Improved Thermal and Electrical Properties of P-I-N-Structured Perovskite Solar Cells Using ZnO-Added PCBM as Electron Transport Layer
Abstract
:1. Introduction
2. Materials and Methods
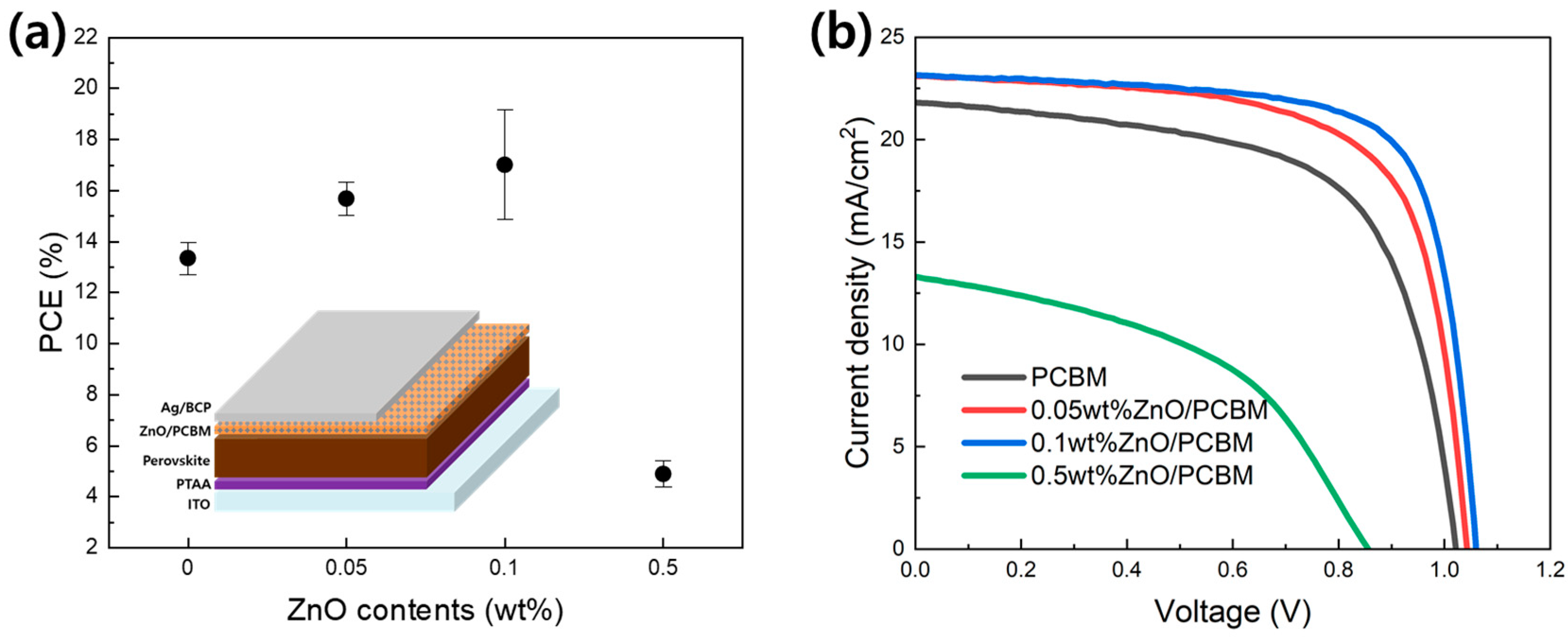
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NREL. Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf (accessed on 13 February 2024).
