Mesoporous Silica MCM-41 from Fly Ash as a Support of Bimetallic Cu/Mn Catalysts for Toluene Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. MCM-41 Synthesis from Fly Ash
2.2. Catalysts Preparation
2.3. Materials Characterization
2.3.1. X-ray Diffraction (XRD)
2.3.2. Nitrogen Adsorption–Desorption at −196 °C
2.3.3. X-ray Fluorescence Spectroscopy (XRF)
2.3.4. Scanning Electron Microscopy (SEM) with Energy Dispersive X-ray Analysis (EDS)
2.3.5. Raman Spectra
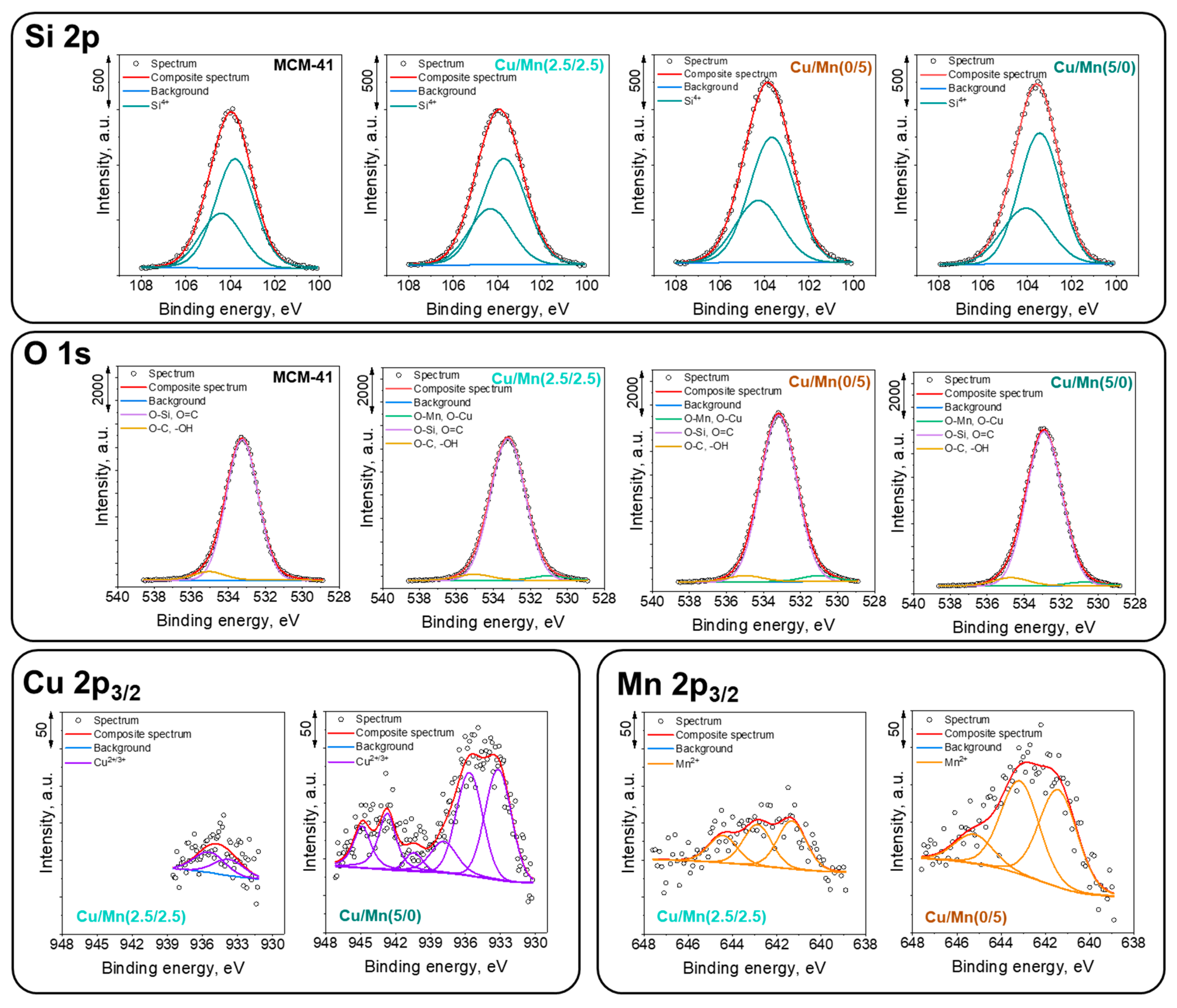
2.3.6. X-ray Photoelectron Spectroscopy (XPS)
2.4. Catalytic Tests
2.4.1. Toluene Combustion
2.4.2. O2 Temperature-Programmed Desorption (O2-TPD)
3. Results and Discussion
3.1. Materials Characterization
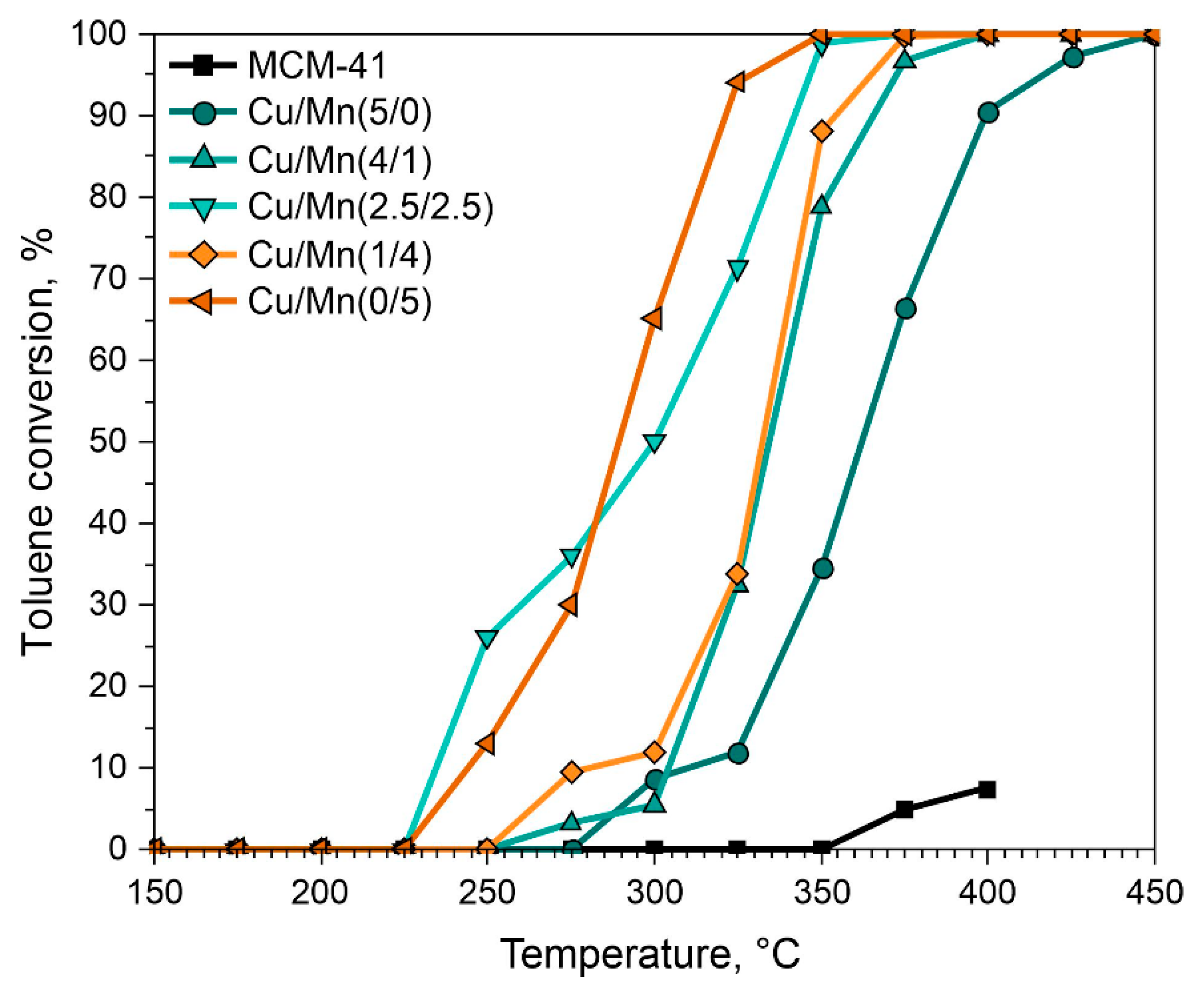
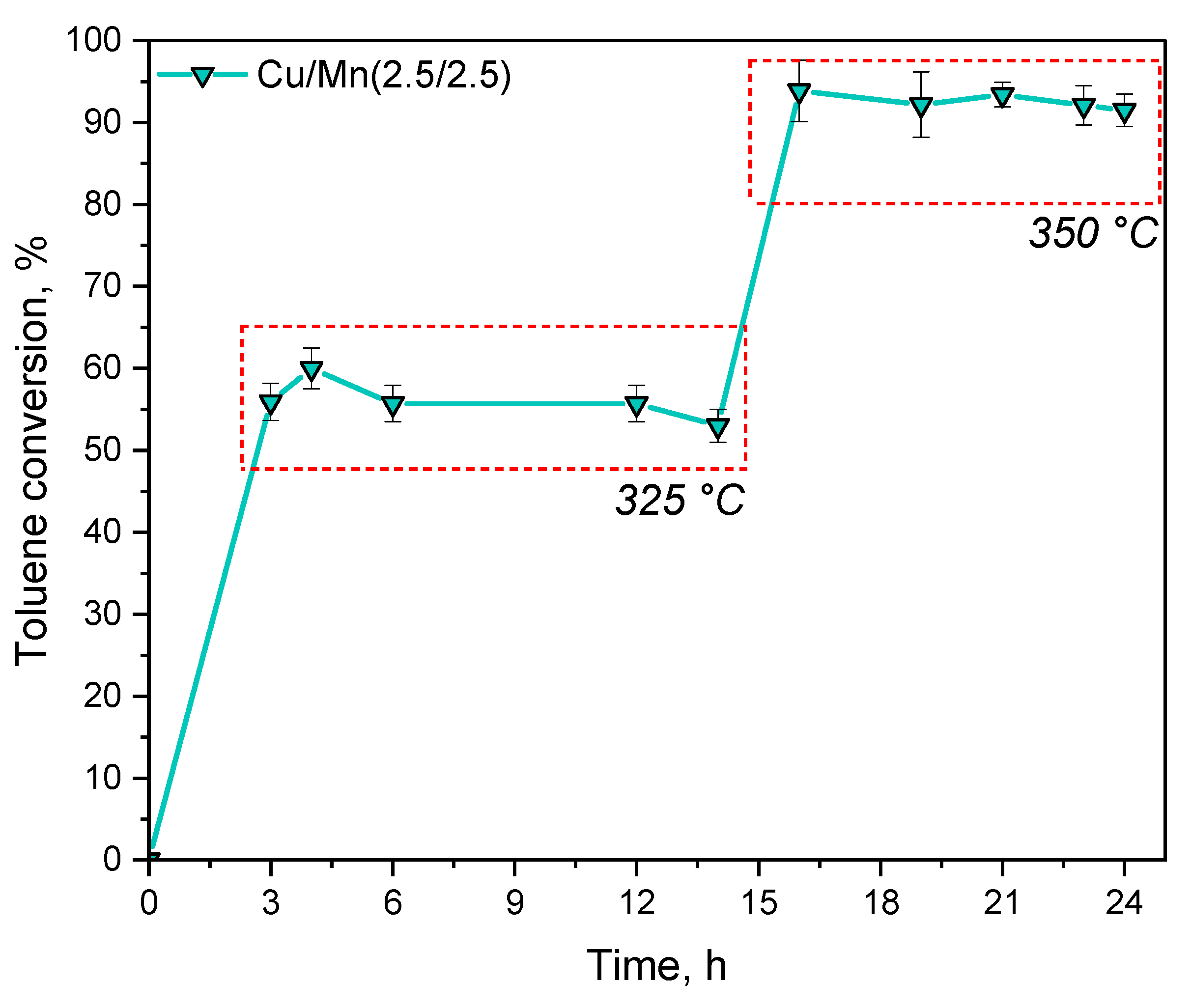
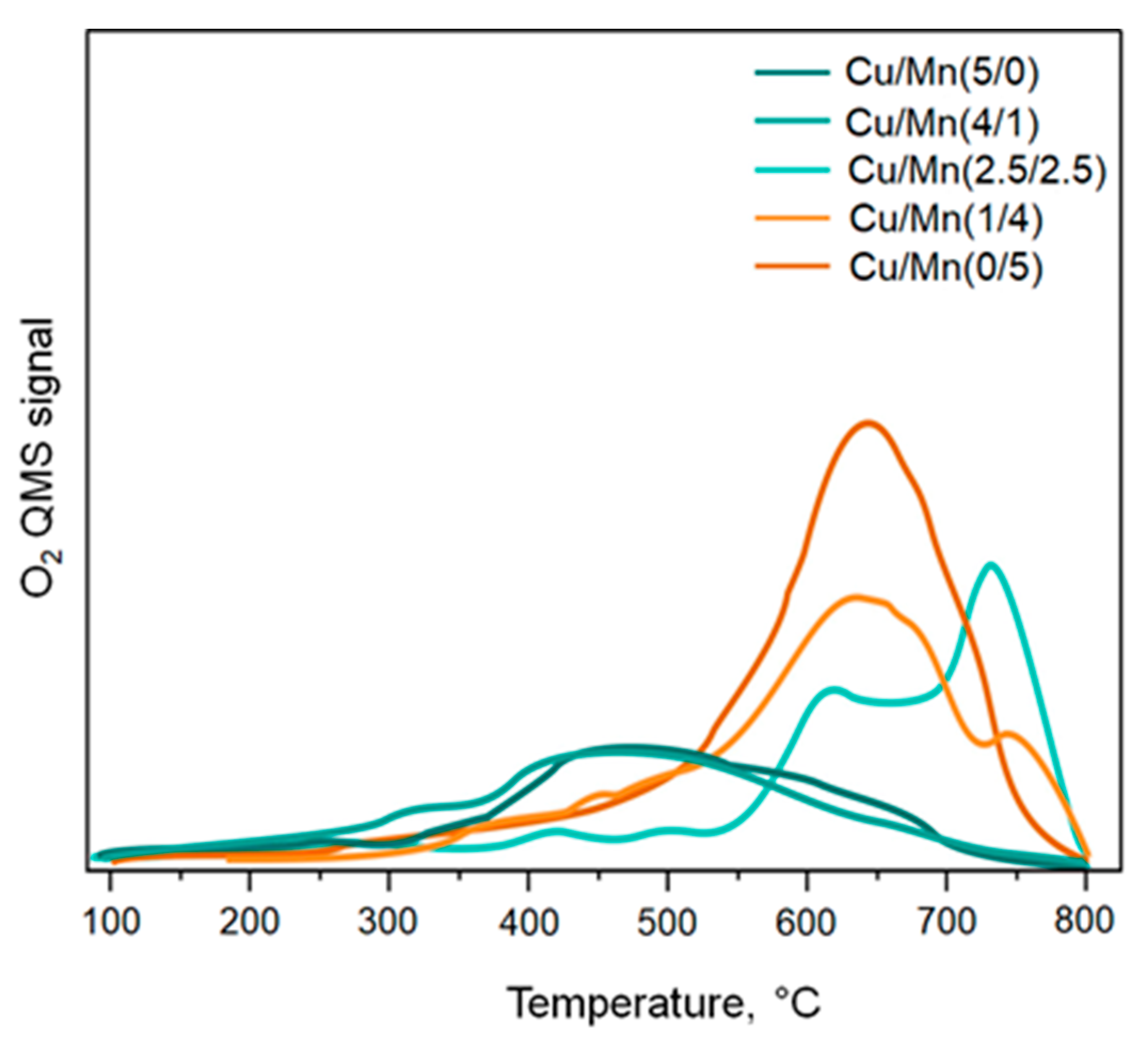
3.2. Catalytic Activity of Materials
3.2.1. Catalytic Combustion of Toluene
3.2.2. O2-TPD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, W.; Zhang, X.; Chen, K.; Fang, J.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Enhanced Adsorption Performance and Governing Mechanisms of Ball-Milled Biochar for the Removal of Volatile Organic Compounds (VOCs). Chem. Eng. J. 2020, 385, 123842. [Google Scholar] [CrossRef]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Shen, Y.; Deng, J.; Impeng, S.; Li, S.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Boosting Toluene Combustion by Engineering Co–O Strength in Cobalt Oxide Catalysts. Environ. Sci. Technol. 2020, 54, 10342–10350. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y.; et al. Engineering Cobalt Oxide with Coexisting Cobalt Defects and Oxygen Vacancies for Enhanced Catalytic Oxidation of Toluene. ACS Catal. 2022, 12, 4906–4917. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Qin, Y.; Fu, Q.; Sun, H.; Duan, X. Revealing the Highly Catalytic Performance of Spinel CoMn2O4 for Toluene Oxidation: Involvement and Replenishment of Oxygen Species Using In Situ Designed-TP Techniques. ACS Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Yang, X.; Zhao, S.; Gao, H.; Yu, Q. Effect of preparation method on active sites variation for catalytic oxidation of toluene over Pt/MCM-48. Appl. Catal. A Gen. 2023, 655, 119114. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Li, G.; Wang, L.; Song, L.; Li, X. Zeolitic acidity as a promoter for the catalytic oxidation of toluene over MnO /HZSM-5 catalysts. Catal. Today 2019, 327, 374–381. [Google Scholar] [CrossRef]
- Dündar-Tekkaya, E.; Yürüm, Y. Mesoporous MCM-41 material for hydrogen storage: A short review. Int. J. Hydrogen Energy 2016, 41, 9789–9795. [Google Scholar] [CrossRef]
- Panek, R.; Wdowin, M.; Franus, W.; Czarna, D.; Stevens, L.A.; Deng, H.; Liu, J.; Sun, C.; Liu, H.; Snape, C. Fly ash-derived MCM-41 as a low-cost silica support for polyethyleneimine in post-combustion CO2 capture. J. CO2 Util. 2017, 22, 81–90. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Baran, P.; Gazda-Grzywacz, M.; Bator, J.; Wróbel, W.; Zarębska, K. Decarbonatization of Energy Sector by CO2 Sequestration in Waste Incineration Fly Ash and Its Utilization as Raw Material for Alkali Activation. Materials 2023, 16, 6094. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, J.; Franus, W.; Panek, R.; Sobczyk, M.; Rusiniak, P.; Szerement, J.; Jarosz, R.; Marcińska-Mazur, L.; Bajda, T.; Mierzwa-Hersztek, M. Zeolite Composite Materials from Fly Ash: An Assessment of Physicochemical and Adsorption Properties. Materials 2023, 16, 2142. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Fedyna, M.; Marzec, M.; Panek, R.; Szerement, J.; Marcińska-Mazur, L.; Jarosz, R.; Bajda, T.; Franus, W.; Mierzwa-Hersztek, M. The influence of zeolite X ion-exchangeable forms and impregnation with copper nitrate on the adsorption of phosphate ions from aqueous solutions. J. Water Process Eng. 2022, 50, 103299. [Google Scholar] [CrossRef]
