1. Introduction
With the continuous improvement in the performance of advanced aerospace engines, there is a growing utilization of lightweight materials with high-temperature resistance and excellent mechanical properties. In this regard, titanium alloys have gained significant popularity in aero-engineering, contributed by their superior properties, such as low density, high specific strength, and high temperature and corrosion resistance [
1]. The incorporation of titanium alloys allows for significant weight reduction in components while simultaneously improving their strength. The amount of titanium alloys utilized has emerged as an important indicator for assessing the advancement of next-generation aero engines [
2]. As a result, there is a pressing demand for the machining of key components and structures made of titanium alloys. However, titanium alloys suffer from certain drawbacks, such as poor room-temperature plasticity, toughness, and thermal conductivity, leading to their subpar mechanical machining performance. This, in turn, leads to issues of shortened tool lifespan and high costs. These processing difficulties have limited the broader applications of titanium alloy in aero engines [
2]. Therefore, addressing the issue of processing titanium alloy efficiently, cost-effectively, and with high surface integrity remains of paramount importance.
Small hole drilling is crucial for the functionalization of titanium alloys, presenting a challenge to the existing processes [
3,
4]. In mechanical-based methods, the occurrence of burrs in the orifice is a common issue [
5,
6,
7]. Moreover, when dealing with small holes with a diameter smaller than 1 mm, tool vibration can lead to poor processing accuracy and tool breakage [
8,
9]. Thermal-based processes, such as electrical discharge machining and laser beam processing, are capable of efficiently removing material using instantaneous high temperatures. However, these processes suffer from issues such as the formation of recast layers, micro-cracks, and heat-affected zones [
2]. Additionally, femtosecond pulsed laser processing also encounters difficulties in altogether avoiding surface thermal damage while processing small holes with a large depth-to-diameter ratio [
10].
Meanwhile, electrochemical machining (ECM) is not limited by the mechanical properties of the conductive material and can achieve high surface integrity without experiencing tool wear [
11,
12]. Unlike other methods, ECM also has the ability to avoid the formation of both the recast layers and heat-affected zones [
13]. Consequently, ECM has been increasingly adopted to process titanium alloys. Along these lines, Chen et al. investigated the electrochemical dissolution characteristics of titanium alloys with different phases, achieving a surface roughness of Ra 0.28 µm through ECM [
11]. Similarly, Xu et al. verified the feasibility of the electrochemical dissolution of titanium alloy, revealing a surface roughness of Ra 0.37 µm, along with a removal rate of 3.1 mm
3/A min [
13]. Additionally, Xu et al. optimized the ECM parameters and successfully processed titanium alloy fan blades at a tool electrode feeding rate of 1.2 mm/min, resulting in a profile accuracy of 0.07 mm [
14]. In a study by Klock et al., it was demonstrated that the machined edges of titanium alloys exhibited no heat-affected zone and no diffusion of foreign atoms [
15]. These findings conclude that titanium alloys can be processed using ECM, resulting in high surface quality.
Shaped tube electrolytic machining (STEM), a variation of ECM, uses a metal tube as the tool electrode, allowing for deep-hole processing. Hung et al. relieved the effects of stray currents during STEM by applying an insulating layer on the sidewall of the tube electrode, which reduced the difference between the entrance and exit of the hole, leading to improved drilling accuracy [
16]. Sakamotoa et al. found that the hole shape accuracy deteriorated when the electrolyte flow rate was decreased during ECM, which was attributed to the difficulties in flowing the electrolytic products away from the machining gap [
17]. In a separate study, Jaina et al. employed a tubular tool electrode to machine a tapered hole with a diameter of 100 µm on the surface of a titanium alloy [
18]. Furthermore, Liu et al. proposed a novel tube electrode structure consisting of an insulating substrate and a conductive film, which was used to process microvias with a diameter of 178 µm and a depth-to-diameter ratio of 3 [
19]. In another attempt, Perla et al. applied Taguchi’s experimental design method to analyze the impact of voltage and the duty cycle on tool tube electrode processing and revealed that the duty cycle had a more significant effect on surface roughness, while the applied voltage greatly affected dimensional deviation [
20]. Meanwhile, Geethapiriyan et al. performed experiments on titanium alloys using STEM and showed a 17% reduction in machining roughness and a 5.3% increase in material removal rate for nickel-plated tube electrodes compared to unplated tube electrodes [
21]. In another interesting study, Anuj et al. indicated that adding sodium hydroxide to sodium chloride and sodium nitrate solutions resulted in a reduction in the hole roundness error [
22]. Zhang et al. confirmed that the utilization of an external rinse solution around the machining area effectively decreased the radius of the stray corrosion-affected area [
23]. Bian et al. investigated the effect of cathode materials on the electrolytic machining of stainless steel with tool tube electrodes and experimentally concluded that the use of 6061 aluminum alloy effectively mitigated the stray current corrosion during the machining process and reduced the hole taper [
24]. Özerkan designed a device for processing small diameter holes in powder metal steel, which achieved a 65% reduction in electrolyte consumption by rotating the tool tube electrode while simultaneously enhancing the efficiency of debris discharge from the side machining gap [
25]. In order to improve the efficiency of titanium alloy drilling via STEM, it is necessary to address passivation by using methods that utilize high-temperature electrolytes (>30 °C) or introduce halogen ions like Cl
–1 and Br
–1 into the electrolyte. However, utilizing these methods may result in compromised machining accuracy and surface roughness. Additionally, the distribution of electric current density may lead to the formation of a protrusion in the central machining zone, hindering the diffusion rate of electrolytic products and deteriorating the machining stability. Consequently, when drilling titanium alloys using STEM, the feeding rate of a tubular electrode is typically kept below 2.0 mm/min to prevent electric short circuits between the tubular electrode and workpiece [
26,
27]. Thus, there is a need to further enhance the electrochemical machining efficiency of titanium alloy drilling while simultaneously preserving machining precision.
