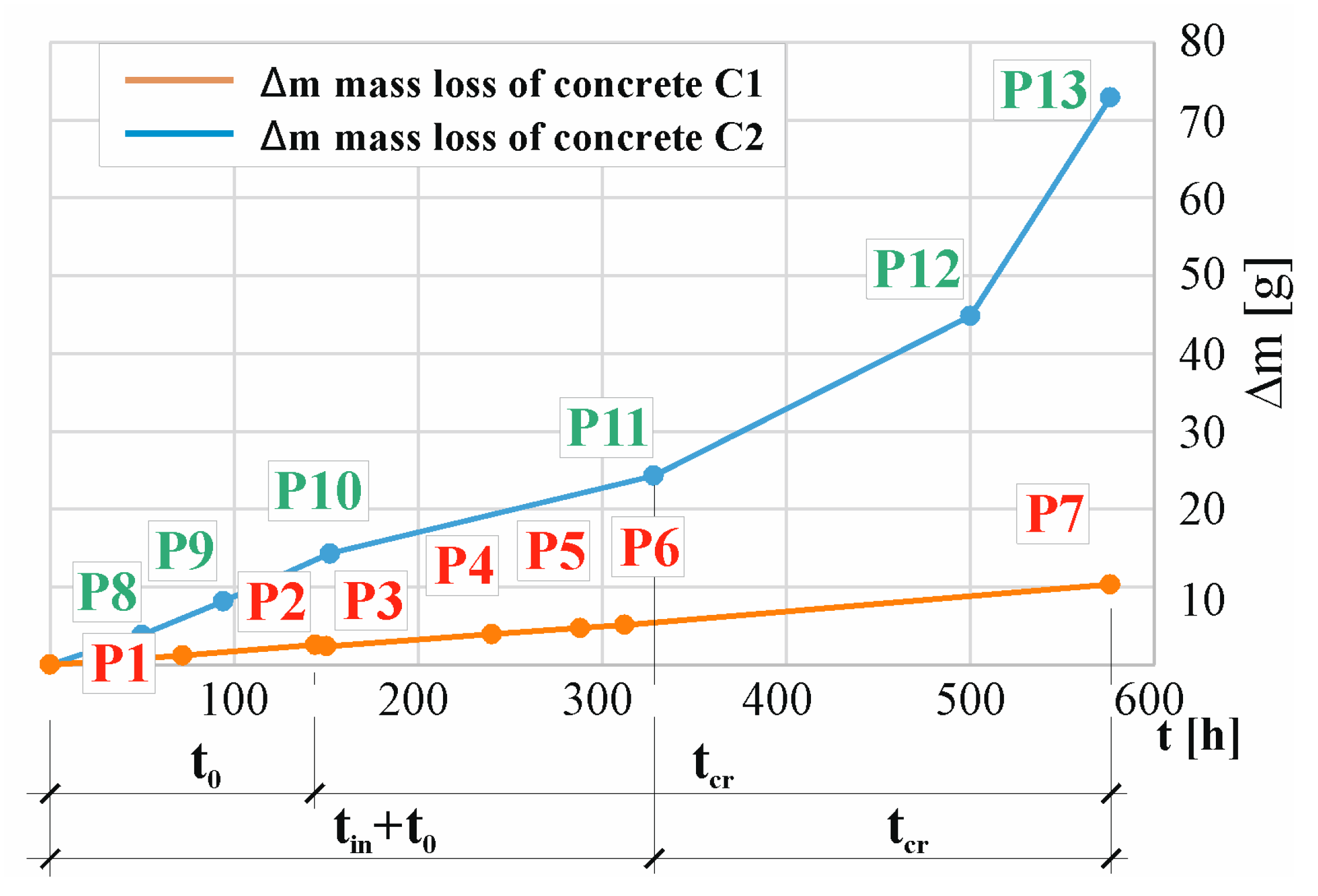
- Preliminary tests carried out using the accelerated electric field penetration of chloride ions into concrete to initiate corrosion and electrolysis to accelerate corrosion (first method in this work) allow for the estimation of the real time for the formation of concrete cracks.