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 27, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic–organic hybrid nanostructured solar cells. Nano Lett. 2013, 10, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Gaspera, E.D.; Peng, Y.; Hou, Q.; Spiccia, L.; Bach, U.; Jasieniak, J.J.; Cheng, Y.B. Ultra-thin high efficiency semitransparent perovskite solar cells. Nano Energy 2015, 1, 249–257. [Google Scholar] [CrossRef]
- Teixeira, C.; Spinelli, P.; Castriotta, L.A.; Muller, D.; Oz, S.; Andrade, L.; Mendes, A.; Carlo, A.D.; Wurfel, U.; Wojciechowski, K.; et al. Charge Extraction in Flexible Perovskite Solar Cell Architectures for Indoor Applications–with up to 31% Efficiency. Adv. Funct. Mater. 2022, 32, 2206761. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef]
- Chen, Q.; Marco, N.D.; Yang, Y.M.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the spotlight: The organic–inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, S.; Bae, S.; Cho, K.; Chung, T.; Mundt, L.E.; Lee, S.; Park, S.; Park, H.; Schubert, M.C.; et al. UV degradation and recovery of perovskite solar cells. Sci. Rep. 2016, 6, 38150. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.; Lee, S.-W.; Cho, K.J.; Park, S.; Lee, S.; Kang, Y.; LEE, H.-S.; Kim, D. Electric-field-induced degradation of methylammonium lead iodide perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Han, G.S.; Park, N.-G.; Ko, M.J. Flexible perovskite solar cells. Joule 2019, 3, 1850–1880. [Google Scholar] [CrossRef]
