Preparation of Ce-Doped Gd3(Al, Ga)5O12 Nanopowders via Microwave-Assisted Homogenization Precipitation for Transparent Ceramic Scintillators
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Ce: GGAG Nanopowders
2.3. Preparation of Ce: GGAG Ceramics
2.4. Characterizations
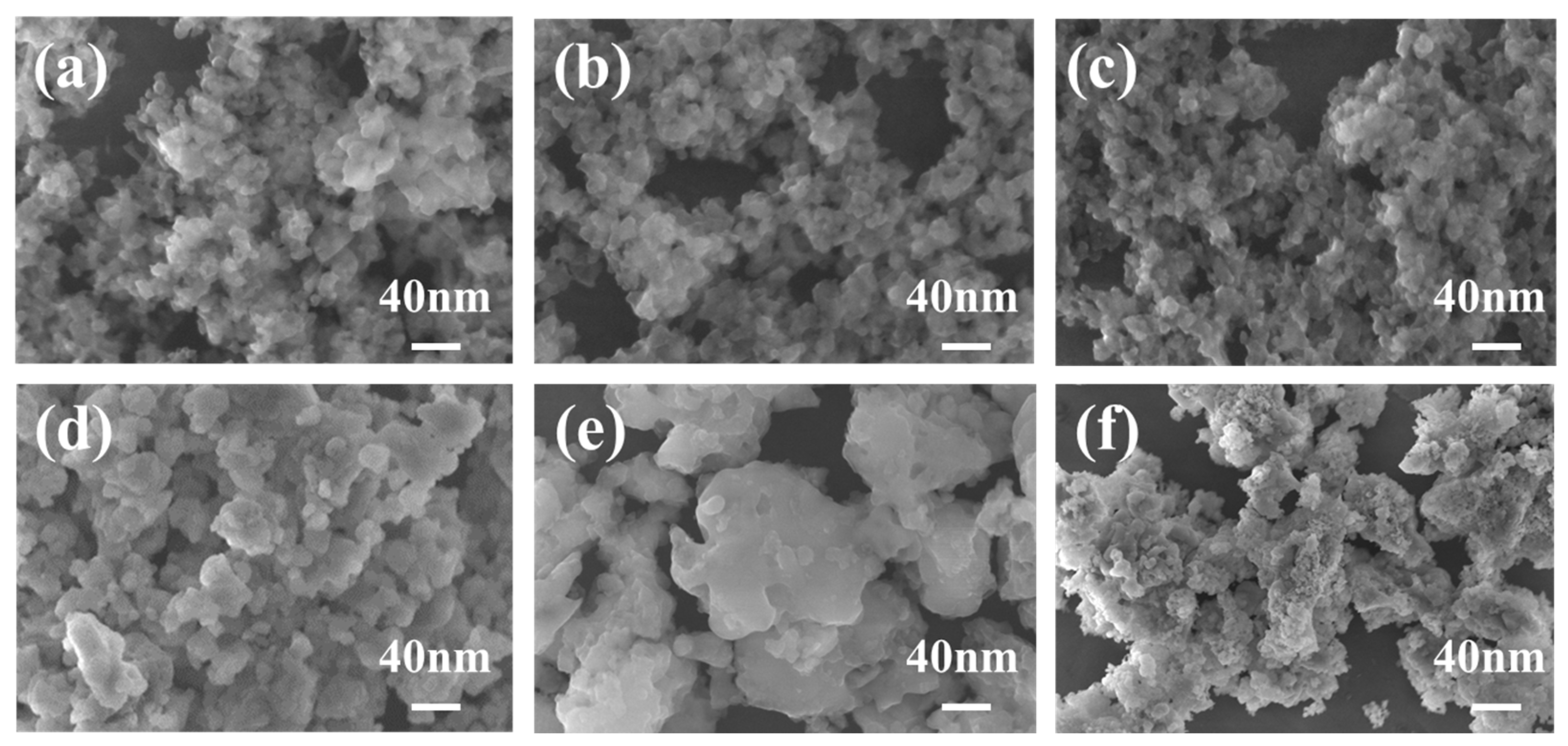
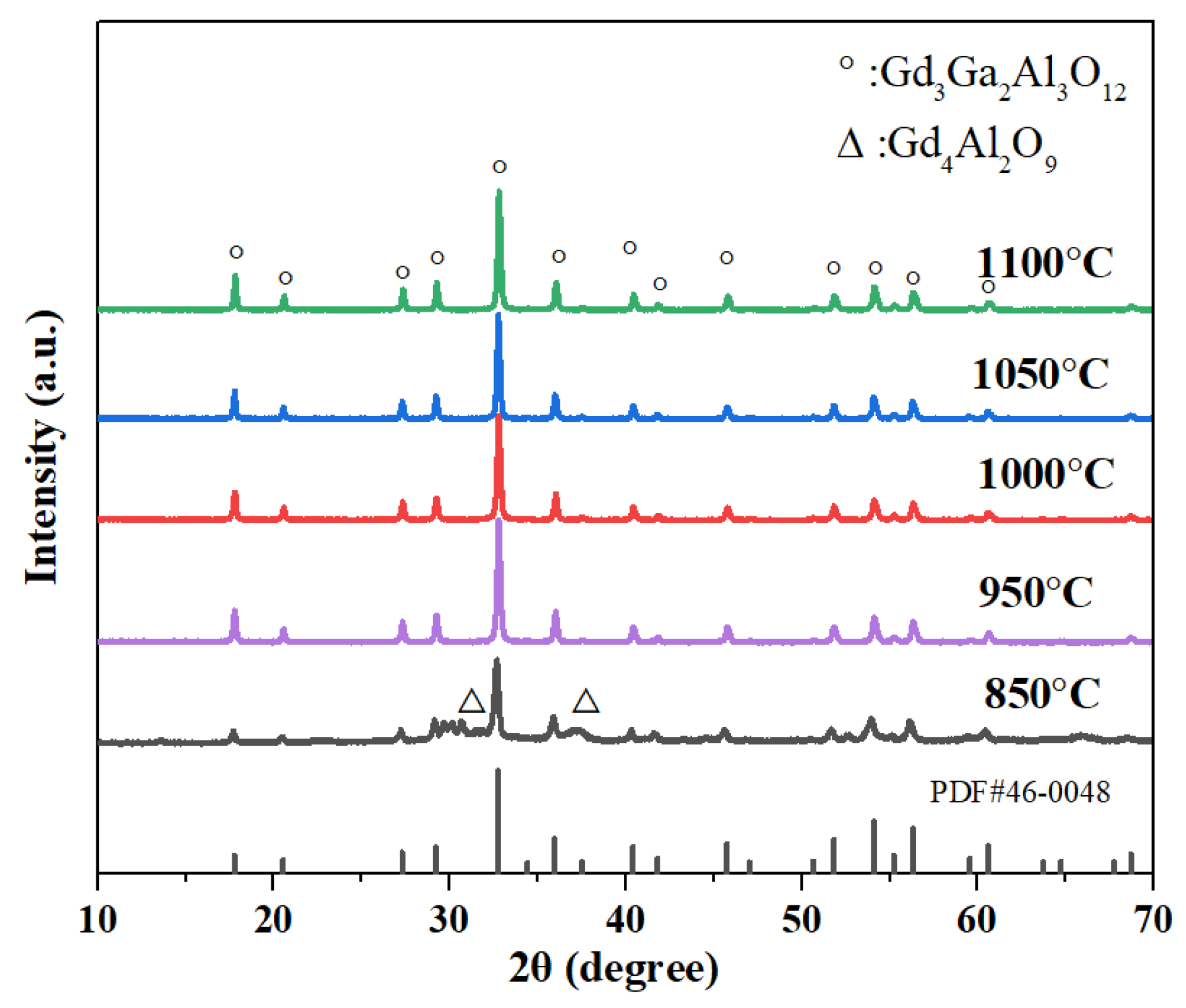
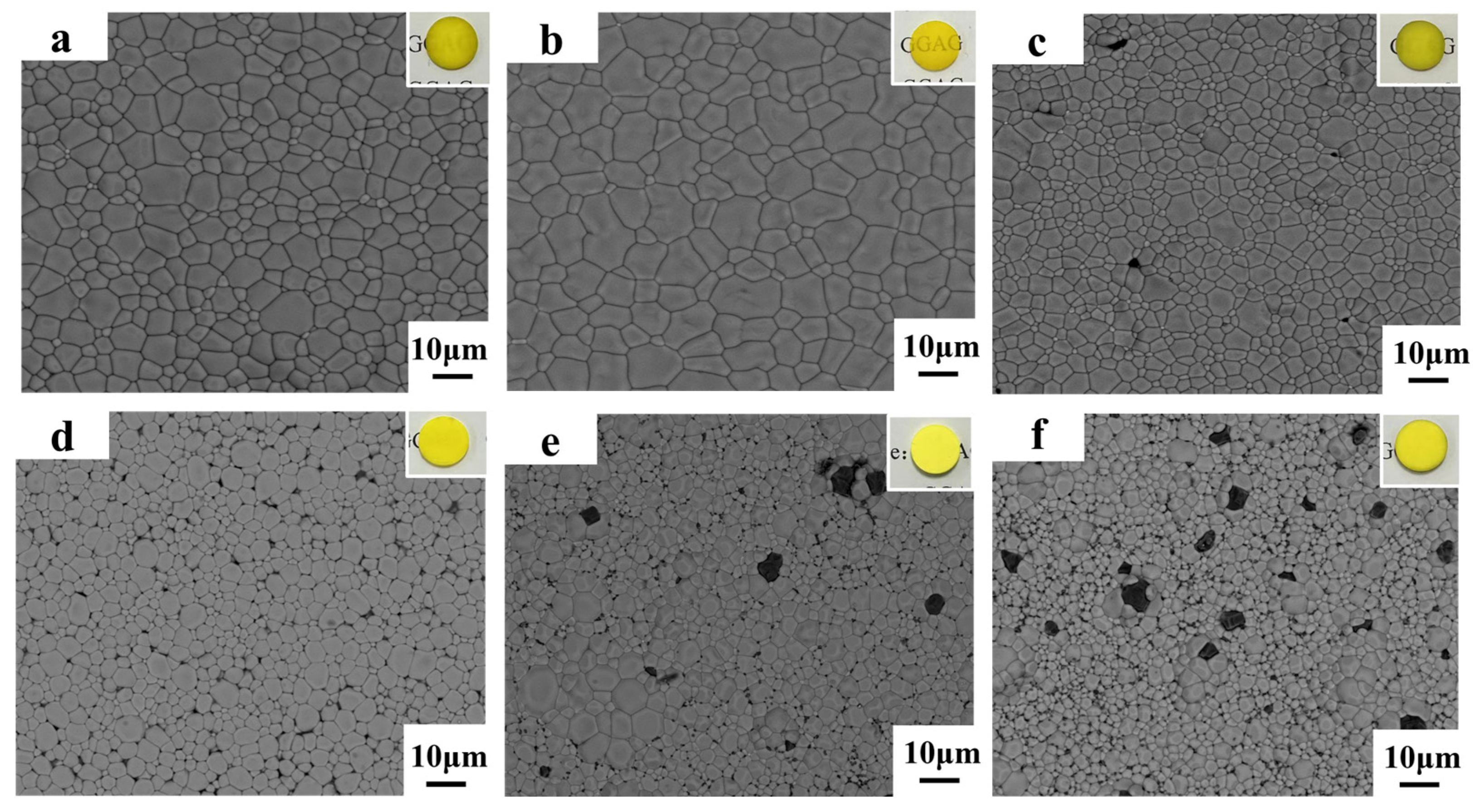
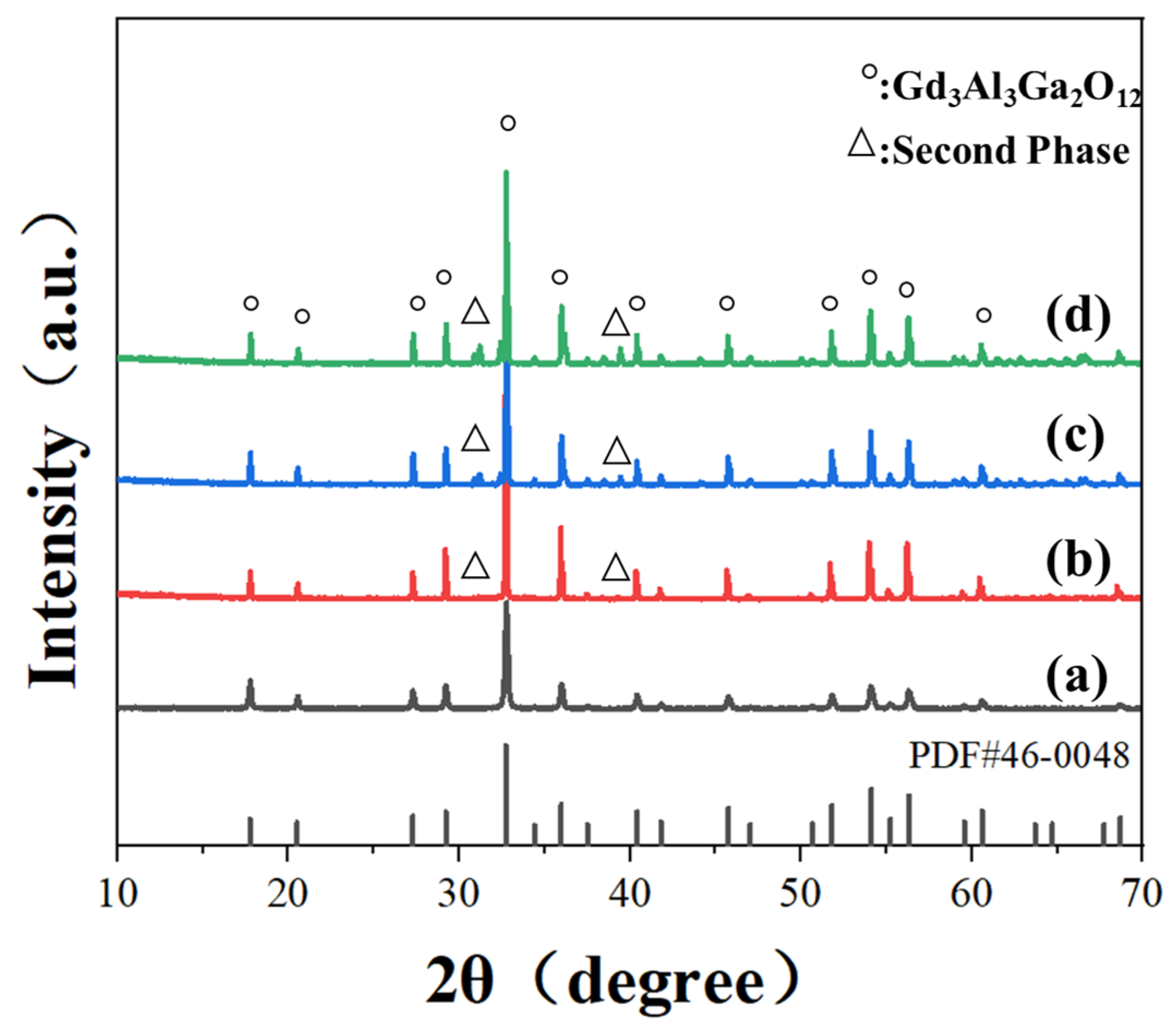
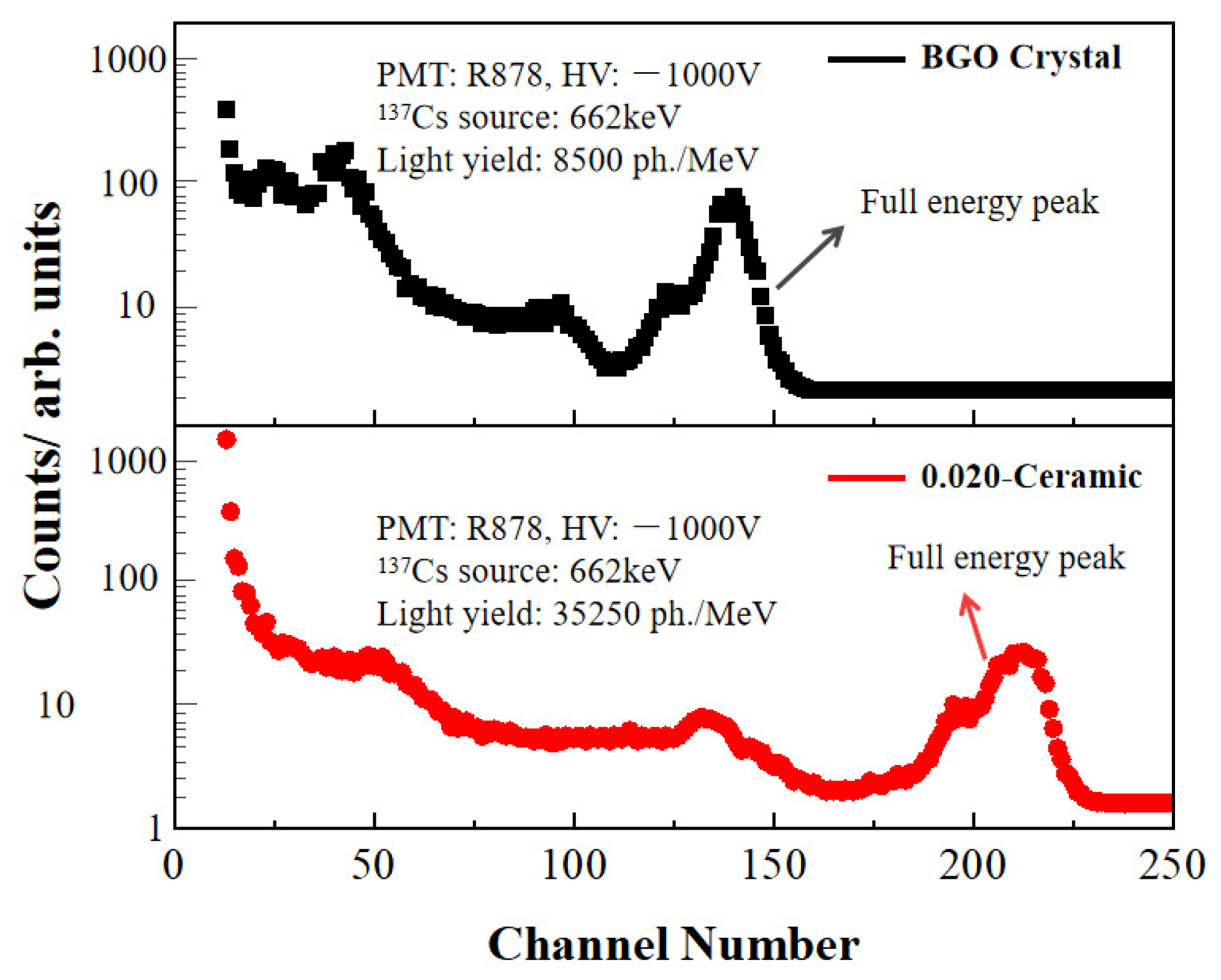
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanagida, T. Inorganic scintillating materials and scintillation detectors. Proc. Jpn. Acad. 2018, 94, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Christ, D.; Kurzeja, M. Comparison of LuYAP, LSO, and BGO as scintillators for high resolution PET detectors. IEEE Trans. Nucl. Sci. 2003, 50, 1370–1372. [Google Scholar] [CrossRef]
- Nakamura, R. Improvements in the X-ray characteristics of Gd2O2S:Pr ceramic scintillators. J. Am. Ceram. Soc. 1999, 82, 2407–2410. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ishibashi, H. A GSO depth of interaction detector for PET. IEEE Trans. Nucl. Sci. 1998, 45, 1078–1082. [Google Scholar] [CrossRef]
- Jasni, A.A.; Yap, Y.S.; Hashim, I.H. Two dimensional array of MPPC and CsI(Tl) for radiation monitoring prototype. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1106, 012–028. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Zhang, Z. Optical and scintillation properties of Ce: Y3Al5O12 single crystal fibers grown by laser heated pedestal growth method. J. Rare Earths 2021, 39, 1533–1539. [Google Scholar] [CrossRef]