- Based on the tests of the accelerated corrosion process (the second method in this study), it can be inferred that it is possible to estimate the real time for the formation of concrete cracks. However, it should be remembered that this time consists of the sum of , the time after which corrosion can be initiated, and , the time of mechanical impact on the cover concrete.
- The first method used, although more labor-intensive, allows for better control of the concentration of chloride ions contained in concrete, which can have a significant impact on the change in the mechanical properties of concrete.
- In the first method, more precise determination of the value of the corrosion current occurring in the natural corrosion process, depending on the concentration of chloride ions in the concrete and the type of materials used, is possible.
- Lack of continuous image measurement during the examination of the first method and the fact that the obtained images do not necessarily coincide with the time read from the current intensity graph based on the disturbances occurring in this graph were disadvantageous for this method.
- However, in the second method, a continuous image was obtained thanks to the use of a camera, but the quality of the obtained images is not sufficient for image analysis with the Gom correlate program. It is necessary to improve the method of recording image changes during the test by using better-quality cameras.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Measure No. | Time Days | Ecorr mV | ba mV | bc mV | Rp kΩ | RpA kΩcm2 | Icorr μA/cm2 | Vr mm/Year |
|---|---|---|---|---|---|---|---|---|
| C1.1-M0 | 0 | −9.3 | 667 | 21.3 | 10.08 | 229.22 | 0.04 | 0.00 |
| C1.1-M1 | 7 | −592 | 125 | 130 | 0.13 | 2.91 | 9.51 | 0.10 |
| C1.1-M2 | 14 | −667 | 75 | 154 | 0.14 | 3.21 | 6.83 | 0.08 |
| C1.2-M0 | 0 | −59.1 | 657 | 25.8 | 12.75 | 289.54 | 0.04 | 0.00 |
| C1.2-M1 | 7 | −578 | 113 | 141 | 0.13 | 2.89 | 9.43 | 0.10 |
| C1.2-M2 | 14 | −650 | 90 | 155 | 0.16 | 3.66 | 6.75 | 0.07 |
| C1.3-M0 | 0 | −9.4 | 502 | 56 | 11.38 | 258.78 | 0.08 | 0.00 |
| C1.3-M1 | 7 | −570 | 112 | 143 | 0.13 | 3.00 | 9.09 | 0.11 |
| C1.3-M2 | 14 | −668 | 86 | 167 | 0.14 | 3.23 | 7.63 | 0.09 |
| C1.3-M3 | 21 | −686 | 87 | 170 | 0.15 | 3.39 | 7.38 | 0.09 |
| C1.3-M4 | 28 | −624 | 81 | 174 | 0.15 | 3.46 | 6.94 | 0.08 |
| C1.4-M0 | 0 | −37.9 | 173 | 109 | 1.63 | 45.26 | 0.64 | 0.00 |
| C1.4-M1 | 7 | −583 | 667 | 21 | 7.58 | 172.28 | 0.05 | 0.12 |
| C1.4-M2 | 14 | −648 | 125 | 134 | 0.12 | 2.68 | 10.47 | 0.09 |
| C1.4-M3 | 21 | −675 | 99 | 149 | 0.14 | 3.23 | 8.00 | 0.09 |
| C1.4-M4 | 28 | −630 | 100 | 146 | 0.15 | 3.32 | 7.76 | 0.10 |
References
- Taerwe, L.; Matthys, S. Fib Model Code for Concrete Structures 2010; Ernst&Sohn: Berlin, Germany, 2013. [Google Scholar]
- EN 1992-1-1; Eurocode 2: Design of Concrete Structures-Part 1-1, General Rules and Rules for Buildings. European Committee for Standardization: Brussels, Belgium, 2004; Volume 1.
