Supercapacitors: An Efficient Way for Energy Storage Application
Abstract
1. Introduction
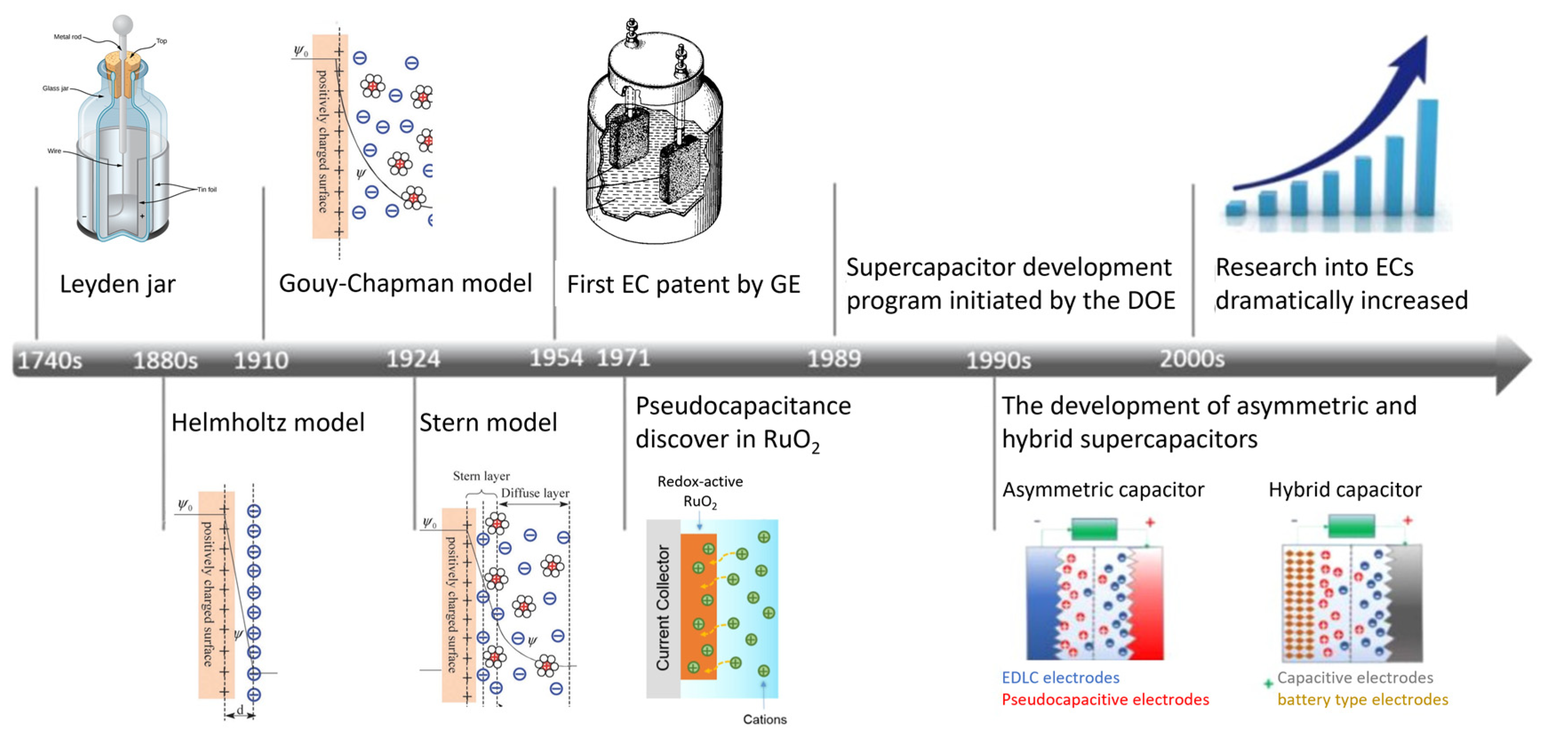
2. Short History of Supercapacitors
3. Fundamentals of Supercapacitors
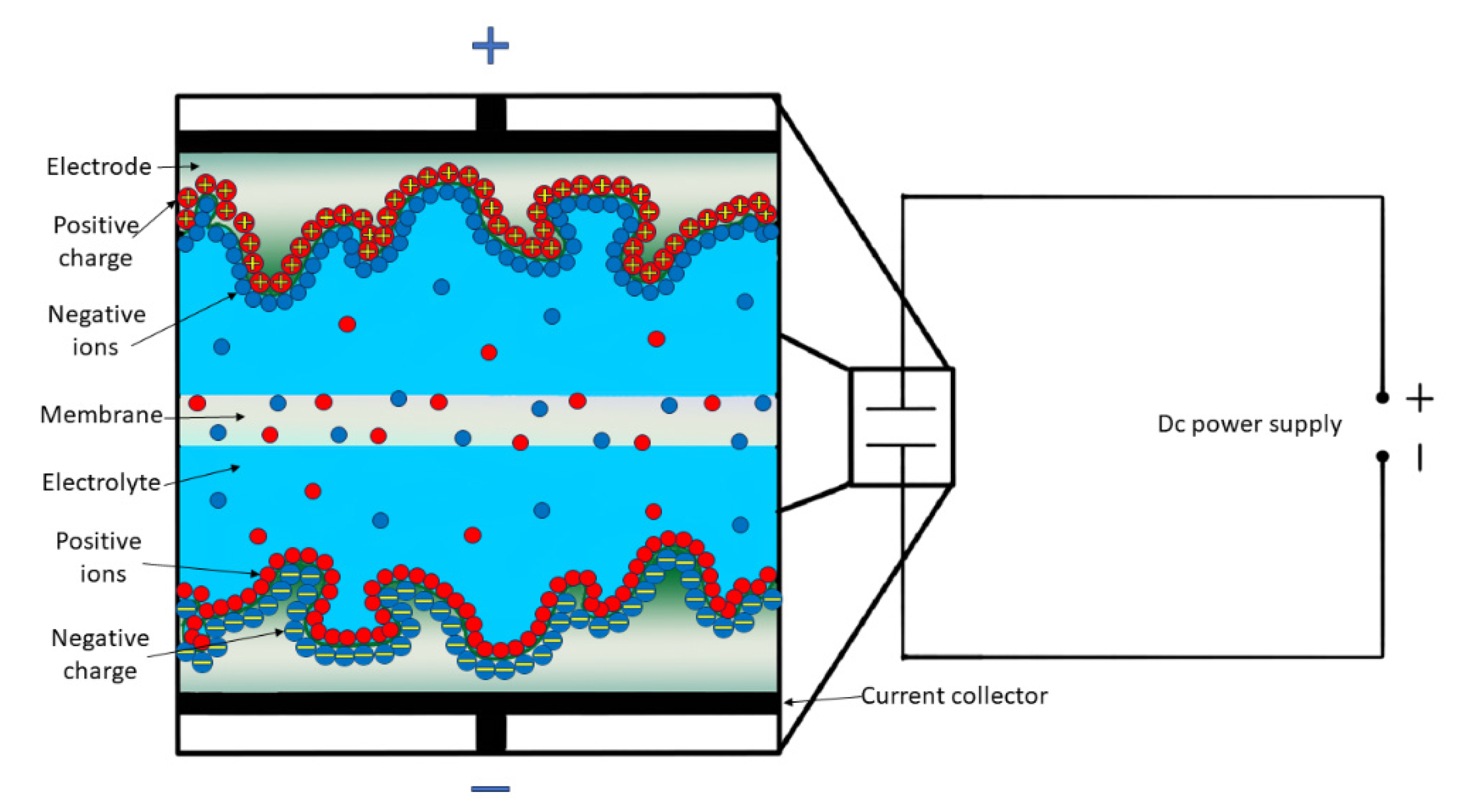
3.1. Principles and Properties
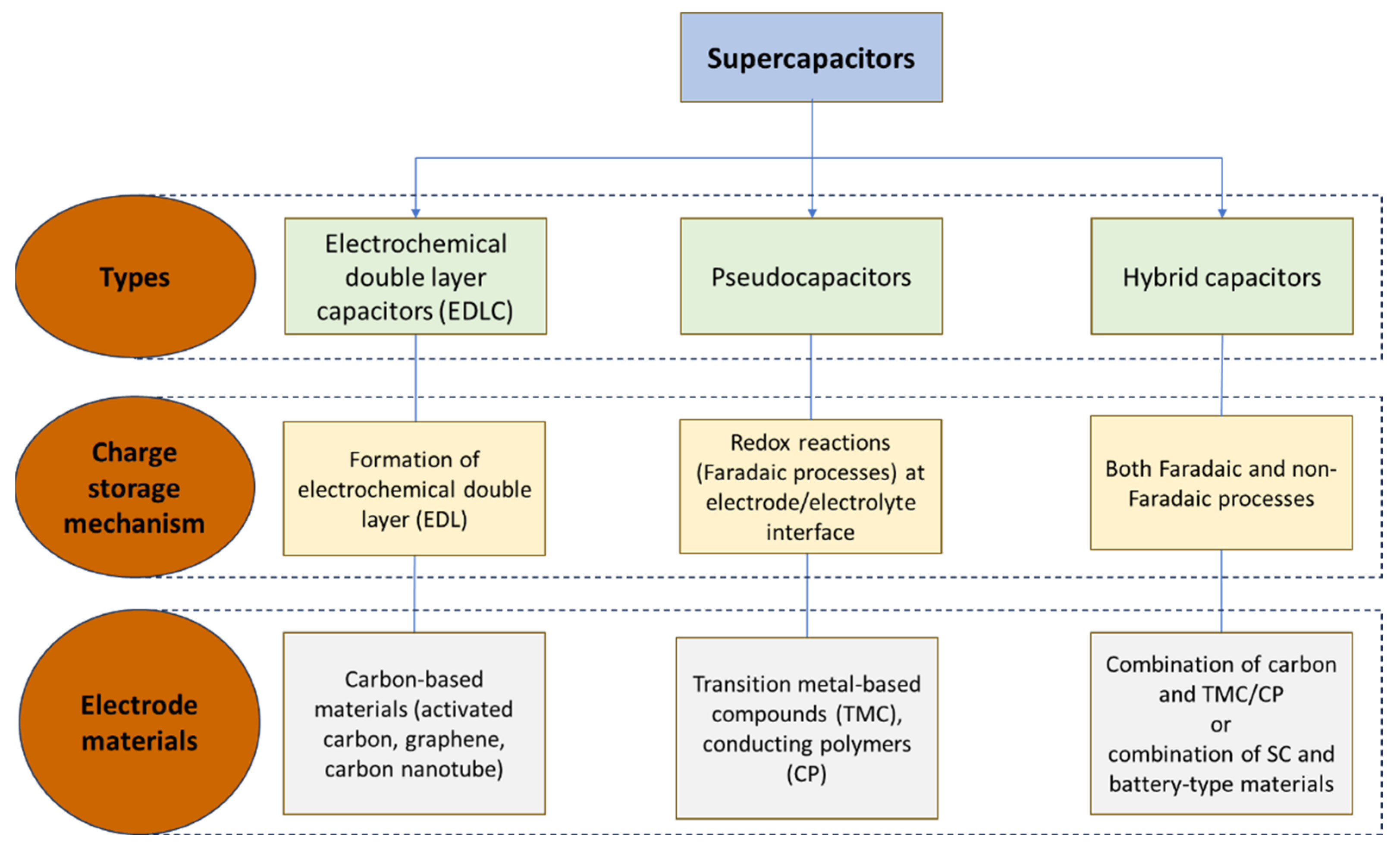
3.2. Classification of Supercapacitors
3.2.1. Electric Double Layer Capacitors (EDLCs)
3.2.2. Pseudocapacitors
3.2.3. Hybrid Capacitors
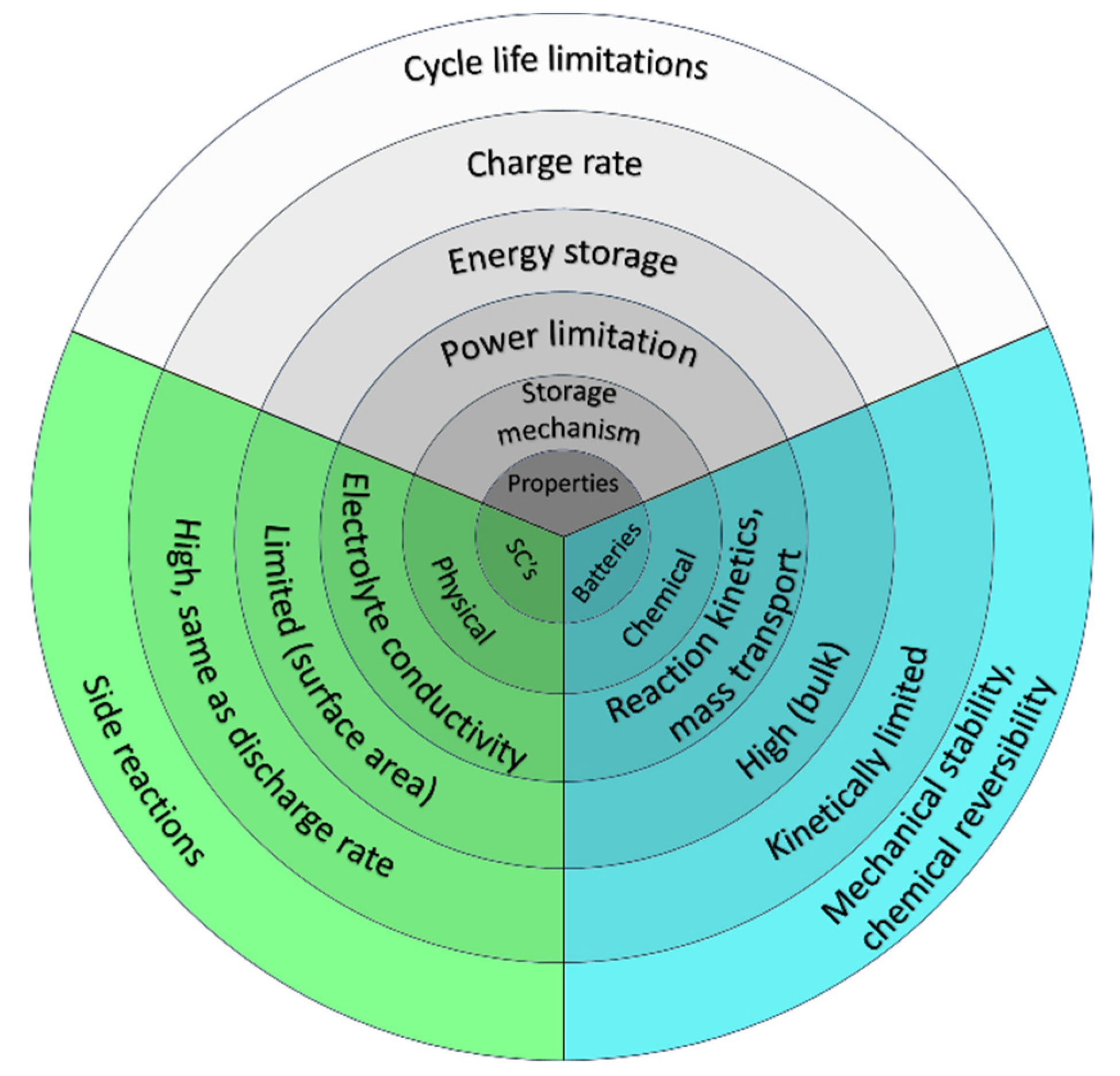
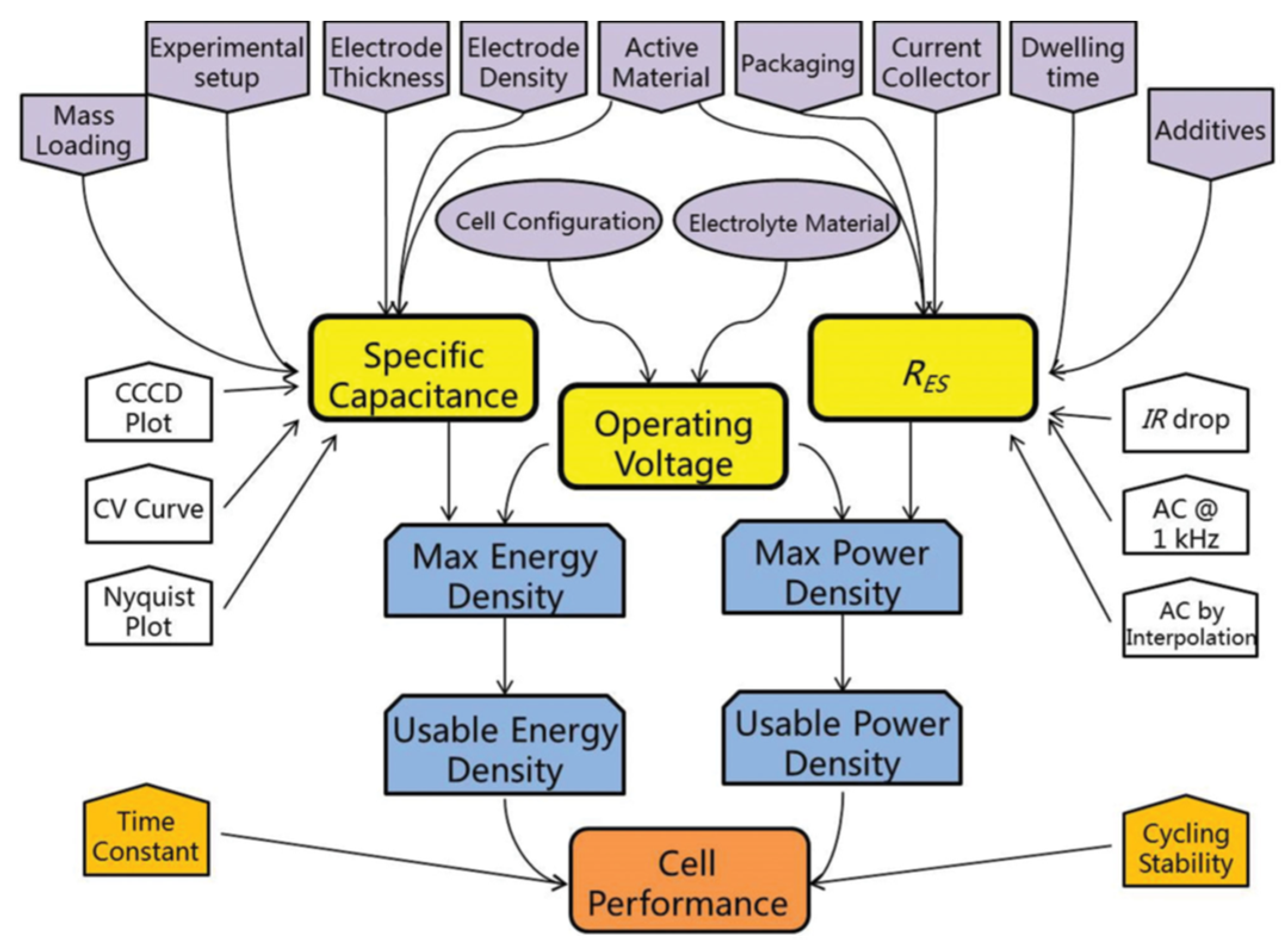
4. Characterization of Supercapacitive Behavior
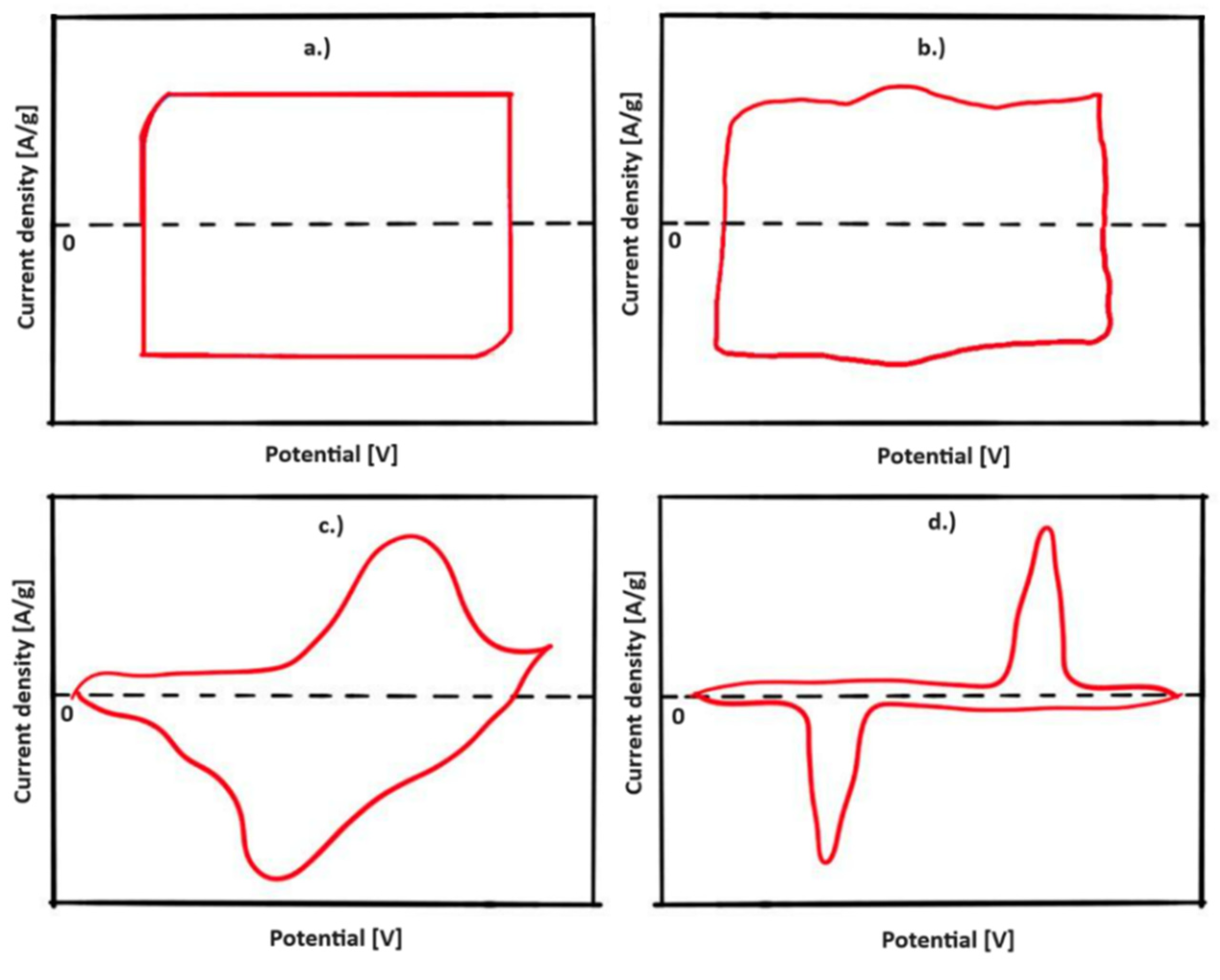
4.1. Cyclic Voltammetry
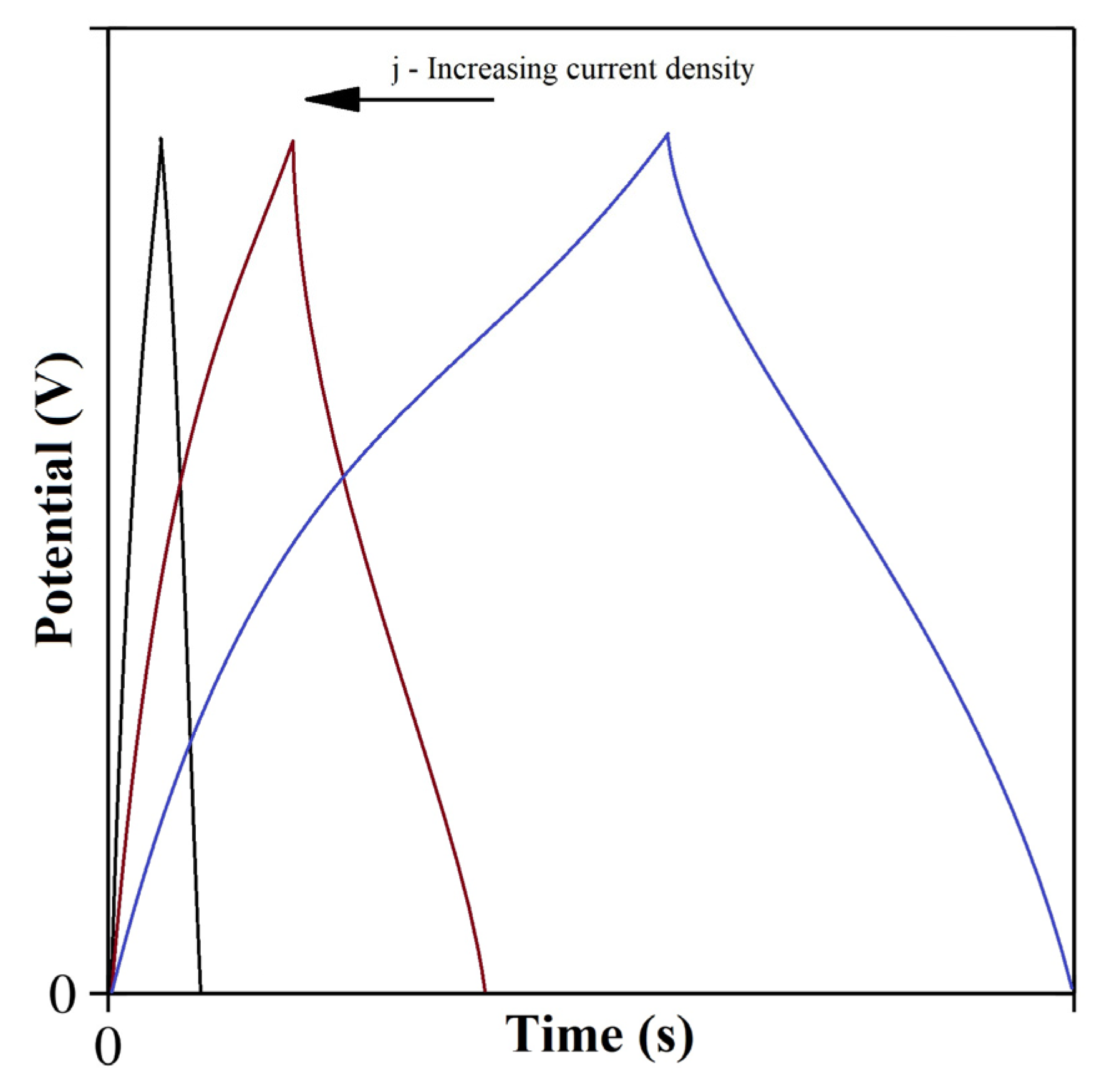
4.2. Galvanostatic Charge-Discharge
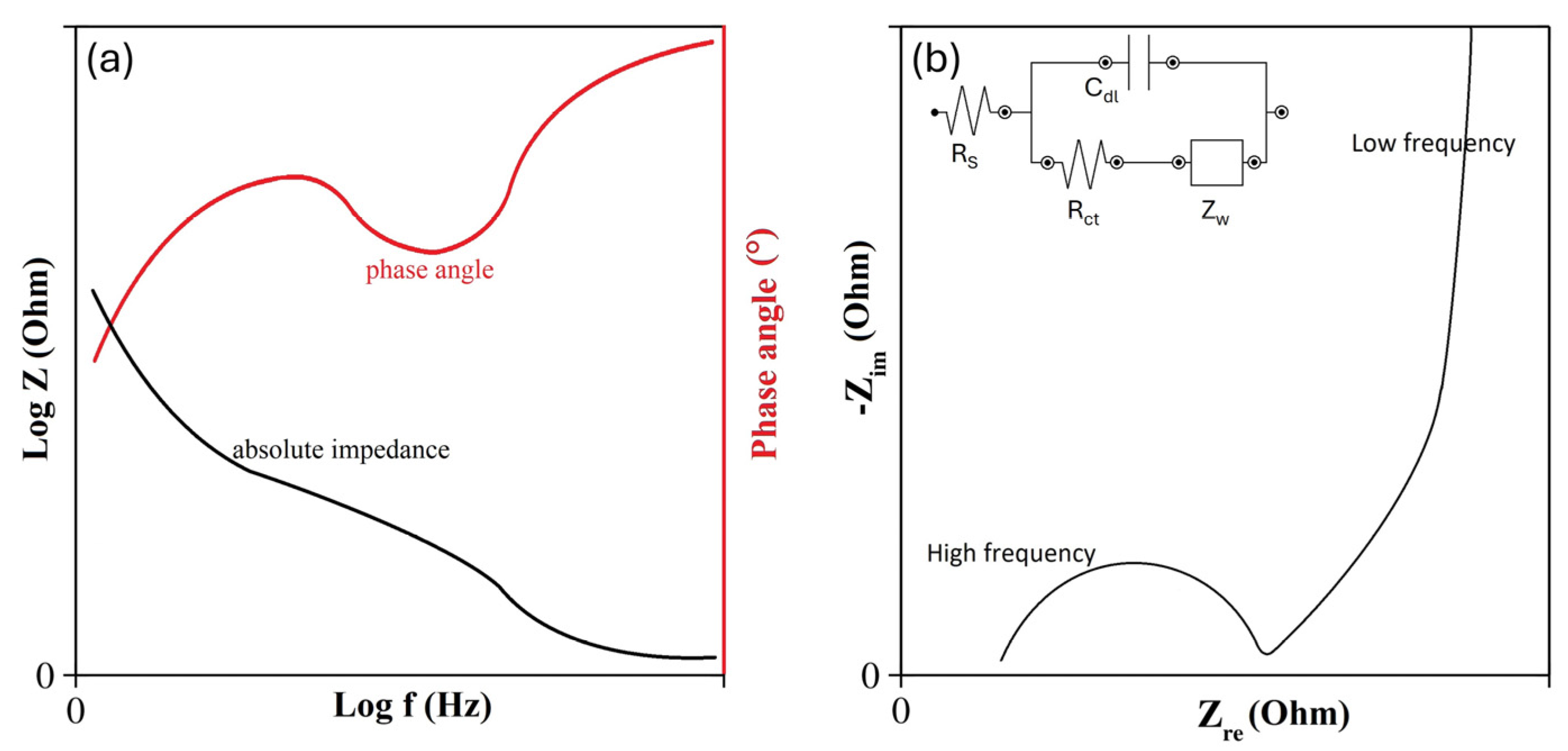
4.3. Electrochemical Impedance Spectroscopy
5. Components of Supercapacitors
5.1. Electrode Materials
5.1.1. Carbon-Based Materials
5.1.2. Transition Metal-Based Compounds
5.1.3. Conducting Polymers
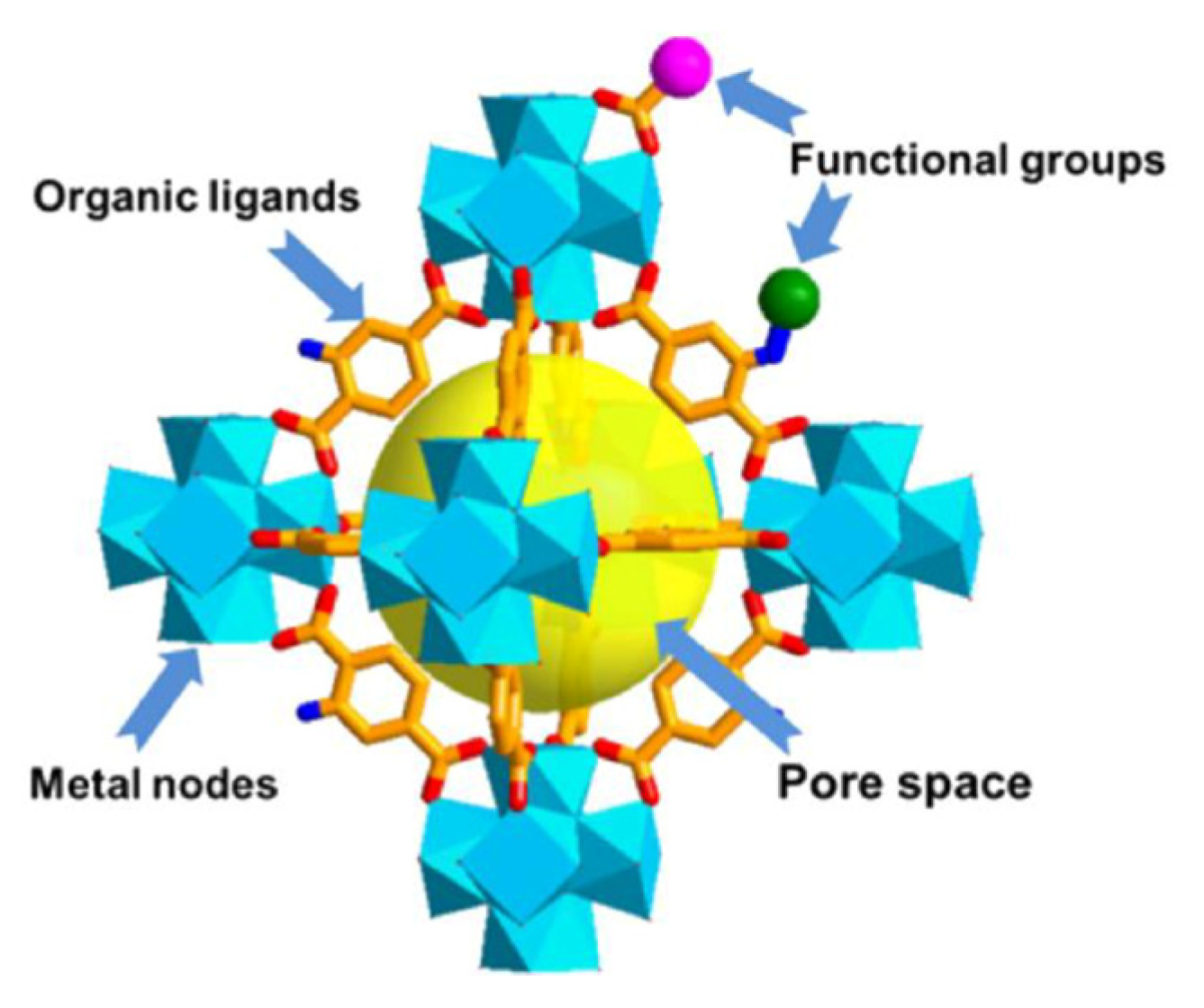
5.1.4. Novel Materials
5.2. Current Collectors
5.3. Electrolytes
5.3.1. Liquid Electrolytes
5.3.2. Solid-State/Quasi-Solid-State Electrolytes
5.4. Separators
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, S.; Prochowicz, D.; Parikh, N.; Mahapatra, A.; Pandey, M.K.; Kalam, A.; Tavakoli, M.M.; Yadav, P. Recent Progress in Growth of Single-Crystal Perovskites for Photovoltaic Applications. ACS Omega 2021, 6, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A Review of Aspects of Additive Engineering in Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Boudghene Stambouli, A.; Traversa, E. Fuel Cells, an Alternative to Standard Sources of Energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Carignano, M.G.; Costa-Castelló, R.; Roda, V.; Nigro, N.M.; Junco, S.; Feroldi, D. Energy Management Strategy for Fuel Cell-Supercapacitor Hybrid Vehicles Based on Prediction of Energy Demand. J. Power Sources 2017, 360, 419–433. [Google Scholar] [CrossRef]
- Song, Z.; Hou, J.; Hofmann, H.; Li, J.; Ouyang, M. Sliding-Mode and Lyapunov Function-Based Control for Battery/Supercapacitor Hybrid Energy Storage System Used in Electric Vehicles. Energy 2017, 122, 601–612. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Behzadi Pour, G.; Fekri Aval, L.; Kianfar, E. Comparative Studies of Nanosheet-Based Supercapacitors: A Review of Advances in Electrodes Materials. Case Stud. Chem. Environ. Eng. 2024, 9, 100584. [Google Scholar] [CrossRef]
- Jayakumar, S.; Santhosh, P.C.; Mohideen, M.M.; Radhamani, A.V. A Comprehensive Review of Metal Oxides (RuO2, Co3O4, MnO2 and NiO) for Supercapacitor Applications and Global Market Trends. J. Alloys Compd. 2024, 976, 173170. [Google Scholar] [CrossRef]
- Hu, H.; Yan, M.; Jiang, J.; Huang, A.; Cai, S.; Lan, L.; Ye, K.; Chen, D.; Tang, K.; Zuo, Q.; et al. A State-of-the-Art Review on Biomass-Derived Carbon Materials for Supercapacitor Applications: From Precursor Selection to Design Optimization. Sci. Total Environ. 2024, 912, 169141. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.H.; Liew, J.; Liu, L.; Goh, Z.L.; Pershaanaa, M.; Kamarulazam, F.; Bashir, S.; Ramesh, K.; Ramesh, S. A Comprehensive Review on Fundamentals and Components of Zinc-Ion Hybrid Supercapacitors. J. Energy Storage 2024, 81, 110370. [Google Scholar] [CrossRef]
- Rahat, S.M.S.M.; Hasan, K.M.Z.; Mondol, M.M.H.; Mallik, A.K. A Comprehensive Review of Carbon Nanotube-Based Metal Oxide Nanocomposites for Supercapacitors. J. Energy Storage 2023, 73, 108847. [Google Scholar] [CrossRef]
- Iversen, P.; Lacks, D.J. A Life of Its Own: The Tenuous Connection between Thales of Miletus and the Study of Electrostatic Charging. J. Electrost. 2012, 70, 309–311. [Google Scholar] [CrossRef]
- Heilbron, J.L. Electricity in the 17th [Seventeenth] and 18th Centuries: A Study of Early Modern Physics; University of California Press: Berkeley, CA, USA, 1979; ISBN 978-0-520-03478-5. [Google Scholar]
- Endo, M. High Power Electric Double Layer Capacitor (EDLC’s); from Operating Principle to Pore Size Control in Advanced Carbons. Carbon. Sci. 2000, 1, 91–97. [Google Scholar]
- Zhang, L.L.; Zhao, X.S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Becker, H.I. Low Voltage Electrolytic Capacitor. U.S. Patent No. 2,800,616, 23 July 1957. [Google Scholar]
- Pershaanaa, M.; Bashir, S.; Ramesh, S. Every Bite of Supercap: A Brief Review on Construction and Enhancement of Supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Subramanian, K.R.V. (Eds.) Nanostructured Ceramic Oxides for Supercapacitor Applications; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-7691-9. [Google Scholar]
- Spyker, R.L.; Nelms, R.M. Optimization of Double-Layer Capacitor Arrays. IEEE Trans. Ind. Appl. 2000, 36, 194–198. [Google Scholar] [CrossRef]
- Namisnyk, A.M.; Zhu, J.G. A Survey of Electrochemical Supercapacitor Technology. In Proceedings of the Australian Universities Power Engineering Conference, Christchurch, New Zealand, 28 September–1 October 2003; p. 6. [Google Scholar]
- Trasatti, S.; Buzzanca, G. Ruthenium Dioxide: A New Interesting Electrode Material. Solid State Structure and Electrochemical Behaviour. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, A1–A5. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Dowgiallo, E.J.; Hardin, J.E. Perspecticve on Ultracapacitors for Electric Vehicles. IEEE AES Syst. Mag. 1995, 10, 26–30. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.; Su, C.; Lu, C.; Dai, Y.; Zhao, S.; Hu, X.; Zhao, F.; Zhang, W.; Parkin, I.P.; et al. Recent Advances in Carbon-Based Nanomaterials for Multivalent-Ion Hybrid Capacitors: A Review. Energy Environ. Sci. 2023, 16, 1364–1383. [Google Scholar] [CrossRef]