- Toshiaki, K.; Kenichi, W.; Prom, K.; Kensei, I.; Daiki, S.; Takumi, K.; Daisuke, N.; Noriaki, K. Dopant concentration dependence on optical and scintillation properties of Eu-doped Gd3Al2Ga3O12 single crystals. Jpn. J. Appl. Phys. 2024, 63, 01SP18. [Google Scholar] [CrossRef]
- Minseok, Y.; Gyung, H.K.; Jae, S.L. Pushing the limit of BGO-based dual-ended Cherenkov PET detectors through photon transit time correction. Phys. Med. Biol. 2024, 69, 025005. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakagawa, H.; Fujii, H.; Kawaguchi, F.; Yamada, H.; Ito, Y.; Takeuchi, H.; Hayakawa, T.; Tsukuda, Y. Application of Gd2O2S ceramic scintillator for X-Ray solid state detector in X-Ray CT. Jpn. J. Appl. Phys. 1988, 27, 1572–1575. [Google Scholar] [CrossRef]
- Greskovich, C.D.; Cusano, D.A.; Dibianca, F.A. Preparation of Yttria Gadolinia Ceramic Scintillators by Sintering and Gas Hot Isostatic Pressing. US Patent 4,518,546, 21 May 1985. [Google Scholar]
- Gorokhova, E.I.; Demidenko, V.A.; Eron’ko, S.B. Spectrokinetic characteristics of the emission of Gd2O2S-Tb(Ce) ceramics. J. Opt. Technol. 2005, 72, 53–57. [Google Scholar] [CrossRef]
- Кравцoв, A.B.; Лапин, B.A.; Tarala, L.V.; Супрунчук, B.E.; Medyanik, E.V. Synthesis and study of the optical and spectral-luminescent properties of LuAG:Ce ceramic. Glass Ceram. 2024, 9, 13–21. [Google Scholar] [CrossRef]
- Sreebunpeng, K.; Chewpraditkul, W.; Chewpraditkul, W. Optical, luminescence and scintillation properties of Mg2+-codoped (Lu, Y)3Al2Ga3O12:Pr garnet crystals: The effect of Y admixture. Radiat. Phys. Chem. 2022, 201, 110400. [Google Scholar] [CrossRef]
