Removal of Plastics from Micron Size to Nanoscale Using Wood Filter
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
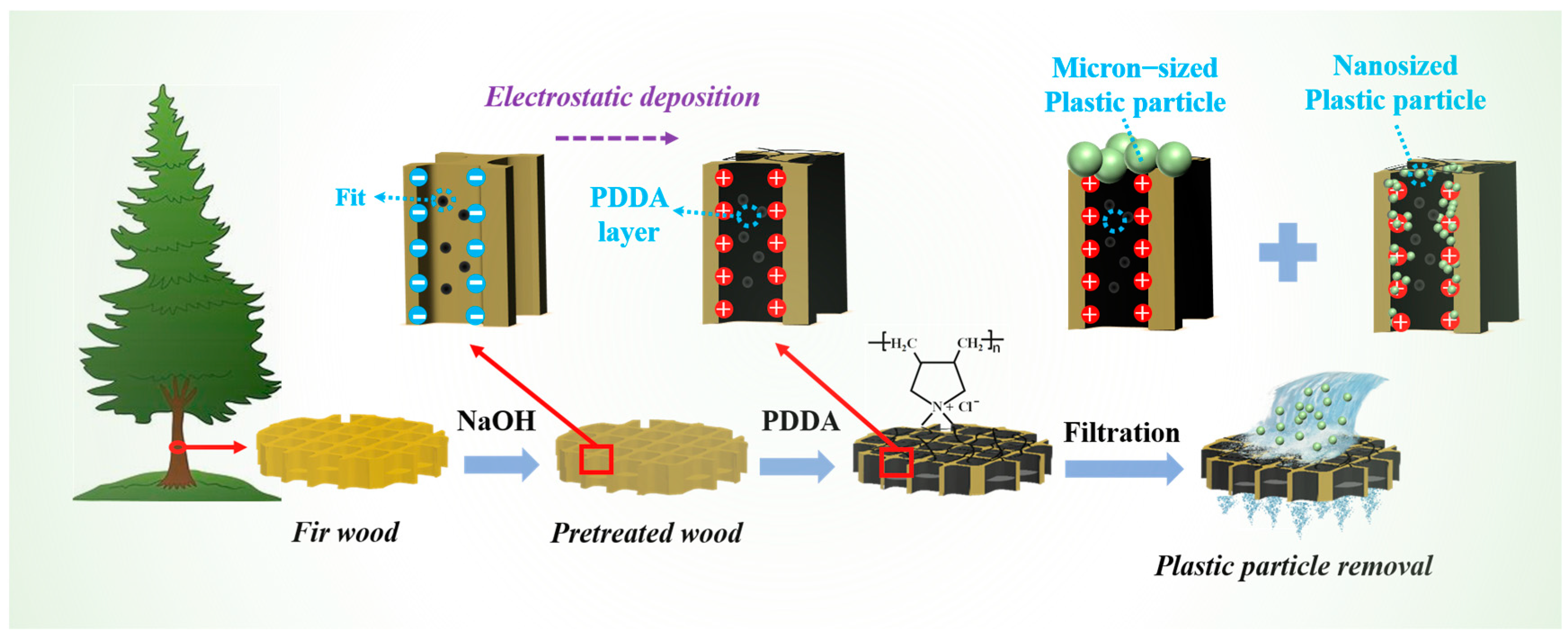
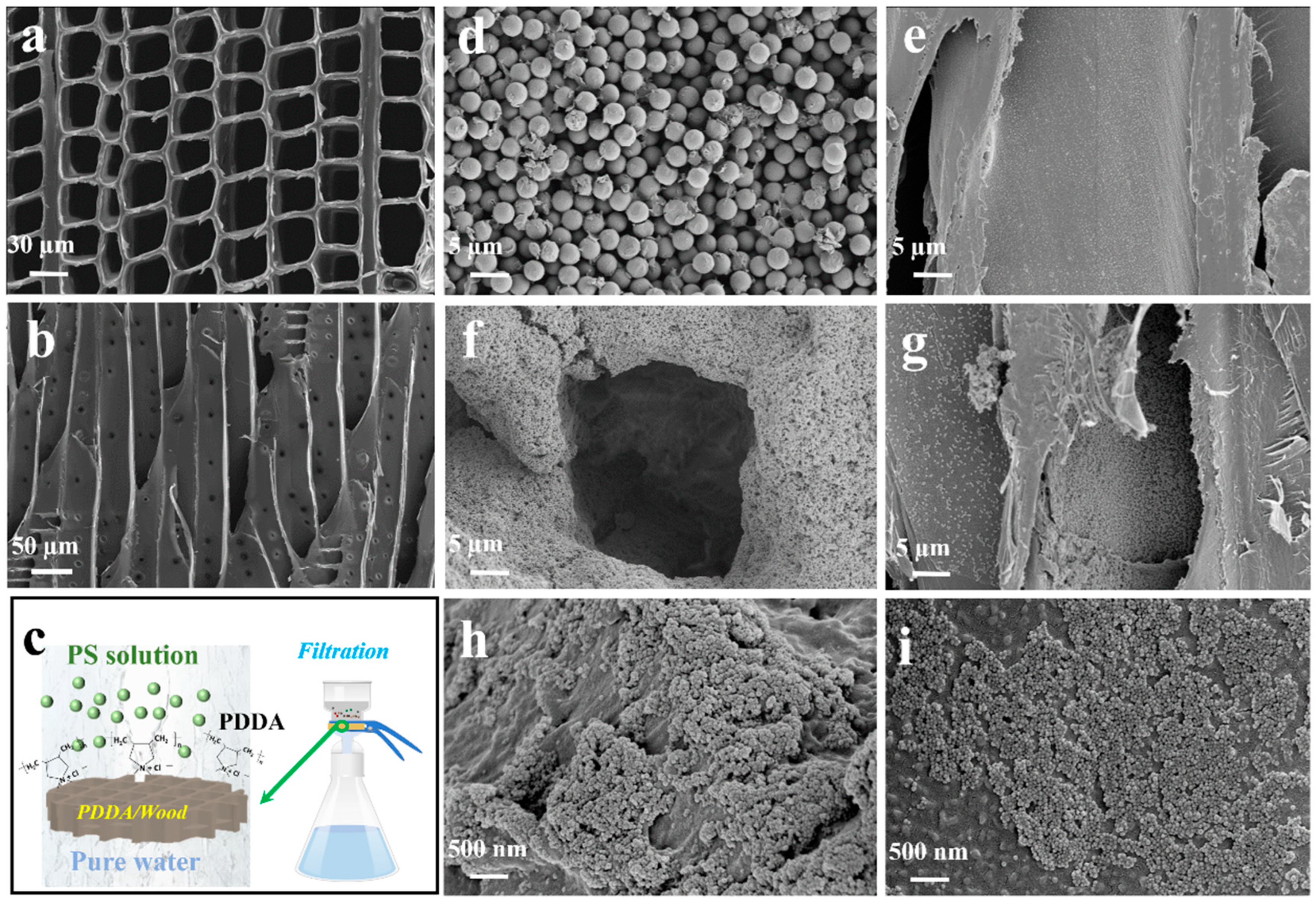
2.2. Fabrication of Wood-Based Filter
2.3. Removal of Nano-/Microplastics from Water
2.4. Characterization Analysis
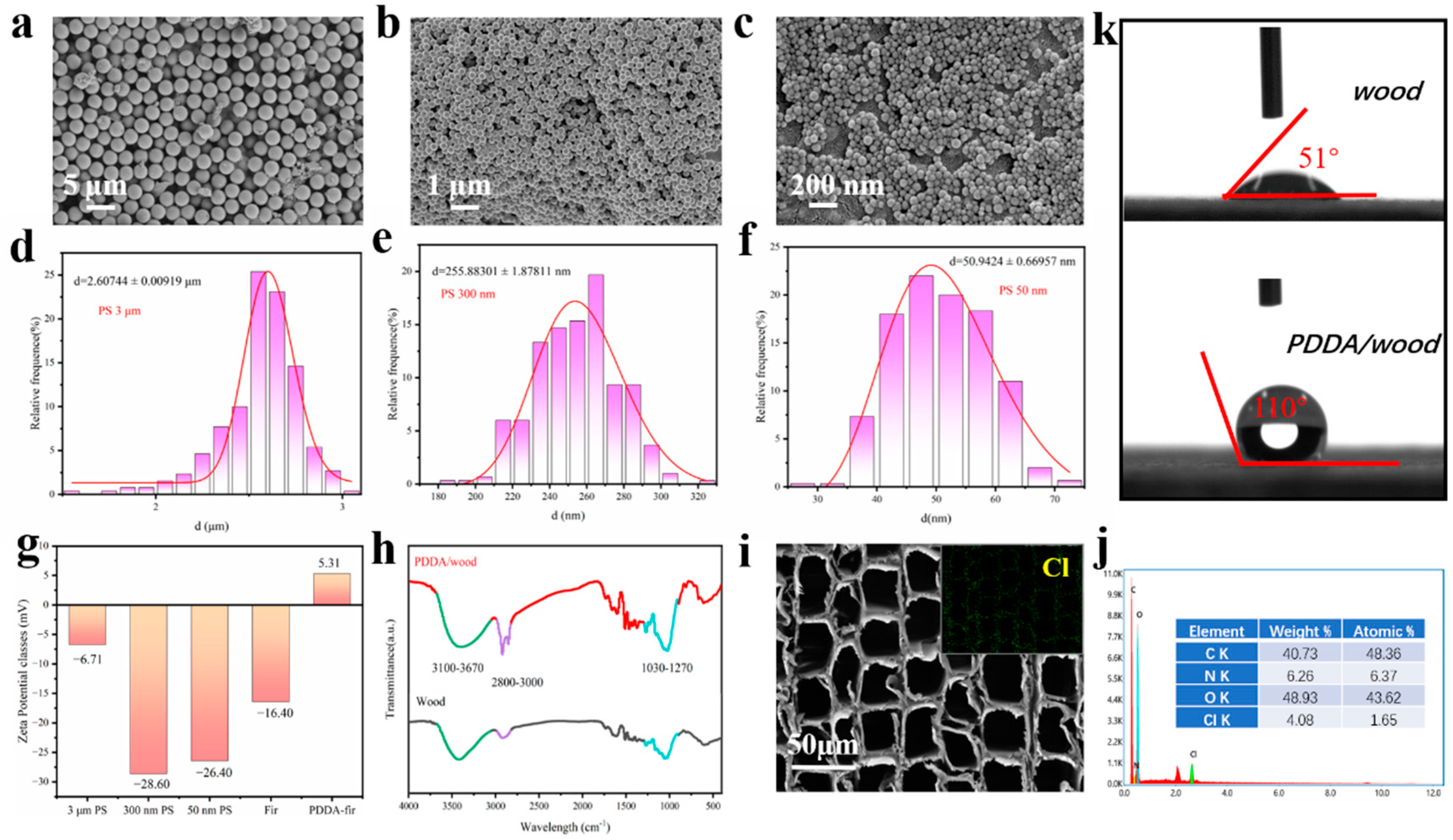
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Zeta Potential Analyzer
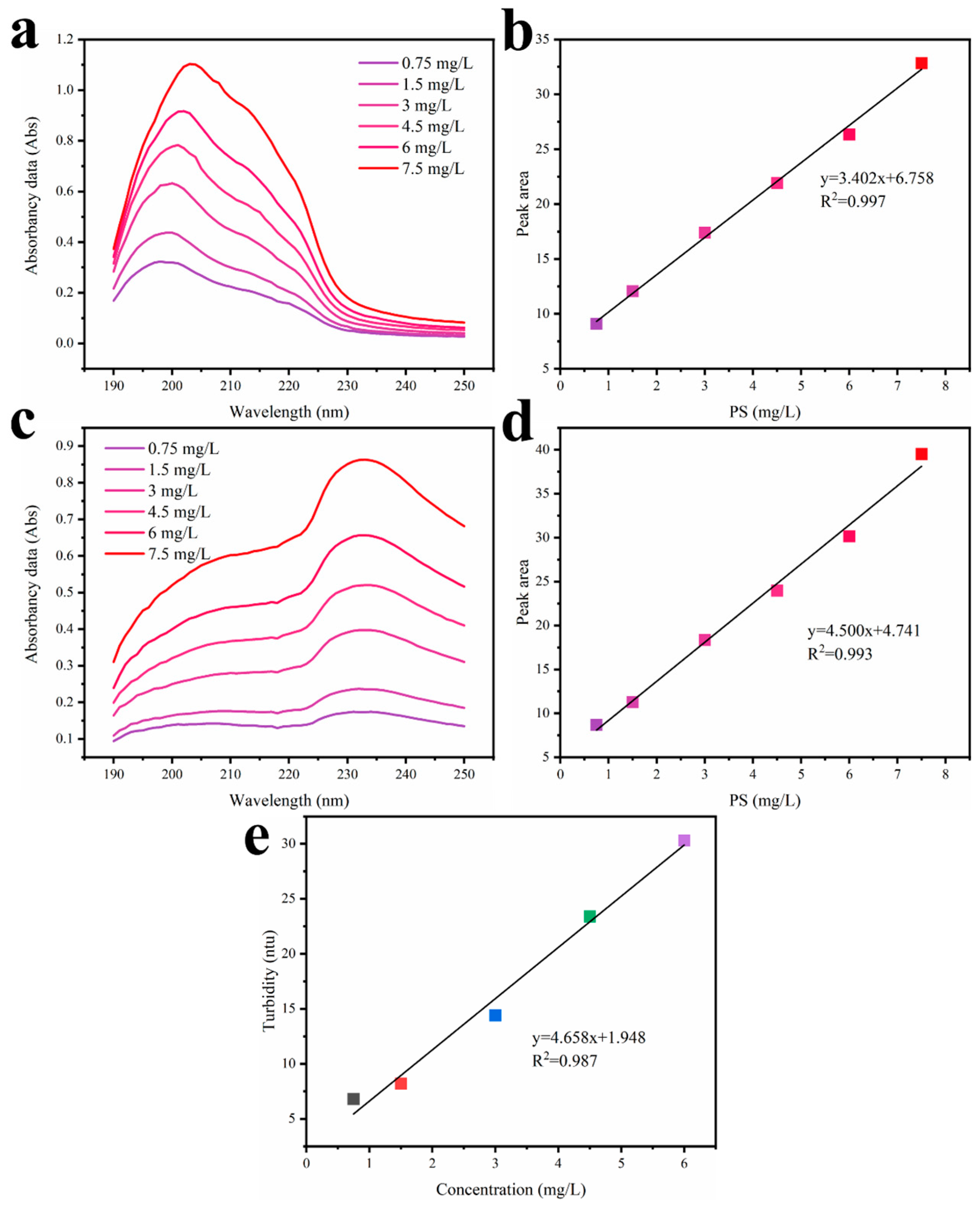
2.4.4. The Absorbance of PS50/PS300 and the Turbidity of PS3000 Solution
2.4.5. Optical Contact Angle Meter
2.4.6. Mechanical Testing Machine
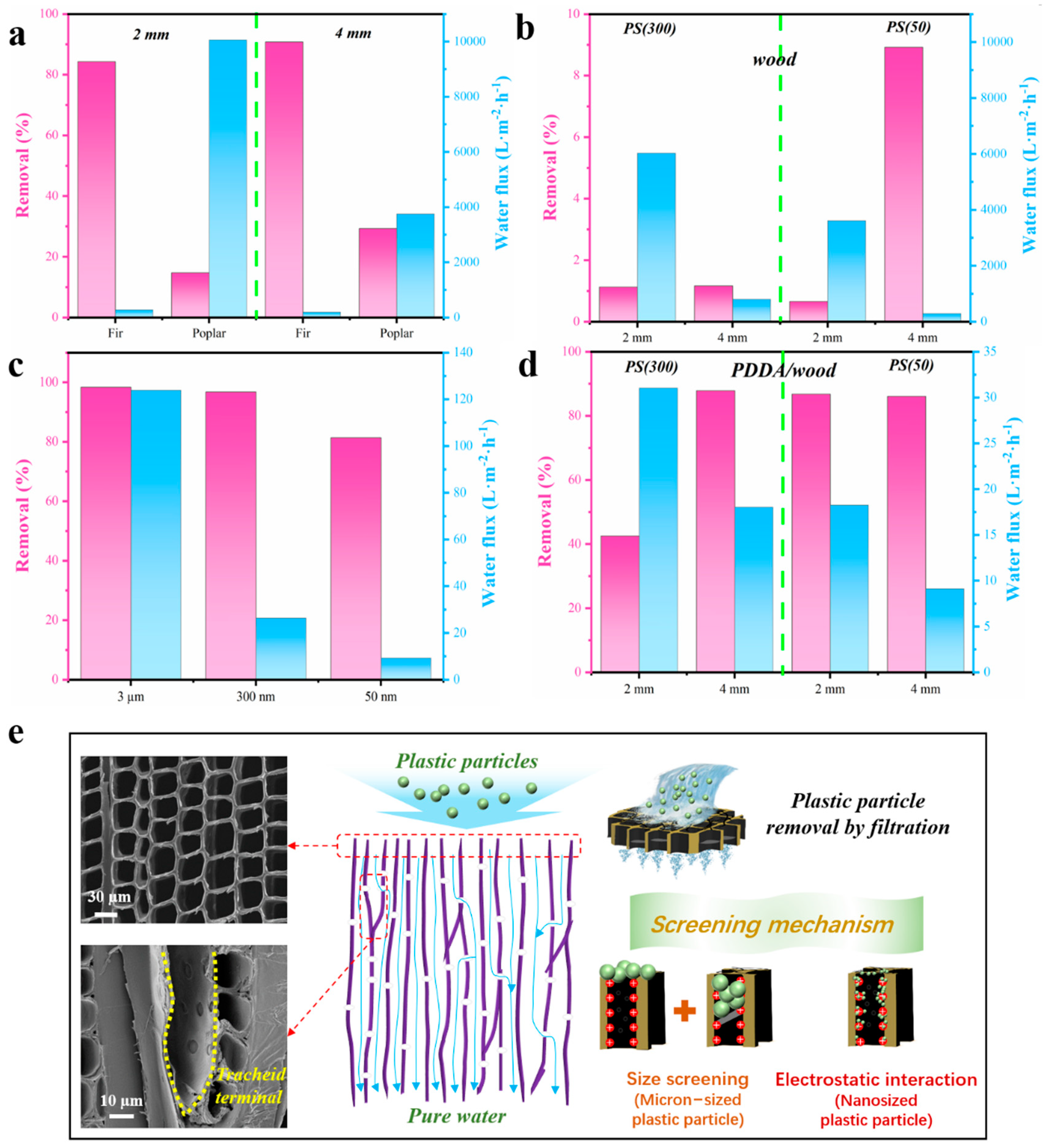
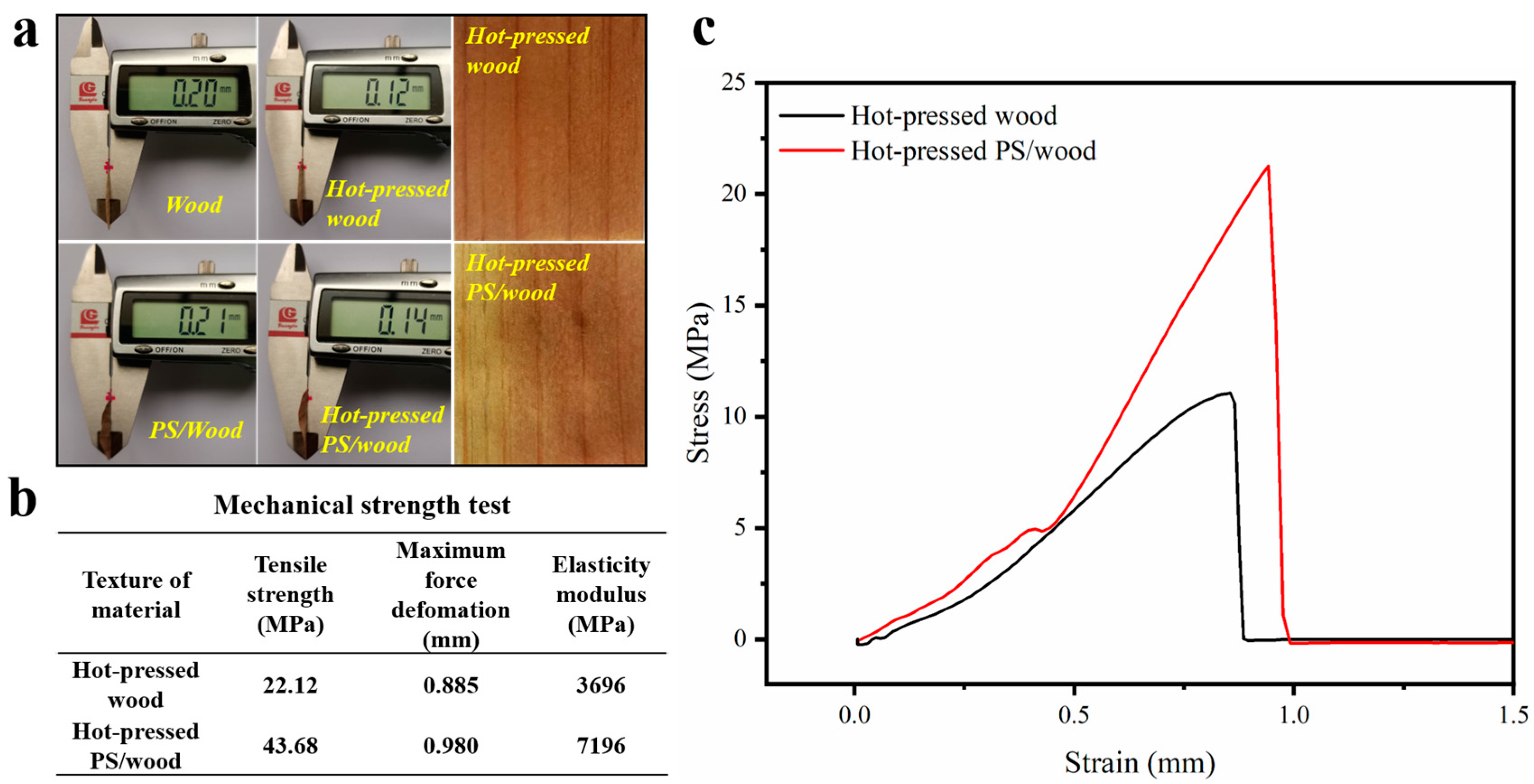
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, K.; Green, D. The Potential Effects of Microplastics on Human Health: What Is Known and What Is Unknown. Ambio 2022, 51, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of Microplastics from the Environment. A Review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Law, K.L. Plastics in the Marine Environment. Annu. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef]
- Singh, J.; Samuel, J.; Hurley, R. Editorial: Plastics, Microplastics, and Nanoplastics: Management and Mitigation of Water Contamination. Front. Environ. Sci. 2021, 9, 803551. [Google Scholar] [CrossRef]