- Xia, J.; Jin, W.-L.; Zhao, Y.-X.; Li, L.-Y. Mechanical performance of corroded steel bars in concrete. Proc. Inst. Civ. Eng. Struct. Build. 2013, 166, 235–246. [Google Scholar] [CrossRef]
- Caré, S.; Nguyen, Q.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Jamali, A.; Angst, U.; Adey, B.; Elsener, B. Modeling of corrosion-induced concrete cover cracking: A critical analysis. Constr. Build. Mater. 2013, 42, 225–237. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, C.; Liu, H.; Yang, J.; Wang, X.; Tian, W.; Cao, K.; Zhang, T. Simulation and analysis of corrosion fracture of reinforced concrete based on phase field method. Case Stud. Constr. Mater. 2022, 17, e01366. [Google Scholar] [CrossRef]
- Suda, K.; Misra, S.; Motohashi, K. Corrosion products of reinforcing bars embedded in concrete. Corros. Sci. 1993, 35, 1543–1549. [Google Scholar] [CrossRef]
- Chehade, F.E.H.; Younes, R.; Mroueh, H.; Chehade, F.H. Time-dependent reliability analysis of reinforced-concrete bridges under the combined effect of corrosion, creep and shrinkage. WIT Trans. Built Environ. 2018, 174, 13–24. [Google Scholar] [CrossRef]
- Vu, K.A.T.; Stewart, M.G. Structural reliability of concrete bridges including improved chloride-induced corrosion models. Struct. Saf. 2000, 22, 313–333. [Google Scholar] [CrossRef]
- Stewart, M.G. Spatial variability of pitting corrosion and its influence on structural fragility and reliability of RC beams in flexure. Struct. Saf. 2004, 26, 453–470. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; CBI Research Report 4:82; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]
- Collepardi, M.; Marcialis, A.; Turriziani, R. Penetration of Chloride Ions into Cement Pastes and Concretes. J. Am. Ceram. Soc. 1972, 55, 534–535. [Google Scholar] [CrossRef]
- Tikalsky, P.J.; Pustka, D.; Marek, P. Statistical Variations in Chloride Diffusion in Concrete Bridges. ACI Struct. J. 2005, 102, 481. [Google Scholar] [CrossRef]
- Konečný, P.; Tikalsky, P.J.; Tepke, D.G. Performance Evaluation of Concrete Bridge Deck Affected by Chloride Ingress: Simulation-based reliability assessment and finite element modeling. Transp. Res. Rec. 2007, 2028, 3–8. [Google Scholar] [CrossRef]
- Vořechovská, D.; Podroužek, J.; Chromá, M.; Rovnaníková, P.; Teplý, B. Modeling of Chloride Concentration Effect on Reinforcement Corrosion. Comput. Civ. Infrastruct. Eng. 2009, 24, 446–458. [Google Scholar] [CrossRef]
- Marsavina, L.; Audenaert, K.; De Schutter, G.; Faur, N.; Marsavina, D. Experimental and numerical determination of the chloride penetration in cracked concrete. Constr. Build. Mater. 2009, 23, 264–274. [Google Scholar] [CrossRef]