- Dou, B.; Whitaker, J.B.; Bruening, K.; Moore, D.T.; Wheeler, L.M.; Ryter, J.; Breslin, N.J.; Berry, J.J.; Garner, S.M.; Barnes, F.S.; et al. Roll-to-roll printing of perovskite solar cells. ACS Energy Lett. 2018, 3, 2558–2565. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, X.; Yang, S. Interface engineering for highly efficient and stable planar p-i-n perovskite solar cells. Adv. Energy Mater. 2018, 8, 1701883. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Wolff, C.M.; Marquez, J.A.; Zhang, S.; Hages, C.J.; Rothhardt, D.; Albrecht, S.; Burn, P.L.; Meredith, P.; Unold, T.; et al. Visualization and suppression of interfacial recombination for high-efficiency large-area pin perovskite solar cells. Nat. Energy 2018, 3, 847–854. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, C.; Gong, C.; Zhang, D.; Zhang, H.; Zhuang, Q.; Yu, X.; Gong, S.; Chen, X.; Yang, J.; et al. 2D/3D heterojunction engineering at the buried interface towards high-performance inverted methylammonium-free perovskite solar cells. Nat. Energy 2023, 8, 946–955. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, S.; Vikraman, D.; Lee, J.; Jaffery, S.H.A.; Jung, J.; Kim, H.-S.; Kang, J. Fabrication of InGaZnO-SnO2/PCBM hybrid electron transfer layer for high-performance Perovskite solar cell and X-ray detector. J. Alloys Compd. 2022, 906, 164399. [Google Scholar] [CrossRef]
