Microstructure and Property Evolution of Diamond/GaInSn Composites under Thermal Load and High Humidity
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Experimental Materials
2.2. Experimental Scheme
3. Results and Discussion
3.1. Experimental Analysis of the High-Temperature Storage Environment
3.2. Experimental Analysis of the High- and Low-Temperature Circulation Environment
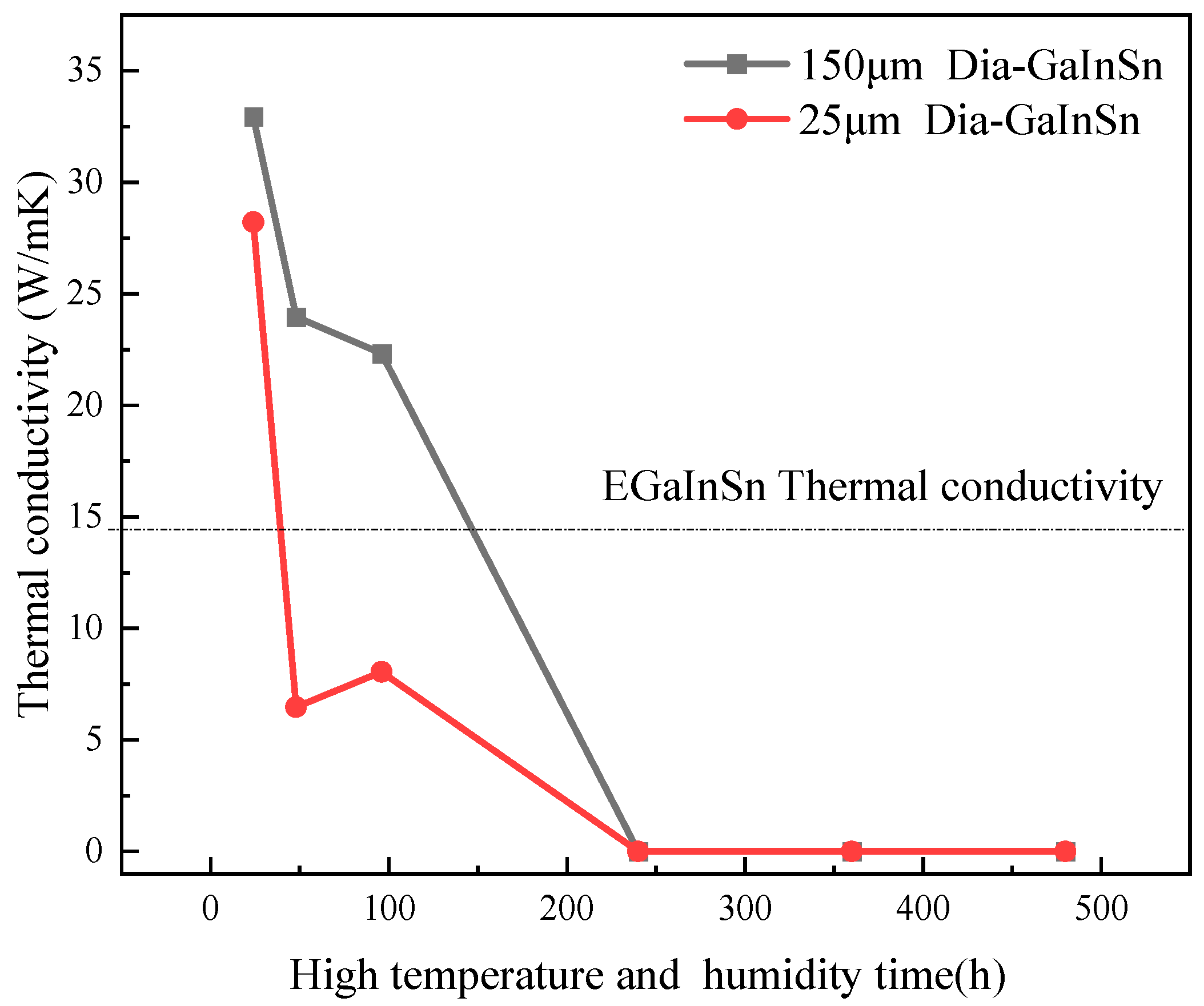
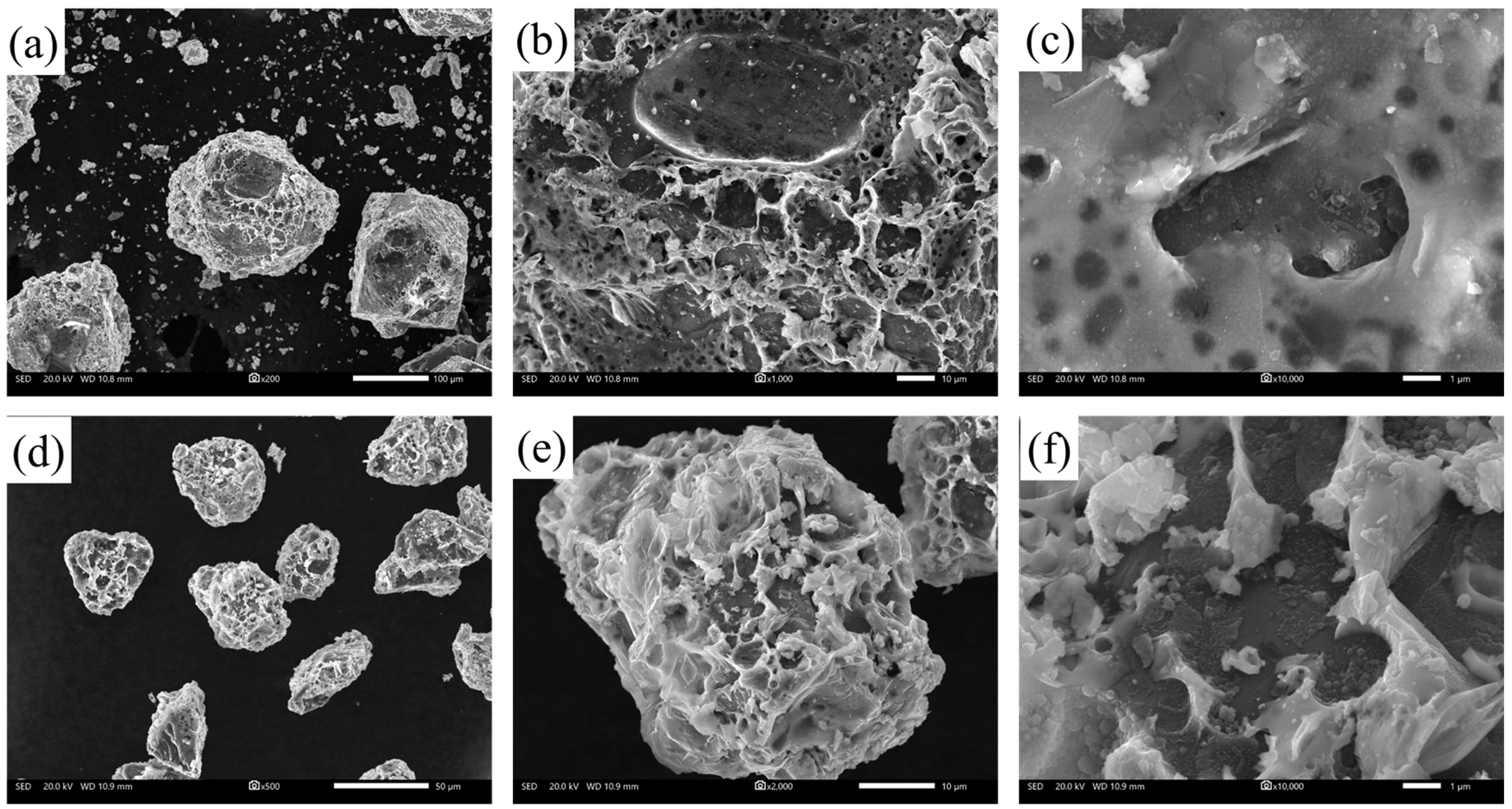
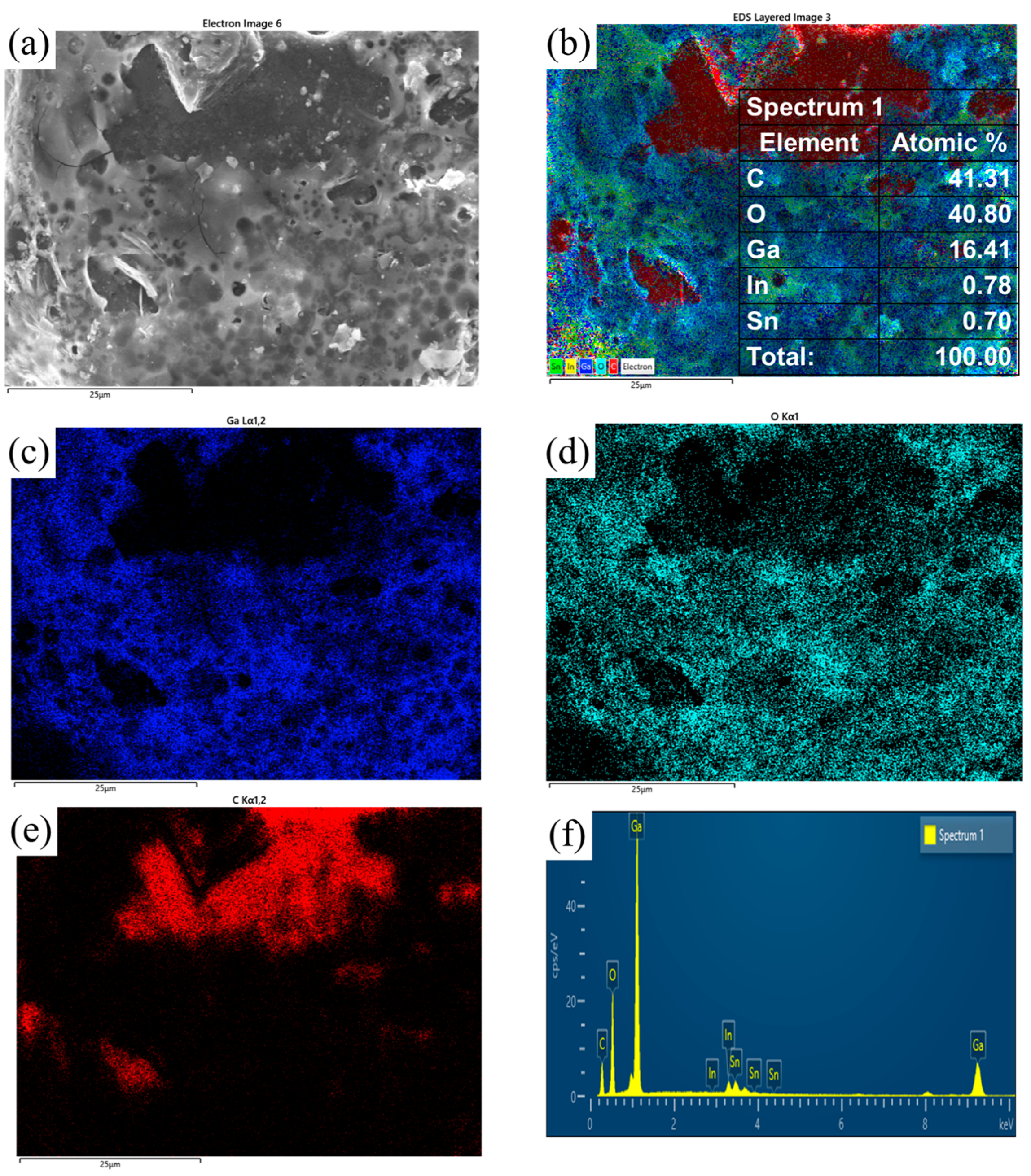