- Shah, S.S.; Niaz, F.; Ehsan, M.A.; Das, H.T.; Younas, M.; Khan, A.S.; Rahman, H.U.; Nayem, S.M.A.; Oyama, M.; Aziz, M.A. Advanced Strategies in Electrode Engineering and Nanomaterial Modifications for Supercapacitor Performance Enhancement: A Comprehensive Review. J. Energy Storage 2024, 79, 110152. [Google Scholar] [CrossRef]
- Ahmad, F.; Zahid, M.; Jamil, H.; Khan, M.A.; Atiq, S.; Bibi, M.; Shahbaz, K.; Adnan, M.; Danish, M.; Rasheed, F.; et al. Advances in Graphene-Based Electrode Materials for High-Performance Supercapacitors: A Review. J. Energy Storage 2023, 72, 108731. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, C.; Kimura, H.; Xie, X.; Jiang, H.; Sun, X.; Yang, X.; Zhang, Y.; Du, W. Recent Advances in the Application of Carbon-Based Electrode Materials for High-Performance Zinc Ion Capacitors: A Mini Review. Adv. Compos. Hybrid. Mater. 2023, 6, 59. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, R.; Tian, L.; Wang, Q. The Development of an Electric Bus with Super-Capacitors as Unique Energy Storage. In Proceedings of the 2006 IEEE Vehicle Power and Propulsion Conference, Windsor, UK, 6–8 September 2006; pp. 1–5. [Google Scholar]
- Tong, H.Y. Development of a Driving Cycle for a Supercapacitor Electric Bus Route in Hong Kong. Sustain. Cities Soc. 2019, 48, 101588. [Google Scholar] [CrossRef]
- Hwang, G.; Lee, K.; Kim, J.; Lee, K.-J.; Lee, S.; Kim, M. Energy Management Optimization of Series Hybrid Electric Bus Using an Ultra-Capacitor and Novel Efficiency Improvement Factors. Sustainability 2020, 12, 7354. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Lajnef, W.; Vinassa, J.-M.; Briat, O.; Azzopardi, S.; Woirgard, E. Characterization Methods and Modelling of Ultracapacitors for Use as Peak Power Sources. J. Power Sources 2007, 168, 553–560. [Google Scholar] [CrossRef]
- Berrueta, A.; Ursua, A.; Martin, I.S.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Chamoli, P.; Banerjee, S.; Raina, K.K.; Kar, K.K. Handbook of Nanocomposite Supercapacitor Materials I: Characteristics; Kar, K.K., Ed.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 300, ISBN 978-3-030-43008-5. [Google Scholar]
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and Opportunities for Supercapacitors. APL Mater. 2019, 7, 100901. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, T.; Zhou, Q.; Sun, Y.; Qu, M.; Zeng, Z.; Ju, Y.; Li, L.; Wang, K.; Chi, F. A Review of Technologies and Applications on Versatile Energy Storage Systems. Renew. Sustain. Energy Rev. 2021, 148, 111263. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E. Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999; ISBN 978-1-4757-3060-9. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 0-471-04372-9. [Google Scholar]
- Paravannoor, A.; Baiju, K.V. Supercapacitors and Their Applications: Fundamentals, Current Trends, and Future Perspectives, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-325838-4. [Google Scholar]
- Arote, S. Electrochemical Energy Storage Devices and Supercapacitors: An Overview; IOP Publishing: Bristol, UK, 2021; ISBN 978-0-7503-3102-9. [Google Scholar]
- Kurzweil, P.; Chwistek, M. Electrochemical Stability of Organic Electrolytes in Supercapacitors: Spectroscopy and Gas Analysis of Decomposition Products. J. Power Sources 2008, 176, 555–567. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and Applications of Electrochemical Capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent Progress in Carbon-Based Materials for Supercapacitor Electrodes: A Review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Conway, B.E.; Angerstein-Kozlowska, H. The Electrochemical Study of Multiple-State Adsorption in Monolayers. Acc. Chem. Res. 1981, 14, 49–56. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Wang, L.; Gao, J.; Li, Q.; Ma, Y.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. High-Rate Capability of Three-Dimensionally Ordered Macroporous T-Nb2O5 through Li+ Intercalation Pseudocapacitance. J. Power Sources 2017, 361, 80–86. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Chakraborty, R.; Maji, P.K.; Koley, S.; Nayak, A.K.; Paul, D.; Pradhan, M. Intercalation Pseudocapacitance in Chemically Stable Au-α-Fe2O3-Mn3O4 Composite Nanorod: Towards Highly Efficient Solid-State Symmetric Supercapacitor Device. Electrochim. Acta 2019, 324, 134865. [Google Scholar] [CrossRef]
- Cook, J.B.; Ko, J.S.; Lin, T.C.; Robertson, D.D.; Kim, H.-S.; Yan, Y.; Yao, Y.; Dunn, B.S.; Tolbert, S.H. Ultrafast Sodium Intercalation Pseudocapacitance in MoS2 Facilitated by Phase Transition Suppression. ACS Appl. Energy Mater. 2023, 6, 99–108. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium Nitride for Aqueous Supercapacitors: A Topic Review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Liu, Z.; Tang, Z.; Yang, Q.; Zhao, Y.; Du, S.; Chen, Q.; Zhi, C. A Highly Elastic and Reversibly Stretchable All-Polymer Supercapacitor. Angew. Chem. 2019, 131, 15854–15858. [Google Scholar] [CrossRef]