- Mardi, S.; Yusupov, K.; Martinez, P.M.; Zakhidov, A.; Vomiero, A.; Reale, A. Enhanced thermoelectric properties of poly (3-hexylthiophene) through the Incorporation of aligned carbon nanotube forest and chemical treatments. ACS Omega 2021, 6, 1073–1082. [Google Scholar] [CrossRef]
- Pohls, J.-H.; Johnson, M.B.; White, M.A. Origins of ultralow thermal conductivity in bulk [6,6]-phenyl-C61-butyric acid methyl ester (PCBM). Phys. Chem. Chem. Phys. 2016, 18, 1185–1190. [Google Scholar] [CrossRef]
- Choi, K.; Lee, J.; Choi, H.; Kim, G.-W.; Kim, H.I. Heat dissipation effects on the stability of planar perovskite solar cells. Energy Environ. Sci. 2020, 13, 5059–5067. [Google Scholar] [CrossRef]
- Jiang, J.; You, J.; Liu, S.F.; Xi, J. Scale-up solutions of 2D perovskite photovoltaics: Insights of multiscale structures. ACS Energy Lett. 2024, 9, 17–29. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Peng, J.; Tang, J.; Zheng, K.; Liang, Z. 2D Ruddlesden-popper perovskites for optoelectronics. Adv. Mat. 2018, 30, 1703487. [Google Scholar] [CrossRef]
- Heo, J.H.; Zhang, F.; Park, J.K.; Lee, H.J.; Lee, D.S.; Heo, S.J.; Luther, J.M.; Berry, J.J.; Zhu, K.; Im, S.H. Surface engineering with oxidized Ti3C2Tx MXene enables efficient and stable p-i-n-structured CsPbI3 perovskite solar cells. Joule 2022, 6, 1672–1688. [Google Scholar] [CrossRef]