- Materić, D.; Holzinger, R.; Niemann, H. Nanoplastics and Ultrafine Microplastic in the Dutch Wadden Sea—The Hidden Plastics Debris? Sci. Total Environ. 2022, 846, 157371. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The Global Threat from Plastic Pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful Effects of the Microplastic Pollution on Animal Health: A Literature Review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Range, D.; Scherer, C.; Stock, F.; Ternes, T.A.; Hoffmann, T.O. Hydro-Geomorphic Perspectives on Microplastic Distribution in Freshwater River Systems: A Critical Review. Water Res. 2023, 245, 120567. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Plastics and the Environment. Annu. Rev. Environ. Resour. 2023, 48, 55–79. [Google Scholar] [CrossRef]
- Helal, M.; Hartmann, N.B.; Khan, F.R.; Xu, E.G. Time to Integrate “One Health Approach” into Nanoplastic Research. Eco-Environ. Health 2023, 2, 18. [Google Scholar] [CrossRef]
- Keerthana Devi, M.; Karmegam, N.; Manikandan, S.; Subbaiya, R.; Song, H.; Kwon, E.E.; Sarkar, B.; Bolan, N.; Kim, W.; Rinklebe, J.; et al. Removal of Nanoplastics in Water Treatment Processes: A Review. Sci. Total Environ. 2022, 845, 157168. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sarcletti, M.; Park, H.; Wirth, J.; Englisch, S.; Eigen, A.; Drobek, D.; Vivod, D.; Friedrich, B.; Tietze, R.; Alexiou, C.; et al. The Remediation of Nano-/Microplastics from Water. Mater. Today 2021, 48, 38–46. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Song, F.; Yan, F.; Yang, Y.; Gao, P.; Ji, P. Removal of Polystyrene Nanoplastics in Multisolute Systems with Metallic Contaminants Using Magnetic Particles. J. Clean. Prod. 2022, 368, 133124. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Kim, C.-M.; Jang, A. Evaluation of Various Factors Influencing Dynamic Membrane Formation and Performance Using Metal Oxide Adsorbent. Desalination 2024, 572, 117117. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Kuttiani Ali, J.; Abi Jaoude, M.; Alhseinat, E. Polyimide Ultrafiltration Membrane Embedded with Reline-Functionalized Nanosilica for the Remediation of Pharmaceuticals in Water. Sep. Purif. Technol. 2021, 266, 118585. [Google Scholar] [CrossRef]
- Carter, J.; Horan, T.; Miller, J.; Madejski, G.; Butler, E.; Amato, C.; Roussie, J. Comparative Evaluation of Filtration and Imaging Properties of Analytical Filters for Microplastic Capture and Analysis. Chemosphere 2023, 332, 138811. [Google Scholar] [CrossRef]