- Franks, L.; James, R.B.; Fiederle, M.; Burger, A. (Eds.) Transparent ceramic scintillators for Gamma spectroscopy and MeV imaging. In Proceedings of the SPIE 9593, Hard X-Ray, Gamma-Ray, & Neutron Detector Physics XVII, San Diego, CA, USA, 9–13 August 2015; SPIE: Bellingham, WA, USA, 2015; pp. 959301–959307. [Google Scholar] [CrossRef]
- Kato, T.; Okada, G.; Fukuda, K.; Yanagida, T. Development of BaF2 transparent ceramics and evaluation of the scintillation properties. Radiat. Meas. 2017, 106, 140–145. [Google Scholar] [CrossRef]
- Wisniewski, D.J.; Boatner, L.A.; Neal, J.S.; Jellison, G.E.; Ramey, J.O.; North, A.; Wisniewska, M.; Payzant, A.E.; Howe, J.Y.; Lempicki, A. Development of novel polycrystalline ceramic scintillators. J. IEEE Trans. Nucl. Sci. 2008, 55, 1501–1508. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Yokota, Y.; Kamada, K.; Yanagida, S.; Yoshikawa, A.; Yagi, H.; Yanagitani, T. Comparative study of transparent ceramic and single crystal Ce doped LuAG scintillators. Radiat. Meas. 2011, 46, 1503–1505. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Seeley, Z.M.; Payne, S.A. Development of transparent ceramic Ce-doped gadolinium garnet gamma spectrometers. Nucl. Sci. Symp. Med. Imag. Conf. 2012, 978, 1692–1697. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Seeley, Z.M.; Payne, S.A.; Swanberg, E.L.; Beck, P.R.; Schneberk, D.J.; Stone, G.; Wihl, B.M.; Fisher, S.E.; Hunter, S.L.; et al. Transparent ceramic scintillators for Gamma spectroscopy and imaging. In Proceedings of the 2017 IEEE Nuclear Science Symposium and Medical Imaging Conference, Atlanta, GA, USA, 21–28 October 2017; pp. 1–2. [Google Scholar] [CrossRef]