- Burke, A. R&D Considerations for the Performance and Application of Electrochemical Capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced Materials and Technologies for Hybrid Supercapacitors for Energy Storage—A Review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A Review on Recent Advances in Hybrid Supercapacitors: Design, Fabrication and Applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Naoi, K.; Nagano, Y. Li-Ion-Based Hybrid Supercapacitors in Organic Medium. In Supercapacitors; Béguin, F., Frąckowiak, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 239–256. ISBN 978-3-527-32883-3. [Google Scholar]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate Methods for Evaluating the Efficiency and Capacitive Behavior of Different Types of Supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent Advancements in Supercapacitor Technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Baig, M.M.; Gul, I.H.; Baig, S.M.; Shahzad, F. The Complementary Advanced Characterization and Electrochemical Techniques for Electrode Materials for Supercapacitors. J. Energy Storage 2021, 44, 103370. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Wang, X.; Zhou, X.; Tao, Y.; Wu, H. Flexible Long-Chain-Linker Constructed Ni-Based Metal-Organic Frameworks with 1D Helical Channel and Their Pseudo-Capacitor Behavior Studies. J. Power Sources 2018, 377, 44–51. [Google Scholar] [CrossRef]
- Tanwilaisiri, A.; Xu, Y.; Zhang, R.; Harrison, D.; Fyson, J.; Areir, M. Design and Fabrication of Modular Supercapacitors Using 3D Printing. J. Energy Storage 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Czagany, M.; Hompoth, S.; Windisch, M.; Baumli, P. Investigation of the Supercapacitive Behavior of Electroless Ni-B Coatings. Metals 2023, 13, 1233. [Google Scholar] [CrossRef]
- Burke, A.; Miller, M. Testing of Electrochemical Capacitors: Capacitance, Resistance, Energy Density, and Power Capability. Electrochim. Acta 2010, 55, 7538–7548. [Google Scholar] [CrossRef]
- Cao, P.; Fan, Y.; Yu, J.; Wang, R.; Song, P.; Xiong, Y. Polypyrrole Nanocomposites Doped with Functional Ionic Liquids for High Performance Supercapacitors. New J. Chem. 2018, 42, 3909–3916. [Google Scholar] [CrossRef]
- Hou, Z.; Lu, H.; Yang, Q.; Zhao, Q.; Liu, J. Micromorphology-Controlled Synthesis of Polypyrrole Films by Using Binary Surfactant of Span80/OP10 via Interfacial Polymerization and Their Enhanced Electrochemical Capacitance. Electrochim. Acta 2018, 265, 601–608. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene Double-Layer Capacitor with Ac Line-Filtering Performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Dupont, M.F.; Hollenkamp, A.F.; Donne, S.W. Large Amplitude Electrochemical Impedance Spectroscopy for Characterizing the Performance of Electrochemical Capacitors. J. Electrochem. Soc. 2014, 161, A648–A656. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, S.; Tripathi; S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Cakici, M.; Kakarla, R.R.; Alonso-Marroquin, F. Advanced Electrochemical Energy Storage Supercapacitors Based on the Flexible Carbon Fiber Fabric-Coated with Uniform Coral-like MnO 2 Structured Electrodes. Chem. Eng. J. 2017, 309, 151–158. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A Review of Carbon Materials for Supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the Nanoporous Texture of Activated Carbons and Their Capacitance Properties in Different Electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Weinstein, L.; Dash, R. Supercapacitor Carbons. Mater. Today 2013, 16, 356–357. [Google Scholar] [CrossRef]
- Nuilek, K.; Wongwiriyapan, W.; Sattayarut, V.; Simon, A.; Koncz-Horváth, D.; Ferenczi, T.; Kristály, F.; Baumli, P. Comparison of Acid Exfoliators in Carbon Nanosheets Synthesis from Stinging Nettle (Urtica Dioica) for Electrochemical Applications. Sci. Rep. 2020, 10, 17270. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.; Wang, W.; Pronkin, S.; Romero, T.; Baaziz, W.; Nguyen-Dinh, L.; Chu, W.; Ersen, O.; Pham-Huu, C. Biosourced Foam-Like Activated Carbon Materials as High-Performance Supercapacitors. Adv. Sustain. Syst. 2018, 2, 1700123. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Zhu, J.; Yang, C.; Huang, H.; Chen, X.; Wang, R.; Yan, P.; Wei, S.; Liu, M.; et al. From Dual-Aerogels with Semi-Interpenetrating Polymer Network Structure to Hierarchical Porous Carbons for Advanced Supercapacitor Electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129356. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, W.; Zhang, R.; Niu, H.; Yang, Q.; Li, F.; Zhou, M.; Zhang, L.; Huang, Y. N,O-Doped Hierarchical Meso/Microporous Carbon Frameworks Enable Efficient Carbon-Based Supercapacitor. Appl. Surf. Sci. 2023, 626, 157148. [Google Scholar] [CrossRef]
- Abdel-Salam, A.I.; Attia, S.Y.; Mohamed, S.G.; El-Hosiny, F.I.; Sadek, M.A.; Rashad, M.M. Designing a Hierarchical Structure of Nickel-Cobalt-Sulfide Decorated on Electrospun N-Doped Carbon Nanofiber as an Efficient Electrode Material for Hybrid Supercapacitors. Int. J. Hydrogen Energy 2023, 48, 5463–5477. [Google Scholar] [CrossRef]
- Zhu, W.; Yan, X.; Huang, X.; Wu, S.; Chen, H.; Pan, J.; Li, T.; Shahnavaz, Z. Three-Dimensional Carbon-Based Endogenous-Exogenous MoO2 Composites as High-Performance Negative Electrode in Asymmetric Supercapacitors and Efficient Electrocatalyst for Oxygen Evolution Reaction. Ceram. Int. 2023, 49, 5646–5656. [Google Scholar] [CrossRef]