- Juraij, K.; Ammed, S.P.; Chingakham, C.; Ramasubramanian, B.; Ramakrishna, S.; Vasudevan, S.; Sujith, A. Electrospun Polyurethane Nanofiber Membranes for Microplastic and Nanoplastic Separation. ACS Appl. Nano Mater. 2023, 6, 4636–4650. [Google Scholar] [CrossRef]
- Hyeon, Y.; Kim, S.; Ok, E.; Park, C. A Fluid Imaging Flow Cytometry for Rapid Characterization and Realistic Evaluation of Microplastic Fiber Transport in Ceramic Membranes for Laundry Wastewater Treatment. Chem. Eng. J. 2023, 454, 140028. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Li, Y.; Li, J.; Yi, Q.; Gao, F.; Wang, Z. Efficient Removal of Micropollutants from Low-Conductance Surface Water Using an Electrochemical Janus Ceramic Membrane Filtration System. Water Res. 2022, 220, 118627. [Google Scholar] [CrossRef]
- Wan, H.; Shi, K.; Yi, Z.; Ding, P.; Zhuang, L.; Mills, R.; Bhattacharyya, D.; Xu, Z. Removal of Polystyrene Nanoplastic Beads Using Gravity-Driven Membrane Filtration: Mechanisms and Effects of Water Matrices. Chem. Eng. J. 2022, 450, 138484. [Google Scholar] [CrossRef]
- Zhao, P.; Hu, Y.; An, X.; Ji, R.; Liu, H.; Zhao, H.; Song, W.; Dong, Y.; Wang, X. Polymeric PDI-Based Photocatalytic Nanoarchitectures Promoting the Performance of Thin Film Composite Membrane for Forward Osmosis Water Purification. Chem. Eng. J. 2023, 476, 146747. [Google Scholar] [CrossRef]