- Szerement, J.; Kowalski, A.; Mokrzycki, J.; Marcińska-Mazur, L.; Mierzwa-Hersztek, M. Restoration of soils contaminated with PAHs by the mixture of zeolite composites mixed with exogenous organic matter and mineral salts. Sci. Rep. 2023, 13, 14227. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Y.; Yao, W.; Wu, Z. One-step synthesis of bimetallic Pt-Pd/MCM-41 mesoporous materials with superior catalytic performance for toluene oxidation. Catal. Commun. 2016, 83, 22–26. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.-M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.-F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Li, W.B.; Zhuang, M.; Xiao, T.C.; Green, M.L.H. MCM-41 Supported Cu-Mn Catalysts for Catalytic Oxidation of Toluene at Low Temperatures. J. Phys. Chem. B 2006, 110, 21568–21571. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Shi, Y.; Zhao, B.; Wang, M.; Liu, Q.; Wang, J.; Long, K.; Duan, Y.; Ning, P. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, Z.Y.; Wang, H.J.; Zhu, J.H. Fabrication of Metal Oxides Occluded in Ordered Mesoporous Hosts via a Solid-State Grinding Route: The Influence of Host–Guest Interactions. Adv. Funct. Mater. 2006, 16, 2374–2386. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Cata Saady, N.M. Desulfurization of actual diesel fuel onto modified mesoporous material Co/MCM-41. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100635. [Google Scholar] [CrossRef]
- Bernal, Y.P.; Alvarado, J.; Juárez, R.L.; Méndez Rojas, M.; de Vasconcelos, E.A.; de Azevedo, W.M.; Iniesta, S.A.; Cab, J.V. Synthesis and characterization of MCM-41 powder and its deposition by spin-coating. Optik 2019, 185, 429–440. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Shi, X.; Wen, X.; Chu, Y.; Yuan, S. Effect of aluminum on the catalytic performance and reaction mechanism of Mn/MCM-41 for NH3-SCR reaction. Appl. Surf. Sci. 2020, 534, 147592. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sudarshan, K.; Sen, D.; Pujari, P. Microenvironment of mesopores of MCM-41 supported CuO catalyst: An investigation using positronium probe. J. Solid State Chem. 2019, 274, 10–17. [Google Scholar] [CrossRef]