- Segovia, E.; de Vera, G.; Miró, M.; Ramis, J.; Climent, M. Cement mortar cracking under accelerated steel corrosion test: A mechanical and electrochemical model. J. Electroanal. Chem. 2021, 896, 115222. [Google Scholar] [CrossRef]
- Castañeda-Valdés, A.; Corvo, F.; Marrero-Águila, R.; Fernández-Domínguez, A.; Del Angel-Meraz, E. The service life of reinforced concrete structures in an extremely aggressive coastal city. Influence of concrete quality. Mater. Struct. Constr. 2023, 56, 1–16. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, R.; Chakraborty, S.; Guadagnini, M.; Pilakoutas, K. Chemical attack and corrosion resistance of concrete prepared with electrolyzed water. J. Mater. Res. Technol. 2021, 11, 1193–1205. [Google Scholar] [CrossRef]
- Ahmad, S. Techniques for Inducing Accelerated Corrosion of Steel in Concrete. Arab. J. Sci. Eng. 2016, 34, 95. [Google Scholar]
- Ballim, Y.; Reid, J. Reinforcement corrosion and the deflection of RC beams—An experimental critique of current test methods. Cem. Concr. Compos. 2003, 25, 625–632. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Zhao, Y.; Wang, S.; Guan, Z. Concrete Cover Cracking and Reinforcement Corrosion Behavior in Concrete with New-to-Old Concrete Interfaces. Materials 2023, 16, 5969. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Wang, Y. Corrosion-Effected Bond Behavior between PVA-Fiber-Reinforced Concrete and Steel Rebar under Chloride Environment. Materials 2023, 16, 2666. [Google Scholar] [CrossRef]
- Caré, S.; Raharinaivo, A. Influence of impressed current on the initiation of damage in reinforced mortar due to corrosion of embedded steel. Cem. Concr. Res. 2007, 37, 1598–1612. [Google Scholar] [CrossRef]
- Yuan, Y.; Ji, Y.; Shah, S.P. Comparison of Two Accelerated Corrosion Techniques for Concrete Structures. ACI Struct. J. 2007, 104, 344–347. [Google Scholar] [CrossRef]
- Geng, C.; Xu, Y.; Weng, D. A new method to quickly assess the inhibitor efficiency. J. Wuhan Univ. Technol. Sci. Ed. 2008, 23, 950–954. [Google Scholar] [CrossRef]
- Krykowski, T.; Jaśniok, T.; Recha, F.; Karolak, M. A Cracking Model for Reinforced Concrete Cover, Taking Account of the Accumulation of Corrosion Products in the ITZ Layer, and Including. Materials 2020, 13, 5375. [Google Scholar] [CrossRef]
- Bažant, Z.P. Physical Model for Steel Corrosion in Concrete Sea Structures—Application. J. Struct. Div. 1979, 105, 1155–1166. [Google Scholar] [CrossRef]
- Khan, I.; François, R.; Castel, A. Prediction of reinforcement corrosion using corrosion induced cracks width in corroded reinforced concrete beams. Cem. Concr. Res. 2014, 56, 84–96. [Google Scholar] [CrossRef]