- Kanai, T.; Satoh, M.; Miur, I. Characteristics of a nonstoichiometric Gd3+δ(Al,Ga)(5-δ)O12: Ce garnet scintillator. J. Am. Ceram. Soc. 2008, 91, 456–462. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, H.; Jiang, J. Synthesis of cerium-doped Gd3(Al,Ga)5O12 powder for ceramic scintillators with ultrasonic-assisted chemical coprecipitation method. J. Am. Ceram. Soc. 2013, 96, 3038–3041. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Chen, X.; Zhang, Y.; Luo, Z.; Jiang, J.; Jiang, H. The Effects of cation concentration in the salt solution on the cerium doped gadolinium gallium aluminum oxide nanopowders prepared by a co-precipitation method. IEEE. Trans. Nucl. Sci. 2014, 61, 301–305. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Zeng, R.; Dou, S.; Sun, X. Microwave synthesis of homogeneous YAG nanopowder leading to a transparent ceramic. J. Am. Ceram. Soc. 2010, 92, 1217–1223. [Google Scholar] [CrossRef]
- Örücü, H. The effect of molar ratio and annealing on crystal structure of gadolinium-gallium garnet nanopowders synthesized by sol-gel method. J. Ceram. Process. Res. 2022, 23, 799–805. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, G.; Chi, R.; Shi, Y.; Zhao, X.; Jiang, H.; Guo, F.; Wang, G.; Guo, J.; Zhang, Z. Preparation of YAG nanopowders and ceramics via coprecipitation: Effects of treatment modes of precipitates. Int. J. Appl. Ceram. Technol. 2022, 19, 2419–2426. [Google Scholar] [CrossRef]
- Wu, P.; Pelton, A.D. Coupled thermodynamic-phase diagram assessment of the rare earth oxide aluminum oxide binary systems. J. Alloys Compd. 1992, 179, 259–287. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, G.; Carloni, D.; Wu, Y. Fabrication, microstructure and optical properties of Ga2O3 transparent ceramics. Ceram. Int. 2020, 46, 21757–21761. [Google Scholar] [CrossRef]