- Obreja, V.V.N.; Dinescu, A.; Obreja, A.C. Activated Carbon Based Electrodes in Commercial Supercapacitors and Their Performance. Int. Rev. Electr. Eng. 2010, 5, 272–281. [Google Scholar]
- Szabó, A.; Nánai, L.; Tóth, Z.R.; Hernadi, K. Simplification of the CCVD Method Used in the Growth of Carbon Nanotube Forests on Titanium Substrate. Solid. State Sci. 2021, 117, 106648. [Google Scholar] [CrossRef]
- Yuksel, R.; Sarioba, Z.; Cirpan, A.; Hiralal, P.; Unalan, H.E. Transparent and Flexible Supercapacitors with Single Walled Carbon Nanotube Thin Film Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 15434–15439. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Kariper, İ.A.; Karaman, C.; Karaman, O. MWCNT/Ruthenium Hydroxide Aerogel Supercapacitor Production and Investigation of Electrochemical Performances. Sci. Rep. 2022, 12, 12862. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Chakraborty, A.K.; Bera, S.; Krishnamurthy, S. Novel Hydrothermal Synthesis of CoS2/MWCNT Nanohybrid Electrode for Supercapacitor: A Systematic Investigation on the Influence of MWCNT. J. Phys. Chem. C 2018, 122, 18237–18246. [Google Scholar] [CrossRef]
- Frackowiak, E.; Jurewicz, K.; Szostak, K.; Delpeux, S.; Béguin, F. Nanotubular Materials as Electrodes for Supercapacitors. Fuel Process. Technol. 2002, 77–78, 213–219. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, Y.; Ubnoske, S.; Zang, J.; Cao, Y.; Henry, P.; Parker, C.B.; Glass, J.T. Highly Stretchable Supercapacitors via Crumpled Vertically Aligned Carbon Nanotube Forests. Adv. Energy Mater. 2019, 9, 1900618. [Google Scholar] [CrossRef]
- Cherusseri, J.; Kar, K.K. Self-Standing Carbon Nanotube Forest Electrodes for Flexible Supercapacitors. RSC Adv. 2015, 5, 34335–34341. [Google Scholar] [CrossRef]
- Avasthi, P.; Kumar, A.; Balakrishnan, V. Aligned CNT Forests on Stainless Steel Mesh for Flexible Supercapacitor Electrode with High Capacitance and Power Density. ACS Appl. Nano Mater. 2019, 2, 1484–1495. [Google Scholar] [CrossRef]
- Liu, C.G.; Liu, M.; Li, F.; Cheng, H.M. Frequency Response Characteristic of Single-Walled Carbon Nanotubes as Supercapacitor Electrode Material. Appl. Phys. Lett. 2008, 92, 143108. [Google Scholar] [CrossRef]
- Hwang, S.-G.; Ryu, S.-H.; Yun, S.-R.; Ko, J.M.; Kim, K.M.; Ryu, K.-S. Behavior of NiO–MnO2/MWCNT Composites for Use in a Supercapacitor. Mater. Chem. Phys. 2011, 130, 507–512. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Arasi, S.E.; Sudhahar, S.; Nallamuthu, N.; Devendran, P.; Lakshmanan, P.; Kumar, M.K. Enhanced Electrochemical Studies of ZnO/CNT Nanocomposite for Supercapacitor Devices. Phys. B Condens. Matter 2019, 568, 51–59. [Google Scholar] [CrossRef]
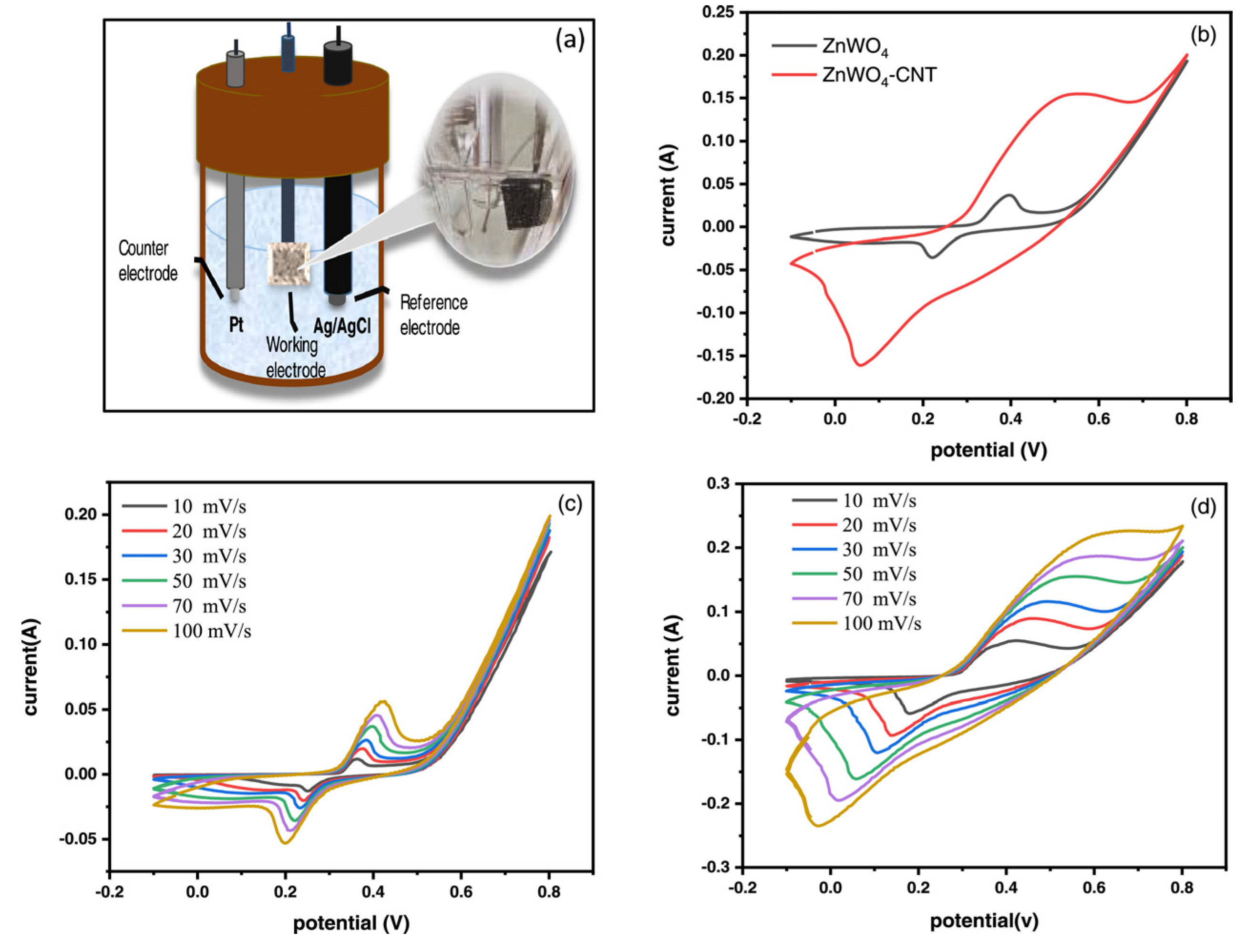
- Tourchi Moghadam, M.T.; Seifi, M.; Jamali, F.; Azizi, S.; Askari, M.B. ZnWO4-CNT as a Superior Electrode Material for Ultra-High Capacitance Supercapacitor. Surf. Interfaces 2022, 32, 102134. [Google Scholar] [CrossRef]
- Mandal, M.; Subudhi, S.; Alam, I.; Subramanyam, B.; Patra, S.; Das, S.; Raiguru, J.; Mahapatra, A.; Mahanandia, P. Simple and Cost-Effective Synthesis of Activated Carbon Anchored by Functionalized Multiwalled Carbon Nanotubes for High-Performance Supercapacitor Electrodes with High Energy Density and Power Density. J. Electron. Mater. 2021, 50, 2879–2889. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An Overview of the Applications of Graphene-Based Materials in Supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Lemine, A.S.; Zagho, M.M.; Altahtamouni, T.M.; Bensalah, N. Graphene a Promising Electrode Material for Supercapacitors—A Review. Int. J. Energy Res. 2018, 42, 4284–4300. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Rout, C.S.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, C.N.R. Graphene-Based Electrochemical Supercapacitors. J. Chem. Sci. 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/Metal Oxide Composite Electrode Materials for Energy Storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Lin, N.; Tian, J.; Shan, Z.; Chen, K.; Liao, W. Hydrothermal Synthesis of Hydrous Ruthenium Oxide/Graphene Sheets for High-Performance Supercapacitors. Electrochim. Acta 2013, 99, 219–224. [Google Scholar] [CrossRef]
- Mandal, M.; Nayak, A.K.; Upadhyay, P.; Patra, S.; Subudhi, S.; Mahapatra, A.; Mahanandia, P. Hydrothermal Synthesis of ZnFe2O4 Anchored Graphene and Activated Carbon as a New Hybrid Electrode for High-Performance Symmetric Supercapacitor Applications. Diam. Relat. Mater. 2023, 139, 110300. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Govindaraj, A.; Rao, C.N.R. Extraordinary Supercapacitor Performance of Heavily Nitrogenated Graphene Oxide Obtained by Microwave Synthesis. J. Mater. Chem. A 2013, 1, 7563. [Google Scholar] [CrossRef]
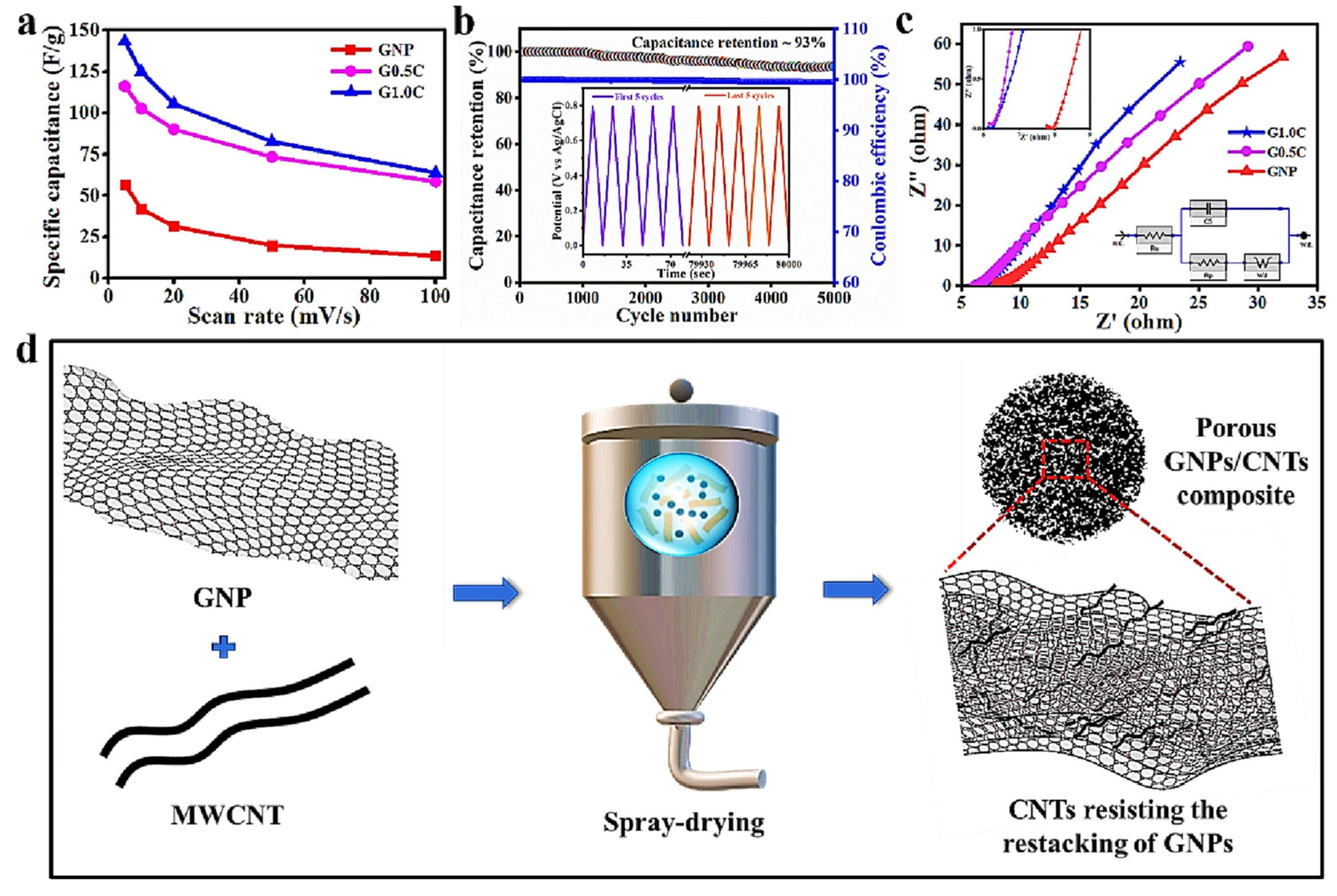