- Glüsen, A.; Stolten, D. Membranen Für Polymerelektrolyt-Brennstoffzellen. Chem. Ing. Tech. 2003, 75, 1591–1597. [Google Scholar] [CrossRef]
- Kook, H.; Cha, M.; Park, C. Transport of Emerging Organic Ultraviolet (UV) Filters in Ceramic Membranes: Role of Polyethylene (PE) Microplastics. Chemosphere 2022, 309, 136570. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Chakrabarty, B.; Barkakati, P. Preparation and Characterization of Novel Ceramic Membranes for Micro-Filtration Applications. Ceram. Int. 2016, 42, 14326–14333. [Google Scholar] [CrossRef]
- Krišťák, Ľ.; Réh, R. Application of Wood Composites. Appl. Sci. 2021, 11, 3479. [Google Scholar] [CrossRef]
- Grubîi, V.; Johansson, J. The Impact of Top-Layer Sliced Lamella Thickness and Core Type on Surface-Checking in Engineered Wood Flooring. Forests 2023, 14, 2250. [Google Scholar] [CrossRef]
- Liu, G.; Chen, D.; Liu, R.; Yu, Z.; Jiang, J.; Liu, Y.; Hu, J.; Chang, S. Antifouling Wood Matrix with Natural Water Transfer and Microreaction Channels for Water Treatment. ACS Sustain. Chem. Eng. 2019, 7, 6782–6791. [Google Scholar] [CrossRef]
- Van Gijn, K.; Chen, Y.L.; Van Oudheusden, B.; Gong, S.; De Wilt, H.A.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Optimizing Biological Effluent Organic Matter Removal for Subsequent Micropollutant Removal. J. Environ. Chem. Eng. 2021, 9, 106247. [Google Scholar] [CrossRef]