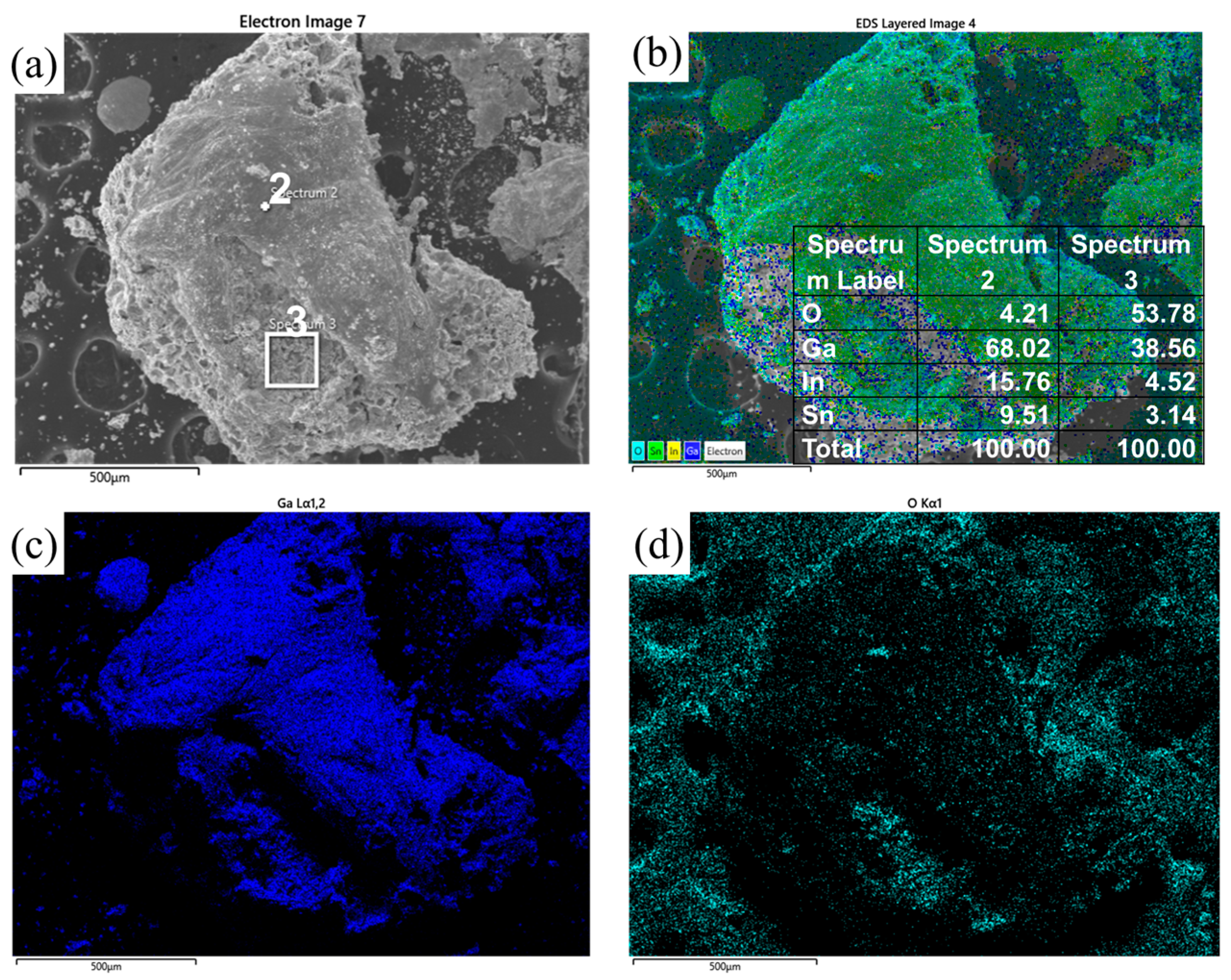
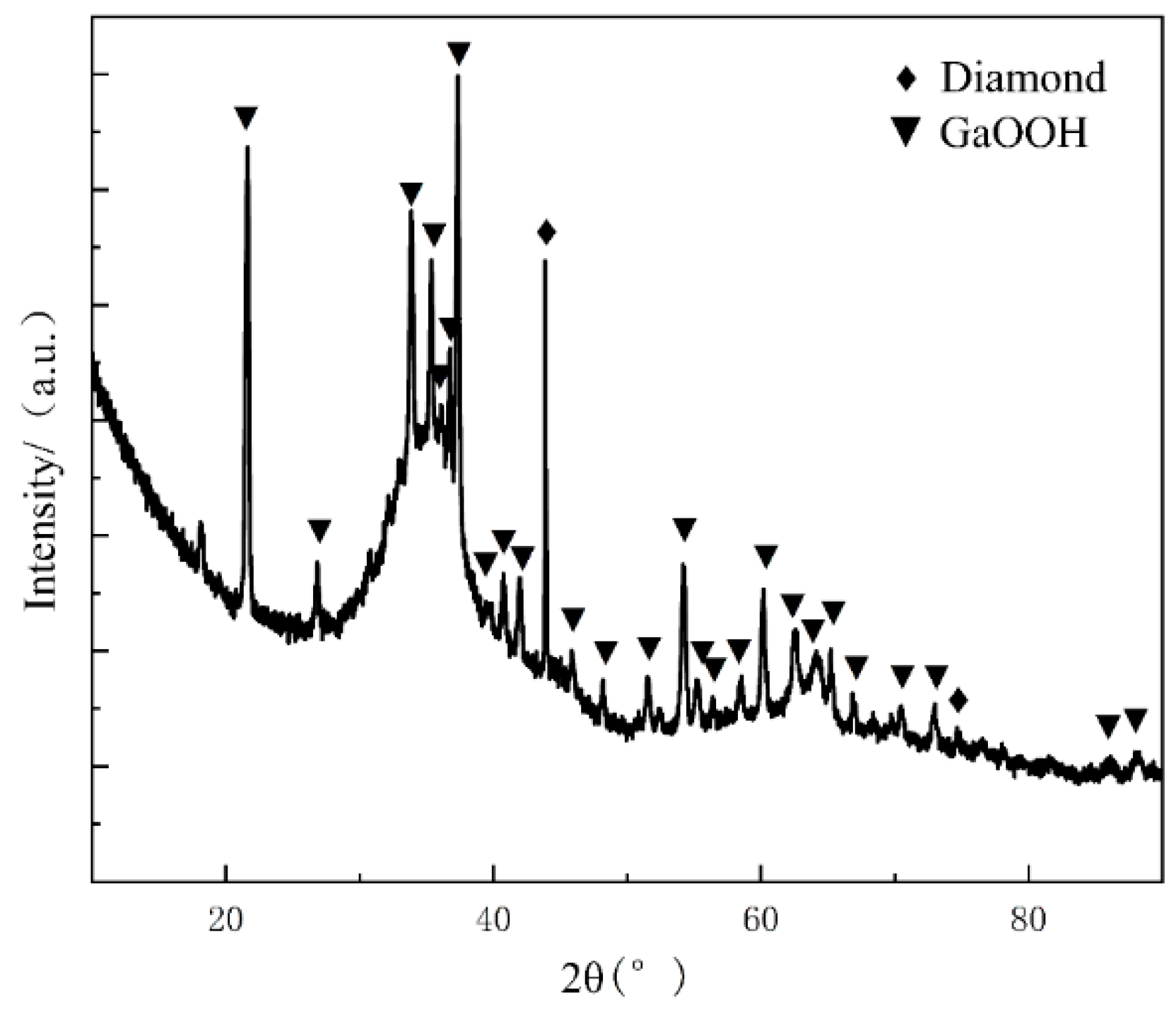
3.3. Experimental Analysis of the High-Temperature and High-Humidity Environment
4. Conclusions
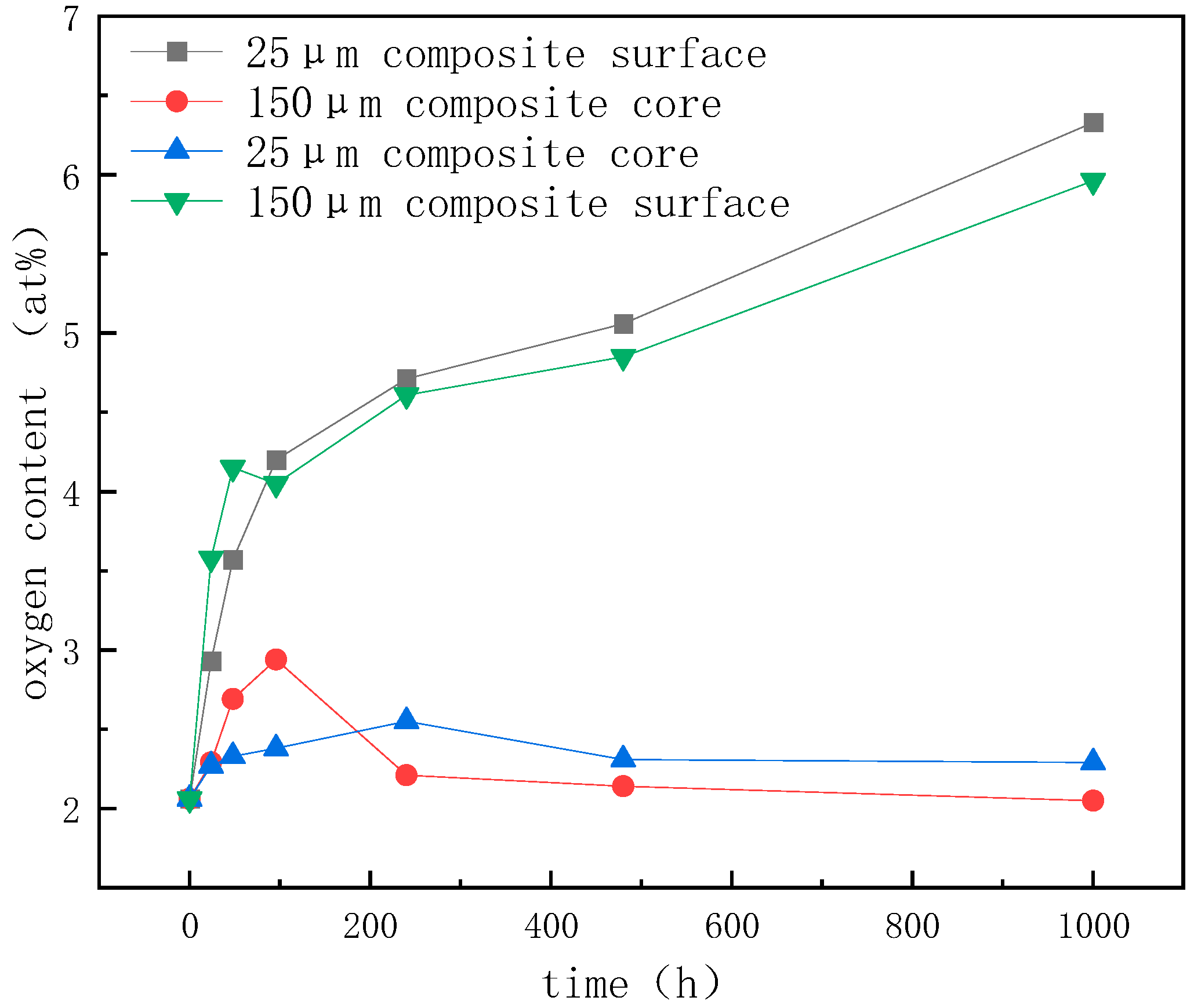
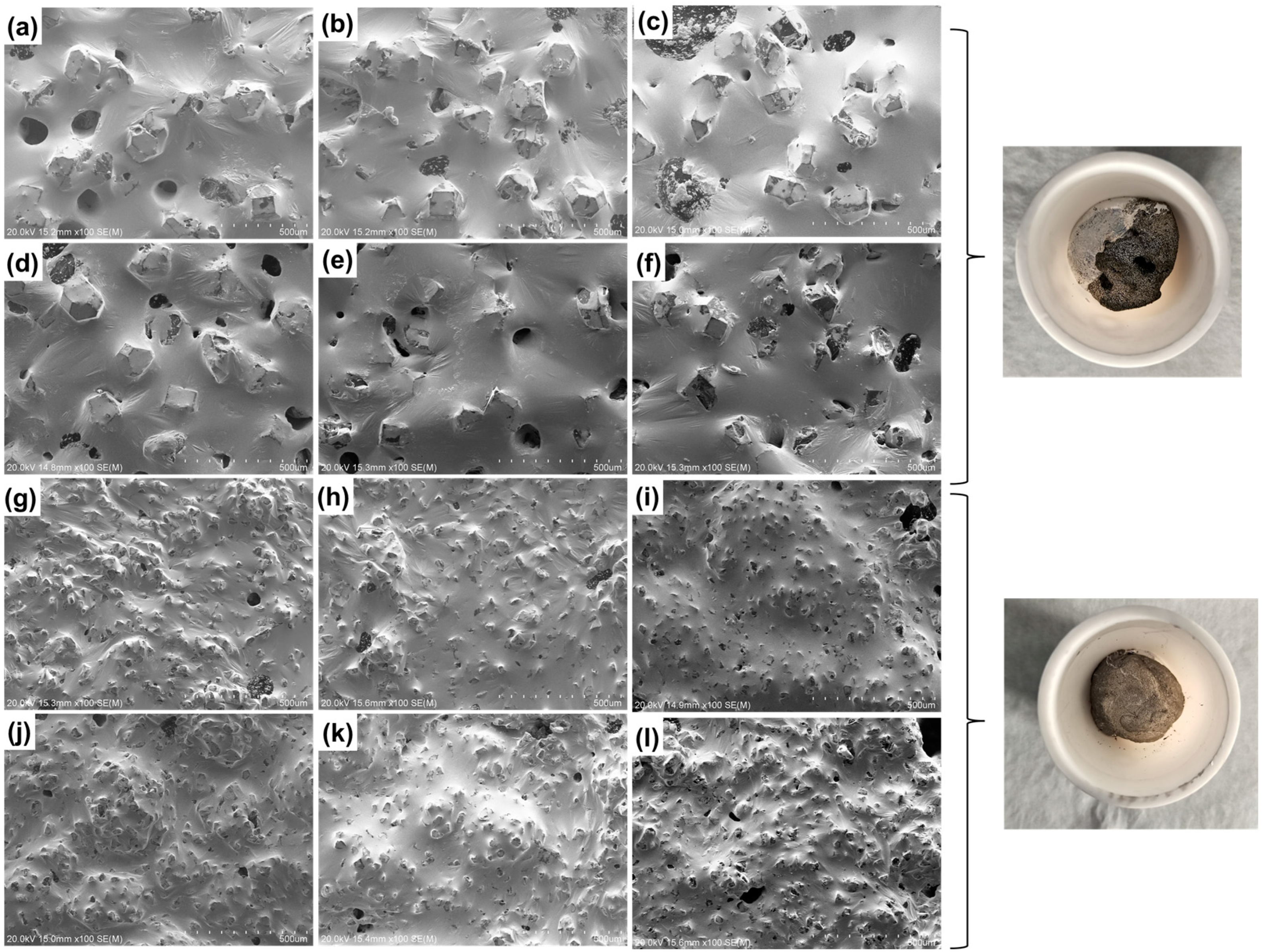
- In the high-temperature (150 °C) storage environment, diamond/GaInSn reacts with oxygen in the air to form Ga2O3. Moreover, the material’s thermal conductivity improves in 24–96 h. The porosity of the 25 μm diamond