- Ding, H.; Liu, Z.; Liu, Y.; Hu, P.; Sun, P.; Luo, Z.; Chao, K.; Jiang, H.; Jiang, J. Gd3Al3Ga2O12:Ce, Mg2+ transparent ceramic phosphors for high-power white LEDs/LDs. Ceram. Int. 2021, 47, 7918–7924. [Google Scholar] [CrossRef]
- Hostaša, J.; Cova, F.; Piancastelli, A.; Fasoli, M.; Zanelli, C.; Vedda, A.; Biasini, V. Fabrication and luminescence of Ce-doped GGAG transparent ceramics, effect of sintering parameters and additives. Ceram. Int. 2019, 45, 23283–23288. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Jiang, J.; Jiang, H. Highly transparent ZrO2-doped (Ce,Gd)3Al3Ga2O12 ceramics prepared via oxygen sintering. J. Eur. Ceram. Soc. 2015, 35, 3879–3883. [Google Scholar] [CrossRef]
- Mendelson, M.I. Average grain size in polycrystalline ceramics. J. Am. Ceram. Soc. 1969, 52, 443–446. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, T.; Yang, Z.; Zhang, H.; Su, Y.; Chen, Z.; Chen, X.; Ma, Y.; Zhu, W.; Yu, X.; et al. Highly resolved and robust dynamic X-Ray imaging using perovskite glass-ceramic scintillator with reduced light scattering. Adv. Sci. 2021, 8, 2003728. [Google Scholar] [CrossRef]
- You, Q.; Lin, H.; Hong, R.; Han, Z.; Zhang, D.; Ding, Y. Structural and scintillation properties of Ce3+:Gd3Al3Ga2O12 translucent ceramics prepared by one-step sintering. Materials 2023, 16, 3373. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Luo, Z.; Jiang, J.; Jiang, H. Preparation and optical properties of transparent (Ce,Gd)3Al3Ga2O12 Ceramic. J. Am. Ceram. Soc. 2015, 98, 2352–2356. [Google Scholar] [CrossRef]
- Mori, M.; Xu, J.; Okada, G.; Yanagida, T.; Ueda, J.; Tanabe, S. Comparative study of optical and scintillation properties of Ce:YAGG, Ce:GAGG and Ce:LuAGG transparent ceramics. J. Ceram. Soc. Jpn. 2016, 124, 569–573. [Google Scholar] [CrossRef]