In the present study, a combination of laser and shaped tube electrolytic machining (Laser-STEM) was adopted for drilling titanium alloys, where the laser was guided to the machining zone via total internal reflection. Two types of electrolytes were utilized in this study, including aggressive and passivating electrolytes. The performance of Laser-STEM was assessed using different kinds of electrolytes. In addition, the experimental investigation focused on examining the effects of laser power, voltage, and the tool electrode feeding rate on machining efficiency, accuracy, and surface roughness. Furthermore, the study explored the different material removal mechanisms that occur when processing titanium alloys with Laser-STEM using different types of electrolytes.
2. Materials and Methods
2.1. Principle of Laser-STEM
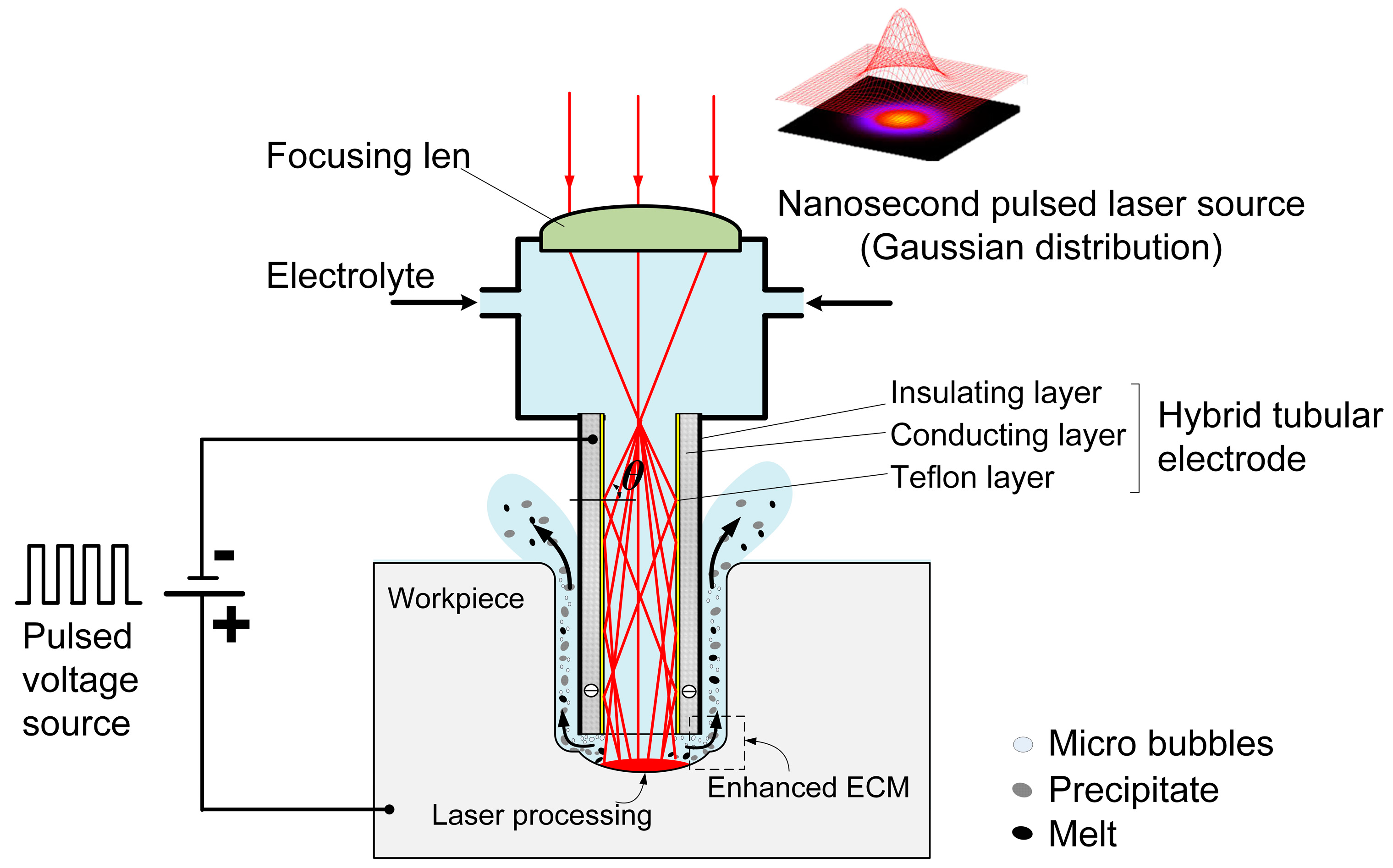
Laser-STEM utilizes total internal reflection to guide the laser beam toward the machining zone. As shown in
Figure 1, the laser is focused at the entrance of the tubular electrode using a focusing lens. The tubular electrode comprises a conducting layer and a Teflon layer with a low optical refractive index (
n2 = 1.24), which is smaller than the refractive index of the electrolyte (
n1 = 1.35). When the incident angle
θ at the interface of the electrolyte/Teflon layer exceeds a critical value of arcsin (
n1/
n2), total internal reflection occurs at the interface. As a result, the laser can effectively transmit to the machining zone with an efficiency greater than 70% [
28].