- Soultati, A.; Nunzi, F.; Fakharuddin, A.; Verykios, A.; Armadorou, K.K.; Tountas, M.; Panagiotakis, S.; Polydorou, E.; Charisiadis, A.; Nikolaou, V.; et al. Functionalized BODIPYs as tailor-made and universal interlayers for efficient and stable organic and perovskite solar cells. Adv. Mater. Interfaces 2022, 9, 2102324. [Google Scholar] [CrossRef]
- Pei, F.; Li, N.; Chen, Y.; Niu, X.; Zhang, Y.; Guo, Z.; Huang, Z.; Zai, H.; Liu, G.; Zhang, Y.; et al. Thermal Management Enables More Efficient and Stable Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 3029–3036. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Lin, K.; Wang, J.; Hu, B.; Dong, Y.; Xia, D.; Yang, Y. Heat diffusion optimization in high performance perovskite solar cells integrated with zeolite. J. Energy Chem. 2023, 86, 308–317. [Google Scholar] [CrossRef]
- Zhang, Q.; Park, K.; Cao, G. Synthesis of ZnO aggregates and their application in dye-sensitized solar cells. Mater. Matters 2010, 5, 32–38. [Google Scholar]
- Ghanbarian, B.; Daigle, H. Thermal conductivity in porous media: Percolation-based effective-medium approximation. Water Resour. Res. 2016, 52, 295–314. [Google Scholar] [CrossRef]
- Gurijala, A.; Zando, R.B.; Faust, J.L.; Barber, J.R.; Zhang, L.; Erb, R.M. Castable and printable dielectric composites exhibiting high thermal conductivity via percolation-enabled phonon transport. Matter 2020, 2, 1015–1024. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; Angelis, F.d.; Kelly, T.L. Origin of the thermal instability in CH3NH3PbI3 thin films deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Yao, K.; Leng, S.; Liu, Z.; Fei, L.; Chen, Y.; Li, S.; Zhou, N.; Zhang, J.; Xu, Y.