- Rehman, K.U.; Zaman, U.; Khan, D.; Khan, W.U. Surfactant assisted CuO/MCM-41 nanocomposite: Ultra efficient photocatalyst for degradation of methylene blue dye and inactivation of highly drug resistant bacteria. Mater. Chem. Phys. 2022, 277, 125454. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 5282. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, X.; Kang, Y.; Shu, Y.; Sun, Q.; Li, L. Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J. Colloid Interface Sci. 2014, 429, 25–33. [Google Scholar] [CrossRef]
- Carta, P.; Cara, C.; Cannas, C.; Scorciapino, M.A. Experiments-Guided Modeling of MCM-41: Impact of Pore Symmetry on Gas Adsorption. Adv. Mater. Interfaces 2022, 9, 2201591. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Robatjazi, Z.S.; Naimi-Jamal, M.R.; Tajbakhsh, M. Synthesis and characterization of highly efficient and recoverable Cu@MCM-41-(2-hydroxy-3-propoxypropyl) metformin mesoporous catalyst and its uses in Ullmann type reactions. Sci. Rep. 2022, 12, 4949. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Abrokwah, R.Y.; Kuila, D. Synthesis of stable Cu-MCM-41 nanocatalysts for H2 production with high selectivity via steam reforming of methanol. Int. J. Hydrogen Energy 2015, 40, 10439–10452. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Villa, A.L. Isomerization of α- and β- pinene epoxides over Fe or Cu supported MCM-41 and SBA-15 materials. Appl. Catal. A Gen. 2019, 580, 17–27. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, W.; Chen, X.; Song, Z.; Gao, C.; Tsubaki, N.; Zhang, X. A novel strategy to adjust the oxygen vacancy of CuO/MnO2 catalysts toward the catalytic oxidation of toluene. Fuel 2022, 312, 122975. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database, Version 3.5. (n.d.). Available online: https://srdata.nist.gov/xps/ (accessed on 17 August 2023).