- He, Z.; Li, Y.; Liu, G.; Wang, C.; Chang, S.; Hu, J.; Zhang, X.; Vasseghian, Y. Fabrication of a Novel Hollow Wood Fiber Membrane Decorated with Halloysite and Metal-Organic Frameworks Nanoparticles for Sustainable Water Treatment. Ind. Crops Prod. 2023, 202, 117082. [Google Scholar] [CrossRef]
- Tu, K.; Büchele, S.; Mitchell, S.; Stricker, L.; Liu, C.; Goldhahn, C.; Allaz, J.; Ding, Y.; Günther, R.; Zhang, Z.; et al. Natural Wood-Based Catalytic Membrane Microreactors for Continuous Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 8417–8426. [Google Scholar] [CrossRef]
- Sun, Y.; He, Z.; Wei, Y.; Liu, G.; Liu, R.; Hu, J.; Liu, H.; Zhang, X.; Yuan, G. Wood-Derived Monolithic Carbon Materials and Their Functional Applications. CLEAN Soil Air Water 2022, 50, 2100420. [Google Scholar] [CrossRef]
- Chen, C.; Berglund, L.; Burgert, I.; Hu, L. Wood Nanomaterials and Nanotechnologies. Adv. Mater. 2021, 33, 2006207. [Google Scholar] [CrossRef] [PubMed]
- Mehrkhah, R.; Goharshadi, E.K.; Lichtfouse, E.; Ahn, H.S.; Wongwises, S.; Yu, W.; Mahian, O. Interfacial Solar Steam Generation by Wood-Based Devices to Produce Drinking Water: A Review. Environ. Chem. Lett. 2023, 21, 285–318. [Google Scholar] [CrossRef]
