Phosphamide-Based Washing-Durable Flame Retardant for Cotton Fabrics
Abstract
1. Introduction
2. Experimental
2.1. Materials
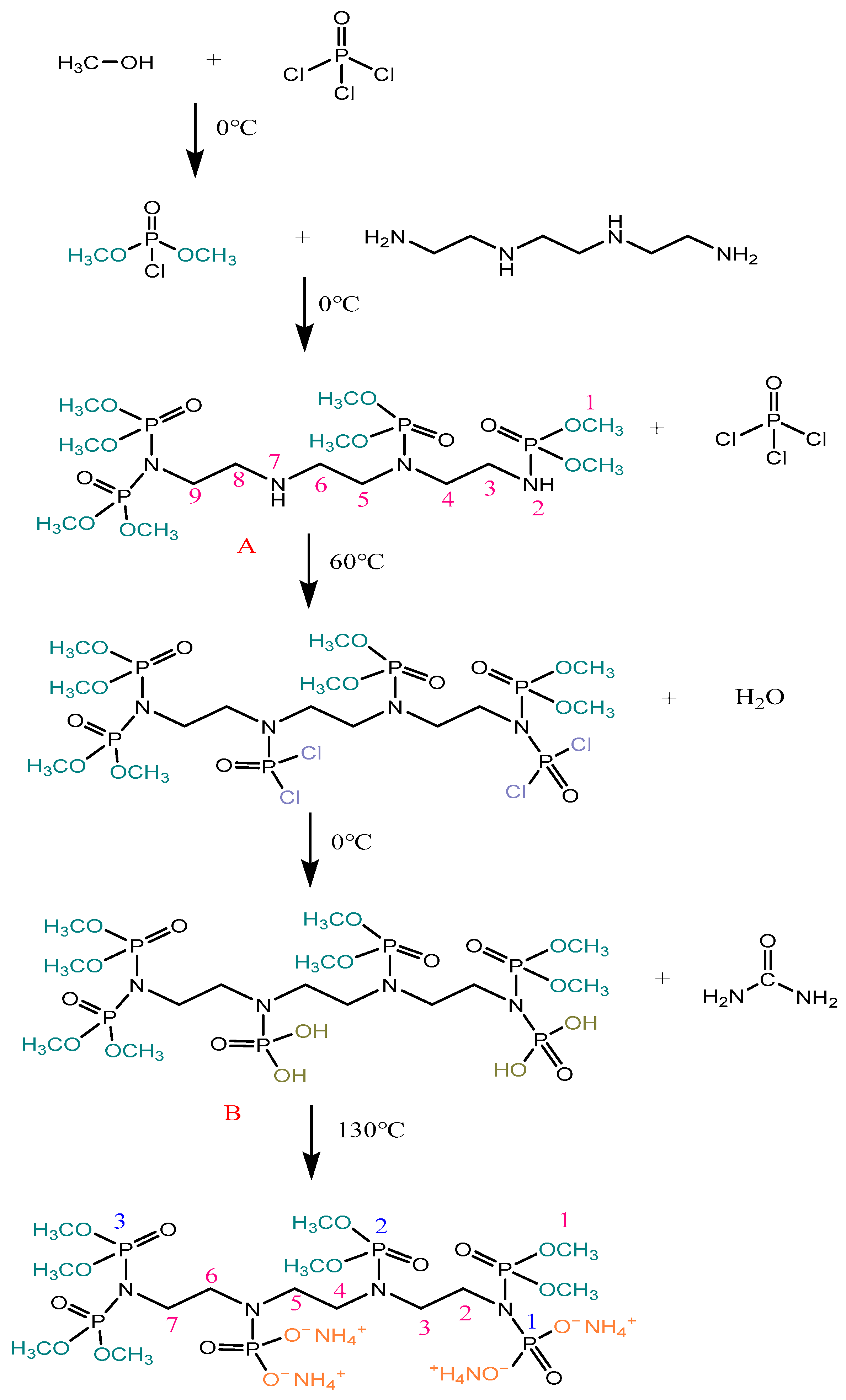
2.2. Synthesis of ATPEPDPA
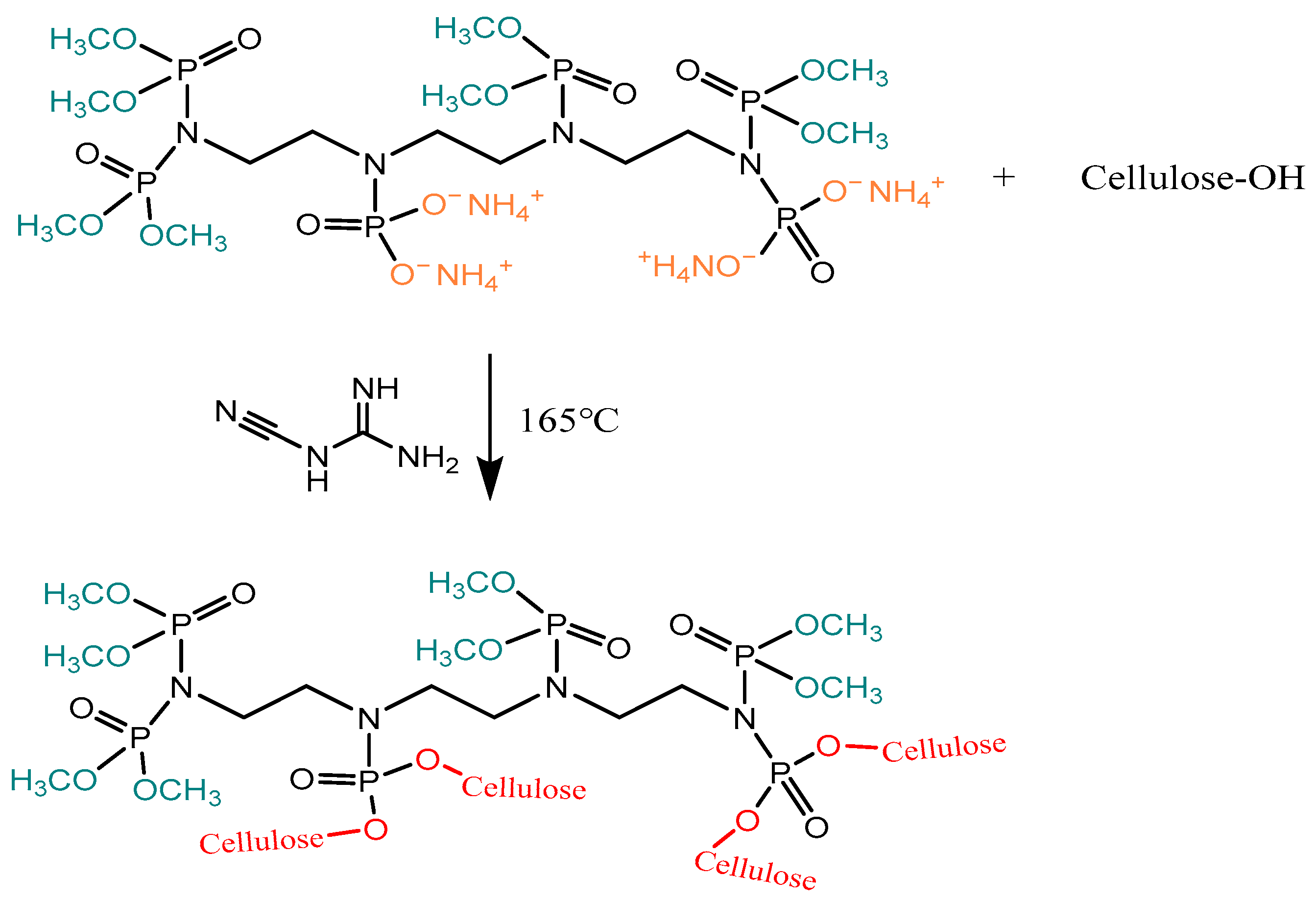
2.3. Preparation for ATPEPDPA-Treated Cotton
2.4. Characterization
3. Results and Discussion
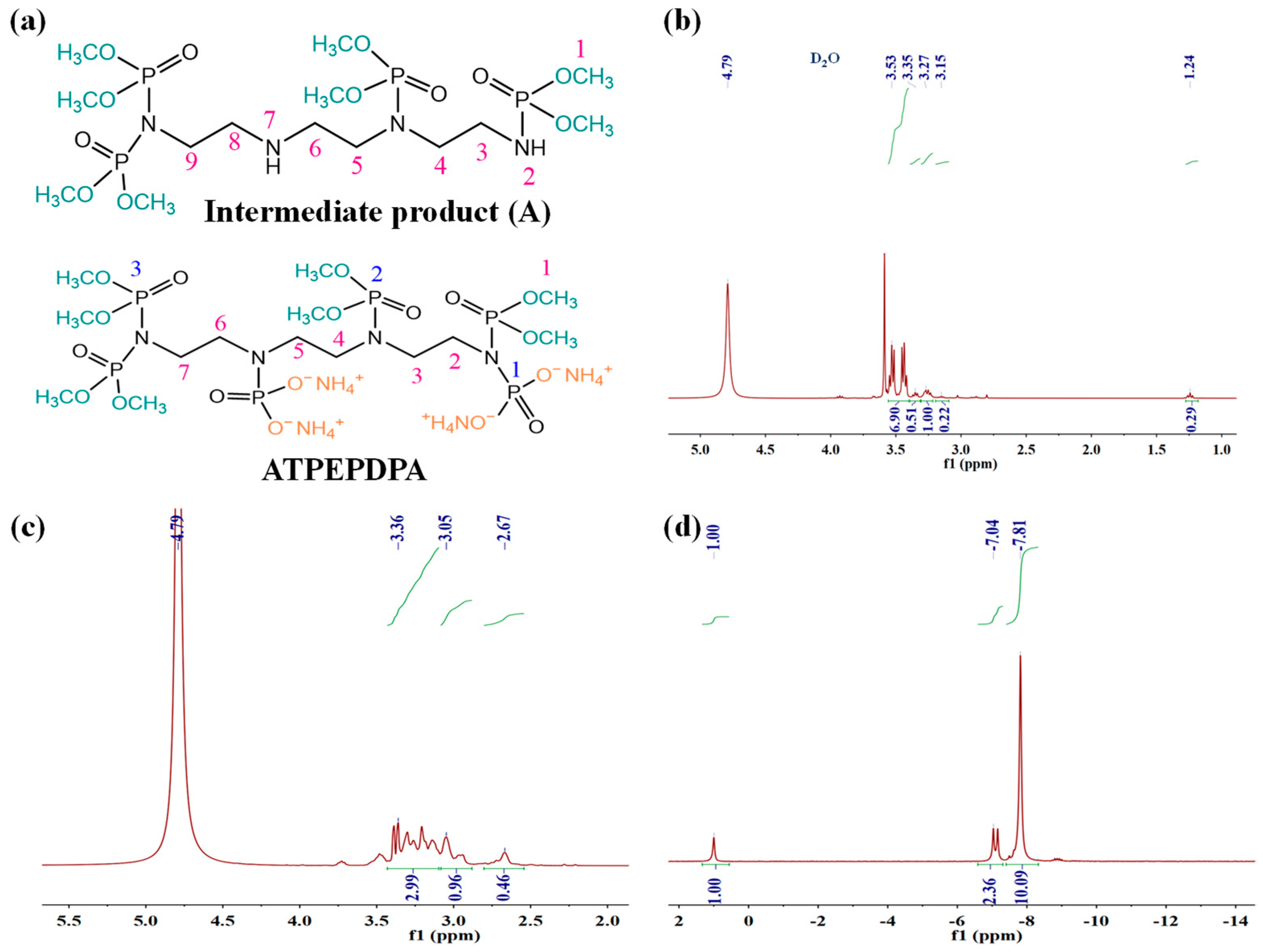
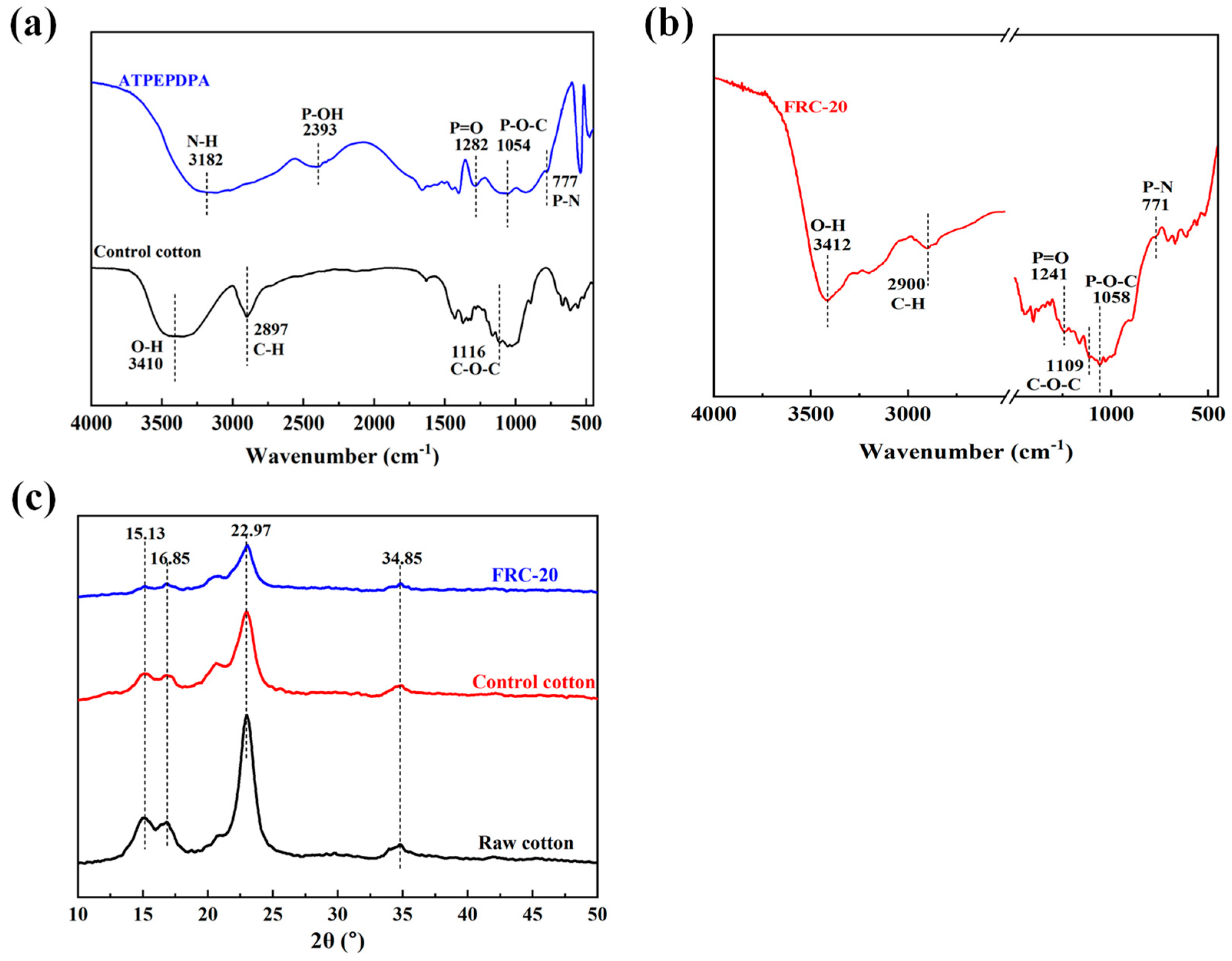
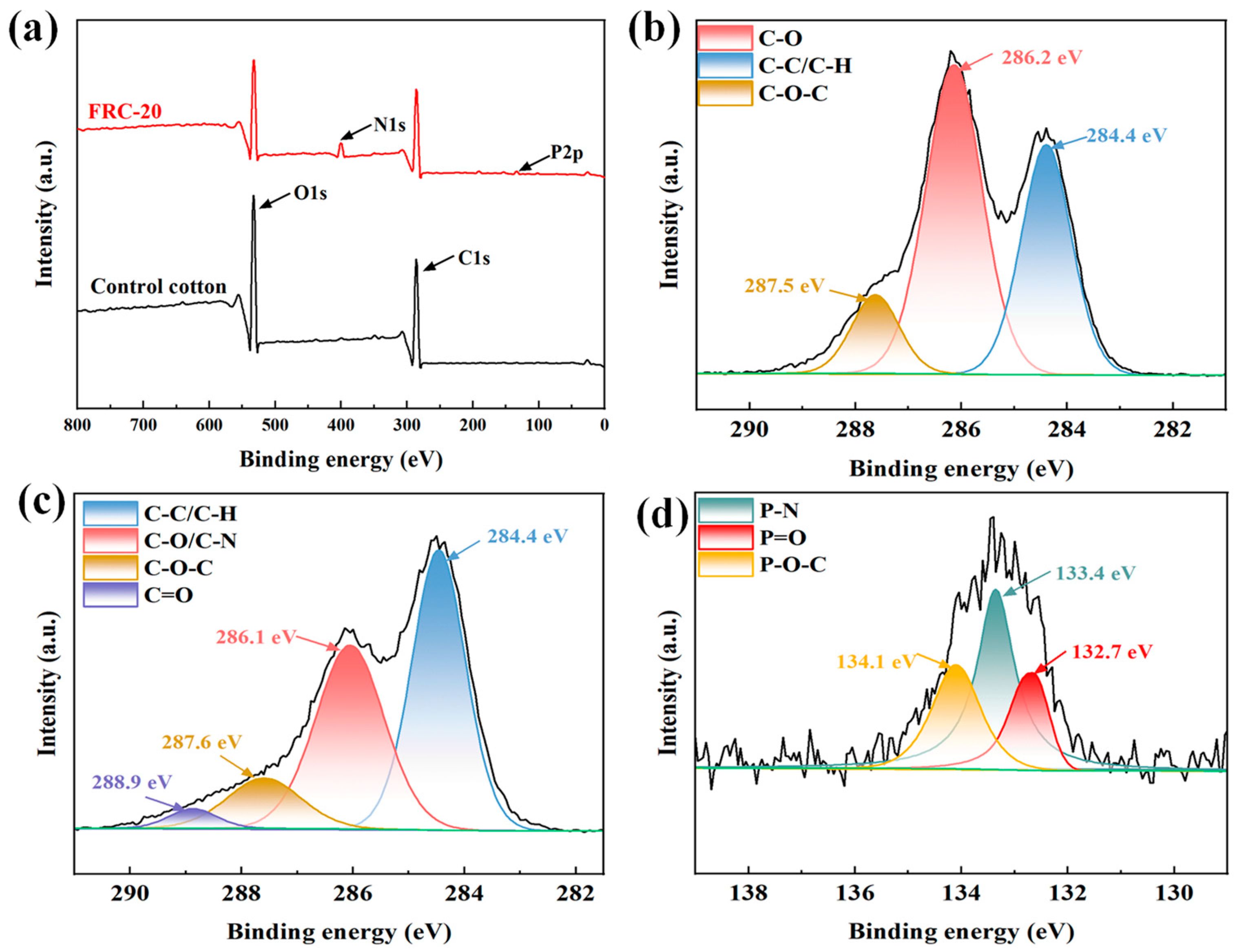
3.1. Structural Analysis of Samples
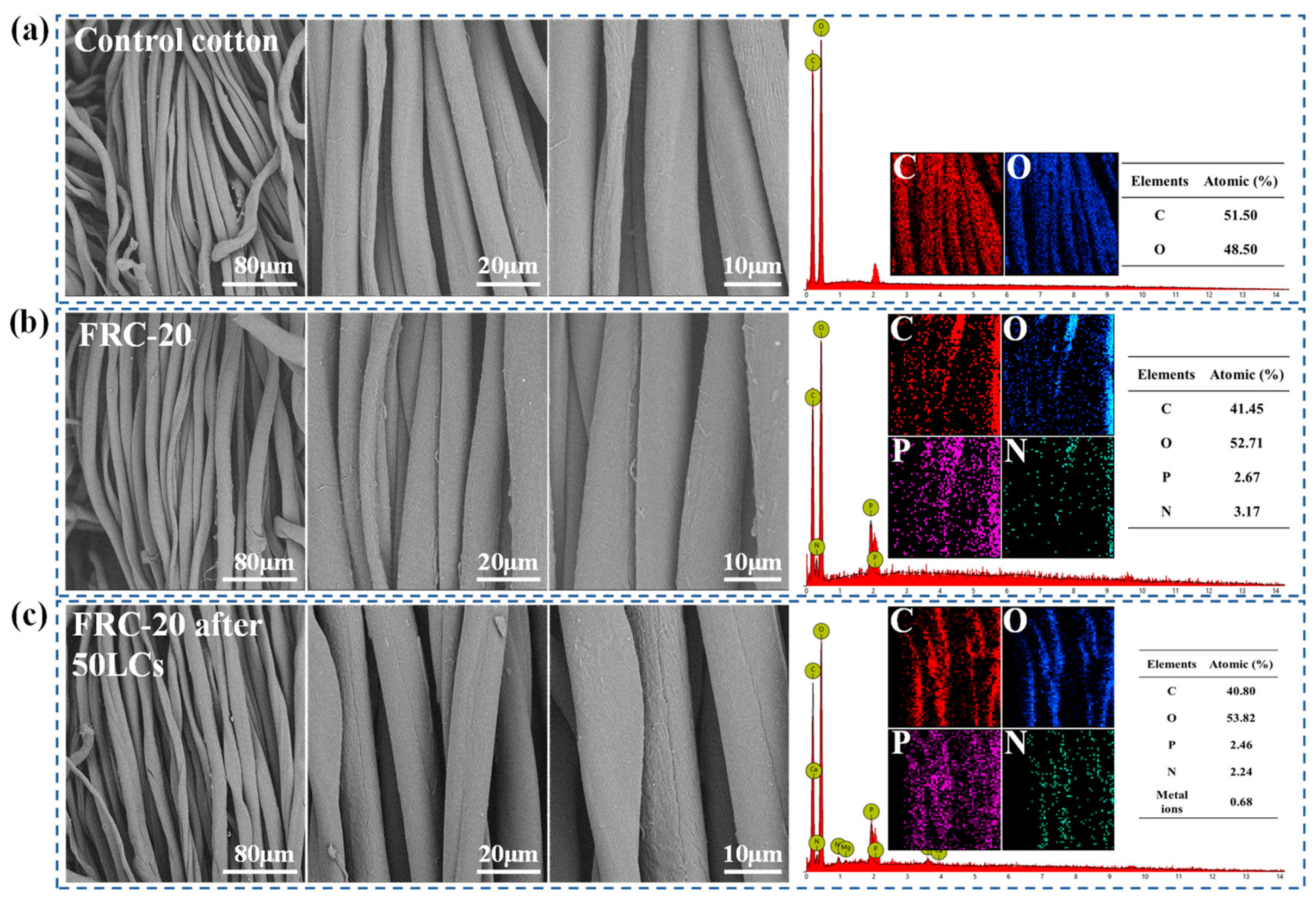
3.2. Characterization of Surface Morphology and Element
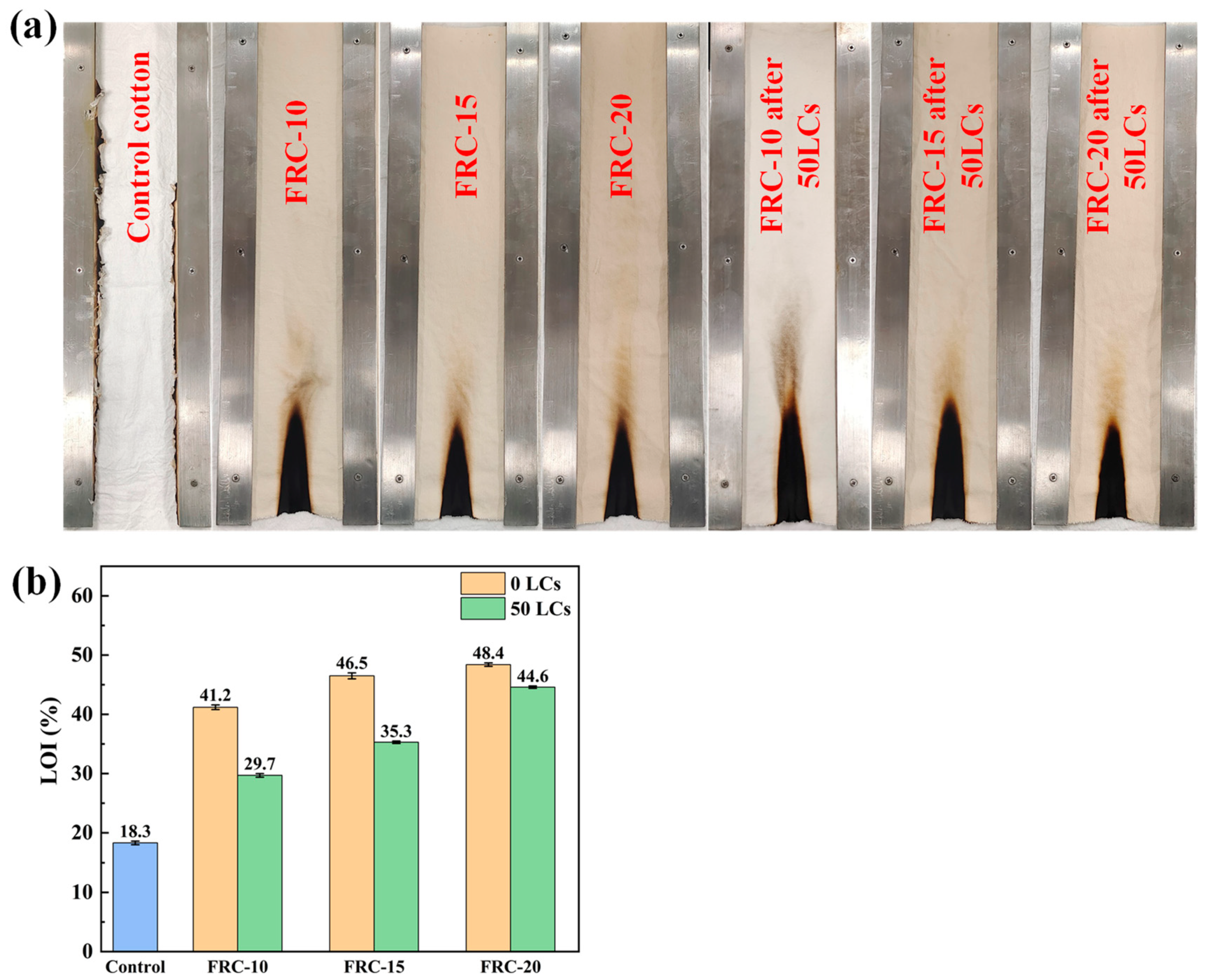
3.3. Flame Resistance and Durability of Samples
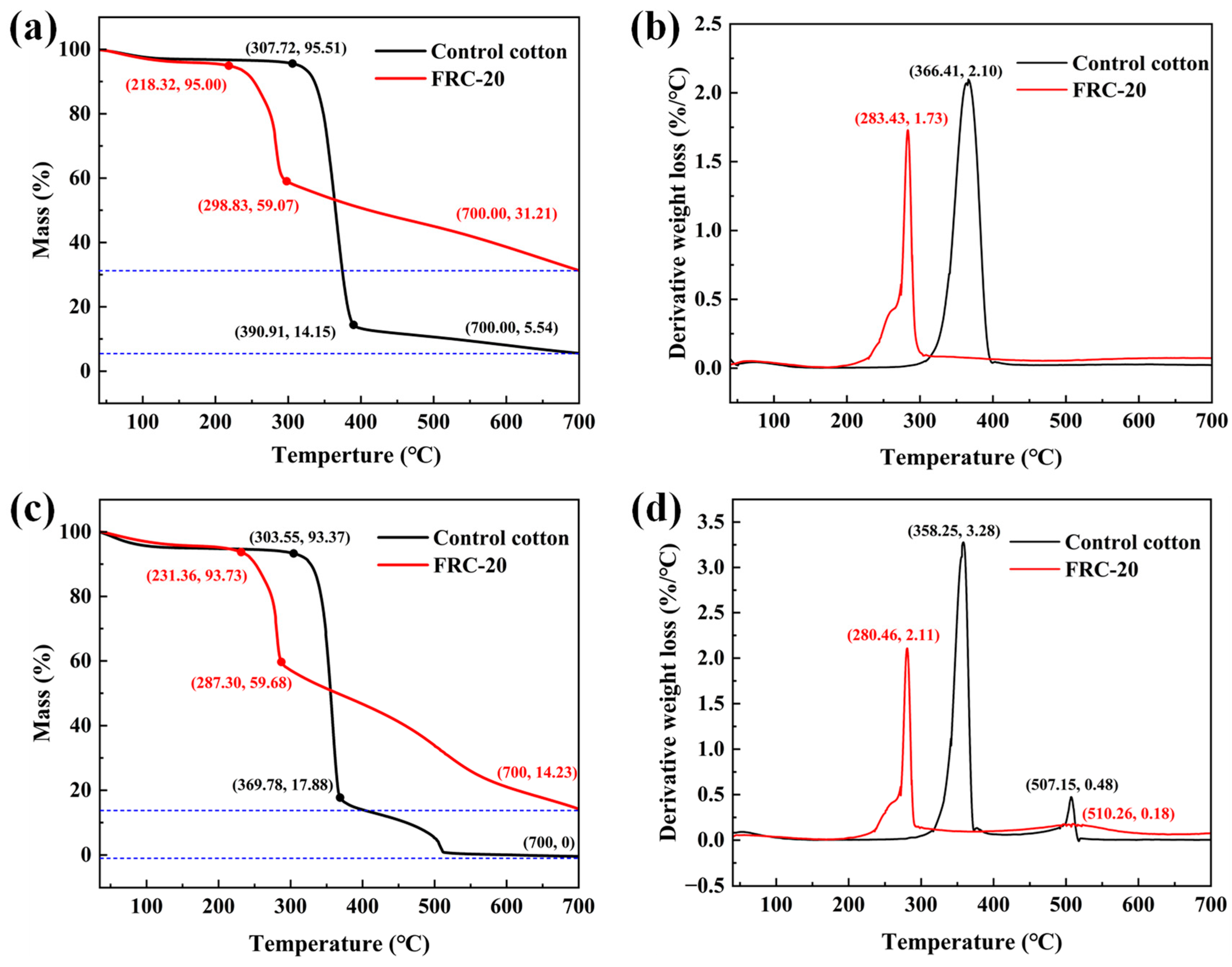
3.4. Thermogravimetric Analysis
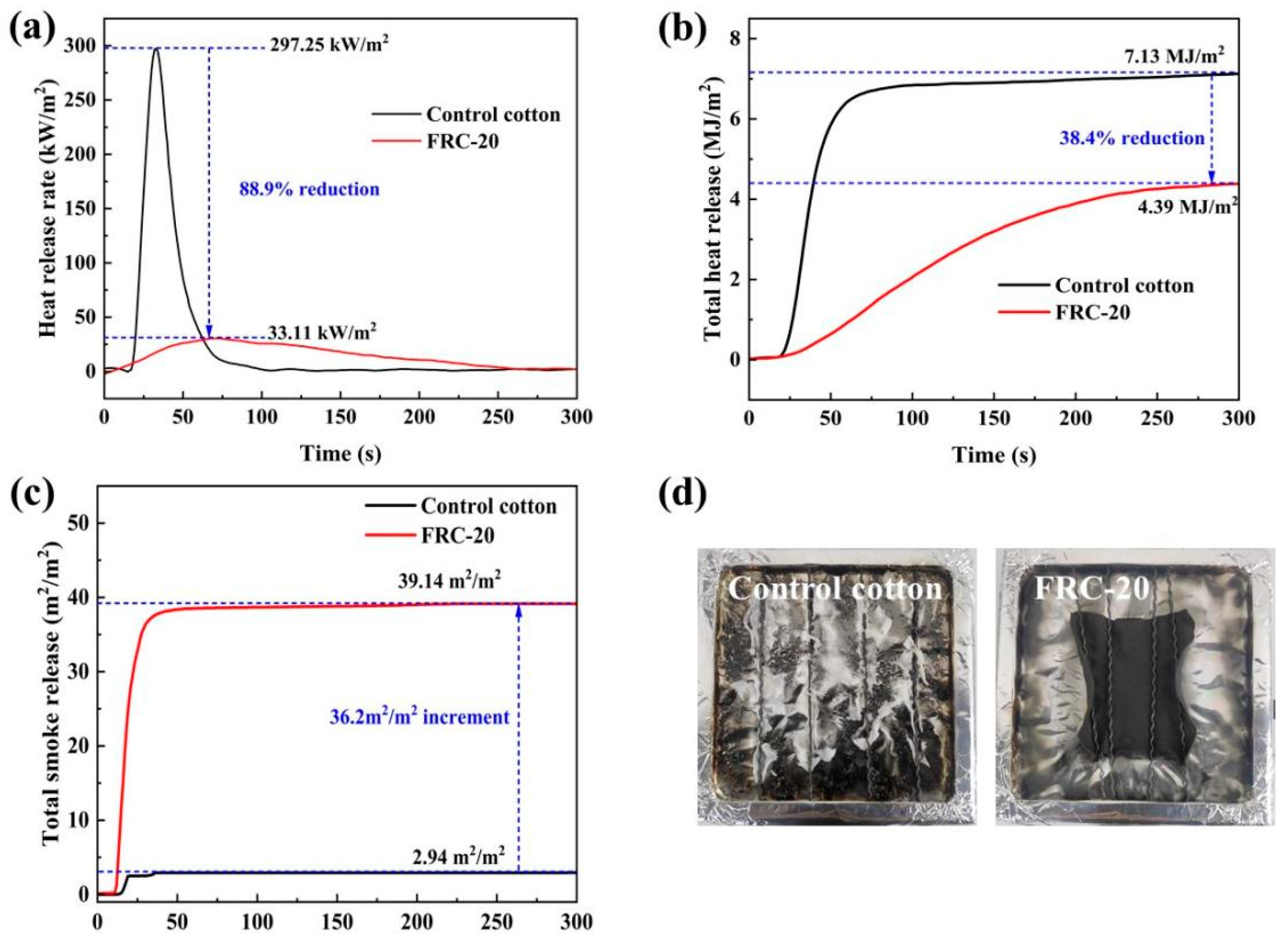
3.5. Combustion Characteristics