- Wagner, C.D.; Passoja, D.E.; Hillery, H.F.; Kinisky, T.G.; Six, H.A.; Jansen, W.T.; Taylor, J.A. Auger and photoelectron line energy relationships in aluminum–oxygen and silicon–oxygen compounds. J. Vac. Sci. Technol. 1982, 21, 933–944. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Q.; Zhang, D.; Wang, J.; Tang, T.; Wang, H.; Liu, X.; Song, Z.; Ning, P. The influence of annealing temperature on copper-manganese catalyst towards the catalytic combustion of toluene: The mechanism study. Appl. Surf. Sci. 2019, 497, 143777. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, J.; Liang, X.; Long, C. Niobium doping enhanced catalytic performance of Mn/MCM-41 for toluene degradation in the NTP-catalysis system. Chemosphere 2019, 230, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Bialas, A.; Niebrzydowska, P.; Dudek, B.; Piwowarska, Z.; Chmielarz, L.; Michalik, M.; Kozak, M.; Kuśtrowski, P. Coprecipitated Co–Al and Cu–Al oxide catalysts for toluene total oxidation. Catal. Today 2011, 176, 413–416. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Chirawatkul, P.; Kamonsuangkasem, K.; Chanlek, N.; Wantala, K. Structural characterizations of copper incorporated manganese oxide OMS-2 material and its efficiencies on toluene oxidation. Chem. Eng. Commun. 2022, 209, 512–528. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Song, Z.; Zhao, H.; Liu, W.; Zhao, M.; Zhao, J. Role of Cryptomelane in Surface-Adsorbed Oxygen and Mn Chemical Valence in MnOx during the Catalytic Oxidation of Toluene. J. Phys. Chem. C 2019, 123, 17255–17264. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, Y.; Leng, X.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Synthesis of CeaMnOx hollow microsphere with hierarchical structure and its excellent catalytic performance for toluene combustion. Appl. Catal. B Environ. 2019, 245, 502–512. [Google Scholar] [CrossRef]
- Legutko, P.; Fedyna, M.; Gryboś, J.; Yu, X.; Zhao, Z.; Adamski, A.; Kotarba, A.; Sojka, Z. Intricate role of doping with d0 ions (Zr4+, V5+, Mo6+, W6+) on cryptomelane (K-OMS-2) performance in the catalytic soot combustion in presence of NO and SO2. Fuel 2022, 328, 125325. [Google Scholar] [CrossRef]
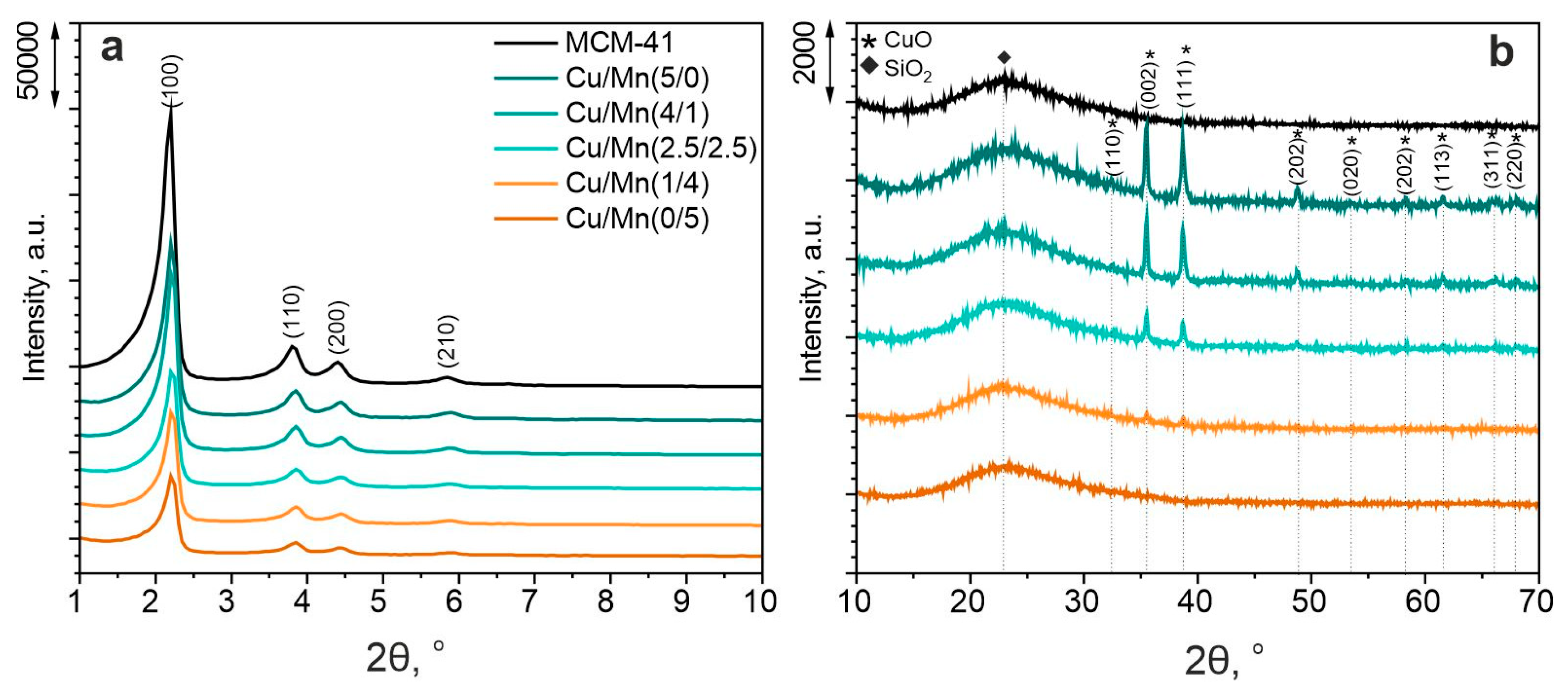
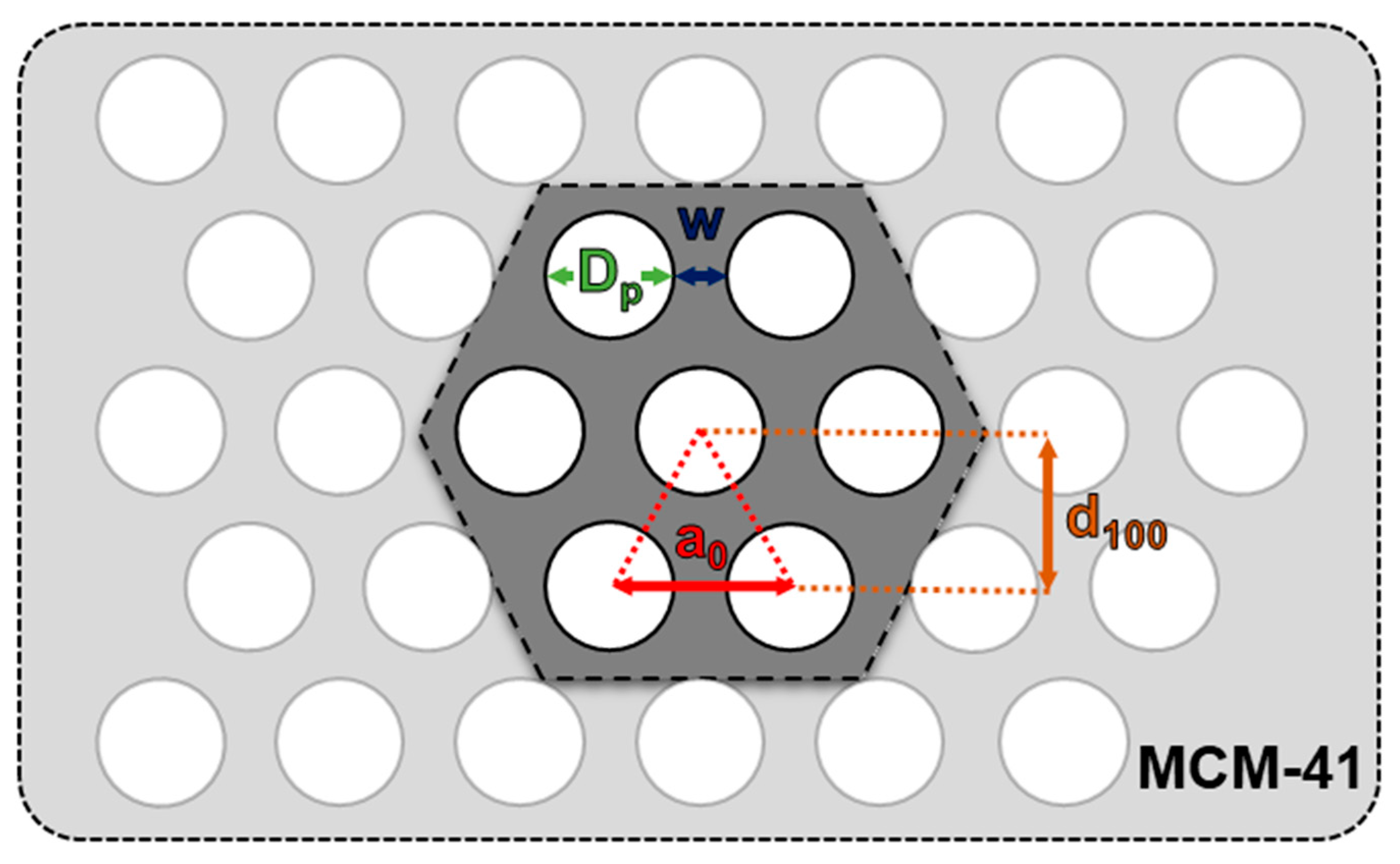
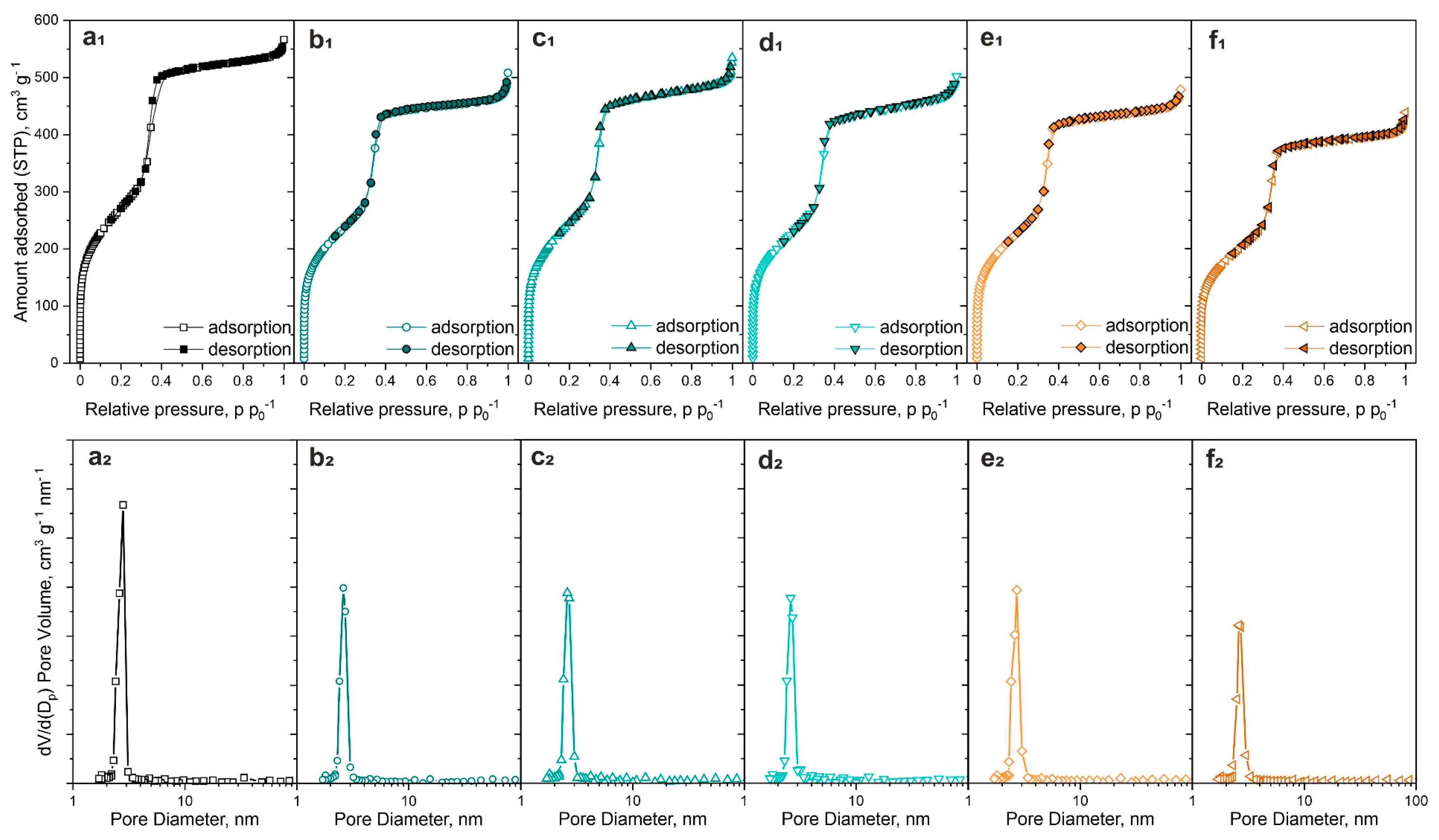
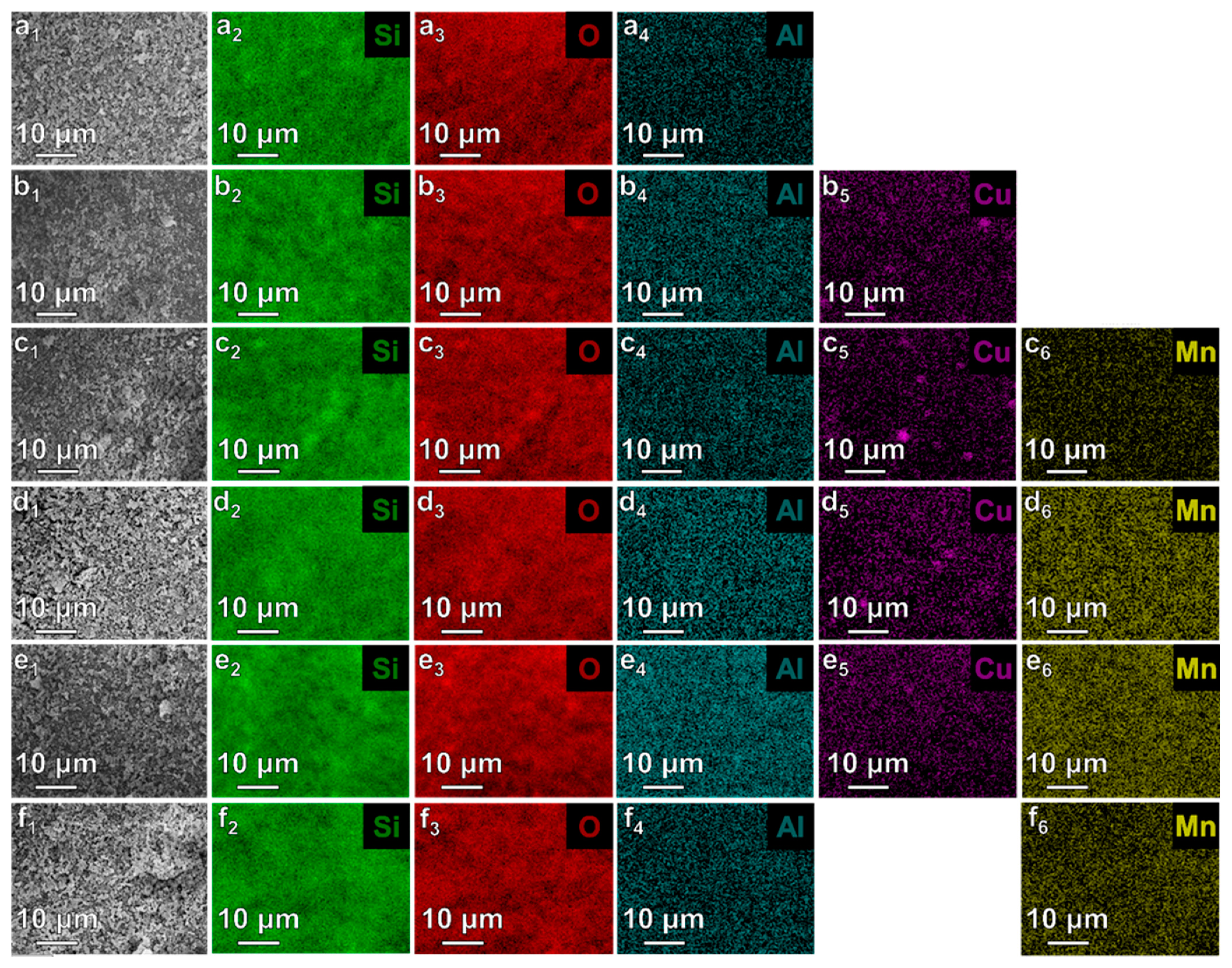

| Sample | SBET, m2 g−1 | Vt, cm3 g−1 | d100, nm | a0, nm | Dp, nm | w, nm |
|---|---|---|---|---|---|---|
| MCM-41 | 981 | 0.84 | 4.02 | 4.64 | 2.86 | 1.78 |
| Cu/Mn(5/0) | 904 | 0.78 | 4.02 | 4.64 | 2.82 | 1.82 |
| Cu/Mn(4/1) | 891 | 0.77 | 4.02 | 4.64 | 2.86 | 1.78 |
| Cu/Mn(2.5/2.5) | 840 | 0.74 | 4.02 | 4.64 | 2.94 | 1.70 |