composite is higher than that of the 150 μm diamond/GaInSn composite, and the oxidation reaction spreads more easily from the surface to the core. At 480 h, the 25 μm diamond composite’s thermal conductivity decreases by up to 7%. The key to the degradation of material properties lies in the oxidation that occurs inside the material.
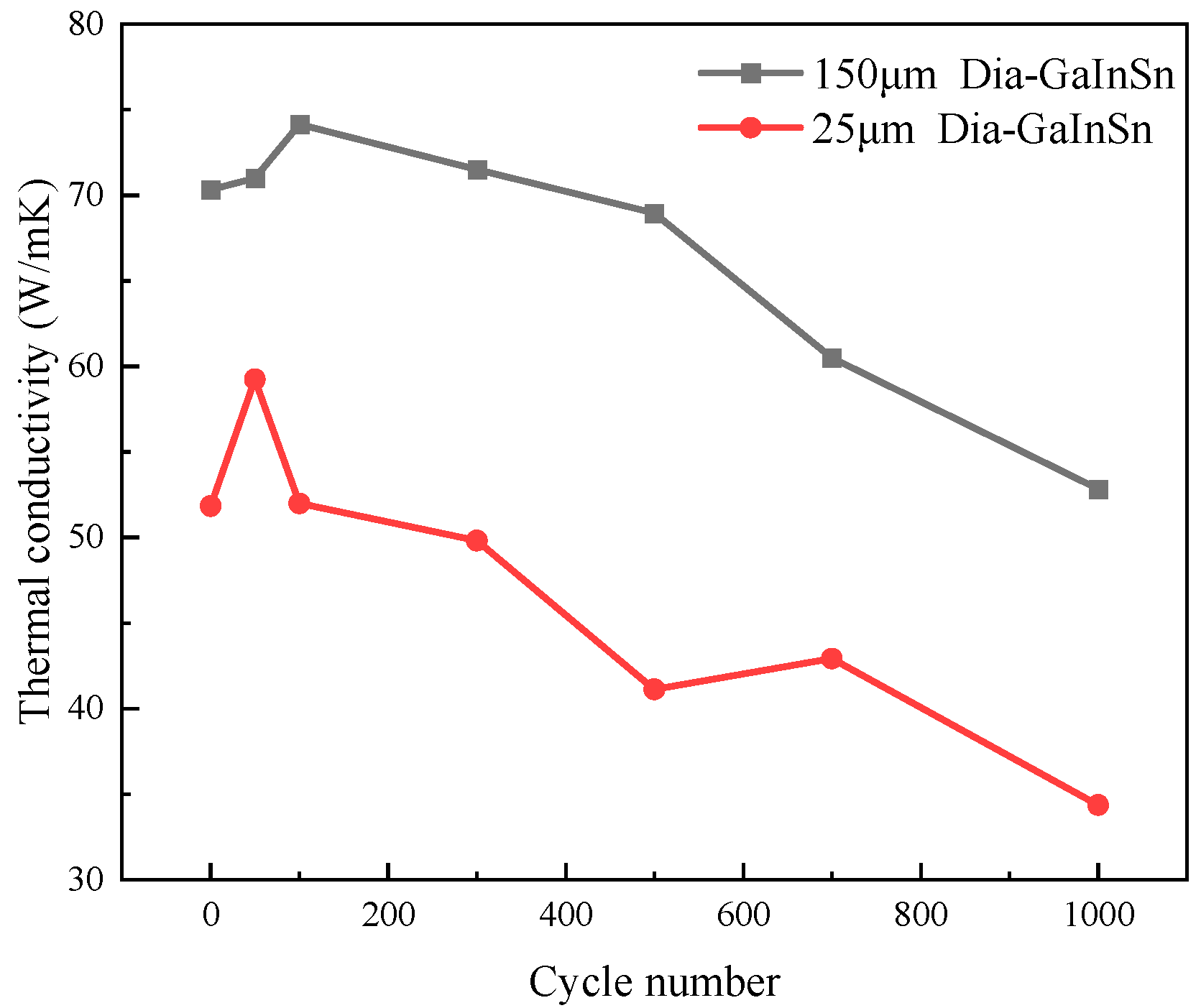
- In the high- and low-temperature cycle experiment, the preliminary oxidation promotes the wetting of the thermal conductivity-enhanced phase and the matrix, and the material’s thermal conductivity is increased. The material undergoes a melting–crystallization–solidification–melting process. The voids in the particle–liquid phase composite continue to move, disappear, and appear in high- and low-temperature cycling. Thus, the oxidation products that originally concentrated on the surface spread to the inner part of the material, resulting in a continuous decrease in the material’s thermal conductivity.