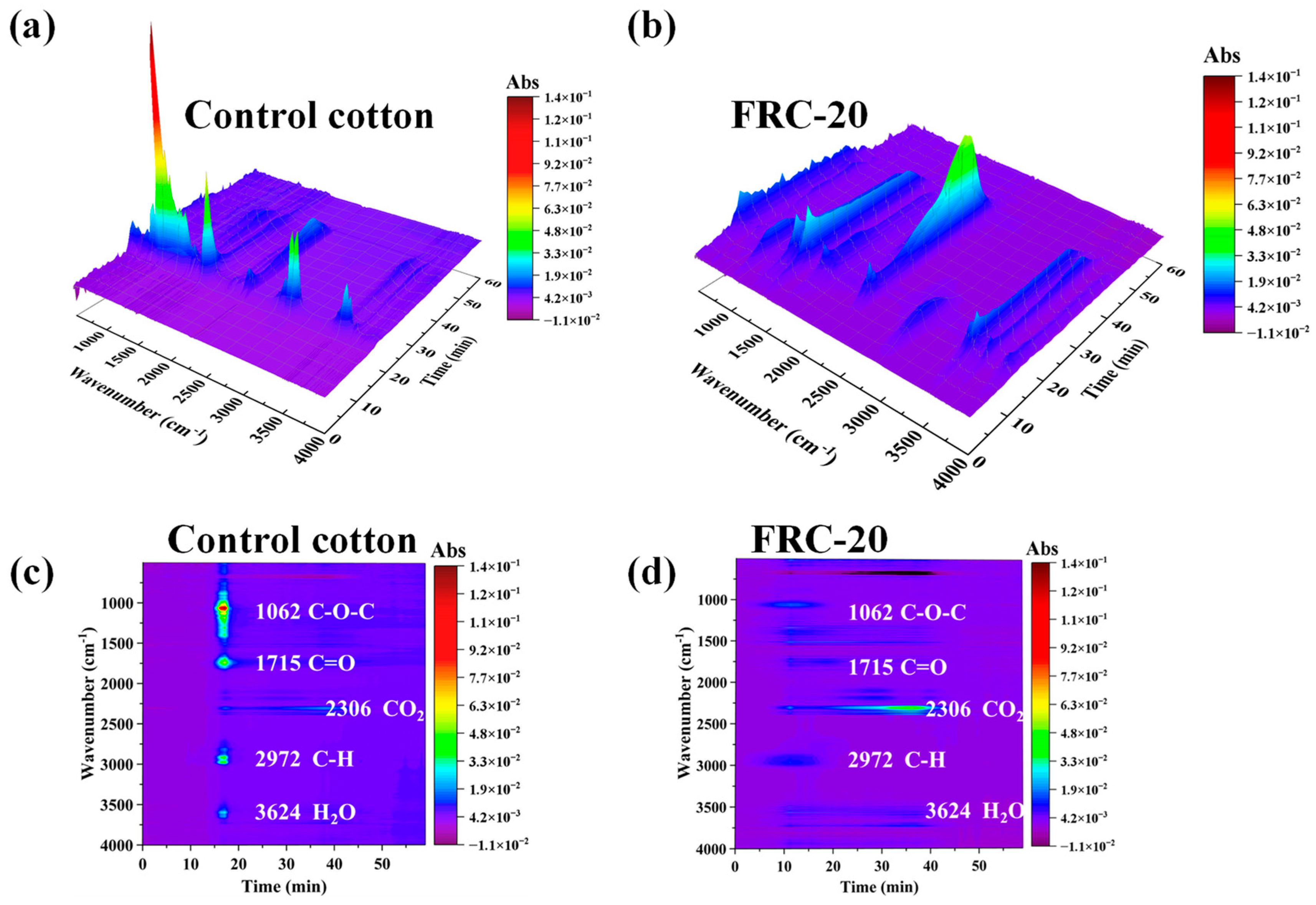
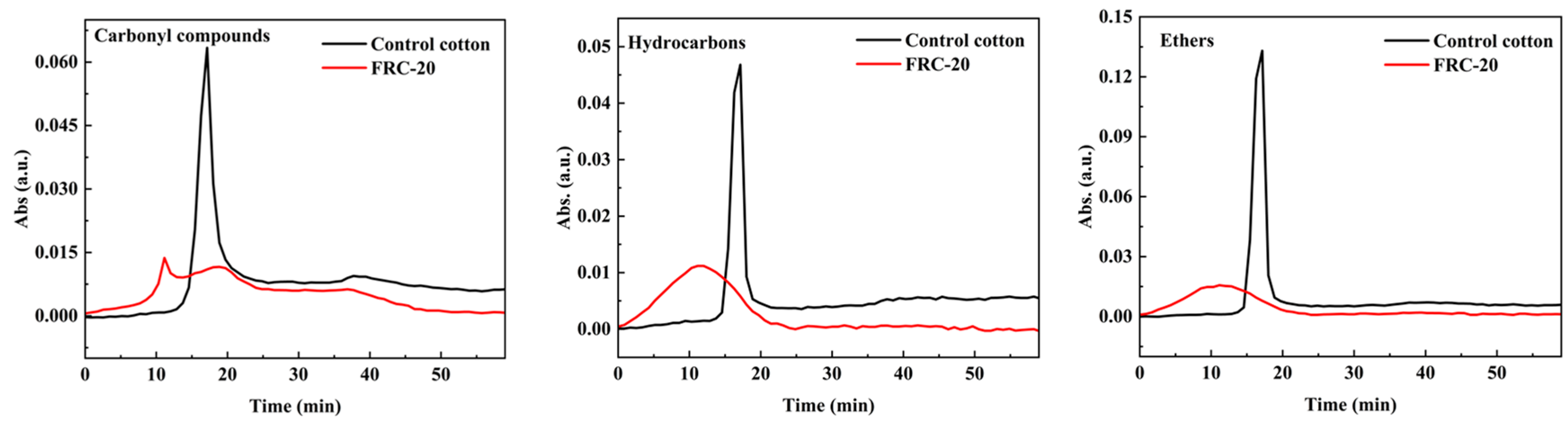
3.6. TG-FTIR Analyses
3.7. Analysis of Char Residues
3.8. Analysis of Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambekar, R.S.; Deshmukh, A.; Suárez-Villagrán, M.Y.; Das, R.; Pal, V.; Dey, S.; Miller, J.H.; Machado, L.D.; Kumbhakar, P.; Tiwary, C.S. 2D Hexagonal Boron Nitride-Coated Cotton Fabric with Self-Extinguishing Property. ACS Appl. Mater. Interfaces 2020, 12, 45274–45280. [Google Scholar] [CrossRef]
- Enwerem, U.E.; Obasi, H.C.; Onuegbu, G.C.; Nwanonenyi, S.C.; Nwuzor, I.C. Effect of Surface Modification of Cotton Weaves on the Mechanical and Morphological Properties of Polyester Composites. J. Text. Inst. 2022, 113, 1497–1508. [Google Scholar] [CrossRef]