- Xu, J.; Ueda, J.; Tanabe, S. Design of deep-red persistent phosphors of Gd3Al5−xGaxO12:Cr3+ transparent ceramics sensitized by Eu3+ as an electron trap using conduction band engineering. Opt. Mater. Express 2015, 5, 963–968. [Google Scholar] [CrossRef]
- Kanai, T.; Satoh, M.; Miura, I. Hot-pressing method to consolidate Gd3(Al,Ga)5O12:Ce garnet scintillator powder for use in an X-ray CT detector. Int. J. Appl. Ceram. Technol. 2013, 10, E1–E10. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Luo, Z.H.; Jiang, H.C.; Jiang, J.; Chen, C.H.; Zhang, J.X.; Gui, Z.Z.; Xiao, N. Highly transparent cerium doped gadolinium gallium aluminum garnet ceramic prepared with precursors fabricated by ultrasonic enhanced chemical co-precipitation. Ultrason. Sonochem. 2017, 39, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Takayuki, Y.; Kamada, K.; Yutaka, F.; Hideki, Y.; Takahiko, Y. Comparative study of ceramic and single crystal Ce:GAGG scintillator. Opt. Mater. 2013, 35, 2480–2485. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, L.; Zhu, R.-Y. Optical and scintillation properties of inorganic scintillators in high energy physics. Nucl. Sci. Symp. Conf. Rec. 2007, 55, 2425–2431. [Google Scholar] [CrossRef]
- Ji, T.; Wang, T.; Li, H.; Peng, Q.; Tang, H. Ce3+-doped yttrium aluminum garnet transparent ceramics for high-resolution X-ray imaging. Adv. Opt. Mater. 2022, 10, 2102056. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nitta, H. Development of an event-by-event based radiation imaging detector using GGAG: A ceramic scintillator for X-ray CT. Nucl. Instrum. Methods Phys. Res. Sect. A 2018, 900, 25–31. [Google Scholar] [CrossRef]