- Pandit, N.; Singh, P.; Prasad, S.; Kumar Keshri, A.; Czagany, M.; Hompoth, S.; Gacsi, Z.; Baumli, P. Electrochemical Behavior of GNP/CNT Porous Composite for Supercapacitor. Chem. Phys. Lett. 2023, 827, 140695. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liang, Q.; Zhao, Q.; Zhao, D.; Si, C. Compressible Cellulose Nanofibrils/Reduced Graphene Oxide Composite Carbon Aerogel for Solid-State Supercapacitor. Adv. Compos. Hybrid. Mater. 2022, 5, 1168–1179. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, J.; Gao, Z.; Li, B.; Xiong, C. Carbonized Cellulose Nanofibril/Graphene Oxide Composite Aerogels for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 1145–1151. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kim, K.-B. Ruthenium Oxide Thin Film Electrodes for Supercapacitors. Electrochem. Solid-State Lett. 2001, 4, A62. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-Nanonet Hollow Structured Co3 O4 for High Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Lu, Z.; Yin, Z.; Sim, D.H.; Xiao, N.; Lim, T.M.; Hng, H.H.; Zhang, H.; Yan, Q. Oriented Molecular Attachments Through Sol–Gel Chemistry for Synthesis of Ultrathin Hydrated Vanadium Pentoxide Nanosheets and Their Applications. Small 2013, 9, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Thalji, M.R.; Ali, G.A.M.; Shim, J.-J.; Chong, K.F. Cobalt-Doped Tungsten Suboxides for Supercapacitor Applications. Chem. Eng. J. 2023, 473, 145341. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary Oxide Nanostructured Materials for Supercapacitors: A Review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Lin, L.-Y.; Li, X. Efficient Battery Supercapacitor Hybrid Devices with Quaternary Metal Oxide Electrodes Based on Nickel and Cobalt. J. Energy Storage 2019, 25, 100826. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Hu, Z.; Miao, Y.; Sui, Y.; Qi, J.; Wei, F.; Ren, Y.; Zhan, Z.; Liu, J.; et al. In-Situ Growth of Core-Shell NiCo2O4@Ni-Co Layered Double Hydroxides for All-Solid-State Flexible Hybrid Supercapacitor. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 607, 125417. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Kim, S.J. Ruthenium Sulfide Nanoparticles as a New Pseudocapacitive Material for Supercapacitor. Electrochim. Acta 2017, 227, 85–94. [Google Scholar] [CrossRef]
- Lichchhavi; Kumar, S.; Srivastava, A.K.; Jha, S.K. Elucidation of Intercalation-Pseudocapacitor Mechanism in Binder-Free Bi2S3@Ni Foam Electrodes towards High-Performance Supercapattery. Electrochim. Acta 2023, 456, 142438. [Google Scholar] [CrossRef]
- Xu, J.-M.; Wang, X.-C.; Cheng, J.-P. Supercapacitive Performances of Ternary CuCo2 S4 Sulfides. ACS Omega 2020, 5, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Kang, R.; Geng, P.; Xiong, X.; Li, D.; Tian, Q.; Pang, H. New Asymmetric and Symmetric Supercapacitor Cells Based on Nickel Phosphide Nanoparticles. Mater. Chem. Phys. 2015, 165, 207–214. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Sun, C.; Shao, Y.; Wu, Y.; Lv, J.; Hao, X. Gallium Nitride Crystals: Novel Supercapacitor Electrode Materials. Adv. Mater. 2016, 28, 3768–3776. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-R.; Li, X.-Z.; Lai, X.-Q.; Xie, Y.; Li, Y.-M.; Yi, T.-F. Construction of Porous NiCo2S4@CeO2 Microspheres Composites for High-Performance Pseudocapacitor Electrode by Morphology Reshaping. Mater. Today Chem. 2021, 20, 100448. [Google Scholar] [CrossRef]
- Zhong, M.; Guo, D.; Meng, X.; Bian, L.; Song, Y.; Sun, X.; Liu, X. Heterostructured Polypyrrole/Hybrid Iron Oxide Composite Film as Highly Stable Anode for Pseudocapacitors. J. Power Sources 2021, 513, 230550. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research Progress on Conducting Polymer Based Supercapacitor Electrode Materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-Polymer-Based Supercapacitor Devices and Electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Han, Y.; Dai, L. Conducting Polymers for Flexible Supercapacitors. Macro Chem. Phys. 2019, 220, 1800355. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Q.; Tang, X.; Du, J.; Yu, T.; Xu, X.; Cai, D.; Guan, C.; Huang, W. 3D-Printed Highly Stretchable Conducting Polymer Electrodes for Flexible Supercapacitors. J. Mater. Chem. A 2021, 9, 19649–19658. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Polyaniline—Graphene Electrodes Prepared by Electropolymerization for High-Performance Capacitive Electrodes: A Brief Review. Batteries 2022, 8, 191. [Google Scholar] [CrossRef]
- Sivakkumar, S. Performance Evaluation of Poly(N-Methylaniline) and Polyisothianaphthene in Charge-Storage Devices. J. Power Sources 2004, 137, 322–328. [Google Scholar] [CrossRef]
- Talbi, H.; Just, P.-E.; Dao, L.H. Electropolymerization of Aniline on Carbonized Polyacrylonitrile Aerogel Electrodes: Applications for Supercapacitors. J. Appl. Electrochem. 2003, 33, 465–473. [Google Scholar] [CrossRef]
- Ryu, K. Redox Supercapacitor Using Polyaniline Doped with Li Salt as Electrode. Solid. State Ion. 2002, 152–153, 861–866. [Google Scholar] [CrossRef]
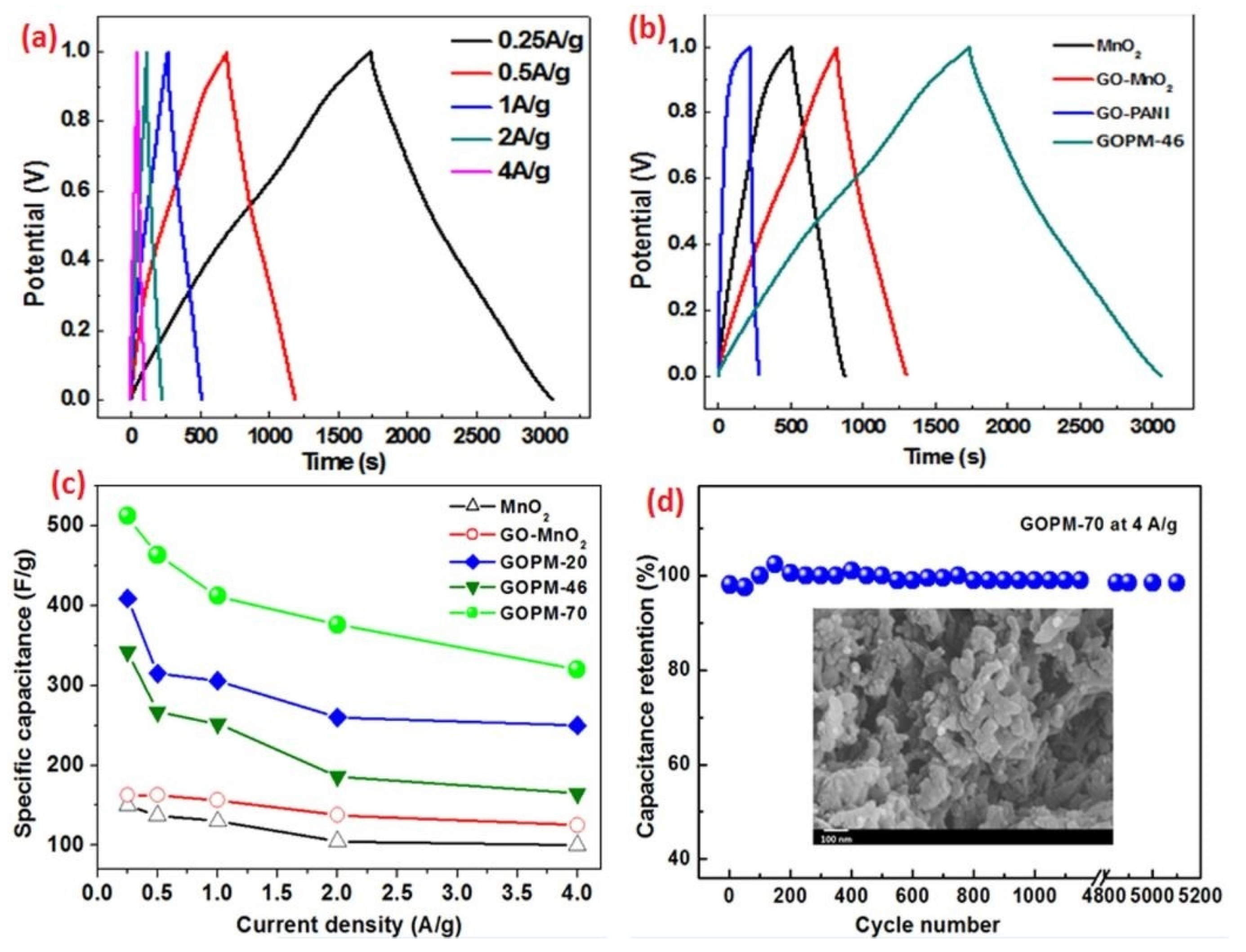
- Han, G.; Liu, Y.; Zhang, L.; Kan, E.; Zhang, S.; Tang, J.; Tang, W. MnO2 Nanorods Intercalating Graphene Oxide/Polyaniline Ternary Composites for Robust High-Performance Supercapacitors. Sci. Rep. 2014, 4, 4824. [Google Scholar] [CrossRef]