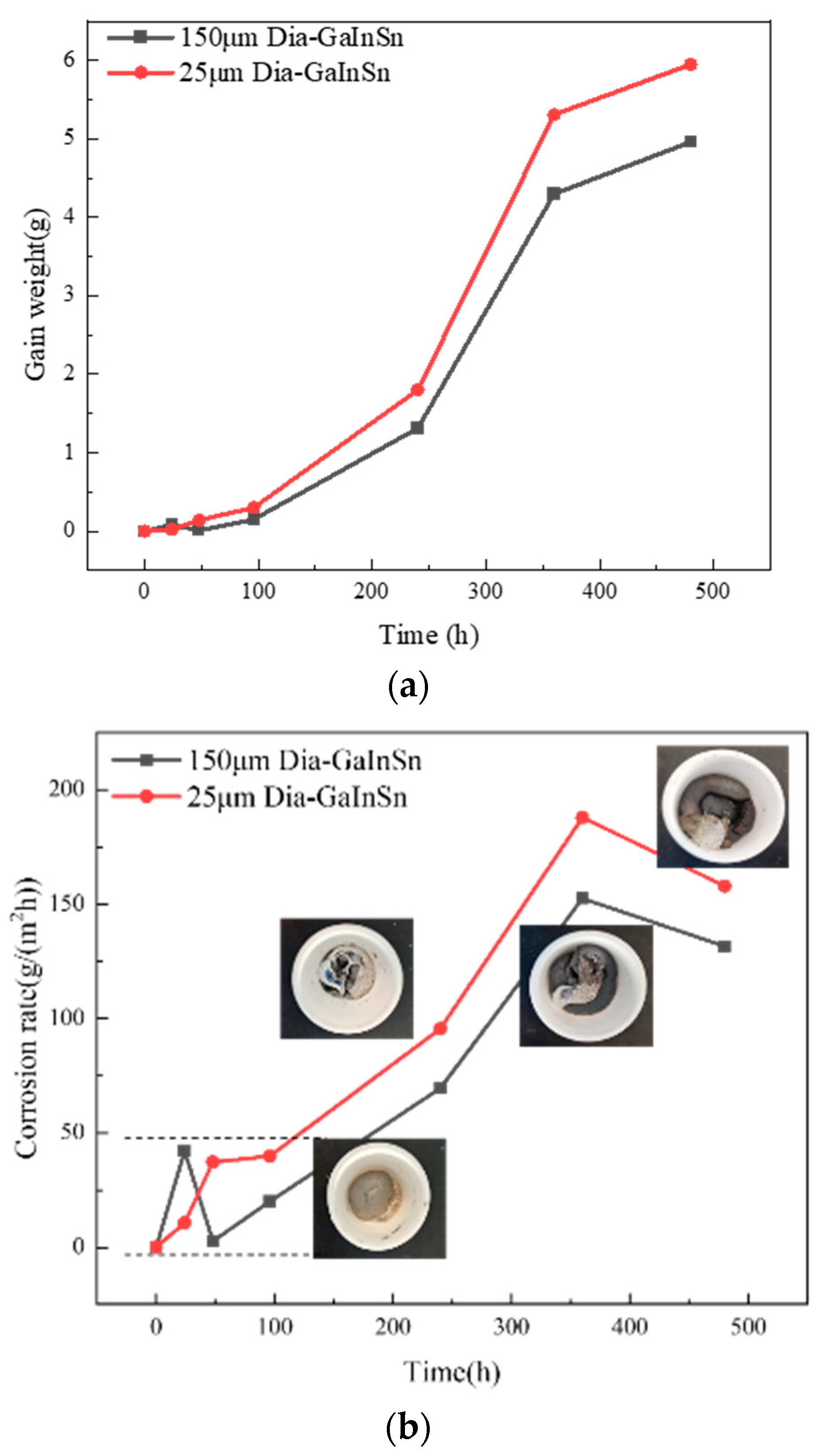
- Under high-temperature and high-humidity conditions, the Ga2O3 in the GaInSn liquid metal reacts with H2O in the air to generate Ga(OH)2. Ga(OH)2 is enriched at the wetting interface between Dia and GaInSn and is dehydrated to generate GaOOH. GaOOH and diamond are tightly combined to form a honeycomb structure. Thus, the wettability of diamond and GaInSn is seriously reduced, and the solid and liquid phases of the composite separate. The liquid phase overflow leads to material failure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Li, J.; Lu, N. Research progress of diamond/copper composites with high thermal conductivity. Diam. Relat. Mater. 2020, 108, 107993. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, Z.; Zhang, X. Thermal expansion behaviour and dimensional stability of Diamond/Cu composites with different diamond content. J. Alloys Compd. 2019, 797, 122–130. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, X.; Huang, S. Enhancing thermal conductivity of Diamond/Cu composites by regulating distribution of bimodal diamond particles. Diam. Relat. Mater. 2019, 100, 107564. [Google Scholar] [CrossRef]
- Pathak, S.K.; Kumar, R.; Goel, V.; Pandey, A.K.; Tyagi, V.V. Recent advancements in thermal performance of nano-fluids charged heat pipes used for thermal management applications: A comprehensive review. Appl. Therm. Eng. 2022, 216, 119023. [Google Scholar] [CrossRef]
- Prasher, R. Thermal Interface Materials: Historical Perspective, Status, and Future Direction. Proc. IEEE 2006, 94, 1571–1586. [Google Scholar] [CrossRef]
- Feng, C.P.; Yang, L.Y.; Yang, J.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Lan, H.B.; Yang, W. Recent advances in polymer-based thermal interface materials for thermal management: A mini-review. Compos. Commun. 2020, 22, 100528. [Google Scholar] [CrossRef]
- Due, J.; Robinson, A.J. Reliability of thermal interface materials: A review. Appl. Therm. Eng. 2013, 50, 455–463. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, S.; Cai, W.; Zhang, Y.; Zhang, X.A. A review of carbon-based thermal interface materials: Mechanism, thermal measurements and thermal properties. Mater. Des. 2021, 209, 109936. [Google Scholar] [CrossRef]
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar] [CrossRef]
- Chung, D.D.L. A critical review of carbon-based thermal interface materials. Mater. Chem. Phys. 2023, 309, 128432. [Google Scholar] [CrossRef]