- Liu, Y.; Weyers, R.E. Modeling the time-to-corrosion cracking in chloride contaminated reinforced concrete structures. Materials 1998, 95, 675–681. [Google Scholar]
- Vidal, T.; Castel, A.; François, R. Analyzing crack width to predict corrosion in reinforced concrete. Cem. Concr. Res. 2004, 34, 165–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, J.; Wu, Y.; Jin, W. Corrosion-induced concrete cracking model considering corrosion. Constr. Build. Mater. 2016, 116, 273–280. [Google Scholar] [CrossRef]
- Bossio, A.; Monetta, T.; Bellucci, F.; Lignola, G.P.; Prota, A. Modeling of concrete cracking due to corrosion process of reinforcement bars. Cem. Concr. Res. 2015, 71, 78–92. [Google Scholar] [CrossRef]
- Recha, F.; Yurkova, K.; Krykowski, T. Application of Interval Analysis to Assess Concrete Cover Degradation in Accelerated Corrosion Tests. Materials 2023, 16, 5845. [Google Scholar] [CrossRef]
- Ranjith, A.; Rao, K.B.; Manjunath, K. Evaluating the effect of corrosion on service life prediction of RC structures—A parametric study. Int. J. Sustain. Built Environ. 2016, 5, 587–603. [Google Scholar] [CrossRef]
- Shi, R.; Pan, Z.; Lun, P.; Zhan, Y.; Nie, Z.; Liu, Y.; Mo, Z.; He, Z. Research on Corrosion Rate Model of Reinforcement in Concrete under Chloride Ion Environments. Buildings 2023, 13, 965. [Google Scholar] [CrossRef]
- Chernin, L.; Val, D.V.; Volokh, K.Y. Analytical modelling of concrete cover cracking caused by corrosion of reinforcement. Mater. Struct. 2010, 43, 543–556. [Google Scholar] [CrossRef]
- Cao, C. 3D simulation of localized steel corrosion in chloride contaminated reinforced concrete. Constr. Build. Mater. 2014, 72, 434–443. [Google Scholar] [CrossRef]
- Guzmán, S.; Gálvez, J.C.; Sancho, J.M. Modelling of corrosion-induced cover cracking in reinforced concrete by an embedded cohesive crack finite element. Eng. Fract. Mech. 2012, 93, 92–107. [Google Scholar] [CrossRef]
- Michel, A.; Pease, B.J.; Peterová, A.; Geiker, M.R.; Stang, H.; Thybo, A.E.A. Penetration of corrosion products and corrosion-induced cracking in reinforced cementitious materials: Experimental investigations and numerical simulations. Cem. Concr. Compos. 2014, 47, 75–86. [Google Scholar] [CrossRef]
- Sola, E.; Ožbolt, J.; Balabanić, G.; Mir, Z. Experimental and numerical study of accelerated corrosion of steel reinforcement in concrete: Transport of corrosion products. Cem. Concr. Res. 2019, 120, 119–131. [Google Scholar] [CrossRef]
- Cabrera, J. Deterioration of concrete due to reinforcement steel corrosion. Cem. Concr. Compos. 1996, 9465, 47–59. [Google Scholar] [CrossRef]
- Mogire, P. Correlation of Accelerated Corrosion and Real Reinforced Concrete Water Structures. J. Mater. Sci. Res. 2023, 12, 88. [Google Scholar] [CrossRef]