- Hughes, M.; Chen, G.Z.; Shaffer, M.S.P.; Fray, D.J.; Windle, A.H. Controlling the Nanostructure of Electrochemically Grown Nanoporous Composites of Carbon Nanotubes and Conducting Polymers. Compos. Sci. Technol. 2004, 64, 2325–2331. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically Synthesized Graphene/Polypyrrole Composites and Their Use in Supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Akbar, A.R.; Saleem, A.; Rauf, A.; Iqbal, R.; Tahir, M.; Peng, G.; Khan, A.S.; Hussain, A.; Ahmad, M.; Akhtar, M.; et al. Integrated MnO2/PEDOT Composite on Carbon Cloth for Advanced Electrochemical Energy Storage Asymmetric Supercapacitors. J. Power Sources 2023, 579, 233181. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal Organic Frameworks for Energy Storage and Conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Kishore Babu, S.; Jayachandran, M.; Maiyalagan, T.; Vijayakumar, T.; Gunasekaran, B. Metal-Organic Framework (MOF-5) Incorporated on NiCo2O4 as Electrode Material for Supercapacitor Application. Mater. Lett. 2021, 302, 130338. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline Metal-Organic Frameworks (MOFs): Synthesis, Structure and Function. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-Organic Framework-Based Materials for Hybrid Supercapacitor Application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yoon, S.J.; Shrestha, N.K.; Lee, S.-H.; Ahn, H.; Han, S.-H. Unusual Energy Storage and Charge Retention in Co-Based Metal–Organic-Frameworks. Microporous Mesoporous Mater. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–Organic Frameworks: A New Promising Class of Materials for a High Performance Supercapacitor Electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Li, Q.; Dai, Z.; Wu, J.; Liu, W.; Di, T.; Jiang, R.; Zheng, X.; Wang, W.; Ji, X.; Li, P.; et al. Fabrication of Ordered Macro-Microporous Single-Crystalline MOF and Its Derivative Carbon Material for Supercapacitor. Adv. Energy Mater. 2020, 10, 1903750. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene Electrochemical Microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; San Baek, D.; Joo, S.H.; Lee, K. MXene: An Emerging Two-Dimensional Material for Future Energy Conversion and Storage Applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Fu, Q.; Wen, J.; Zhang, N.; Wu, L.; Zhang, M.; Lin, S.; Gao, H.; Zhang, X. Free-Standing Ti3C2Tx Electrode with Ultrahigh Volumetric Capacitance. RSC Adv. 2017, 7, 11998–12005. [Google Scholar] [CrossRef]
- Syamsai, R.; Grace, A.N. Ta4C3 MXene as Supercapacitor Electrodes. J. Alloys Compd. 2019, 792, 1230–1238. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Cheng, R.; Yang, J.; Cui, C.; Zhang, C.; Wang, X. MXene-Coated Silk-Derived Carbon Cloth toward Flexible Electrode for Supercapacitor Application. J. Energy Chem. 2018, 27, 161–166. [Google Scholar] [CrossRef]
- Adil, M.; Olabi, A.G.; Abdelkareem, M.A.; Alawadhi, H.; Bahaa, A.; El Said, K.; Rodriguez, C. In-Situ Grown Bimetallic FeCu MOF-MXene Composite for Solid-State Asymmetric Supercapacitors. J. Energy Storage 2023, 68, 107817. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, X.; Ding, B.; Zou, L.; Wang, P.; Li, C.; Hong, X.; Wang, Z. MXene/PANI Composite Fiber-Based Asymmetric Supercapacitors for Self-Powered Energy Storage System. Mater. Lett. 2024, 355, 135494. [Google Scholar] [CrossRef]
- Etman, A.E.-S.; Ibrahim, A.M.; Darwish, F.A.-Z.M.; Qasim, K.F. A 10 Years-Developmental Study on Conducting Polymers Composites for Supercapacitors Electrodes: A Review for Extensive Data Interpretation. J. Ind. Eng. Chem. 2023, 122, 27–45. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, W.; Xu, G.; Chou, T.-W. Structural Supercapacitor Composites: A Review. Compos. Sci. Technol. 2021, 204, 108636. [Google Scholar] [CrossRef]
- Dhandapani, E.; Thangarasu, S.; Ramesh, S.; Ramesh, K.; Vasudevan, R.; Duraisamy, N. Recent Development and Prospective of Carbonaceous Material, Conducting Polymer and Their Composite Electrode Materials for Supercapacitor—A Review. J. Energy Storage 2022, 52, 104937. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, A.; Zhang, X.; Mu, J.; Che, H.; Wang, Y.; Wang, T.-T.; Zhang, Z.; Kou, Z. Fundamentals, Advances and Challenges of Transition Metal Compounds-Based Supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar] [CrossRef]
- Cui, M.; Meng, X. Overview of Transition Metal-Based Composite Materials for Supercapacitor Electrodes. Nanoscale Adv. 2020, 2, 5516–5528. [Google Scholar] [CrossRef] [PubMed]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.-H.; Deep, A. Metal-Organic Frameworks and Their Composites as Efficient Electrodes for Supercapacitor Applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Panda, S.; Deshmukh, K.; Khadheer Pasha, S.K.; Theerthagiri, J.; Manickam, S.; Choi, M.Y. MXene Based Emerging Materials for Supercapacitor Applications: Recent Advances, Challenges, and Future Perspectives. Coord. Chem. Rev. 2022, 462, 214518. [Google Scholar] [CrossRef]
- Meena, D.; Kumar, R.; Gupta, S.; Khan, O.; Gupta, D.; Singh, M. Energy Storage in the 21st Century: A Comprehensive Review on Factors Enhancing the next-Generation Supercapacitor Mechanisms. J. Energy Storage 2023, 72, 109323. [Google Scholar] [CrossRef]
- Lei, Z.; Christov, N.; Zhao, X.S. Intercalation of Mesoporous Carbon Spheres between Reduced Graphene Oxide Sheets for Preparing High-Rate Supercapacitor Electrodes. Energy Environ. Sci. 2011, 4, 1866. [Google Scholar] [CrossRef]
- Abdisattar, A.; Yeleuov, M.; Daulbayev, C.; Askaruly, K.; Tolynbekov, A.; Taurbekov, A.; Prikhodko, N. Recent Advances and Challenges of Current Collectors for Supercapacitors. Electrochem. Commun. 2022, 142, 107373. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Flexible Supercapacitors Based on Paper Substrates: A New Paradigm for Low-Cost Energy Storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Y.; Li, N.; Zhang, H.; Zhi, C. Recent Progress on Flexible and Wearable Supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef]