| Cu/Mn(1/4) | 826 | 0.71 | 4.02 | 4.64 | 2.88 | 1.76 |
| Cu/Mn(0/5) | 744 | 0.64 | 4.02 | 4.64 | 2.84 | 1.80 |
| Sample | ||||||
|---|---|---|---|---|---|---|
| Element | MCM-41 | Cu/Mn(5/0) | Cu/Mn(4/1) | Cu/Mn(2.5/2.5) | Cu/Mn(1/4) | Cu/Mn(0/5) |
| Chemical composition as obtained from XRF, wt.% | ||||||
| Si | 45.76 ± 0.31 | 42.80 ± 0.24 | 44.07 ± 0.33 | 43.37 ± 0.11 | 44.19 ± 0.29 | 44.09 ± 0.19 |
| Mn | - | - | 1.12 ± 0.03 | 2.28 ± 0.08 | 4.07 ± 0.08 | 4.93 ± 0.09 |
| Cu | - | 4.94 ± 0.01 | 4.29 ± 0.05 | 2.52 ± 0.00 | 0.85 ± 0.02 | - |
| Mg | 1.26 ± 0.10 | 1.03 ± 0.08 | 1.31 ± 0.00 | 1.13 ± 0.02 | - | - |
| Chemical composition as obtained from EDS, wt.% | ||||||
| O | 62.9 ± 0.2 | 56.8 ± 0.2 | 62.2 ± 0.2 | 60.4 ± 0.3 | 61.0 ± 0.1 | 55.4 ± 0.1 |
| Si | 35.6 ± 0.1 | 36.9 ± 0.3 | 31.9 ± 0.3 | 33.0 ± 0.1 | 32.2 ± 0.2 | 36.7 ± 0.2 |
| Al | 1.4 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.2 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.2 |
| Cu | - | 4.7 ± 0.2 | 3.4 ± 0.2 | 2.9 ± 0.1 | 1.2 ± 0.1 | - |
| Mn | - | - | 0.9 ± 0.2 | 2.6 ± 0.1 | 3.9 ± 0.1 | 6.1 ± 0.3 |
| Element | C | O | Si | Mn | Cu | ||||
|---|---|---|---|---|---|---|---|---|---|
| Binding Energy, eV | 285.0 | 286.2 | 289.2 | 531.0 | 533.2 | 535.0 | 102.7 | 640.2 | 933.3 |
| MCM-41 | 6.7 | 1.2 | 0.3 | 1.2 | 57.4 | 4.0 | 29.2 | 0 | 0 |
| Cu/Mn(5/0) | 1.2 | 0.2 | 0 | 1.5 | 62.0 | 3.7 | 30.3 | 0 | 1.1 |