- Özer, M.S.; Gaan, S. Recent Developments in Phosphorus Based Flame Retardant Coatings for Textiles: Synthesis, Applications and Performance. Prog. Org. Coat. 2022, 171, 107027. [Google Scholar] [CrossRef]
- Suryaprabha, T.; Sethuraman, M.G. Fabrication of a Superhydrophobic and Flame-Retardant Cotton Fabric Using a DNA-Based Coating. J. Mater. Sci. 2020, 55, 11959–11969. [Google Scholar] [CrossRef]
- Dalal, A.; Bagotia, N.; Sharma, K.K.; Chatterjee, K.N.; Bansal, P.; Kumar, S. One Pot Facile Synthesis of Self-Extinguishable Metal Based Flame Retardant for Cotton Fabric. J. Nat. Fibers 2022, 19, 10475–10489. [Google Scholar] [CrossRef]
- Passauer, L. Thermal Characterization of Ammonium Starch Phosphate Carbamates for Potential Applications as Bio-Based Flame-Retardants. Carbohydr. Polym. 2019, 211, 69–74. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Yu, D. Preparation of a Reactive Flame Retardant and Its Finishing on Cotton Fabrics Based on Click Chemistry. RSC Adv. 2017, 7, 2044–2050. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Javeri, N.; Shekarriz, S. Flame Retardant Cotton Fibers Produced Using Novel Synthesized Halogen-Free Phosphoramide Nanoparticles. Carbohydr. Polym. 2015, 118, 183–198. [Google Scholar] [CrossRef]
- Lee, D.H.; Jang, J. Synergistic Flame-Retardant Finishing of Cotton Using Dichlorotriazinyl Phosphonate and Triethanolamine. Fibers Polym. 2020, 21, 343–349. [Google Scholar] [CrossRef]
- Battig, A.; González, K.I.G.; Schartel, B. Valorizing “Non-Vegan” Bio-Fillers: Synergists for Phosphorus Flame Retardants in Epoxy Resins. Polym. Degrad. Stab. 2022, 198, 109875. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Blosi, M.; Zanoni, I.; Costa, A.L. NanoTiO2@DNA Complex: A Novel Eco, Durable, Fire Retardant Design Strategy for Cotton Textiles. J. Colloid Interface Sci. 2019, 546, 174–183. [Google Scholar] [CrossRef]
