Supercapacitor Electrodes: Is Nickel Foam the Right Substrate for Active Materials?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Preparation
2.3. Methods
3. Results
3.1. Electrooxidation of Nickel—Experimental Results and Literature Consideration
3.2. Nickel Foam “Cleaning”—Experimental Results and Literature Consideration
3.3. Miscellaneous Problems Tied to Ni Foam Use
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talaie, E.; Bonnick, P.; Sun, X.; Pang, Q.; Liang, X.; Nazar, L.F. Methods and protocols for electrochemical energy storage materials research. Chem. Mater. 2017, 29, 90–105. [Google Scholar] [CrossRef]
- Majumdar, D.; Mandal, M.; Bhattacharya, S.K. Journey from supercapacitors to supercapatteries: Recent advancements in electrochemical energy storage systems. Emergent Mater. 2020, 3, 347–367. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical supercapacitors for energy storage and conversion. In Handbook of Clean Energy Systems; Yan, J., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Available online: https://www.amazon.co.uk/VARTA-Single-Use-Battery-Nickel-Oxyhydroxide/dp/B0016FQAMK (accessed on 27 December 2023).
- Tsais, P.-J.; Chan, L.I. 11—Nickel-based batteries: Materials and chemistry. In Electricity Transmission, Distribution and Storage Systems; Woodhead Publishing Series in Energy; Melhem, Z., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 309–397. [Google Scholar] [CrossRef]
- Reddy, T.B.; Linden, D. Linden’s Handbook of Batteries, 4th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Young, K.-H.; Yasuoka, S. Capacity degradation mechanisms in nickel/metal hydride batteries. Batteries 2016, 2, 3. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Na, H.; Liu, H.; Zhou, H. Rechargeable Ni-Li battery integrated aqueous/nonaqueous system. J. Am. Chem. Soc. 2009, 131, 15098–15099. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, H.; Li, G.; Wu, Z. Nickel-cobalt bimetallic anode catalysts for direct urea fuel cell. Sci. Rep. 2014, 4, 5863. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wang, T.; Héroguel, F.; Krammer, A.; Lee, S.; Yao, L.; Schüler, A.; Luterbacher, J.S.; Yan, Y.; Hu, X. An efficient nickel hydrogen oxidation catalyst for hydroxide exchange membrane fuel cells. Nat. Mater. 2022, 21, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Zaiman, N.F.H.N.; Shaari, N. Review on flower-like structure nickel based catalyst in fuel cell application. J. Ind. Eng. Chem. 2023, 119, 1–76. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T.; Kowalski, I.M. Hydrogen evolution at catalytically-modified Ni foam in alkaline solution. J. Power Sources 2014, 271, 231–238. [Google Scholar] [CrossRef]