- Sreekumar, E.N.; Senthil Saravanan, M.S. A review on Aluminium based thermal interface materials for heat transfer application. Mater. Today Proc. 2023, 72, 3036–3039. [Google Scholar] [CrossRef]
- Lv, L.; Dai, W.; Yu, J.; Jiang, N.; Lin, C.T. A mini review: Application of graphene paper in thermal interface materials. New Carbon Mater. 2021, 36, 930–938. [Google Scholar] [CrossRef]
- McNamara, A.J.; Joshi, Y.; Zhang, Z.M. Characterization of nanostructured thermal interface materials—A review. Int. J. Therm. Sci. 2012, 62, 2–11. [Google Scholar] [CrossRef]
- Du, S.J.; Guo, H.; Xie, Z.N.; Zhang, J.; Huang, S.H.; Wu, N.; Mi, X.-J.; He, X.-B.; Yang, H. Unveiling thermal properties and pump-out blocking in diamond/GaInSn composites as thermal interface materials. Rare Met. 2023, 42, 3969–3976. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Z.; Liu, J. High performance liquid metal thermal interface materials. Nanotechnology 2021, 32, 092001. [Google Scholar] [CrossRef]
- Gwinn, J.P.; Saini, M.; Webb, R.L. Apparatus for accurate measurement of interface resistance of high performance thermal interface materials. In Proceedings of the ITHERM 2002. The Eighth Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–1 June 2002. [Google Scholar] [CrossRef]
- Ding, Y.; Deng, Z.; Cai, C.; Yang, Z.; Yang, Y.; Lu, J.; Gao, Y. Bulk Expansion Effect of Gallium-Based Thermal Interface Material. Int. J. Thermophys. 2017, 38, 91. [Google Scholar] [CrossRef]
- Yang, B.; Li, D.; Yang, H.; Hu, Y.; Yang, P. Vibrational fatigue and reliability of package-on-package stacked chip assembly. Microelectron. J. 2019, 92, 104609.1–104609.6. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, H.; Zhou, C.; Liu, X.; Zhu, W.; Chen, Z. The thermal cycling reliability of copper pillar solder bump in flip chip via thermal compression bonding. Microelectron. Reliab. 2020, 104, 113543. [Google Scholar] [CrossRef]
- Jeong, H.; Min, K.D.; Lee, C.J.; Kim, J.H.; Jung, S.B. Mechanical reliability of Cu cored solder ball in flip chip package under thermal shock test. Microelectron. Reliab. 2020, 112, 113918. [Google Scholar] [CrossRef]
- Frisk, L.; Saarinen-Pulli, K. Reliability of adhesive joined thinned chips on flexible substrates under humid conditions. Microelectron. Reliab. 2014, 54, 2058–2063. [Google Scholar] [CrossRef]
- La, J.H. Thermal Stress and Strain in Microelectronic Packaging; Van Nostrand Reinhold: New York, NY, USA, 1993; pp. 234–256. [Google Scholar]