-X.; Zhou, L.; et al. Fullerene-Anchored Core-Shell ZnO Nanoparticles for Efficient and Stable Dual-Sensitized Perovskite Solar Cells. Joule 2019, 3, 417–431. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Huang, W.-K.; Chang, Y.-C.; Lee, K.-T.; Chen, C.-T. A solution-processed n-doped fullerene cathode interfacial layer for efficient and stable large-area perovskite solar cells. J. Mater. Chem. A. 2016, 4, 640–648. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Huang, Z.; Dai, R.; Sheng, W.; Gong, C.; Tan, L.; Chen, Y. A Regularity-Based Fullerene Interfacial Layer for Efficient and Stable Perovskite Solar Cells via Blade-Coating. Adv. Funct. Mater. 2022, 32, 2105917. [Google Scholar] [CrossRef]
- Bin, Z.; Li, J.; Wang, L.; Duan, L. Efficient n-type dopants with extremely low doping ratios for high performance inverted perovskite solar cells. Energy Environ. Sci. 2016, 9, 3424–3428. [Google Scholar] [CrossRef]
- Chang, T.-C.; Liao, C.-Y.; Lee, C.-T.; Lee, H.-Y. Investigation of the Performance of Perovskite Solar Cells with ZnO-Covered PC61BM Electron Transport Layer. Materials 2023, 16, 5061. [Google Scholar] [CrossRef]

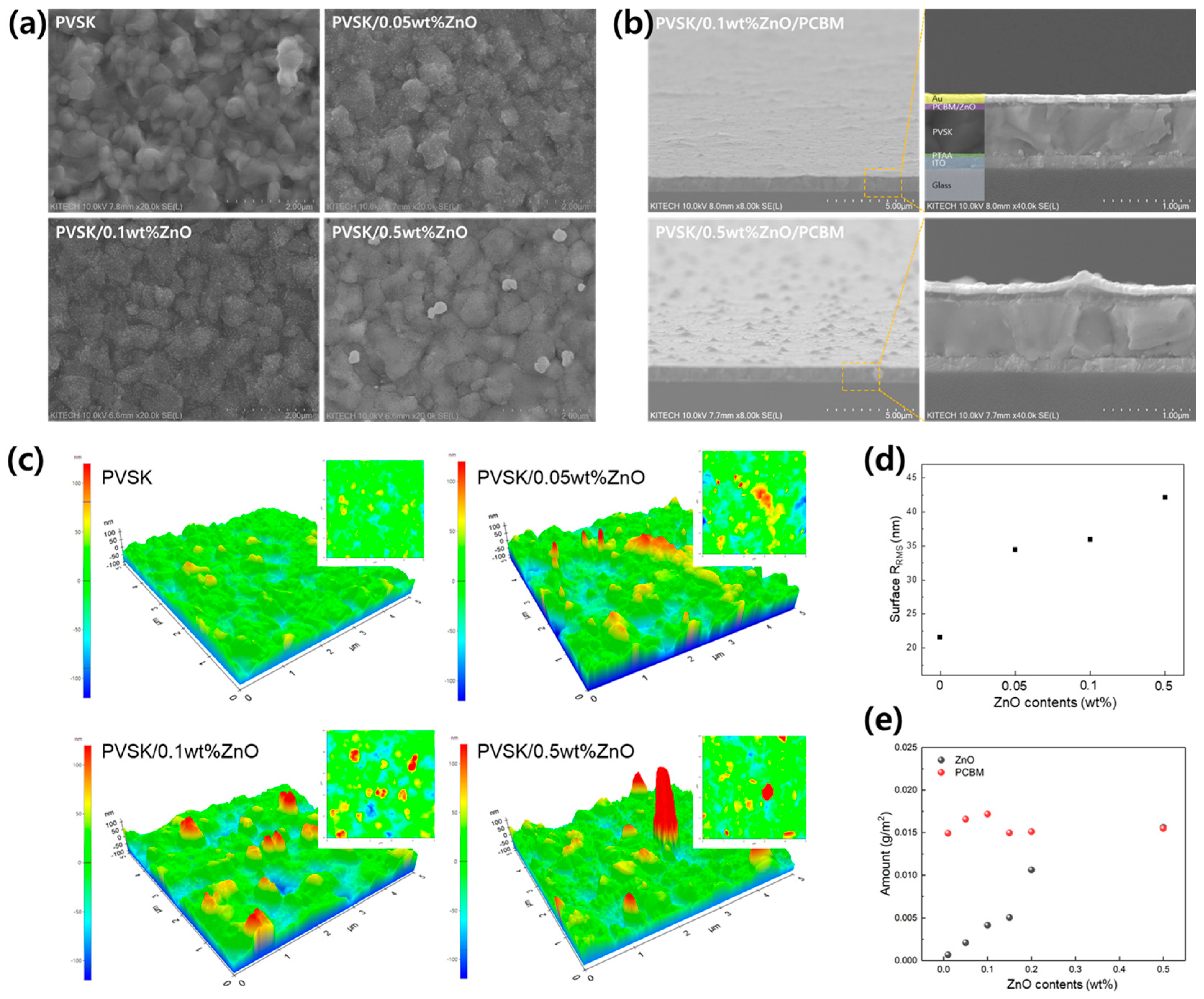
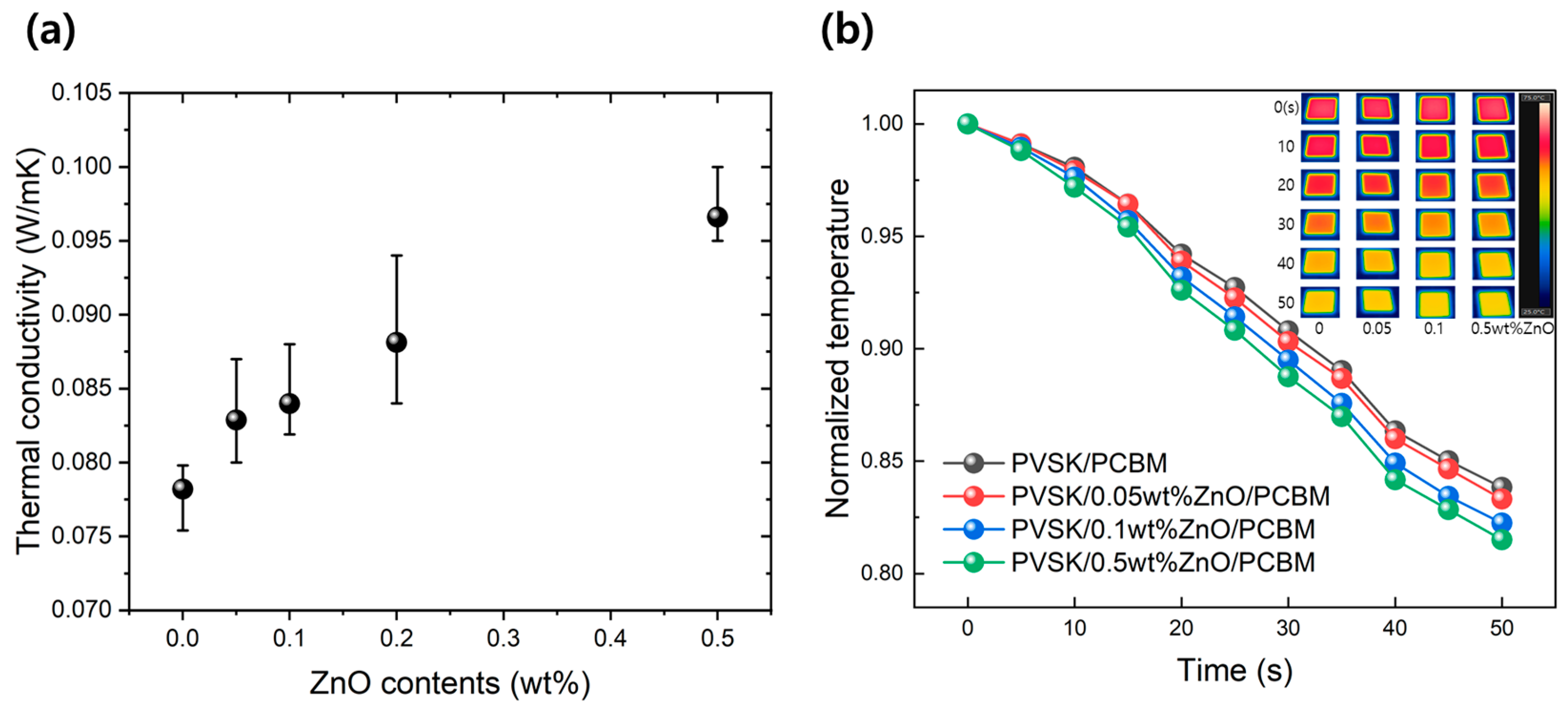
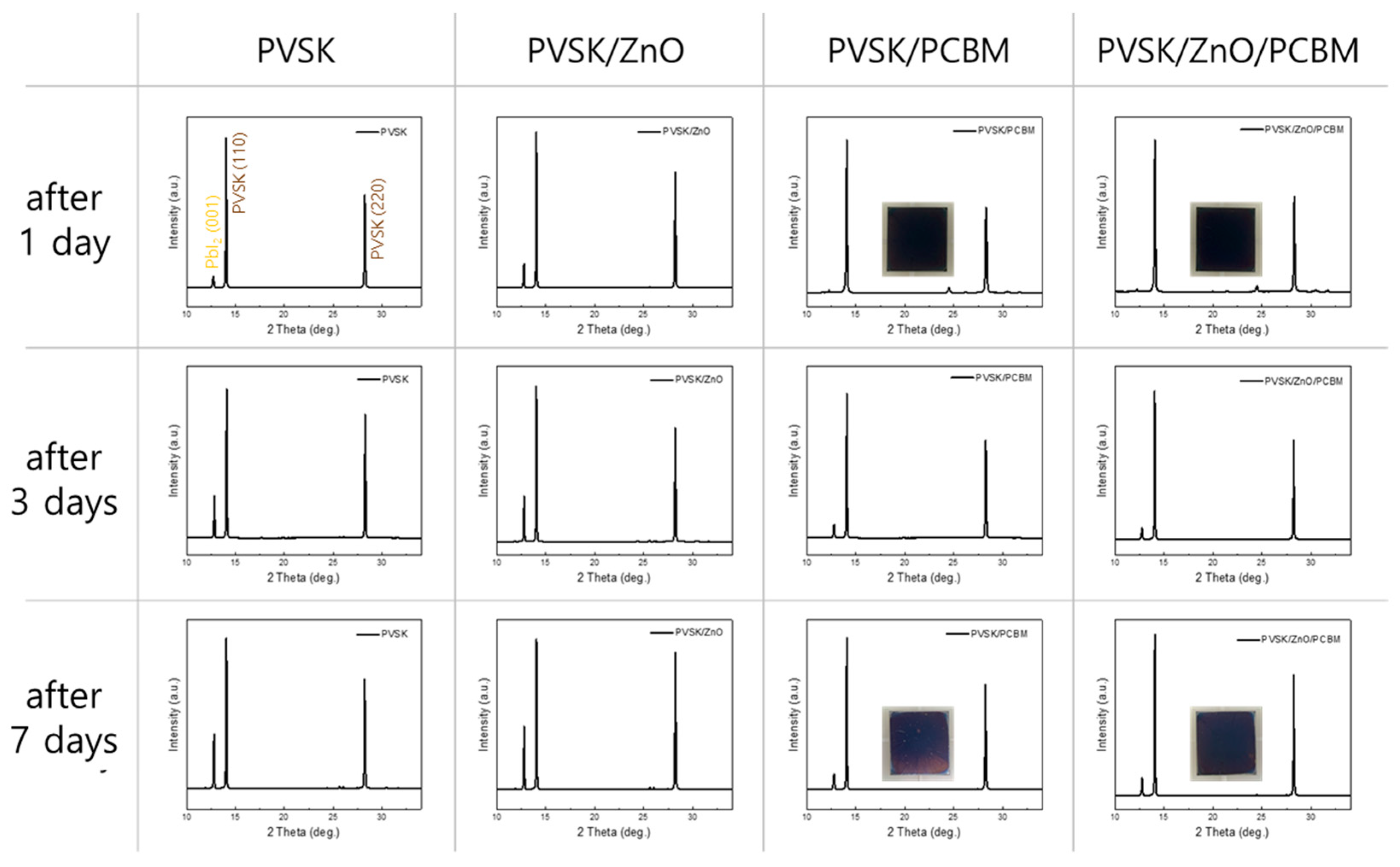

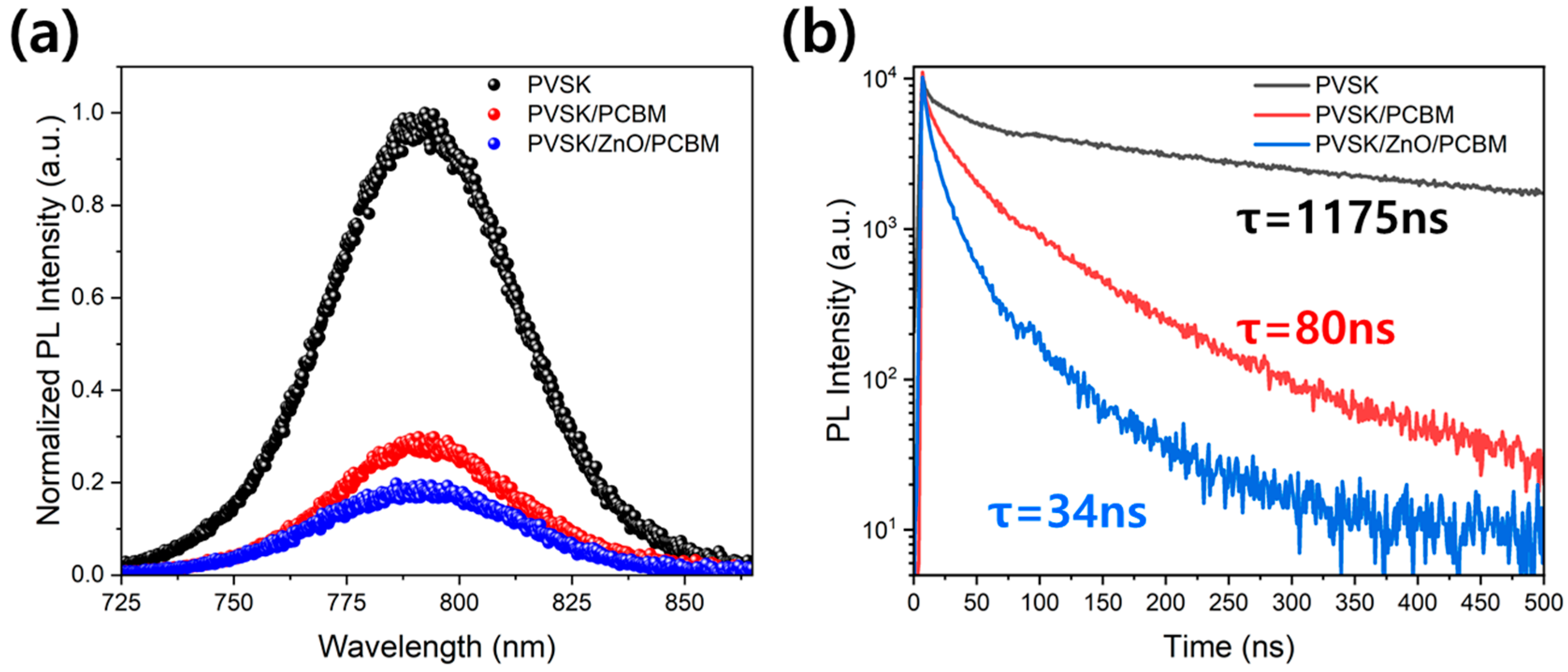