- Park, S.S.; Kwon, S.-J.; Song, H.-W. Analysis technique for restrained shrinkage of concrete containing chlorides. Mater. Struct. 2011, 44, 475–486. [Google Scholar] [CrossRef]
- Jain, S.; Pradhan, B. Fresh, mechanical, and corrosion performance of self-compacting concrete in the presence of chloride ions. Constr. Build. Mater. 2020, 247, 118517. [Google Scholar] [CrossRef]
- Szweda, Z.; Mazurkiewicz, J.; Konečný, P.; Ponikiewski, T. Effect of Imperial Smelting Process Slag Addition in Self Compacting Concrete Concrete on the Efficiency of Electrochemical Chloride Extraction. Materials 2023, 16, 5159. [Google Scholar] [CrossRef] [PubMed]
- Perkowski, Z.; Szweda, Z. The “Skin Effect” Assessment of Chloride Ingress into Concrete Based on the Identification of Effective and Apparent Diffusivity. Appl. Sci. 2022, 12, 1730. [Google Scholar] [CrossRef]
- Szweda, Z. Evaluating the Impact of Concrete Design on the Effectiveness of the Electrochemical Chloride Extraction Process. Materials 2023, 16, 666. [Google Scholar] [CrossRef]
- Szweda, Z.; Gołaszewski, J.; Ghosh, P.; Lehner, P.; Konečný, P. Comparison of Standardized Meth-ods for Determining the Diffusion Coefficient of Chloride in Concrete with Thermodynamic Model of Migration. Materials 2023, 16, 637. [Google Scholar] [CrossRef]
- Szweda, Z.; Jaśniok, T.; Jaśniok, M. Evaluation of the effectiveness of electrochemical chloride extraction from concrete on the basis of testing reinforcement polarization and chloride concentra-tion. Ochr. Przed Korozją 2018, 61, 3–9. [Google Scholar] [CrossRef]
- Szweda, Z.; Zybura, A. Theoretical Model and Experimental Tests on Chloride Diffusion and Migration Processes in Concrete. Procedia Eng. 2013, 57, 1121–1130. [Google Scholar] [CrossRef]
- Torres-Acosta, A.A.; Navarro-Gutierrez, S.; Terán-Guillén, J. Residual flexure capacity of corroded reinforced concrete beams. Eng. Struct. 2007, 29, 1145–1152. [Google Scholar] [CrossRef]
- Dixit, M.; Gupta, A.K. Assessment of Corrosion in Rebars by Impressed Current Technique. Lect. Notes Civ. Eng. 2021, 143, 89–97. [Google Scholar] [CrossRef]
- Auyeung, Y.; Balaguru, P.N.; Chung, L. Bond Behavior Of Corroded Reinforce-Ment Bars. Aci Mater. J. 2000, 97, 214–220. [Google Scholar]
- Szweda, Z.; Skórkowski, A.; Konečný, P. The influence of corrosion processes on the degradation of concrete cover, [Data set]. Zenodo 2022. [CrossRef]
- Szweda, Z.; Kuziak, J.; Sozańska-Jędrasik, L.; Czachura, D. Analysis of the Effect of Protective Properties of Concretes with Similar Composition on the Corrosion Rate of Reinforcing Steel Induced by Chloride Ions. Materials 2023, 16, 3889. [Google Scholar] [CrossRef]