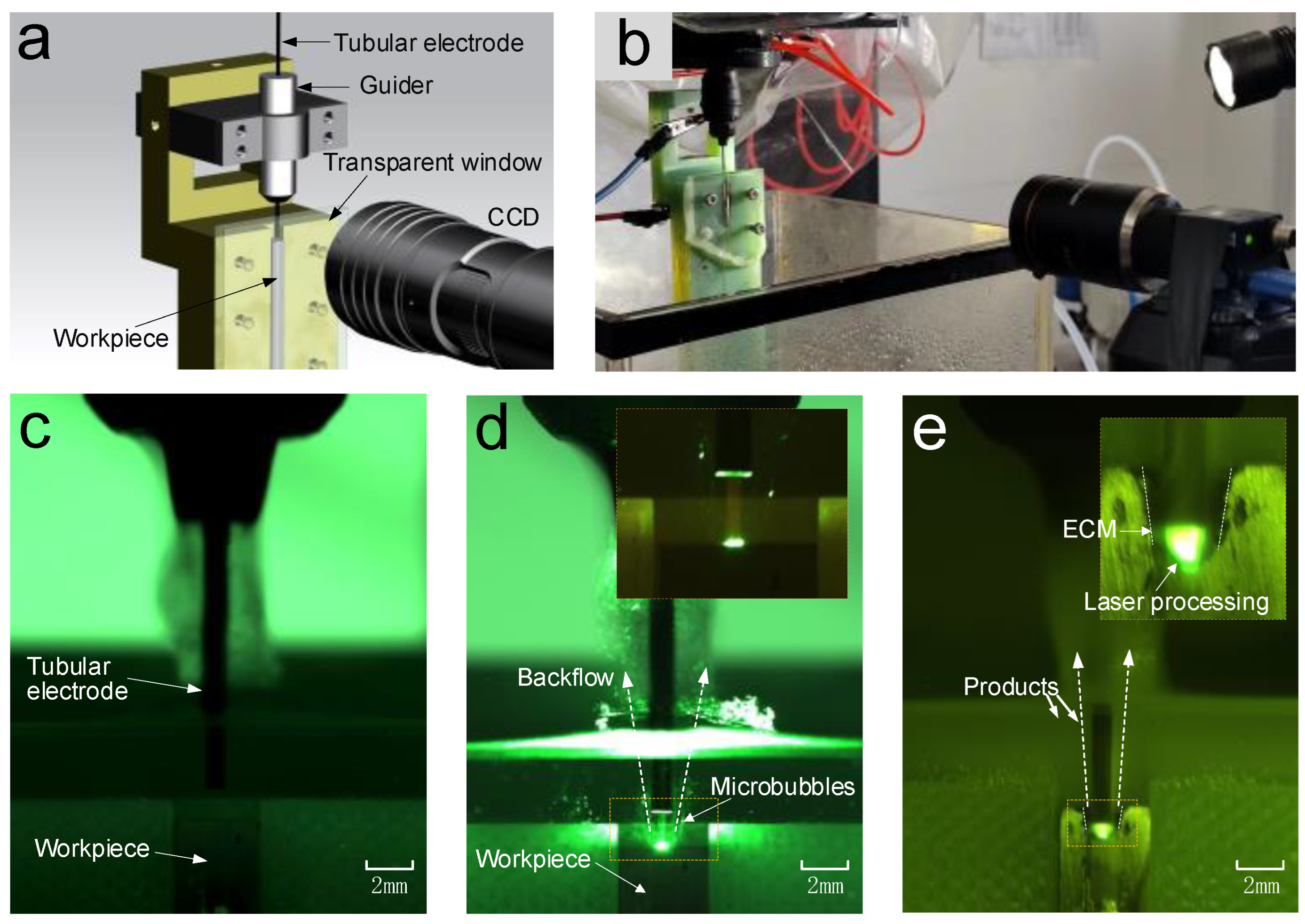
In Laser-STEM, the laser is directed to the central machining area through the inner hole of the tubular electrode. By feeding the tubular electrode, the laser can be transmitted to the large-depth machining zone, making it suitable for deep-hole drilling applications. When the laser intensity surpasses a threshold value, the workpiece materials can be effectively removed through laser processing. The materials in both the central machining zone and the side machining gap can be synchronously removed via ECM. Employing ECM at the side machining gap guarantees the attainment of the high surface quality of the processed microholes. Meantime, the laser-induced temperature rise leads to an increase in the electrolyte temperature within the processing area, thereby improving the ECM rate. During Laser-STEM, an electrolyte is flushed to the machining area from the inner hole of the tubular electrode and subsequently flushed out from the side gap between the tubular electrode and the workpiece, enabling the effective removal of microbubbles, electrolytic products, plasma, melt, and heat from the machining gap. Therefore, Laser-STEM allows the processing of holes with greater depth while maintaining high surface quality.