- Samantara, A.K.; Ratha, S. Materials Development for Active/Passive Components of a Supercapacitor: Background, Present Status and Future Perspective; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-981-10-7263-5. [Google Scholar]
- Gong, X.; Cheng, J.P.; Liu, F.; Zhang, L.; Zhang, X. Nickel–Cobalt Hydroxide Microspheres Electrodepositioned on Nickel Cobaltite Nanowires Grown on Ni Foam for High-Performance Pseudocapacitors. J. Power Sources 2014, 267, 610–616. [Google Scholar] [CrossRef]
- Ryu, I.; Yang, M.; Kwon, H.; Park, H.K.; Do, Y.R.; Lee, S.B.; Yim, S. Coaxial RuO2–ITO Nanopillars for Transparent Supercapacitor Application. Langmuir 2014, 30, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.H.; Shao, L.; Zhao, P.C.; Wang, B.Y.; Cao, G.P.; Yang, Y.S. Carbon Coated Stainless Steel Mesh as a Low-Cost and Corrosion-Resistant Current Collector for Aqueous Rechargeable Batteries. J. Mater. Chem. A 2017, 5, 15752–15758. [Google Scholar] [CrossRef]
- Lei, C.; Markoulidis, F.; Ashitaka, Z.; Lekakou, C. Reduction of Porous Carbon/Al Contact Resistance for an Electric Double-Layer Capacitor (EDLC). Electrochim. Acta 2013, 92, 183–187. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Miller, M. The Power Capability of Ultracapacitors and Lithium Batteries for Electric and Hybrid Vehicle Applications. J. Power Sources 2011, 196, 514–522. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Guay, D. Asymmetric and Hybrid Devices in Aqueous Electrolytes. In Supercapacitors; Béguin, F., Frąckowiak, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 257–288. ISBN 978-3-527-32883-3. [Google Scholar]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte Selection for Supercapacitive Devices: A Critical Review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; Jiang, L.; Wu, C.; Zhao, Q.; Liu, X.; Hu, B.; Yi, L. The Effects of Electrolyte on the Supercapacitive Performance of Activated Calcium Carbide-Derived Carbon. J. Power Sources 2013, 226, 202–209. [Google Scholar] [CrossRef]
- Jiménez-Cordero, D.; Heras, F.; Gilarranz, M.A.; Raymundo-Piñero, E. Grape Seed Carbons for Studying the Influence of Texture on Supercapacitor Behaviour in Aqueous Electrolytes. Carbon. 2014, 71, 127–138. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, M.I.; Cheng, F.; Lu, W. A Review on Selection Criteria of Aqueous Electrolytes Performance Evaluation for Advanced Asymmetric Supercapacitors. J. Energy Storage 2021, 40, 102729. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Direct Synthesis of Highly Porous Interconnected Carbon Nanosheets and Their Application as High-Performance Supercapacitors. ACS Nano 2014, 8, 5069–5078. [Google Scholar] [CrossRef]
- Jung, N.; Kwon, S.; Lee, D.; Yoon, D.; Park, Y.M.; Benayad, A.; Choi, J.; Park, J.S. Synthesis of Chemically Bonded Graphene/Carbon Nanotube Composites and Their Application in Large Volumetric Capacitance Supercapacitors. Adv. Mater. 2013, 25, 6854–6858. [Google Scholar] [CrossRef]
- Barranco, V.; Lillo-Rodenas, M.A.; Linares-Solano, A.; Oya, A.; Pico, F.; Ibañez, J.; Agullo-Rueda, F.; Amarilla, J.M.; Rojo, J.M. Amorphous Carbon Nanofibers and Their Activated Carbon Nanofibers as Supercapacitor Electrodes. J. Phys. Chem. C 2010, 114, 10302–10307. [Google Scholar] [CrossRef]
- Hanlon, D.; Backes, C.; Higgins, T.M.; Hughes, M.; O’Neill, A.; King, P.; McEvoy, N.; Duesberg, G.S.; Mendoza Sanchez, B.; Pettersson, H.; et al. Production of Molybdenum Trioxide Nanosheets by Liquid Exfoliation and Their Application in High-Performance Supercapacitors. Chem. Mater. 2014, 26, 1751–1763. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, B.; Wang, J.; Shao, Z. Non-Aqueous Hybrid Supercapacitors Fabricated with Mesoporous TiO2 Microspheres and Activated Carbon Electrodes with Superior Performance. J. Power Sources 2014, 253, 80–89. [Google Scholar] [CrossRef]
- Rogers, R.D.; Voth, G.A. Ionic Liquids. Acc. Chem. Res. 2007, 40, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. GSC 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Aravindan, V.; Ulaganathan, M.; Madhavi, S. Research Progress in Na-Ion Capacitors. J. Mater. Chem. A 2016, 4, 7538–7548. [Google Scholar] [CrossRef]
- McEwen, A.B.; McDevitt, S.F.; Koch, V.R. Nonaqueous Electrolytes for Electrochemical Capacitors: Imidazolium Cations and Inorganic Fluorides with Organic Carbonates. J. Electrochem. Soc. 1997, 144, L84–L86. [Google Scholar] [CrossRef]
- Orita, A.; Kamijima, K.; Yoshida, M. Allyl-Functionalized Ionic Liquids as Electrolytes for Electric Double-Layer Capacitors. J. Power Sources 2010, 195, 7471–7479. [Google Scholar] [CrossRef]
- Lin, R.; Huang, P.; Ségalini, J.; Largeot, C.; Taberna, P.L.; Chmiola, J.; Gogotsi, Y.; Simon, P. Solvent Effect on the Ion Adsorption from Ionic Liquid Electrolyte into Sub-Nanometer Carbon Pores. Electrochim. Acta 2009, 54, 7025–7032. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-Based Materials for Flexible All-Solid-State Supercapacitors. Adv. Mater. 2018, 30, 1705489. [Google Scholar] [CrossRef]