- Mori, M.; Xu, J.; Okada, G.; Yanagida, T.; Ueda, J.; Tanabe, S. Scintillation and optical properties of Ce-doped YAGG transparent ceramics. J. Rare Earths 2016, 34, 763–768. [Google Scholar] [CrossRef]
- Singh, G.; Thomas, V.; Tiwari, V.S.; Karnal, A.K. Effect of cerium doping on optical and scintillation properties of transparent YAG ceramic. Ceram. Int. 2017, 43, 9032–9040. [Google Scholar] [CrossRef]




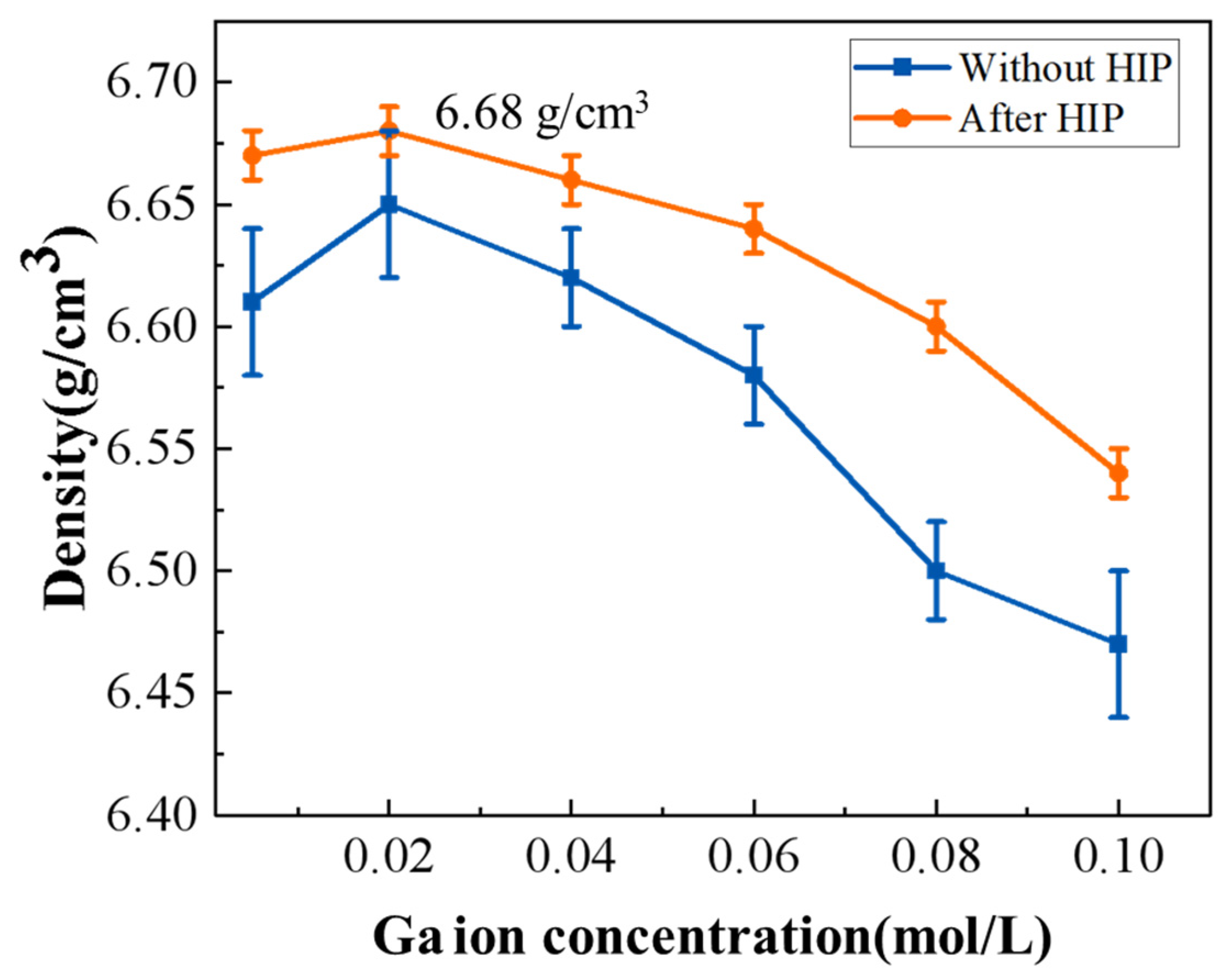

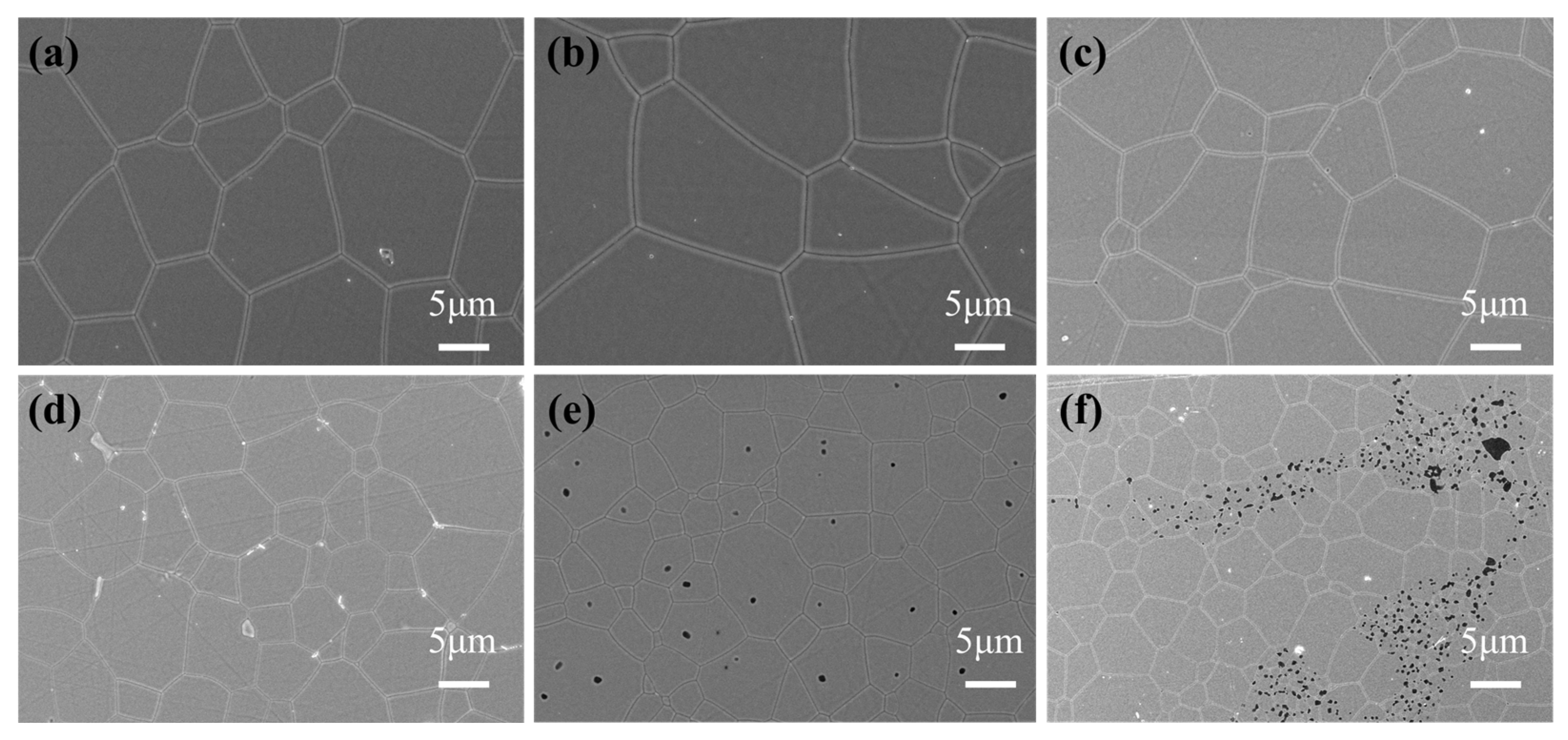
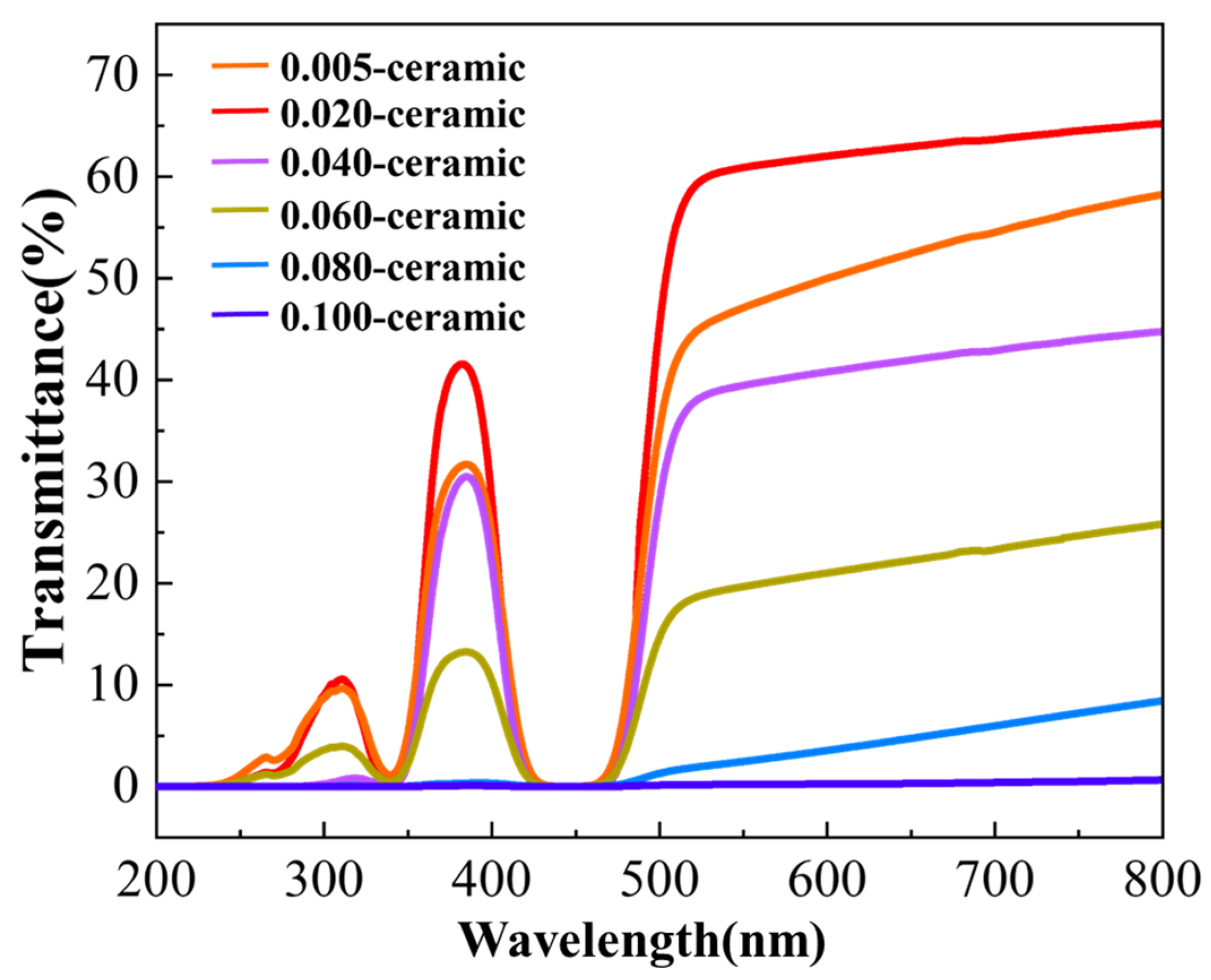
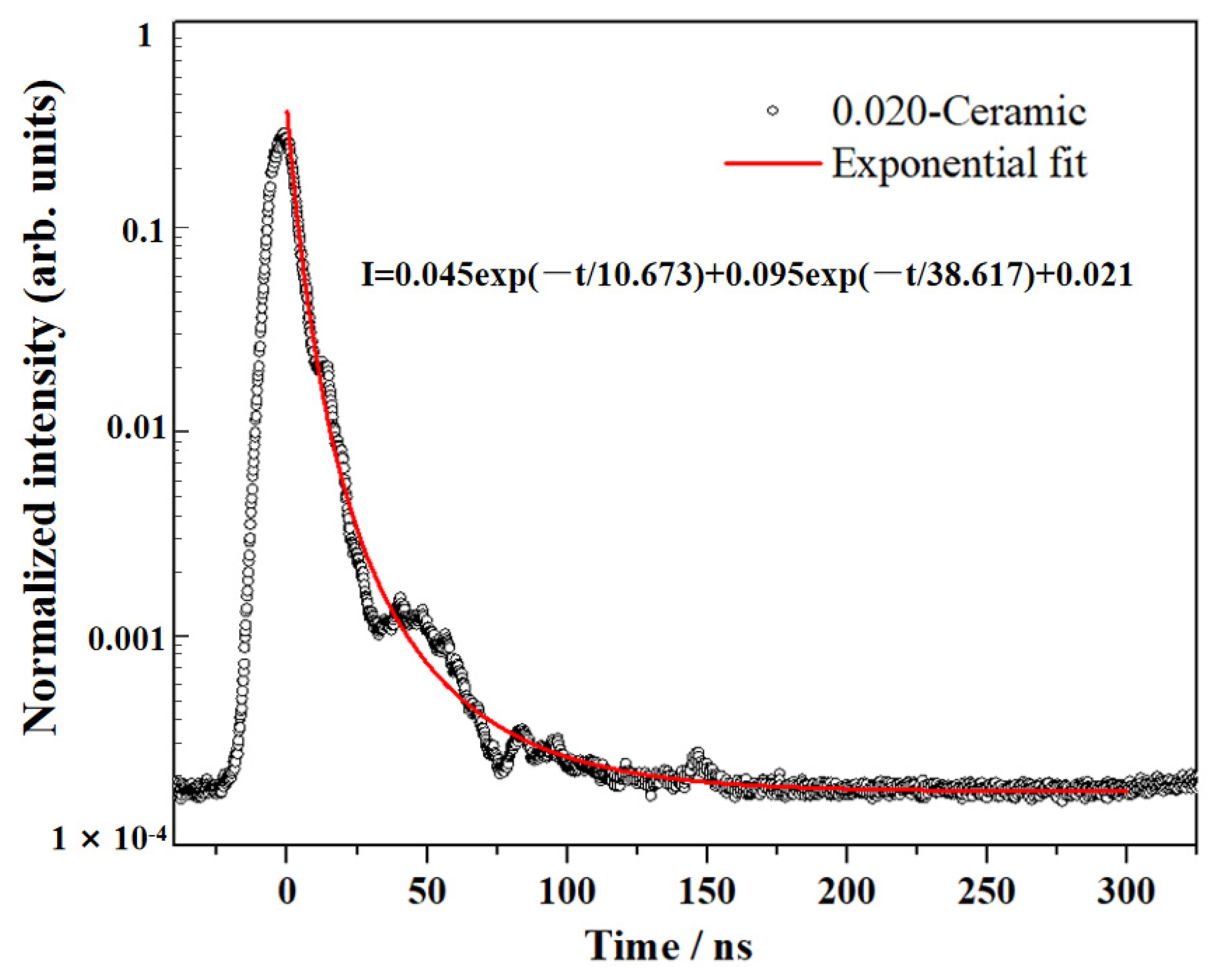
| Composition | Fabrication Method | Sintering Aids | Pre-Sintering Atmosphere | HIP Parameter | Thickness | Highest Transmittance | Reference |
|---|---|---|---|---|---|---|---|
| Gd3Al3Ga2O12:Ce | Solid state reaction | MgO | Oxygen | NON | Information absence | ~45%@545 nm | [33] |
| Gd3Al2Ga3O12:Ce | Solid state reaction | TEOS, MgO, CaO | Air | 200 MPa 1500 °C | 1.13 mm | 50.1%@550 nm | [29] |
| Gd3Al3Ga2O12:Ce | Solid state reaction | Information absence | Oxygen | NON | 1 mm | 62%@558 nm | [34] |
| Gd3Al3Ga2O12:Ce | Solid state reaction | TEOS | Vacuum | NON | 1.5 mm | ~70@600 nm | [35] |
| Gd3Al3Ga2O12:Ce | Solid state reaction | ZrO2 | Oxygen | NON | 1 mm | 73%@558 nm | [30] |
| Gd3Al2Ga3O12:Cr/Eu | Solid state reaction | Information absence | Vacuum | NON | 1 mm | 75.3%@800 nm | [36] |
| Gd3Al3Ga2O12:Ce | Solid state reaction | MgO | Oxygen | 1500 °C | 1 mm | 78.6%@500–800 nm | [28] |
| Gd3(Al,Ga)5O12:Ce | Hot-pressing | NON | Vacuum | NON | 1.8 mm | 33%@550 nm | [37] |
| Gd3(Al,Ga)5O12:Ce | Ultrasonic chemical co-precipitation | Information absence | Oxygen | Yes | 1 mm | 51%@545 nm | [38] |