- Roy, C.K.; Harris, D.K.; Bhavnani, S.; Johnson, R.W.; Knight, R.W.; Harris, D.K. Durability of Low Melt Alloys as Thermal Interface Materials. J. Electron. Packag. 2015, 138, 010913. [Google Scholar] [CrossRef]
- Roy, C.K.; Bhavnani, S.; Hamilton, M.C.; Johnson, R.W.; Nguyen, J.L.; Knight, R.W.; Harris, D.K. Investigation into the application of low melting temperature alloys as wet thermal interface materials. Int. J. Heat Mass Transf. 2015, 85, 996–1002. [Google Scholar] [CrossRef]
- Yang, E.; Guo, H.; Guo, J.; Shang, J.; Wang, M. Thermal Performance of Low-Melting-Temperature Alloy Thermal Interface Materials. Acta Metologica Sin. (Engl. Lett.) 2014, 27, 290–294. [Google Scholar] [CrossRef]
- Plevachuk, Y.; Sklyarchuk, V.; Eckert, S.; Gerbeth, G.; Novakovic, R. Thermophysical Properties of the Liquid Ga–In–Sn Eutectic Alloy. J. Chem. Eng. Data 2014, 59, 757. [Google Scholar] [CrossRef]
- Kim, D.; Thissen, P.; Viner, G.; Lee, D.; Choi, W.; Chabal, Y.; Lee, J. Recovery of nonwetting characteristics by surface modification of gallium-based liquid metal droplets using hydrochloric acid vapor. ACS Appl. Mater. Interfaces 2013, 5, 179–185. [Google Scholar] [CrossRef]
- Bharatham, L.; Fong, W.S.; Torresola, J.; Koang, C.C. Qualification of phase change thermal interface material for wave solder heat sink on FCBGA package. In Proceedings of the 2005 7th Electronic Packaging Technology Conference, Singapore, 7–9 December 2005. [Google Scholar] [CrossRef]
- JESD22-A103E; High Temperature Storage Life. JEDEC: Columbus, OH, USA, 2015.
- JESD22-A104D; Temperature Cycling. JEDEC: Columbus, OH, USA, 2009.
- JESD22-A105C; Power and Temperature Cycling. JEDEC: Columbus, OH, USA, 2011.
- Gowda, A.; Esler, D.; Paisner, S.N.; Tonapi, S. Reliability testing of silicone-based thermal greases [IC cooling applications]. In Proceedings of the Semiconductor Thermal Measurement and Management IEEE Twenty First Annual IEEE Symposium, San Jose, CA, USA, 15–17 March 2005; pp. 64–71. [Google Scholar] [CrossRef]
- Ramaswamy, C.; Shinde, S.; Pompeo, F.; Sablinski, W.; Bradley, S. Phase change materials as a viable thermal interface material for high-power electronic applications. In Proceedings of the Ninth Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (IEEE Cat. No.04CH37543), Las Vegas, NV, USA, 1–4 June 2004; Volume 2, pp. 687–691. [Google Scholar] [CrossRef]