2.2. Experimental Setup
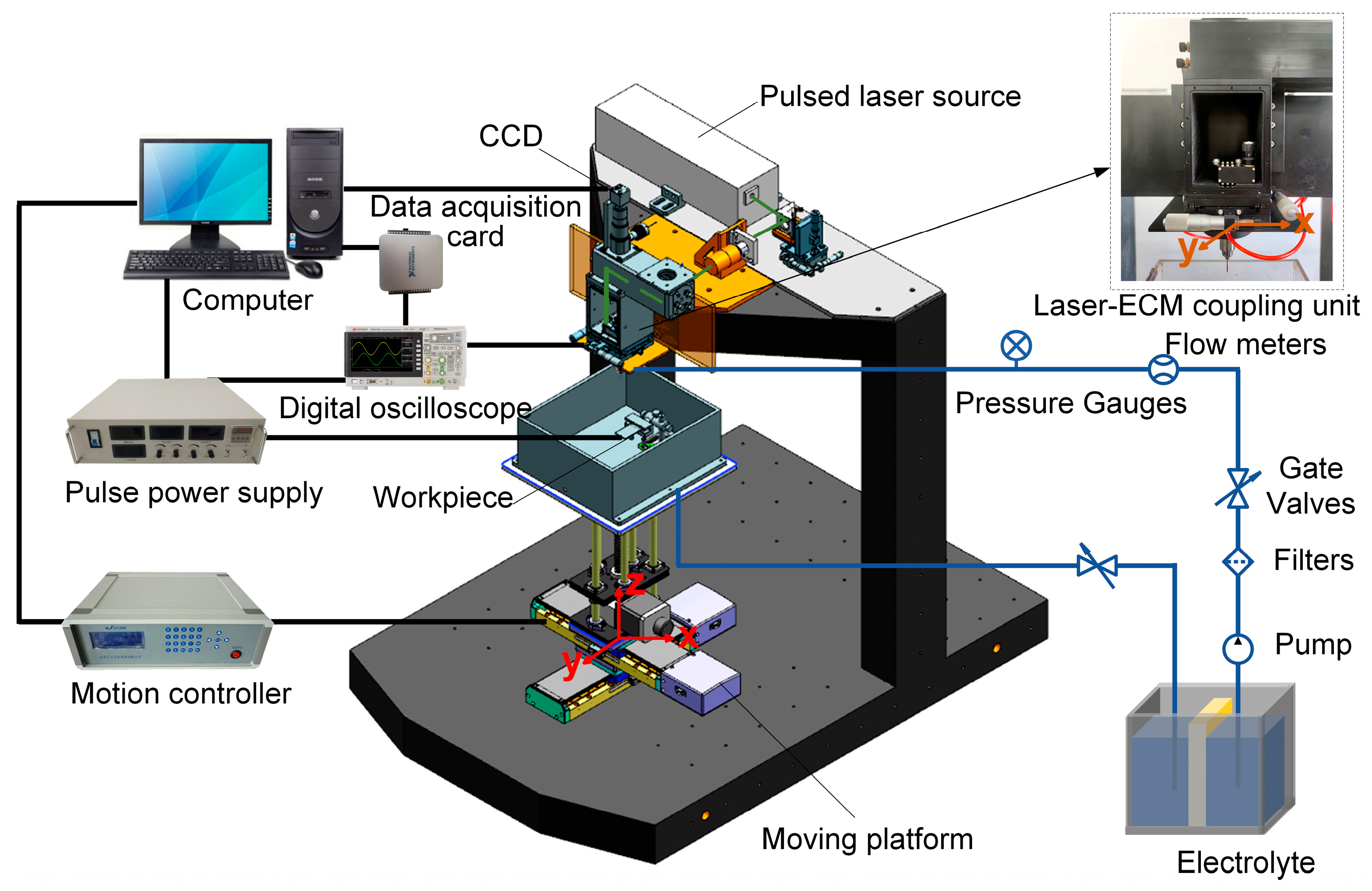
Figure 2 illustrates the schematic diagram of the experimental setup developed for Laser-STEM, consisting of a laser power source, an optical guide system, an ECM system, a liquid transportation system, a laser-ECM coupling unit, a motion control system, and a data acquisition system.