- Zhang, W.; Li, D.; Zhang, L.; She, X.; Yang, D. NiFe-based nanostructures on Ni foam as highly efficiently electrocatalysts for oxygen and hydrogen evolution reactions. J. Energy Chem. 2019, 39, 39–53. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, T.; Zhou, W.; Yang, L.; Tang, Z.; Chen, S. Metal Ni foam as an efficient and stable electrode for hydrogen evolution reaction in acidic electrolyte under reasonable overpotentials. ACS Appl. Mater. Interfaces 2016, 8, 5065–5069. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. Ni foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.E.G.; Doyle, R.L.; Godwin, I.; O’Brien, M.; Russell, L. Hydrous nickel oxide: Redox switching and the oxygen evolution reaction in aqueous alkaline solution. J. Electrochem. Soc. 2012, 159, H932–H934. [Google Scholar] [CrossRef]
- Liu, A.; Lv, Y.; Mu, J.; Guo, Z.; Pei, Z.; Zhang, X.; Bai, Y.; Xie, H.; Che, H. Facile synthesis of hollow Ni0.2Mn0.8O1.5 twin microspheres for electrochemical energy storage. J. Appl. Electrochem. 2018, 48, 15–26. [Google Scholar] [CrossRef]
- Karmakar, S.; Behera, D. Small polaron hopping conduction in NiMnO3/NiMn2O4 nano-cotton and its emerging energy application with MWCNT. Ceram. Int. 2019, 45, 13052–13066. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Hubali, K.M.; Shembade, U.V.; Abitkar, S.B.; Waikar, M.R.; Sonkawade, R.G.; Agawane, G.L.; Moholkar, A.V. Hydrothermal synthesis of mesoporous NiMnO3 nanostructures for supercapacitor application: Effect of electrolyte. J. Energy Storage 2021, 35, 102277. [Google Scholar] [CrossRef]
- Vamsi Krishna, B.N.; Bhagwan, J.; Yu, J.S. Sol-gel routed NiMn2O4 nanofabric electrode materials for supercapacitors. J. Electrochem. Soc. 2019, 166, A1950. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Yu, L.; Zhang, Y.; Hou, D.; Li, T. Facile synthesis of NiMn2O4 nanosheet arrays grown on Ni foam as novel electrode materials for high-performance supercapacitors. Ceram. Int. 2016, 42, 14963–14969. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Z.; Liu, H.; Ma, T. Biomass-derived highly porous nitrogen-doped graphene orderly supported NiMn2O4 nanocrystals as efficient electrode materials for asymmetric supercapacitors. Appl. Surf. Sci. 2020, 507, 145065. [Google Scholar] [CrossRef]
- Du, X.; Yang, L.; Fu, Y.; Liu, S.; Huang, N.; Wang, S. Microwave-assisted synthesis of NiMn2O4 grown on Ni foam as electrode material for high-performance supercapacitors. ChemistrySelect 2021, 6, 5567. [Google Scholar] [CrossRef]
- Yan, H.; Li, T.; Qiu, K.; Lu, Y.; Cheng, J.; Liu, Y.; Xu, J.; Luo, Y. Growth and electrochemical performance of porous NiMn2O4 nanosheets with high specific surface areas. J. Solid State Electrochem. 2015, 19, 3169–3175. [Google Scholar] [CrossRef]
- Khaja Hussain, S.; Nagaraju, G.; Chandra Sekhar, S.; Yu, J.S. Morphological synergistic behavior on electrochemical performance of battery-type spinel nickel manganese oxides for aqueous hybrid supercapacitors. J. Power Sources 2019, 439, 227088. [Google Scholar] [CrossRef]