- Khuu, V.; Osterman, M.; Bar-Cohen, A.; Pecht, M. Effects of Temperature Cycling and Elevated Temperature/Humidity on the Thermal Performance of Thermal Interface Materials. IEEE Trans. Device Mater. Reliab. 2009, 9, 379–391. [Google Scholar] [CrossRef]
- Chiu, C.P.; Chandran, B.; Mello, K.; Kelley, K. An accelerated reliability test method to predict thermal grease pump-out in flip-chip applications. In Proceedings of the 2001 51st Electronic Components and Technology Conference (Cat. No.01CH37220), Orlando, FL, USA, 29 May–1 June 2001; pp. 91–97. [Google Scholar] [CrossRef]
- Chen, W.; Gui, X.; Yang, L.; Zhu, H.; Tang, Z. Wrinkling of two-dimensional materials: Methods, properties and applications. Nanoscale Horiz. 2019, 2, 291–320. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Guo, H.; Hui, Y.; Zhang, J.; Xie, Z.N.; Wu, N. Interface regulation of diamond-doped GaInSn composites. Diam. Relat. Mater. 2024, 141, 110655. [Google Scholar] [CrossRef]
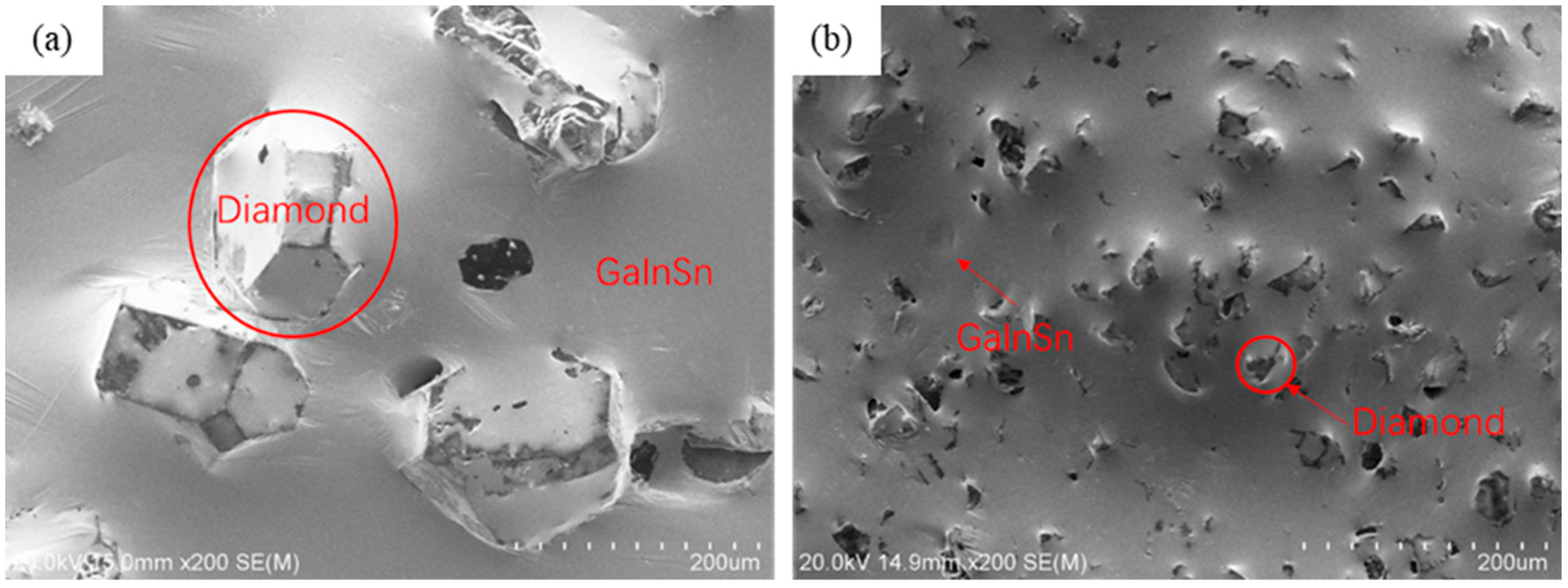
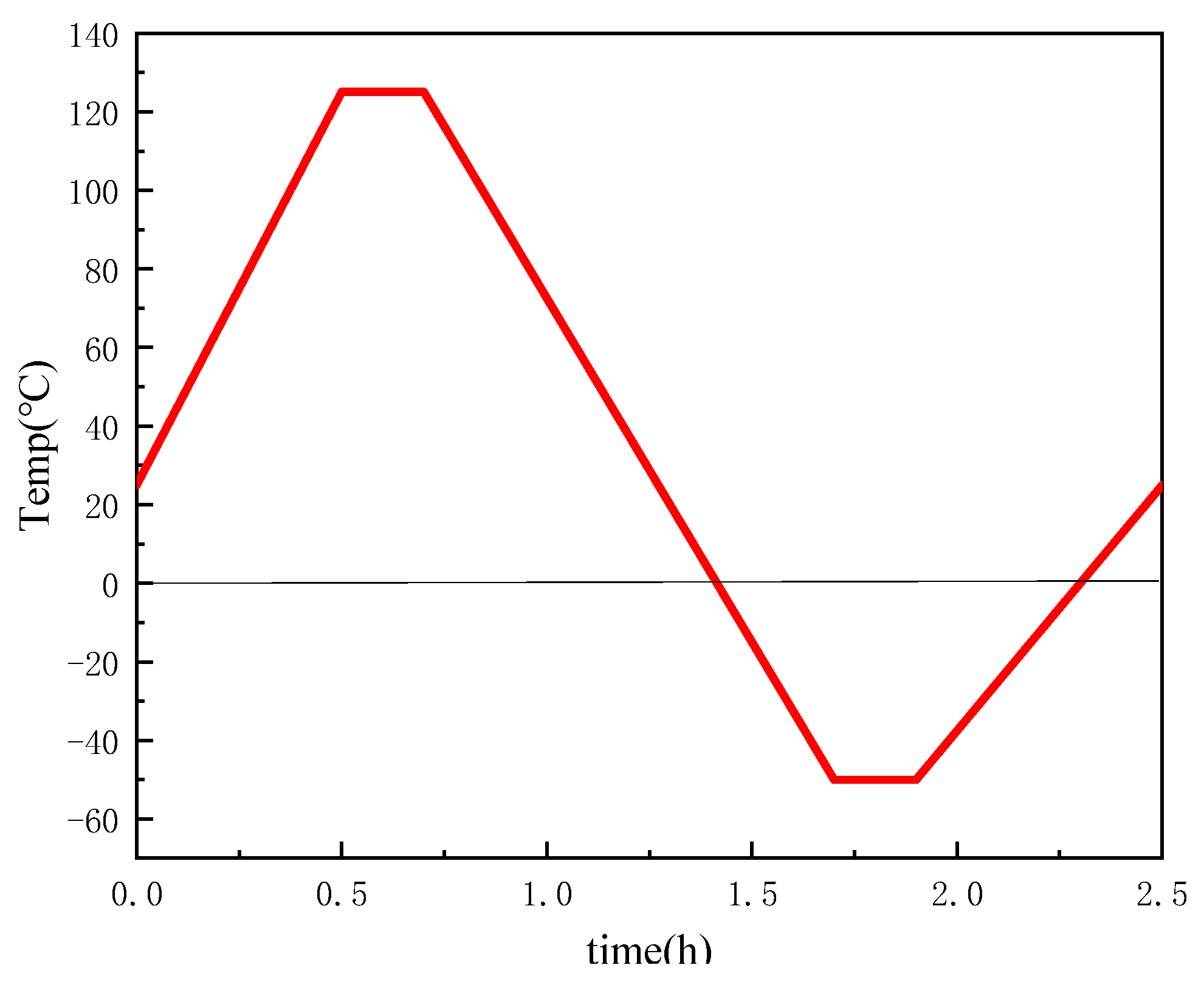
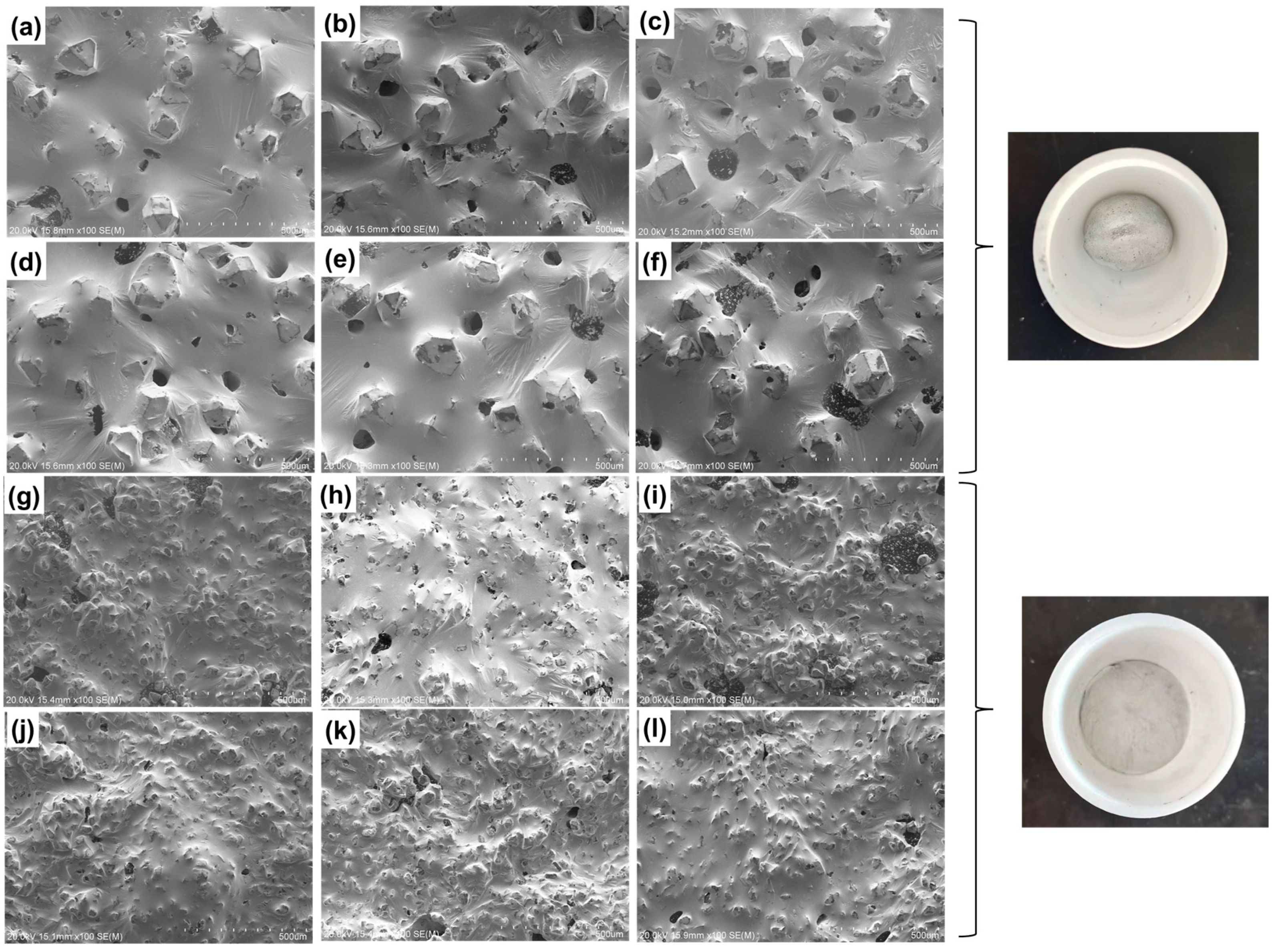
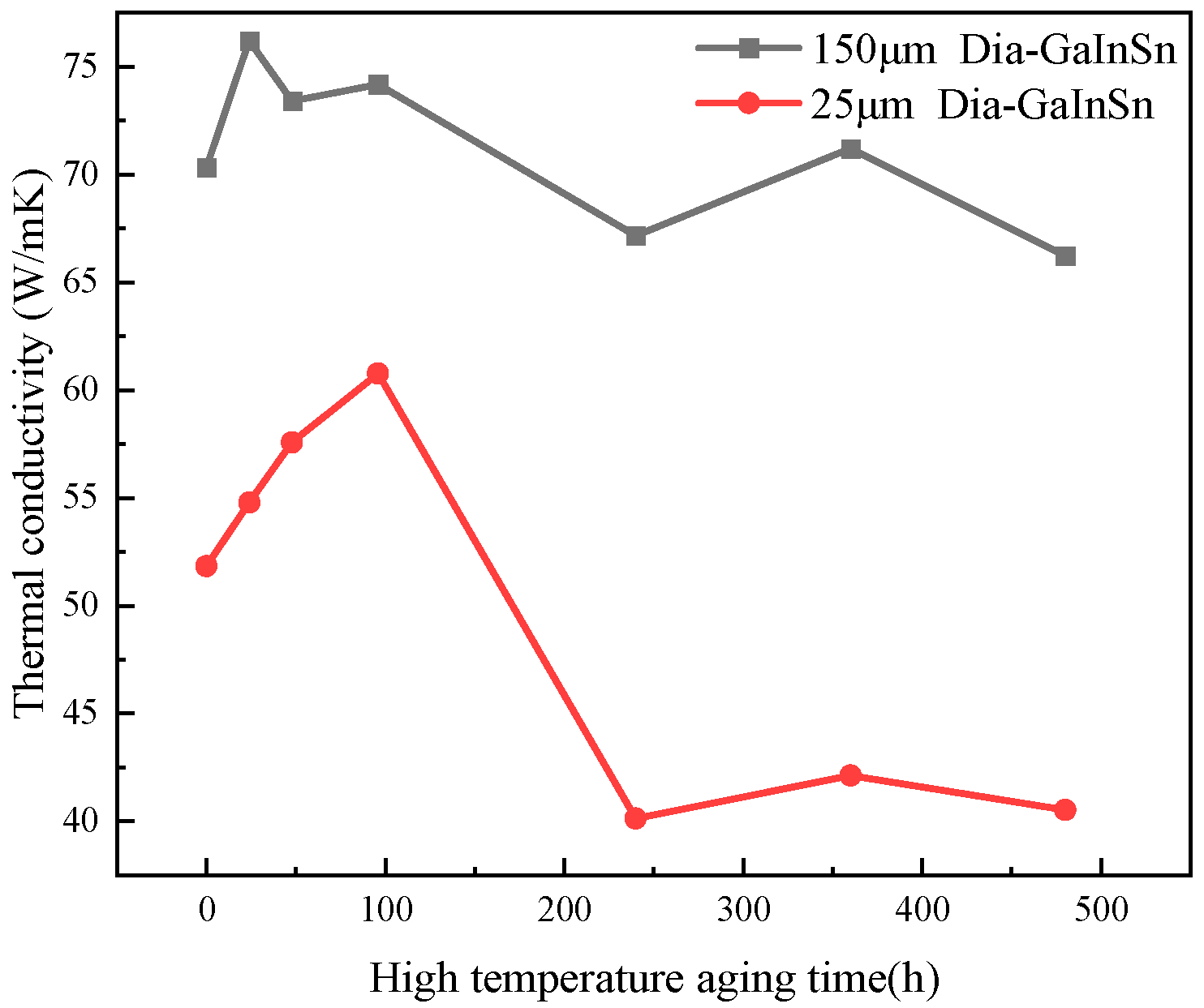



| Material Property | GaInSn Liquid Metal | GaInSn/Diamond (13 μm) | GaInSn/Diamond (150 μm) |
|---|---|---|---|
| Melting point (°C) | 9 | 9 | 9 |
| Density (g/cm3) | 6.17 | 4.36 | 4.83 |
| Specific heat (J/gK) | 0.38 | 0.42 | 0.50 |
| Thermal conductivity (W/mK) | 14.94 | 51.84 | 70.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Guo, H.; Zhang, J.; Xie, Z.; Yang, H.; Wu, N.; Liu, Y. Microstructure and Property Evolution of Diamond/GaInSn Composites under Thermal Load and High Humidity. Materials 2024, 17, 1152. https://doi.org/10.3390/ma17051152
Du S, Guo H, Zhang J, Xie Z, Yang H, Wu N, Liu Y. Microstructure and Property Evolution of Diamond/GaInSn Composites under Thermal Load and High Humidity. Materials. 2024; 17(5):1152. https://doi.org/10.3390/ma17051152
Chicago/Turabian StyleDu, Shijie, Hong Guo, Jie Zhang, Zhongnan Xie, Hui Yang, Nan Wu, and Yulin Liu. 2024. "Microstructure and Property Evolution of Diamond/GaInSn Composites under Thermal Load and High Humidity" Materials 17, no. 5: 1152. https://doi.org/10.3390/ma17051152
APA StyleDu, S., Guo, H., Zhang, J., Xie, Z., Yang, H., Wu, N., & Liu, Y. (2024). Microstructure and Property Evolution of Diamond/GaInSn Composites under Thermal Load and High Humidity. Materials, 17(5), 1152. https://doi.org/10.3390/ma17051152