The pulsed laser emitted from the laser generator is directed toward the entrance of the tubular electrode using reflective mirrors and a focusing lens. In this setup, a pulsed laser source with a wavelength of 532 nm, a pulse duration of 16.3 ns, and a pulse repletion frequency of 8 kHz is used. The laser beam is focused at the entrance of the tube electrode through the use of a focusing lens with a focal length of 100 mm. The position of the laser focal point and the entrance of the tubular electrode are continuously monitored using a CCD monitoring system. The electrolyte supply system uses a diaphragm pump to deliver electrolytes to the laser-ECM coupling unit. After the machining process, the electrolyte flows into a waste tank, where it can undergo settling and filtering before being reused for further operations. The metal tube in the tubular electrode and the workpiece are connected to the positive and negative polarities of a pulse power supply, respectively, operating at a pulse frequency of 20 kHz and a duty ratio of 50%. The machining current is acquired and analyzed in real-time, using a data acquisition card (PCI-6221, NI) to monitor the status of the Laser-STEM. The electrolytic cell used for securing the workpiece is mounted on an X–Y–Z motion platform, which offers a travel range of 250 mm × 250 mm × 250 mm and a motion accuracy of 0.001 mm.
2.3. Materials and Measurement
A polished specimen made of TC4 titanium alloy with dimensions of 50 mm × 50 mm × 2 mm was utilized in the experiments. Before conducting experiments, the material underwent a cleaning process in an ultrasonic cleaning machine for 10 min using anhydrous ethanol. The electrolytes employed in this study included sodium nitrate (NaNO3) and sodium chloride (NaCl), with NaNO3 serving as the aggressive electrolyte and NaCl as the passivating electrolyte. A brass tube with an outer diameter of 1.25 mm and an inner diameter of 0.8 mm was used to prepare the tubular electrode. A Teflon layer was installed inside the tube, while an insulating layer was applied to the outside wall of the tube. Following the completion of the experiments, the specimen was immersed in anhydrous ethanol and ultrasonically cleaned for 30 min.
Before the processing of the specimen, its weight was measured using an electric balance (EX125ZH, Ohaus, Parsippany, NJ, USA) with a resolution of 0.001 mg. Once the experiments were completed, the workpiece was weighed again to determine the quality of the material after processing. To ensure accuracy, three holes were machined using each set of machining parameters, and the average value of the three holes was taken into consideration. The material removal rate (MRR) was determined by calculating the ratio of the mass of material removed to the processing time, which can be expressed as:
where
m0 represents the mass of the material before processing,
ma represents the mass of the material after processing, and
t denotes the processing time. The machined hole was measured using a laser confocal microscope (VK-X200K, Keyence, Tokyo, Japan). Furthermore, a scanning electron microscope (SEM, SU5000, Hitachi, Tokyo, Japan) along with an energy dispersive spectrometer (EDS) was used to examine the microscale morphology and element distribution of the machined surface. The machining accuracy was quantified using the lateral/side machining gap (Δ
s), which can be expressed as:
where
Da denotes the average hole diameter, and
D signifies the outer diameter of the tubular electrode. The front machining gap (Δ
b) is defined as the difference between the hole depth and the feed depth and can be calculated using the following expression:
where
ha represents the distance from the workpiece surface to the bottom of the hole,
h denotes the tube electrode feeding depth, and Δ
0 is the initial front machining gap.
4. Discussions
The results showed that the laser played distinct roles in affecting the machining precision and efficiency of Laser-STEM while processing titanium alloys with different electrolytes. To gain insights into the material removal mechanisms, the polarization curves of titanium alloys in NaCl and NaNO
3 electrolytes were tested and subsequently analyzed.
Figure 12 displays the polarization curves of titanium alloys in different electrolytes at a temperature of 18 °C and a mass fraction of 12.5 wt.%. Noticeably, the electric current density in the NaNO
3 solution was almost zero within the voltage range of 0–13 V due to the passivation effects on the surface of titanium alloys. The passivation layer on the surface of titanium alloys could be broken with a lower voltage in NaCl electrolyte owing to the corrosion caused by chloride ions disrupting the oxide layer [
29]. Meanwhile, processing titanium alloys in the NaNO
3 solution required a higher decomposition voltage of 13.8 V compared to 6.1 V in the NaCl solution. Thus, it was concluded that the effects of the passivation layer were more pronounced when using the NaCl electrolyte during Laser-STEM.