- Talluri, B.; Aparna, M.L.; Sreenivasulu, N.; Bhattacharya, S.S.; Thomas, T. High entropy spinel metal oxide (CoCrFeMnNi)3O4 nanoparticles as a high-performance supercapacitor electrode material. J. Energy Storage 2021, 42, 103004. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, B.; Yu, G.; Hou, X.; Chen, D.; Shen, G. NiCo2O4 nanowire arrays supported on Ni foam for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2013, 1, 2468–2473. [Google Scholar] [CrossRef]
- Nagaraju, M.; Chandra Sekhar, S.; Arbaz, S.J.; Yu, J.S. Solvothermal-derived nanoscale spinel bimetallic oxide particles rationally bridged with conductive vapor-grown carbon fibers for hybrid supercapacitors. Appl. Surf. Sci. 2021, 563, 150223. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Mu, Y.; Bai, Y.; Wang, Y. Binary cobalt ferrite nanomesh arrays as the advanced binder-free electrode for applications in oxygen evolution reaction and supercapacitors. J. Power Sources 2016, 327, 599–609. [Google Scholar] [CrossRef]
- Dam, D.T.; Huang, T.; Lee, J.-M. Ultra-small and low crystalline CoMoO4 nanorods for electrochemical capacitors. Sustain. Energy Fuels 2017, 1, 324–335. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, Y.; Wang, H.; Huang, B.; Sun, P.; Chen, T.; Zhou, J.; Xie, E.; Zhang, Z. A high energy density asymmetric supercapacitor from ultrathin manganese molybdate nanosheets. Electrochim. Acta 2016, 211, 217–224. [Google Scholar] [CrossRef]
- Li, L.; Shi, F.; Zhang, Y.; Zhang, J.; Gu, C.; Wang, X.; Tu, J. Spinel manganese–nickel–cobalt ternary oxide nanowire array for high-performance electrochemical capacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 18040–18047. [Google Scholar] [CrossRef]
- Gao, H.; Xiang, J.; Cao, Y. Hierarchically porous CoFe2O4 nanosheets supported on Ni foam with excellent electrochemical properties for asymmetric supercapacitors. Appl. Surface Sci. 2017, 413, 351–359. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile synthesis of three-dimensional ternary ZnCO2O4/reduced graphene oxide/NiO composite film on Ni foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, G.; Du, Q.; Su, L.; Ma, Z.; Qin, X.; Shao, G. Co3O4@MnO2 core shell arrays on Ni foam with excellent electrochemical performance for aqueous asymmetric supercapacitor. Ionics 2017, 23, 1637–1643. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Zhang, C.; Xu, S.; Wei, M.; Duan, X. Co3O4@layered double hydroxide core/shell hierarchical nanowire arrays for enhanced supercapacitance performance. Nano Energy 2014, 7, 134–142. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-nanonet hollow structured Co3O4 for high capacity supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 18040–18047. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, R.; Wei, X.; Zou, L.; Qin, N.; Bao, D. CoNi2S4 nanosheet arrays supported on Ni foams with ultrahigh capacitance for aqueous asymmetric supercapacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 19318–19326. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, J.; Yan, P.; Miao, C.; Ye, K.; Cheng, K.; Yin, J.; Cao, D.; Li, K.; Wang, G. Preparation of porous cadmium sulphide on Ni foam: A novel electrode material with excellent supercapacitor performance. J. Mater. Chem. A 2016, 4, 4920–4928. [Google Scholar] [CrossRef]