| Gd3(Al,Ga)5O12:Ce | Ultrasonic chemical co-precipitation | Information absence | Oxygen | Yes | 1 mm | 68%@545 nm | [38] |
| Gd3Al3Ga2O12:Ce | MAHP | NON | Oxygen | 200 MPa 1480 °C | 1 mm | 65.2%@800 nm | This work |
| Materials | Appearances | Light Yield/ph/MeV | Reference |
|---|---|---|---|
| Y3Al5O12:0.4%Ce | Transparent | 24,600 | [41] |
| Y3Al2Ga3O12:0.8%Ce | Transparent | 13,700 ± 1400 | [35] |
| Lu3Al2Ga3O12:0.8%Ce | Transparent | 18,700 ± 1900 | [35] |
| Gd3Al2Ga3O12:0.8%Ce | Transparent | 15,500 ± 1600 | [35] |
| GYGAG:Ce | Transparent | 50,000 | [19] |
| Gd3Al3Ga2O12:0.35%Ce | Translucent | 31,500 | [33] |
| Gd3(Al, Ga)5O12:Ce | Translucent | 48,000 | [42] |
| GAGG:1%Ce | Opaque | 70,000 | [39] |
| Gd3Al3Ga2O12:0.33%Ce | Transparent | 35,000 ± 1250 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, Y.; Hu, S.; Zhou, G.; Qin, X.; Wang, S. Preparation of Ce-Doped Gd3(Al, Ga)5O12 Nanopowders via Microwave-Assisted Homogenization Precipitation for Transparent Ceramic Scintillators. Materials 2024, 17, 1258. https://doi.org/10.3390/ma17061258
Liu M, Zhang Y, Hu S, Zhou G, Qin X, Wang S. Preparation of Ce-Doped Gd3(Al, Ga)5O12 Nanopowders via Microwave-Assisted Homogenization Precipitation for Transparent Ceramic Scintillators. Materials. 2024; 17(6):1258. https://doi.org/10.3390/ma17061258
Chicago/Turabian StyleLiu, Min, Yansen Zhang, Song Hu, Guohong Zhou, Xianpeng Qin, and Shiwei Wang. 2024. "Preparation of Ce-Doped Gd3(Al, Ga)5O12 Nanopowders via Microwave-Assisted Homogenization Precipitation for Transparent Ceramic Scintillators" Materials 17, no. 6: 1258. https://doi.org/10.3390/ma17061258