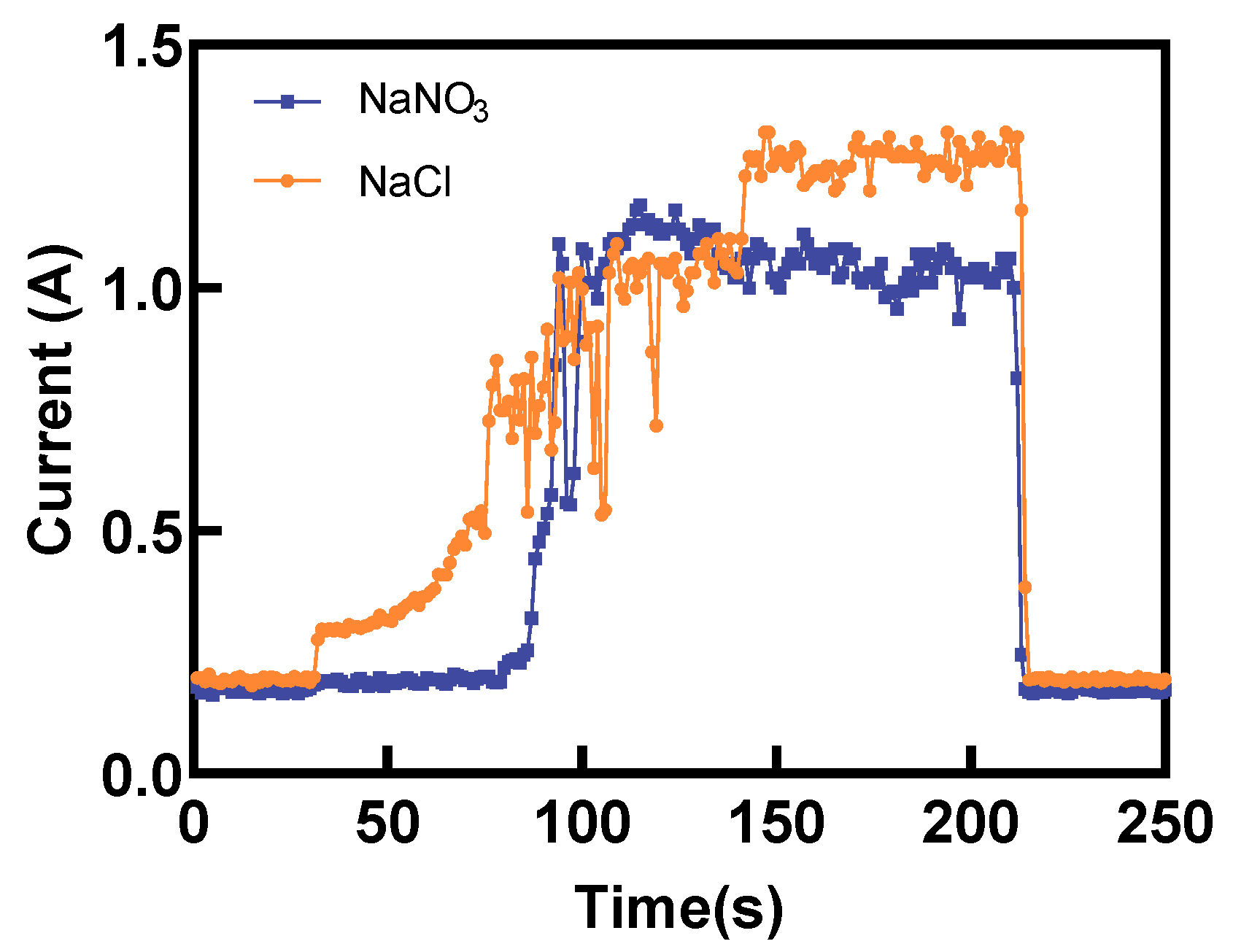
Figure 13 presents a comparison of the electric current during Laser-STEM using NaCl and NaNO
3 electrolytes. The electric current using NaCl increased gradually in the initial stage. Conversely, the electric current using NaNO
3 exhibited a sudden increase when the distance between the tube electrode and the workpiece was reduced to a certain range. When utilizing NaCl as the electrolyte during Laser-STEM, the chloride ions can penetrate the passivation layer, thereby minimizing its impact on the ECM process. As the tube electrode was fed toward the workpiece, the distance between the electrode and the workpiece surface gradually decreased, leading to an increase in the electric current. Moreover, when processing titanium alloy in the NaNO
3 solution, the resistance was mainly concentrated in the passivation layer in the initial stage. However, as the tube electrode was fed to a certain range, the voltage penetrated the passivation layer, resulting in an abrupt increase in the electric current, eventually reaching a stable processing state [
30]. In addition, the electric current observed during Laser-STEM using the NaCl solution was higher than that while using the NaNO
3 solution due to the effects of the passivation layer.
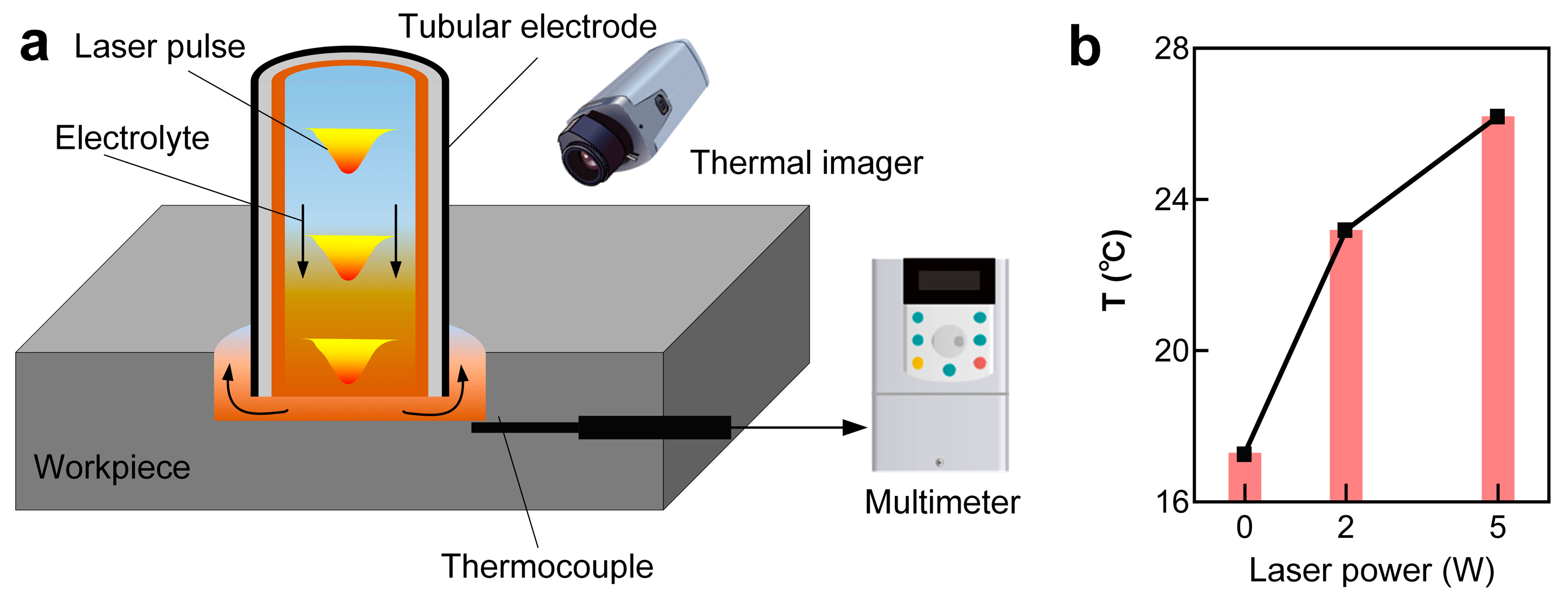
To study the impact of the laser on the temperature rise of the local electrolyte in the machining zone, the temperature of the electrolyte in proximity to the machining zone was measured using a thermocouple and thermal imager, as shown in
Figure 14a. The results suggested that the local electrolyte temperature increased with the rise in laser power, as depicted in
Figure 14b. Interestingly, a temperature rise of around 10 °C was observed with a laser power of 5 W. Theoretically, a higher laser power would result in a higher temperature rise in the local electrolyte.
In the process of using titanium alloys with NaNO
3 electrolyte using ECM, the passivation layer was formed through the following reaction:
Further, during Laser-STEM with the NaNO
3 electrolyte, the laser-induced temperature rise enhanced the production of the passivation layer, which was more pronounced with the increase in laser power. As shown in
Figure 15b, the thickness of the oxide layer,
δ(