- Yuan, A.; Wang, X.; Wang, Y.; Hu, J. Textural and capacitive characteristics of MnO2 nanocrystals derived from a novel solid-reaction route. Electrochim. Acta 2009, 54, 1021–1026. [Google Scholar] [CrossRef]
- Wongpratat, U.; Tipsawat, P.; Khajonrit, J.; Swatsitang, E.; Maensiri, S. Effects of Nickel and Magnesium on electrochemical performances of partial substitution in spinel ferrite. J. Alloys Compd. 2020, 831, 154718. [Google Scholar] [CrossRef]
- Grdeń, M.; Alsabet, M.; Jerkiewwicz, G. Surface science and electrochemical analysis of Ni foams. ACS Appl. Mater. Interfaces 2012, 4, 3012–3021. [Google Scholar] [CrossRef]
- Conway, B.E.; Sattar, M.A.; Gilroy, D. Electrochemistry of the nickel-oxide Electrode-v. Self passivation effects in oxygen-evolution Kinetics. Electrochim. Acta 1969, 14, 677–694. [Google Scholar] [CrossRef]
- Schrebler Guzmán, R.S.; Vilche, J.R.; Arvía, A.J. Rate processes related to the hydrated nickel hydroxide electrode in alkaline solutions. J. Electrochem. Soc. 1978, 125, 1578. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; de Guibert, A. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Hahn, F.; Beden, B.; Croissant, M.J.; Lamy, C. In situ UV visible reflectance spectroscopic investigation of the nickel electrode-alkaline solution interface. Electrochim. Acta 1986, 31, 335–342. [Google Scholar] [CrossRef]
- Visscher, W.; Barendrecht, E. Anodic oxide films of nickel in alkaline electrolyte. Surf. Sci. 1983, 135, 436–452. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, M.; Lee, Y.S. Best practices in using foam-type electrodes for electrocatalytic performance benchmark. ACS Energy Lett. 2020, 5, 3260–3264. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Brandon, P. A comparative study of the oxygen evolution reaction on oxidised nickel, cobalt and iron electrodes in base. J. Electroanal. Chem. 2010, 641, 119–130. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Liu, S.; Fu, Y.; Huang, N. In-Situ Grown NiMn2O4/GO Nanocomposite Material on Nickel Foam Surface by Microwave-Assisted Hydrothermal Method and Used as Supercapacitor Electrode. Nanomaterials 2023, 13, 2487. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Shanmugavadivel, M. Fabrication of NiMn2O4 nanospheres and its hybrid with polyaniline for high energy and high power supercapacitor with long cycle stability. Mater. Sci. Eng. B 2023, 294, 116553. [Google Scholar] [CrossRef]
- Lee, K.-C.; Hsiao, Y.-S.; Sung, M.-Y.; Chen, Y.-L.; Wu, N.-J.; Huang, J.-H.; Cho, E.-C.; Weng, H.C.; Hsu, S.-C. MOF-derived spinel NiMn2O4/CoMn2O4 heterojunction and its application in a high-performance photocatalyst and supercapacitor. J. Environ. Chem. Eng. 2023, 11, 110762. [Google Scholar] [CrossRef]
- Kanagambal, P.; Ahamed, A.J.; Rajeswaran, P.; Kamatchi, T. Hybrids of porous NiMn2O4@reduced graphene oxide composites for asymmetric supercapacitor applications. J. Mater. Sci. Mater. Electron. 2023, 34, 1873. [Google Scholar] [CrossRef]