- Ramalingam, B.; Das, S.K. Biomimetic Strategy for Fabrication of Bifunctional Graphene Oxide-Biomaterial Aerogel as Highly Porous Antifouling Material for Oil/Water Separation. Chem. Eng. J. 2023, 475, 145906. [Google Scholar] [CrossRef]
- Bansal, R.; Barshilia, H.C.; Pandey, K.K. Nanotechnology in Wood Science: Innovations and Applications. Int. J. Biol. Macromol. 2024, 262, 130025. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, J.; Liu, G.; Xu, D.; Wei, Y.; Li, D.; Chang, S.; Li, X.; Liu, Y. Unique 3D Interpenetrating Capillary Network of Wood Veneer for Highly Efficient Cross Flow Filtration. J. Mater. Sci. 2021, 56, 3155–3167. [Google Scholar] [CrossRef]
- Otto, D.P.; De Villiers, M.M. Coarse-Grained Molecular Dynamics (CG-MD) Simulation of the Encapsulation of Dexamethasone in PSS/PDDA Layer-by-Layer Assembled Polyelectrolyte Nanocapsules. AAPS PharmSciTech 2020, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.V.M.; Orlando, R.M.; Pereira, F.V. Layer-by-Layer Assembly of PDDA/MWCNTs Thin Films as an Efficient Strategy for Extraction of Organic Compounds from Complex Samples. J. Chromatogr. A 2024, 1717, 464705. [Google Scholar] [CrossRef]
- Zakharova, J.A.; Zansokhova, M.F.; Karpushkin, E.A.; Sergeyev, V.G. Significant Improving H+/VO2+ Permselectivity of Nafion Membrane by Modification with PDDA in Aqueous Isopropanol. Mendeleev Commun. 2021, 31, 839–841. [Google Scholar] [CrossRef]