r), significantly increased with the rise in laser power. In contrast, the thickness of the passivation layer became thinner as the radius (
r) increased, owing to the distribution of the laser intensity on the workpiece surface. Under these conditions, the resistance of the passivation layer (
R0) on the surface of the titanium alloys increased with the rise in laser power. Consequently, the machining side gap was decreased due to the reduced effects of stray corrosion, resulting in an improvement in the machining precision of Laser-STEM with an increase in laser power. These findings align with the experimental results presented in
Section 3.2.
Furthermore, when using the NaCl solution as the electrolyte, the increased local electrolyte temperature facilitated the movement rate of chloride ions, enhancing the removal rate of the oxide layer [
31]. Meanwhile, the electrolyte resistance (
Re(
T)) decreased with the increase in the local temperature of the electrolyte, as shown in
Figure 15b. Moreover, the MRR and machining gap showed an increase with the rise in laser power, consequently deteriorating the machining precision of Laser-STEM using the NaCl electrolyte. At the same time, the absence of a passivation layer to stop stray current corrosion resulted in a broader range of stray corrosion in the NaCl solution. Thus, due to the resistance of the oxide layer, the electric current was higher in the NaCl solution than that using the NaNO
3 electrolyte.
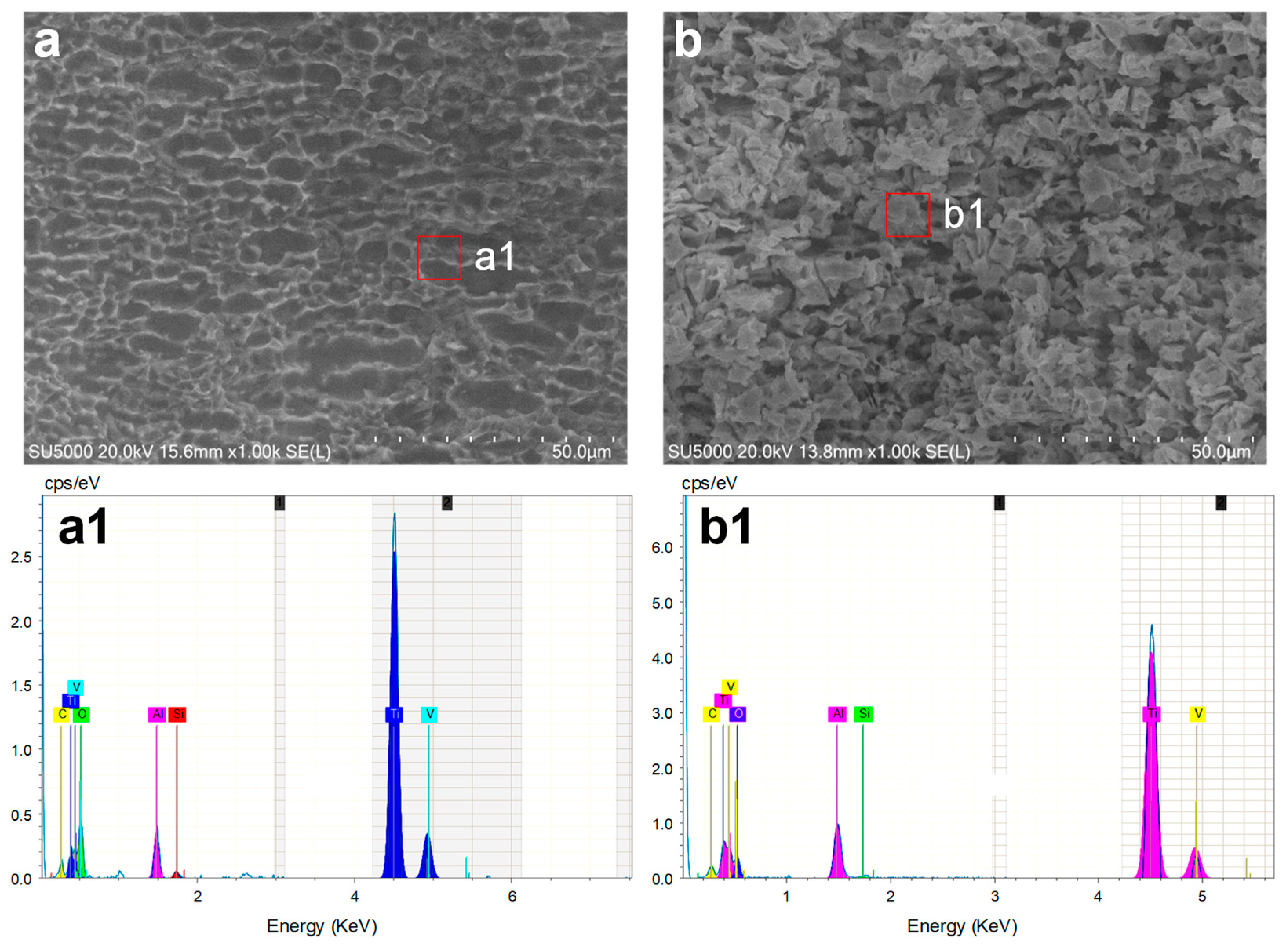
Figure 16 illustrates the comparison of the machined surfaces obtained using Laser-STEM. The surface processed with NaCl electrolyte exhibited numerous micro pits caused by the corrosion of chloride ions. On the contrary, the surface processed with the NaNO
3 electrolyte was covered with an oxide layer. In conclusion, the use of the NaNO
3 electrolyte during Laser-STEM enhanced the machining precision with increasing laser power, whereas the use of the NaCl electrolyte improved machining efficiency.
5. Conclusions
In this work, the machining performance of Laser-STEM using two different types of electrolytes was compared, and the corresponding material removal mechanisms were studied. The effects of laser power and voltage on MRR, surface roughness, and the machining side were thoroughly investigated. The study also explored the maximum feeding rate of the tubular tool electrode using different electrolytes. Additionally, the polarization curve and local electrolyte temperature were measured to reveal the material removal mechanisms. Based on the findings, the following conclusions can be drawn:
(1) The laser exerted both a temperature-increasing effect and directly removed material, leading to a substantial increase in MRR with laser powers of 3–5 W. Specifically, the MRR with the NaCl electrolyte increased from 3.99 mg/min to 4.65 mg/min. Meanwhile, when using the NaNO3 electrolyte in Laser-STEM, the laser-induced thermal effects promoted the formation of the passivation layer on the surface of the titanium alloys, reducing the stray current corrosion and enhancing the machining precision of ECM. As a result, the side gap with the NaNO3 solution decreased from 90.66 µm to 73.08 µm as the laser power was increased from 0 W to 5 W.
(2) With a voltage of 20 V and a laser power of 4 W, the maximum processing speed of Laser-STEM using the NaCl solution reached 6.0 mm/min; meanwhile, the maximum processing speed was 3 mm/min with the NaNO3 solution. These values were increased by 200% and 333%, respectively, compared to the maximum processing speed without laser action.
(3) The study achieved small holes with a diameter of 1.5 mm, a depth of 38 mm, and a surface roughness below Ra 2 µm. Moreover, the cross-inner flow channels in the titanium alloys were also effectively processed. The SEM and EBSD results demonstrated that the processed surface showed no presence of a recast layer, microcracks, or heat-affected zones.
(4) The study further utilized polarization curves and measured local electrolyte temperature to reveal the material removal mechanisms in different electrolytes during Laser-STEM. A model was established to illustrate these mechanisms. The laser-induced thermal effect was found to enhance the movement rate of ions within the electrolyte, resulting in a reduced equivalent resistance of the electrolyte and an increased processing current. When using the NaNO3 solution, the laser-induced thermal effects led to an increased passivation layer thickness, thereby improving the machining accuracy of Laser-STEM. On the other hand, with the NaCl solution, the increased electric current caused by the laser-induced thermal effects enhanced the MRR of Laser-STEM.