- Malucelli, G. Flame-Retardant Systems Based on Chitosan and Its Derivatives: State of the Art and Perspectives. Molecules 2020, 25, 4046. [Google Scholar] [CrossRef]
- Song, F.; Zhao, Q.; Zhu, T.; Bo, C.; Zhang, M.; Hu, L.; Zhu, X.; Jia, P.; Zhou, Y. Biobased Coating Derived from Fish Scale Protein and Phytic Acid for Flame-Retardant Cotton Fabrics. Mater. Des. 2022, 221, 110925. [Google Scholar] [CrossRef]
- Alongi, J.; Di Blasio, A.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Kandola, B.; Camino, G. Thermal Degradation of DNA, an All-in-One Natural Intumescent Flame Retardant. Polym. Degrad. Stab. 2015, 113, 110–118. [Google Scholar] [CrossRef]
- Barbalini, M.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Phytic Acid and Biochar: An Effective All Bio-Sourced Flame Retardant Formulation for Cotton Fabrics. Polymers 2020, 12, 811. [Google Scholar] [CrossRef]
- Gou, T.; Wu, X.; Zhao, Q.; Chang, S.; Wang, P. Novel Phosphorus/Nitrogen-Rich Oligomer with Numerous Reactive Groups for Durable Flame-Retardant Cotton Fabric. Cellulose 2021, 28, 7405–7419. [Google Scholar] [CrossRef]
- Ling, C.; Guo, L. Preparation of a Flame-Retardant Coating Based on Solvent-Free Synthesis with High Efficiency and Durability on Cotton Fabric. Carbohydr. Polym. 2020, 230, 115648. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, F.; Chen, Y.; Huang, T.; Zhang, G. A Novel Durable Flame Retardant for Cotton Fabrics Based on Diethylenetriamine. Polym. Degrad. Stab. 2022, 195, 109796. [Google Scholar] [CrossRef]
- Ding, D.; Liu, Y.; Lu, Y.; Liao, Y.; Chen, Y.; Zhang, G.; Zhang, F. Highly Effective and Durable P-N Synergistic Flame Retardant Containing Ammonium Phosphate and Phosphonate for Cotton Fabrics. Polym. Degrad. Stab. 2022, 200, 109964. [Google Scholar] [CrossRef]
- ASTM D2863-2000; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM: West Conshohocken, PA, USA, 2000.
- ASTM D6413-99; Standard Test Method for Flame Resistance of Textiles (Vertical Test). ASTM: West Conshohocken, PA, USA, 1999.
- ISO 5660-1:2015; Reaction-to-Fire Tests: Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- ASTM D5035-06; Standard Test Method for Breaking Force and Elongation of Textile Fabrics (Strip Method). ASTM: West Conshohocken, PA, USA, 2006.
- ASTM D1388-96(2002); Standard Test Method for Stiffness of Fabrics. ASTM: West Conshohocken, PA, USA, 2002.