| Cu/Mn(2.5/2.5) | 6.9 | 1.7 | 0.7 | 1.8 | 57.4 | 3.0 | 27.9 | 0.4 | 0.1 |
| Cu/Mn(0/5) | 5.8 | 2.5 | 0.6 | 2.1 | 58.2 | 2.5 | 27.5 | 0.8 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrzycki, J.; Fedyna, M.; Duraczyńska, D.; Marzec, M.; Panek, R.; Franus, W.; Bajda, T.; Karcz, R. Mesoporous Silica MCM-41 from Fly Ash as a Support of Bimetallic Cu/Mn Catalysts for Toluene Combustion. Materials 2024, 17, 653. https://doi.org/10.3390/ma17030653
Mokrzycki J, Fedyna M, Duraczyńska D, Marzec M, Panek R, Franus W, Bajda T, Karcz R. Mesoporous Silica MCM-41 from Fly Ash as a Support of Bimetallic Cu/Mn Catalysts for Toluene Combustion. Materials. 2024; 17(3):653. https://doi.org/10.3390/ma17030653
Chicago/Turabian StyleMokrzycki, Jakub, Monika Fedyna, Dorota Duraczyńska, Mateusz Marzec, Rafał Panek, Wojciech Franus, Tomasz Bajda, and Robert Karcz. 2024. "Mesoporous Silica MCM-41 from Fly Ash as a Support of Bimetallic Cu/Mn Catalysts for Toluene Combustion" Materials 17, no. 3: 653. https://doi.org/10.3390/ma17030653
APA StyleMokrzycki, J., Fedyna, M., Duraczyńska, D., Marzec, M., Panek, R., Franus, W., Bajda, T., & Karcz, R. (2024). Mesoporous Silica MCM-41 from Fly Ash as a Support of Bimetallic Cu/Mn Catalysts for Toluene Combustion. Materials, 17(3), 653. https://doi.org/10.3390/ma17030653