- Yi, F.; Ren, H.; Shan, J.; Sun, X.; Wei, D.; Liu, Z. Wearable Energy Sources Based on 2D Materials. Chem. Soc. Rev. 2018, 47, 3152–3188. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.M.; Venâncio, R.; Peterlevitz, A.C.; Zanin, H. Recent Advances on Quasi-Solid-State Electrolytes for Supercapacitors. J. Energy Chem. 2022, 67, 697–717. [Google Scholar] [CrossRef]
- Rodríguez, J.; Navarrete, E.; Dalchiele, E.A.; Sánchez, L.; Ramos-Barrado, J.R.; Martín, F. Polyvinylpyrrolidone–LiClO4 Solid Polymer Electrolyte and Its Application in Transparent Thin Film Supercapacitors. J. Power Sources 2013, 237, 270–276. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, S.; Yan, Y.; Wang, L.; Xu, G.; Yarlagadda, S.; Chou, T.-W. High-Performance Structural Supercapacitors Based on Aligned Discontinuous Carbon Fiber Electrodes and Solid Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 11774–11782. [Google Scholar] [CrossRef]
- Sumboja, A.; Liu, J.; Zheng, W.G.; Zong, Y.; Zhang, H.; Liu, Z. Electrochemical Energy Storage Devices for Wearable Technology: A Rationale for Materials Selection and Cell Design. Chem. Soc. Rev. 2018, 47, 5919–5945. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Chun, S.-J.; Lee, S.-S.; Kim, B.-Y.; Kim, J.H.; Chung, H.; Lee, S.-Y.; Kim, W. All-Solid-State Flexible Supercapacitors Fabricated with Bacterial Nanocellulose Papers, Carbon Nanotubes, and Triblock-Copolymer Ion Gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-Based Gel Polymer Electrolytes in Flexible Solid-State Supercapacitors: Opportunities and Challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Qin, G.; Wang, M.; Fan, L.; Fang, X.; Zhang, D.; Liu, J.; Qin, J.; Shi, J.; Yang, J.; Chen, Q. Multifunctional Supramolecular Gel Polymer Electrolyte for Self-Healable and Cold-Resistant Supercapacitor. J. Power Sources 2020, 474, 228602. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, S.S.; Lee, E.; Yoon, J.; Lee, H.S. Kinetics of Hydrazine Reduction of Thin Films of Graphene Oxide and the Determination of Activation Energy by the Measurement of Electrical Conductivity. RSC Adv. 2015, 5, 102567–102573. [Google Scholar] [CrossRef]
- Ahankari, S.; Lasrado, D.; Subramaniam, R. Advances in Materials and Fabrication of Separators in Supercapacitors. Mater. Adv. 2022, 3, 1472–1496. [Google Scholar] [CrossRef]
- Saunier, J.; Alloin, F.; Sanchez, J.Y.; Maniguet, L. Plasticized Microporous Poly(Vinylidene Fluoride) Separators for Lithium-ion Batteries. III. Gel Properties and Irreversible Modifications of Poly(Vinylidene Fluoride) Membranes under Swelling in Liquid Electrolytes. J. Polym. Sci. B Polym. Phys. 2004, 42, 2308–2317. [Google Scholar] [CrossRef]
- Karabelli, D.; Leprêtre, J.-C.; Alloin, F.; Sanchez, J.-Y. Poly(Vinylidene Fluoride)-Based Macroporous Separators for Supercapacitors. Electrochim. Acta 2011, 57, 98–103. [Google Scholar] [CrossRef]


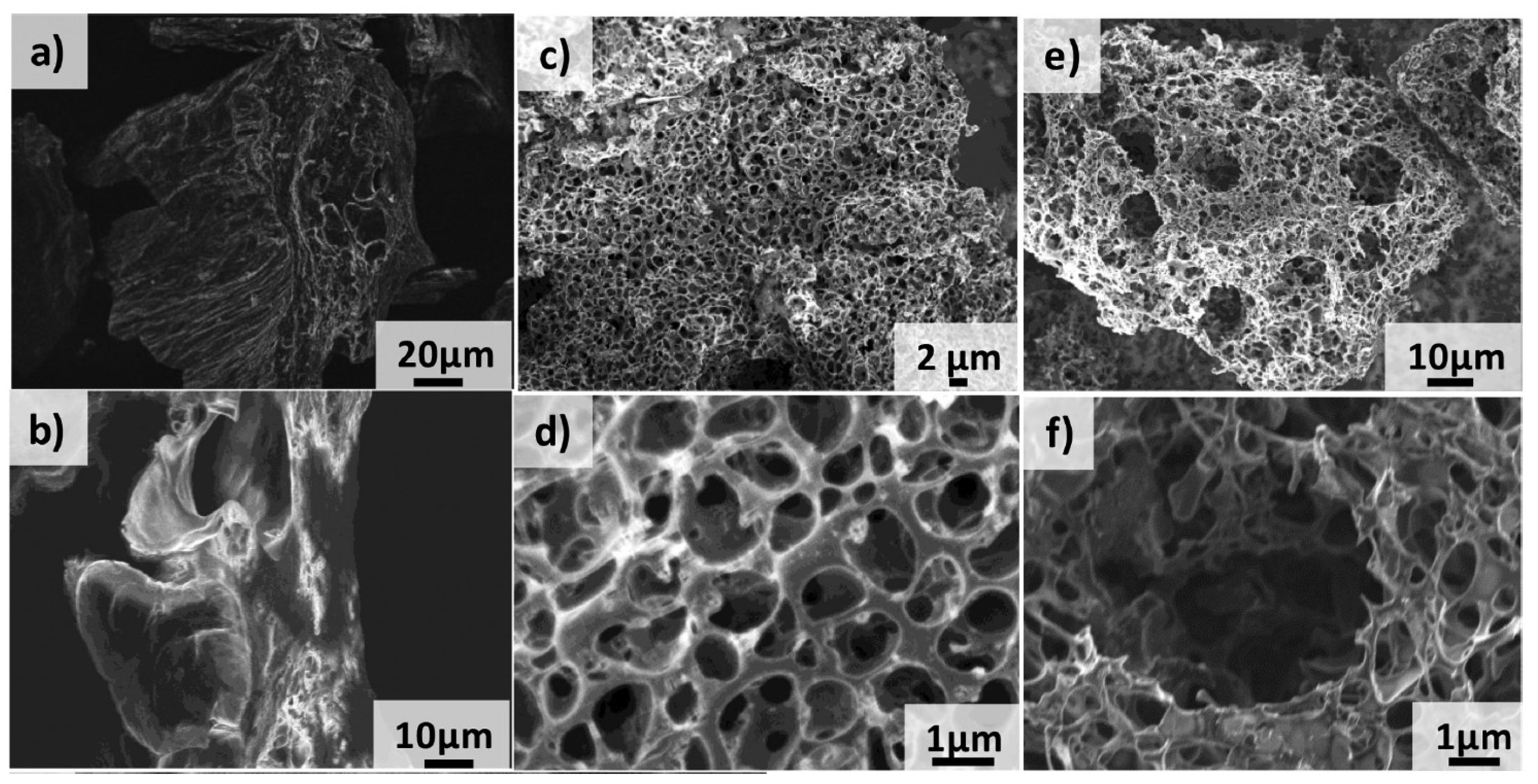


| Technique | Merits and Demerits |
|---|---|
| CV | Merits |
| |
| Demerits | |
| |
| GCD | Merits |
| |
| Demerits | |
| |
| EIS | Merits |
| |
| Demerits | |
|
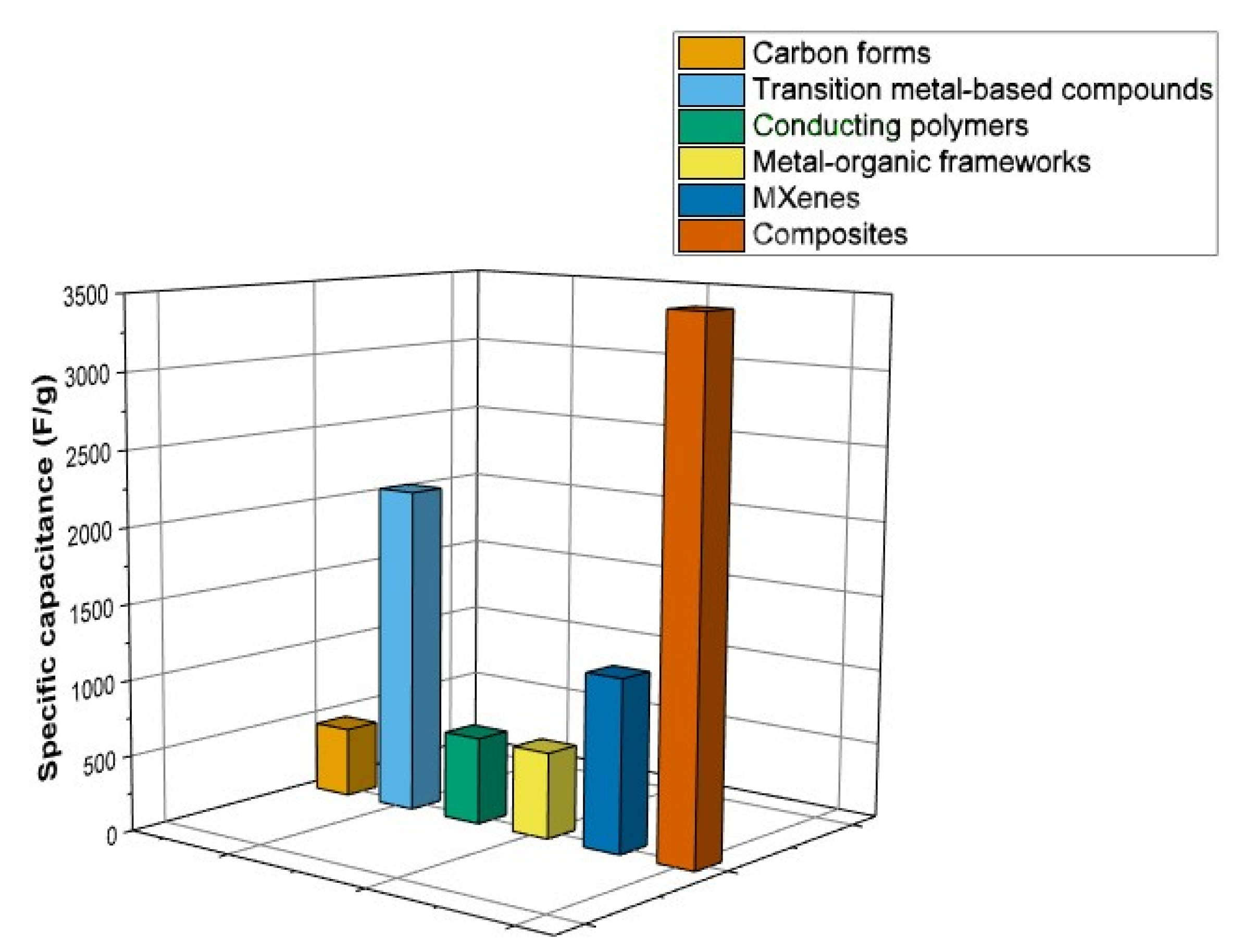
| Material Group | Material Types | Features | Importance for SCs |
|---|---|---|---|
| Carbon-based materials | Activated Carbon | High SSA, porous structure, physisorption | EDL capacitance, large amount of charge to be stored at electrode/electrolyte interface, high CS |
| Carbon Nanotubes (CNTs) | Unique tubular nanostructure, high electrical conductivity, high SSA | EDL capacitance, high CS, high mechanical stability, | |
| Graphene | Excellent electrical conductivity, large SSA | EDL capacitance, high CS, fast charge/discharge rates | |
| Carbon aerogel | Interconnected highly porous network | EDL capacitance, efficient charge transport, high CS | |
| Transitin metal based compounds | Transition metal oxides/hydroxides | Pseudocapacitive electrode materials, redox reactions | Fast, reversible faradaic charge-storage, CS higher than with EDLCs |
| Transition metal sulfides, phosphides, nitrides | Pseudocapacitive electrode materials, redox reactions, easy availability | Fast, reversible faradaic charge-storage, CS higher than with EDLCs | |
| Conducting polymers | Electrical conductivity, pseudocapacitive behavior | Redox charge-storage reactions, enhanced energy density | |
| Metal organic frameworks | Porous materials, tunable structures, High SSA | Ability to incorporate redox-active metal centers | |
| MXenes (Metal Nitrides and Carbides) | New class of 2D materials, high electrical conductivity, high SSA | Combination of EDLC and pseudocapacitance | |
| Hybrid composite materials | Combine the advantages of different materials | Enhanced capacitance, rate capability and cyclic stability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. https://doi.org/10.3390/ma17030702
Czagany M, Hompoth S, Keshri AK, Pandit N, Galambos I, Gacsi Z, Baumli P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials. 2024; 17(3):702. https://doi.org/10.3390/ma17030702
Chicago/Turabian StyleCzagany, Mate, Szabolcs Hompoth, Anup Kumar Keshri, Niranjan Pandit, Imre Galambos, Zoltan Gacsi, and Peter Baumli. 2024. "Supercapacitors: An Efficient Way for Energy Storage Application" Materials 17, no. 3: 702. https://doi.org/10.3390/ma17030702
APA StyleCzagany, M., Hompoth, S., Keshri, A. K., Pandit, N., Galambos, I., Gacsi, Z., & Baumli, P. (2024). Supercapacitors: An Efficient Way for Energy Storage Application. Materials, 17(3), 702. https://doi.org/10.3390/ma17030702