- Shirzad Choubari, M.; Rahmani, S.; Mazloom, J. Boosted electrochemical performance of magnetic caterpillar-like Mg0.5Ni0.5Fe2O4 nanospinels as a novel pseudocapacitive electrode material. Sci. Rep. 2023, 13, 7822. [Google Scholar] [CrossRef]
- Pelani, H.; Rastogi, A. Effect of annealing temperature on structural and electrochemical behaviour on MgFe2O4 as electrode material in neutral aqueous electrolyte for supercapacitors. Nanotechnology 2024, 35, 175401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mei, Y.; Su, Q.; Wang, Z.; Guo, G. Fe2O3/MgFe2O4 Nanosheet on nickel foam for high-performance asymmetric supercapacitors. Crystals 2023, 13, 1561. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Dojcinovic, M.P.; Tadic, N.B.; Radovanovic, M.; Stojanovic, G.M. Nanocrystalline nickel manganite synthesis by sol-gel combustion for flexible temperature sensors. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Dojcinovic, M.P.; Vasiljevic, Z.Z.; Rakocevic, L.; Pavlovic, V.P.; Ammar-Merah, S.; Vujancevic, J.D.; Nikolic, M.V. Humidity and temperature sensing of mixed nickel–magnesium spinel ferrites. Chemosensors 2023, 11, 34. [Google Scholar] [CrossRef]
- Malaie, K.; Ganjali, M.R.; Alizadeh, T.; Norouzi, P. Hydrothermal growth of magnesium ferrite rose nanoflowers on Ni foam; application in high-performance asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 650–657. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, J.; Huang, W.; Liu, X. Electrocatalytic oxidation of cyclohexanol on a nickel oxyhydroxide modified nickel electrode in alkaline solutions. Catal. Commun. 2007, 8, 1017–1022. [Google Scholar] [CrossRef]
- Xing, W.; Qiao, S.; Wu, X.; Gao, X.; Zhou, J.; Zhuo, S.; Budi Hartono, S.; Hulicova-Jurcakova, D. Exaggerated capacitance using electrochemically active nickel foam as current collector in electrochemical measurement. J. Power Sources 2011, 196, 4123–4127. [Google Scholar] [CrossRef]
- Malaie, K.; Heydari, Z.; Brousse, T. Methods—On the reliability of the electrochemical data recorded on nickel foam in alkaline solution: The illusive surface oxide layer. J. Electrochem. Soc. 2021, 168, 120547. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Manavalan, S.; Thanasekaran, P.; Lin, K.-C. Research progress on porous carbon supported metal/metal oxide nanomaterials for supercapacitor electrode applications. Ind. Eng. Chem. Res. 2020, 59, 6347–6374. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Prabhakar Vattikuti, S.V.; Kim, K.H. Electrochemical investigation on nickel-doped spinel magnesium ferrite nanoparticles for supercapacitor applications. Mater. Chem. Phys. 2023, 301, 127601. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma Ghrera, A.; Rana, A.; Devi, A. Evaluating the role of current collectors in supercapacitor electrodes with NiCo2O4 nanospheres. J. Phys. Chem. Solids 2023, 178, 111347. [Google Scholar] [CrossRef]