| ZnO Concentration (wt%) | Amount of ZnO (g/m2) | Amount of PCBM (g/m2) | Amount of ZnO in PCBM | |
|---|---|---|---|---|
| wt% | vol% | |||
| 0.01 | 0.00069 | 0.01493 | 4.44 | 1.33 |
| 0.05 | 0.00211 | 0.01658 | 11.28 | 3.56 |
| 0.10 | 0.00416 | 0.01718 | 19.48 | 6.57 |
| 0.15 | 0.00507 | 0.01497 | 25.31 | 8.97 |
| 0.20 | 0.01063 | 0.01511 | 41.30 | 16.98 |
| 0.50 | 0.01562 | 0.01549 | 49.79 | 22.38 |
| ETL Structures | Voltage (V) | Current Density (mA/cm2) | Fill Factor (%) | PCE (%) |
|---|---|---|---|---|
| PCBM | 1.02 | 21.73 | 63.60 | 14.12 |
| 0.05wt%ZnO/PCBM | 1.04 | 23.00 | 68.81 | 16.51 |
| 0.1wt%ZnO/PCBM | 1.06 | 23.03 | 73.58 | 17.97 |
| 0.5wt%ZnO/PCBM | 0.86 | 13.25 | 46.34 | 5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Han, D.; Kim, S.; Mo, C.B. Improved Thermal and Electrical Properties of P-I-N-Structured Perovskite Solar Cells Using ZnO-Added PCBM as Electron Transport Layer. Materials 2024, 17, 1376. https://doi.org/10.3390/ma17061376
Jeong Y, Han D, Kim S, Mo CB. Improved Thermal and Electrical Properties of P-I-N-Structured Perovskite Solar Cells Using ZnO-Added PCBM as Electron Transport Layer. Materials. 2024; 17(6):1376. https://doi.org/10.3390/ma17061376
Chicago/Turabian StyleJeong, Younghun, Dongwoon Han, Seongtak Kim, and Chan Bin Mo. 2024. "Improved Thermal and Electrical Properties of P-I-N-Structured Perovskite Solar Cells Using ZnO-Added PCBM as Electron Transport Layer" Materials 17, no. 6: 1376. https://doi.org/10.3390/ma17061376