- Miarka, P.; Seitl, S.; Horňáková, M.; Lehner, P.; Konečný, P.; Sucharda, O.; Bílek, V. Influence of chlorides on the fracture toughness and fracture resistance under the mixed mode I/II of high-performance concrete. Theor. Appl. Fract. Mech. 2020, 110, 102812. [Google Scholar] [CrossRef]
- Sungkono, K.K.D.; Satyarno, I.; Priyosulistyo, H.; Perdana, I. Corrosion Resistance of High Calcium Fly Ash Based Reinforced Geopolymer Concrete in Marine Environment. Civ. Eng. Arch. 2023, 11, 3175–3189. [Google Scholar] [CrossRef]
- Miranda, J.; Cobo, A.; Otero, E.; González, J. Limitations and advantages of electrochemical chloride removal in corroded reinforced concrete structures. Cem. Concr. Res. 2007, 37, 596–603. [Google Scholar] [CrossRef]
- Tang, F.; Lin, Z.; Chen, G.; Yi, W. Three-dimensional corrosion pit measurement and statistical mechanical degradation analysis of deformed steel bars subjected to accelerated corrosion. Constr. Build. Mater. 2014, 70, 104–117. [Google Scholar] [CrossRef]






| No. | Compressive Strength [MPa] | Density [kg/m3] | Porosity [%] |
|---|---|---|---|
| C1 | 54.2 | 2271 | 12 |
| C2 | 49.5 | 2269 | 7 |
| Constituent % mass | Concrete | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | Eq. Na2O | SO3 | Cl |
| C1 | 19.38 | 4.57 | 3.59 | 63.78 | 1.38 | 0.58 | 0.21 | 0.59 | 3.26 | 0.069 | |
| C2 | 29.08 | 6.30 | 1.37 | 48.82 | 4.36 | 0.73 | 0.34 | 0.82 | 2.74 | 0.066 |
| Mixture ID. | Sand (0–2) * mm [kg/m3] | Gravel (2–8) * mm [kg/m3] | Gravel (8–16) * mm [kg/m3] | Type of Cement | Cement [kg/m3] | w/c |
|---|---|---|---|---|---|---|
| C1 | 722 | 512 | 2271 | CEM I 42.5 R * | 681 | 0.3 |
| C2 | CEM III/A 42.5 N-LH/HSR/NA * |
| P1 (72 h) | P2 (144 h) | P3 (150 h) | P4 (240 h) | P5 (288 h) | P6 (312 h) | P7 (576 h) |
|---|---|---|---|---|---|---|
| 1.19 (0.0) | 2.27 (0.11) | 2.38 (0.16) | 3.93 (0.89) | 4.71 (1.27) | 5.12 (1.45) | 10.32 (3.55) |
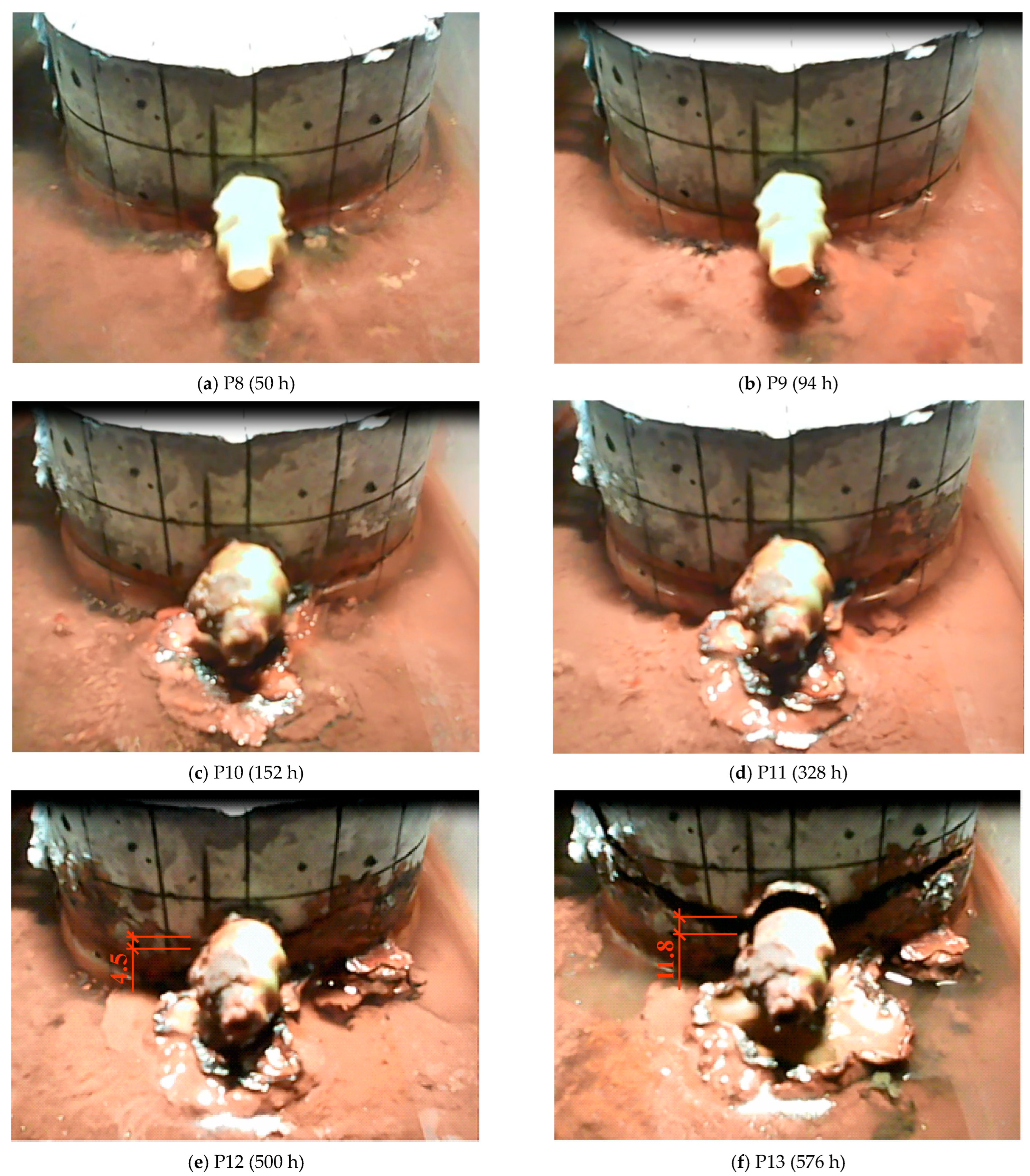
| P8 (50 h) | P19 (94 h) | P10 (152 h) | P11 (328 h) | P12 (500 h) | P13 (576 h) | - |
| 3.83 (0.0) | 8.14 (0.0) | 14.27 (0.0) | 24.28 (0.0) | 44.82 (4.5) | 72.95 (11.84) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szweda, Z.; Skórkowski, A.; Konečný, P. The Influence of Corrosion Processes on the Degradation of Concrete Cover. Materials 2024, 17, 1398. https://doi.org/10.3390/ma17061398
Szweda Z, Skórkowski A, Konečný P. The Influence of Corrosion Processes on the Degradation of Concrete Cover. Materials. 2024; 17(6):1398. https://doi.org/10.3390/ma17061398
Chicago/Turabian StyleSzweda, Zofia, Artur Skórkowski, and Petr Konečný. 2024. "The Influence of Corrosion Processes on the Degradation of Concrete Cover" Materials 17, no. 6: 1398. https://doi.org/10.3390/ma17061398