- Seghiouer, A.; Chevalet, J.; Barhoun, A.; Lantelme, F. Electrochemical oxidation of nickel in alkaline solutions: A voltammetric study and modelling. J. Electroanal. Chem. 1998, 442, 113–123. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 0-8493-0487-3. [Google Scholar]
- Alsabet, M.; Grdeń, M.; Jerkiewicz, G. Electrochemical growth of surface oxides on nickel. Part 1: Formation of α-Ni(OH)2 in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 2011, 2, 317–330. [Google Scholar] [CrossRef]
- Alsabet, M.; Grdeń, M.; Jerkiewicz, G. Electrochemical growth of surface oxides on nickel. Part 2: Formation of β-Ni(OH)2 and NiO in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 2014, 5, 136–147. [Google Scholar] [CrossRef]
- Alsabet, M.; Grdeń, M.; Jerkiewicz, G. Electrochemical growth of surface oxides on nickel. Part 3: Formation of β-NiOOH in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 2015, 6, 60–71. [Google Scholar] [CrossRef]
- Dmochowska, M.; Czerwiński, A. Behavior of a nickel electrode in the presence of carbon monoxide. J. Solid State Electrochem. 1998, 2, 16–23. [Google Scholar] [CrossRef]
- Hahn, F.; Floner, D.; Beden, B.; Lamy, C. In situ investigation of the behaviour of a nickel electrode in alkaline solution by UV-vis and IR reflectance spectroscopies. Electrochim. Acta 1987, 32, 1631–1636. [Google Scholar] [CrossRef]
- Vuković, M. Voltammetry and anodic stability of a hydrous oxide film on a nickel electrode in alkaline solution. J. Appl. Electrochem. 1994, 24, 878–882. [Google Scholar] [CrossRef]
- Seyeux, A.; Maurice, V.; Klein, L.H.; Marcus, P. In situ scanning tunnelling microscopic study of the initial stages of growth and of the structure of the passive film on Ni(111) in 1 mM NaOH(aq). J. Solid State Electrochem. 2005, 9, 337–346. [Google Scholar] [CrossRef]
- Constantin, D.M.; Rus, E.M.; Oniciu, L.; Ghergari, L. The influence of some additives on the electrochemical behaviour of sintered nickel electrodes in alkaline electrolyte. J. Power Sources 1998, 74, 188–197. [Google Scholar] [CrossRef]
- Snook, G.A.; Duffy, N.W.; Pandolfo, A.G. Evaluation of the effects of oxygen evolution on the capacity and cycle life of nickel hydroxide electrode materials. J. Power Sources 2007, 168, 513–521. [Google Scholar] [CrossRef]
- Godwin, I.J.; Lyons, M.E.G. Enhanced oxygen evolution at hydrous nickel oxide electrodes via electrochemical ageing in alkaline solution. Electrochem. Commun. 2013, 32, 39–42. [Google Scholar] [CrossRef]
- Hall, D.S.; Bock, C.; MacDougall, B.R. The Electrochemistry of metallic nickel: Oxides, hydroxides, hydrides and alkaline hydrogen evolution. J. Electrochem. Soc. 2013, 160, F235. [Google Scholar] [CrossRef]
- Su, D.; Kim, H.-S.; Kim, W.-S.; Wang, G. Mesoporous nickel oxide nanowires: Hydrothermal synthesis, characterisation and applications for lithium-ion batteries and supercapacitors with superior performance. Chem. Eur. J. 2012, 18, 8224–8229. [Google Scholar] [CrossRef] [PubMed]