- Ratajski, T.; Kalemba-Rec, I.; Indyka, P.; Ledwig, P.; Szczerba, M.J.; Dubiel, B. Effect of PDDA Surfactant on the Microstructure and Properties of Electrodeposited SiO2/Ni Nanocomposites. Mater. Charact. 2020, 163, 110229. [Google Scholar] [CrossRef]
- Bayarkhuu, B.; Byun, J. Optimization of Coagulation and Sedimentation Conditions by Turbidity Measurement for Nano- and Microplastic Removal. Chemosphere 2022, 306, 135572. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; Li, Y.; Liu, A.; Zhang, H.; Zhou, Y. Facile Route to Preparation of Positively Charged GO Using Poly (Diallyldimethylammoniumchloride). Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 394–401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, G.; Wang, C.; Chang, S.; Hu, J. Removal of Plastics from Micron Size to Nanoscale Using Wood Filter. Materials 2024, 17, 1361. https://doi.org/10.3390/ma17061361
Li M, Liu G, Wang C, Chang S, Hu J. Removal of Plastics from Micron Size to Nanoscale Using Wood Filter. Materials. 2024; 17(6):1361. https://doi.org/10.3390/ma17061361
Chicago/Turabian StyleLi, Min, Gonggang Liu, Chongqing Wang, Shanshan Chang, and Jinbo Hu. 2024. "Removal of Plastics from Micron Size to Nanoscale Using Wood Filter" Materials 17, no. 6: 1361. https://doi.org/10.3390/ma17061361