- Paravannoor, A.; Ranjusha, R.; Asha, A.M.; Vani, R.; Kalluri, S.; Subramanian, K.R.V.; Sivakumar, N.; Kim, T.N.; Nair, S.V.; Balakrishnan, A. Chemical and structural stability of porous thin film NiO nanowire based electrodes for supercapacitors. Chem. Eng. J. 2013, 220, 360–366. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Kim, Y.S.; Pawar, S.M.; Kim, J.H.; Im, H.; Kim, H. Chemically grown, porous, nickel oxide thin-film for electrochemical supercapacitors. J. Power Sources 2011, 196, 2393–2397. [Google Scholar] [CrossRef]
- Chang, J.; Park, M.; Ham, D.; Ogale, S.B.; Mane, R.S.; Han, S.-H. Liquid-phase synthesized mesoporous electrochemical supercapacitors of nickel hydroxide. Electrochim. Acta 2008, 53, 5016–5021. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, T.; Chen, J.S.; Wang, Z.; Yuan, C.; Lou, X.W. Controlled synthesis of hierarchical NiO nanosheet hollow spheres with enhanced supercapacitive performance. J. Mater. Chem. 2011, 21, 6602. [Google Scholar] [CrossRef]
- Cai, G.; Wang, X.; Cui, M.; Darmawan, P.; Wang, J.; Eh, A.L.S.; Lee, P.S. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Babu, I.M.; Sethuraman, B.; Muralidharan, G. Nanosheet-assembled NiO microstructures for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10767–10773. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, L.; You, T.; Wang, F.; Wen, Z. Preparation of chestnut-like porous NiO nanospheres as electrodes for supercapacitors. RSC Adv. 2015, 5, 96165. [Google Scholar] [CrossRef]
- Liu, M.; Chang, J.; Sun, J.; Gao, L. A facile preparation of NiO/Ni composites as high performance pseudocapacitor materials. RSC Adv. 2013, 3, 8003. [Google Scholar] [CrossRef]
- Yan, X.; Tong, X.; Wang, J.; Gong, C.; Zhang, M.; Liang, L. Synthesis of mesoporous NiO nanoflake array and its enhanced electrochemical performance for supercapacitor application. J. Alloys Compd. 2014, 593, 184–189. [Google Scholar] [CrossRef]
- Steimecke, M.; Seiffarth, G.; Schneemann, C.; Oehler, F.; Förster, S.; Bron, M. Higher-valent nickel oxides with improved oxygen evolution activity and stability in alkaline media prepared by high-temperature treatment of Ni(OH)2. ACS Catal. 2020, 10, 3595–3603. [Google Scholar] [CrossRef]
- Ali, B.A.; Ahmed, N.; Allam, N.K. Deciphering the hype effect of Ni-foam substrate in electrochemical supercapacitors: Is there a way out? Mater. Today Commun. 2022, 32, 103972. [Google Scholar] [CrossRef]
- Bakar NA, B.; Salleh, N.A.; Hamid NA, A.; Abdullah CA, C.; Rahiman, W.; Basirun, W.J.; Kheawhom, S.; Mohamad, A.A. The effect different of hydrochloric acid concentrations on the cleaning of Ni foam substrate: Structural and morphological studies. Mat. Today Proc. 2022, 60, 1036–1041. [Google Scholar] [CrossRef]
- Fouda, A.S.; Tawfik, H.; Abdallah, N.M.; Ahmd, A.M. Corrosion Inhibition of Nickel in HCl Solution by Some Indole Derivatives. Int. J. Electrochem. Sci. 2013, 8, 3390–3405. [Google Scholar] [CrossRef]
- El Aal, E.E.A.; Zakria, W.; Diab, A.; Abd El Haleem, S.M. Anodic dissolution of nickel in acidic chloride solutions. J. Materi. Eng. Perform. 2003, 12, 172–178. [Google Scholar] [CrossRef]
- Drodten, P. Hydrochloric acid. In Corrosion Resistance of Nickel Alloys against Acids and Lyes; Shültze, M., Rebak, R.B., Bender, R., Eds.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-3-527-33849-8. [Google Scholar]
- Yu, M.; Wang, W.; Li, C.; Zhai, T.; Lu, X.; Tong, Y. Scalable self-growth of Ni@NiO core-shell electrode with ultrahigh capacitance and super-long cyclic stability for supercapacitors. NPG Asia Mater. 2014, 6, e129. [Google Scholar] [CrossRef]
- Şahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A comprehensive review on supercapacitor applications and developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective—Guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Lee, K. Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Wei, R.; Cao, Z.; Quan, Q.; Zhang, H.; Wang, W.; Li, F.; Yip, S.P.; Meng, Y.; Chan, K.S.; et al. More than physical support: The effect of Ni foam corrosion on electrocatalytic performance. Appl. Surf. Sci. 2021, 538, 147977. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Jiang, M.; Kuang, Y.; Sun, X.; Duan, X. Ternary NiCoP nanosheet arrays: An excellent bifunctional catalyst for alkaline overall water splitting. Nano Res. 2016, 9, 2251–2259. [Google Scholar] [CrossRef]
- Huang, C.; Chu, P.K. Recommended practices and benchmarking of foam electrodes in water splitting. Trends Chem. 2022, 4, 1065–1077. [Google Scholar] [CrossRef]
- Rakocevic, L.; Strbac, S.; Potocnik, J.; Popovic, M.; Jugovic, D.; Stojkovic Simatovic, I. The NaxMnO2 materials prepared by a glycine-nitrate method as advanced cathode materials for aqueous sodium-ion rechargeable batteries. Ceram. Int. 2021, 47, 4595–4603. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V.; Kovalenko, I. Activation of the nickel foam as a current collector for application in supercapacitors. East.-Eur. J. Enterp. 2018, 3, 56–62. [Google Scholar] [CrossRef]
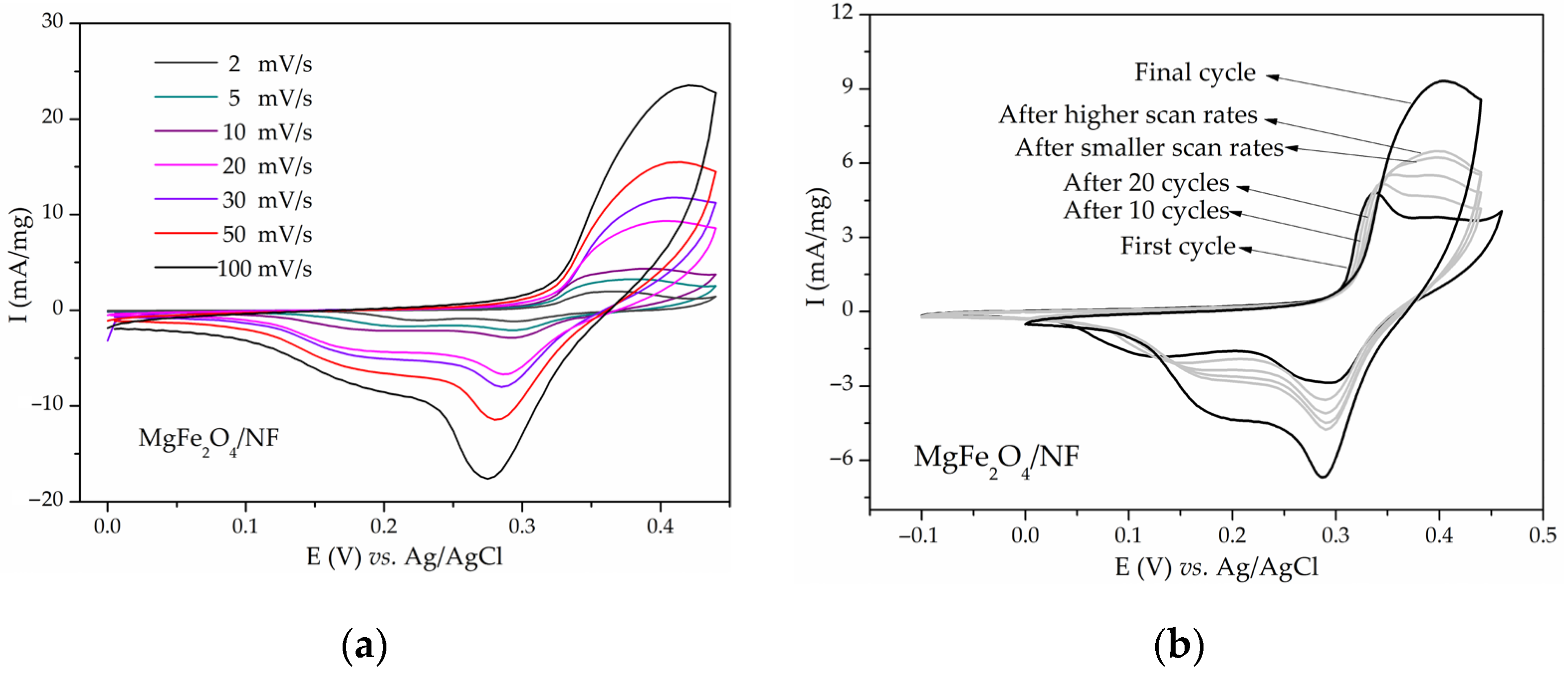
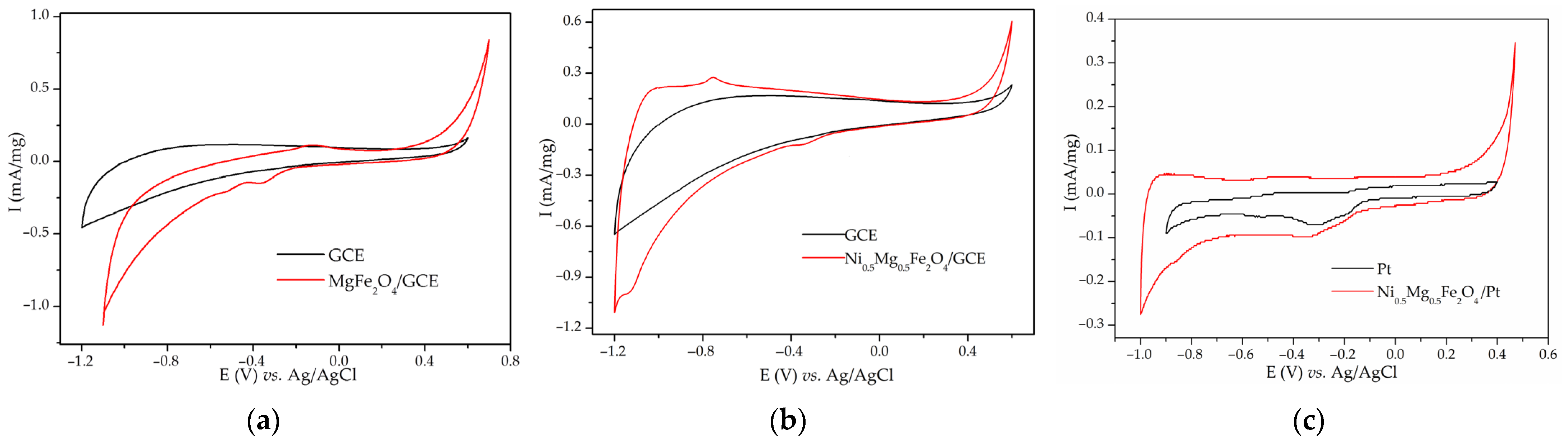

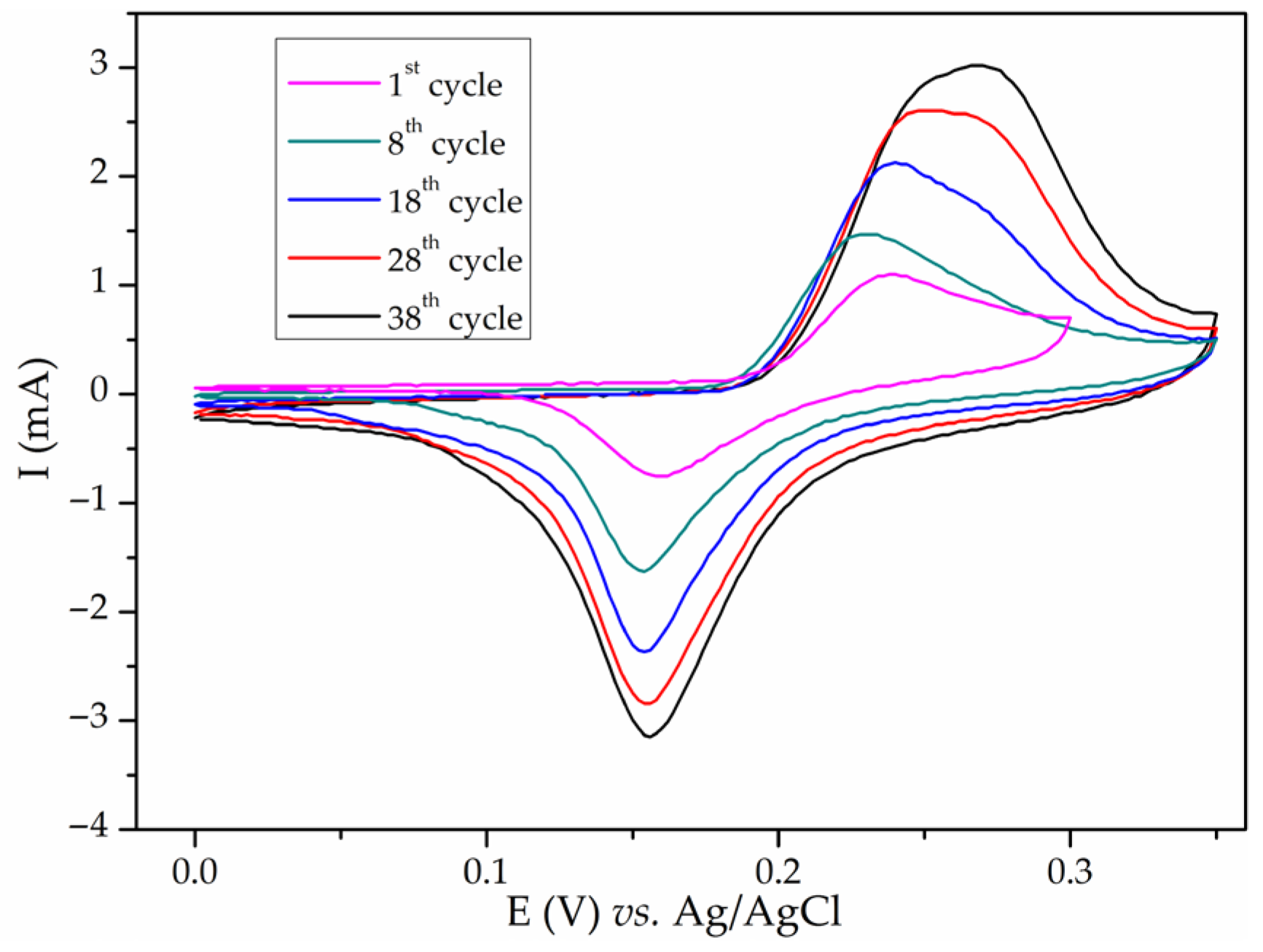

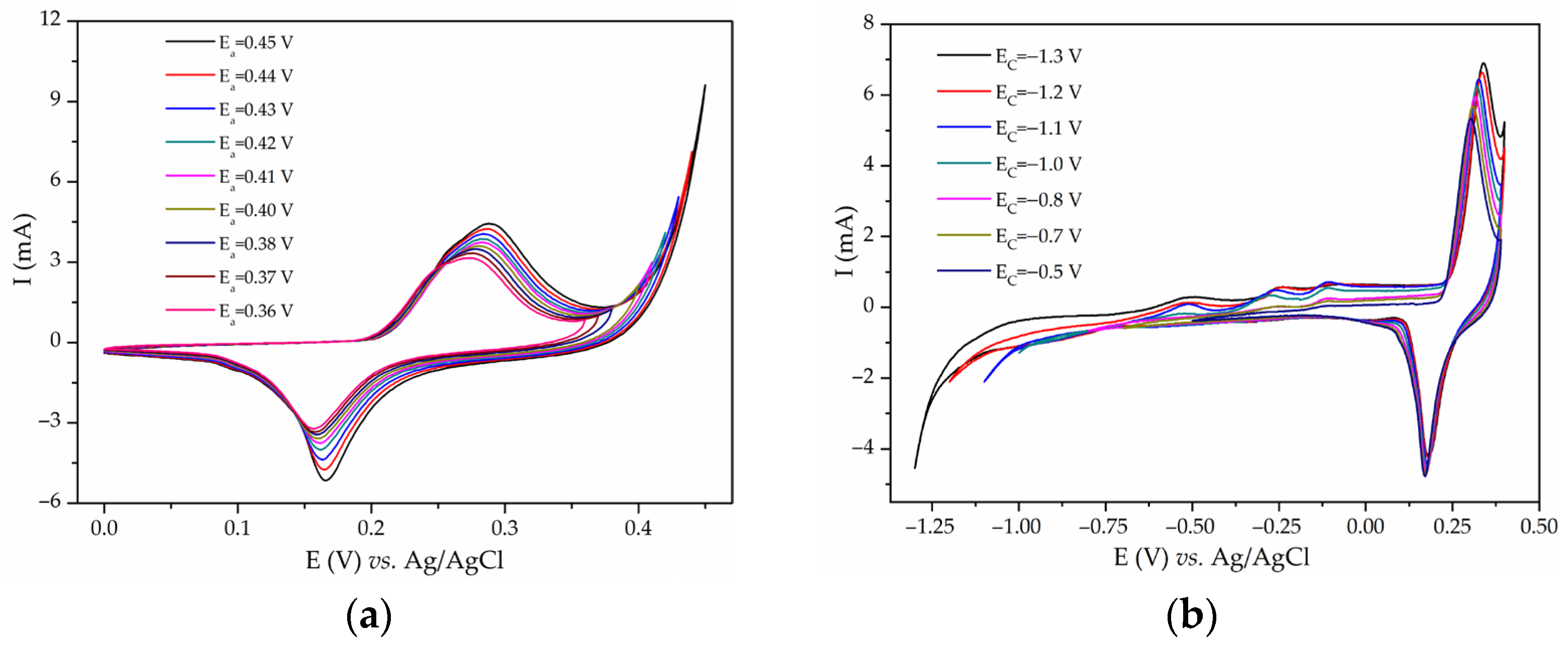

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dojčinović, M.P.; Stojković Simatović, I.; Nikolić, M.V. Supercapacitor Electrodes: Is Nickel Foam the Right Substrate for Active Materials? Materials 2024, 17, 1292. https://doi.org/10.3390/ma17061292
Dojčinović MP, Stojković Simatović I, Nikolić MV. Supercapacitor Electrodes: Is Nickel Foam the Right Substrate for Active Materials? Materials. 2024; 17(6):1292. https://doi.org/10.3390/ma17061292
Chicago/Turabian StyleDojčinović, Milena P., Ivana Stojković Simatović, and Maria Vesna Nikolić. 2024. "Supercapacitor Electrodes: Is Nickel Foam the Right Substrate for Active Materials?" Materials 17, no. 6: 1292. https://doi.org/10.3390/ma17061292