- Makhlouf, G.; Abdelkhalik, A.; Ameen, H. Preparation of Highly Efficient Chitosan-Based Flame Retardant Coatings with Good Antibacterial Properties for Cotton Fabrics. Prog. Org. Coat. 2022, 163, 106627. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Li, W.; Zhang, J.; Zhang, Z.; Lu, Z.; Zhu, P.; Dong, C. Preparation, Characterization and Testing of Flame Retardant Cotton Cellulose Material: Flame Retardancy, Thermal Stability and Flame-Retardant Mechanism. Cellulose 2021, 28, 3789–3805. [Google Scholar] [CrossRef]
- Zain, G.; Jordanov, I.; Bischof, S.; Magovac, E.; Šišková, A.O.; Vykydalová, A.; Kleinová, A.; Mičušík, M.; Mosnáčková, K.; Nováčiková, J.; et al. Flame-Retardant Finishing of Cotton Fabric by Surface-Initiated Photochemically Induced Atom Transfer Radical Polymerization. Cellulose 2023, 30, 2529–2550. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, C.; Liu, J.; Kong, D.; Sun, L.; Lu, Z. Preparation of a Synergistic Reactive Flame Retardant Based on Silicon, Phosphorus and Nitrogen and Its Application to Cotton Fabrics. Cellulose 2020, 27, 1799–1815. [Google Scholar] [CrossRef]
- Hokayem, K.A.; Hage, R.E.; Svecova, L.; Otazaghine, B.; Le Moigne, N.; Sonnier, R. Flame Retardant-Functionalized Cotton Cellulose Using Phosphonate-Based Ionic Liquids. Molecules 2020, 25, 1629. [Google Scholar] [CrossRef]
- Miao, Z.; Yan, D.; Zhang, T.; Yang, F.; Zhang, S.; Liu, W.; Wu, Z. High-Efficiency Flame Retardants of a P-N-Rich Polyphosphazene Elastomer Nanocoating on Cotton Fabric. ACS Appl. Mater. Interfaces 2021, 13, 32094–32105. [Google Scholar] [CrossRef]
- Xavier, J.R. Investigation of Anticorrosion, Flame Retardant and Mechanical Properties of Polyurethane/GO Nanocomposites Coated AJ62 Mg Alloy for Aerospace/Automobile Components. Diam. Relat. Mater. 2023, 136, 110025. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, B.T.; Phan, H.T.Q.; Pham, L.H.; Hoang, C.N.; Nguyen, N.N.; Lee, P.C.; Kang, S.J.; Kim, J.; Hoang, D.Q. Highly Efficient Fire Retardant Behavior, Thermal Stability, and Physicomechanical Properties of Rigid Polyurethane Foam Based on Recycled Poly(Ethylene Terephthalate). J. Appl. Polym. Sci. 2020, 137, 49110. [Google Scholar] [CrossRef]
- Ali, W.; Zilke, O.; Danielsiek, D.; Salma, A.; Assfour, B.; Shabani, V.; Caglar, S.; Phan, H.M.; Kamps, L.; Wallmeier, R.; et al. Flame-Retardant Finishing of Cotton Fabrics Using DOPO Functionalized Alkoxy- and Amido Alkoxysilane. Cellulose 2023, 30, 2627–2652. [Google Scholar] [CrossRef]
- Wu, X.; Gou, T.; Zhao, Q.; Chen, L.; Wang, P. High-Efficiency Durable Flame Retardant with Ammonium Phosphate Ester and Phosphine Oxide Groups for Cotton Cellulose Biomacromolecule. Int. J. Biol. Macromol. 2022, 194, 945–953. [Google Scholar] [CrossRef]
- Piao, J.; Ren, J.; Wang, Y.; Feng, T.; Wang, Y.; Liu, W.; Dong, H.; Chen, W.; Jiao, C.; Chen, X. Green P–N Coating by Mechanochemistry: Efficient Flame Retardant for Cotton Fabric. Cellulose 2022, 29, 2711–2729. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Zheng, D.; Zhang, G.; Zhang, F.; Liang, Y. Synthesis and Evaluation of an Efficient, Durable, and Environmentally Friendly Flame Retardant for Cotton. Cellulose 2017, 24, 1159–1170. [Google Scholar] [CrossRef]
- Ding, D.; Liu, Y.; Lu, Y.; Chen, Y.; Liao, Y.; Zhang, G.; Zhang, F. A Formaldehyde-Free P-N Synergistic Flame Retardant Containing Phosphonate and Ammonium Phosphate for Cotton Fabrics. J. Nat. Fibers 2022, 19, 13337–13347. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, P.; Chen, Y.; Huang, T.; Liu, Y.; Ding, D.; Zhang, G. A Bio-Based Macromolecular Phosphorus-Containing Active Cotton Flame Retardant Synthesized from Starch. Carbohydr. Polym. 2022, 298, 120076. [Google Scholar] [CrossRef]
- Tu, J.; Mao, T.; Xie, S.; Guan, J.; Xiao, H.; Wang, P. Environmentally Benign Flame-Retardant Cotton Fabric with Superior Anti-Wrinkle Performance and Restorable Flame Retardancy. Ind. Crops Prod. 2022, 189, 115856. [Google Scholar] [CrossRef]
- Li, M.; Prabhakar, M.N.; Song, J.-l. Synthesis of Novel Si–P–N Complex Coating for the Fabrication of Flame-Retardant and Hydrophobic Cotton Textile Potentially Suitable for Rail Seat Cover. Prog. Org. Coatings 2022, 172, 107144. [Google Scholar] [CrossRef]
- Gu, J.; Yan, X.; Li, J.; Qian, Y.; Zhu, C.; Qi, D. Durable Flame-Retardant Behavior of Cotton Textile with a Water-Based Ammonium Vinyl Phosphonate. Polym. Degrad. Stab. 2021, 191, 109658. [Google Scholar] [CrossRef]
- Li, H.; Wen, D.; Wang, S.; Jiang, Z.; Zhu, P. Durable Multifunctional Cotton Fabric with Superior Biocidal Efficacy and Flame Retardancy Based on an Ammonium Phosphate N-Halamine. Int. J. Biol. Macromol. 2023, 253, 126812. [Google Scholar] [CrossRef]
- Furtana, S.; Mutlu, A.; Dogan, M. Thermal Stability and Flame Retardant Properties of Calcium- and Magnesium-Hypophosphite-Finished Cotton Fabrics and the Evaluation of Interaction with Clay and POSS Nanoparticles. J. Therm. Anal. Calorim. 2020, 139, 3415–3425. [Google Scholar] [CrossRef]
- Gholivand, K.; Faraghi, M.; Barzegari, A.; Fallah, N.; Dusek, M.; Eigner, V. Synthesis, Characterisation, Investigation of Flame Retardancy Properties and Thermal Stability of Two New Phosphoramides with Cotton Fabric. J. Anal. Appl. Pyrolysis 2022, 165, 105568. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, H.; Duan, C.; Wen, B.; Li, Z. Sulfathiazole Derivative with Phosphaphenanthrene Group: Synthesis, Characterization and Its High Flame-Retardant Activity on Epoxy Resin. Polym. Degrad. Stab. 2020, 173, 109078. [Google Scholar] [CrossRef]
- Jo, C.H.; Jang, Y.M.; Mun, D.H.; Yu, C.J.; Choe, C.G.; Ri, S.G. Preparation of Acrylic Emulsion Coating with Melamine Polyphosphate, Pentaerythritol and Titanium Dioxide for Flame Retardant Cotton/Polyethylene Terephthalate Blend Fabrics. Polym. Degrad. Stab. 2023, 214, 110366. [Google Scholar] [CrossRef]
- Sut, A.; Metzsch-Zilligen, E.; Großhauser, M.; Pfaendner, R.; Schartel, B. Synergy between Melamine Cyanurate, Melamine Polyphosphate and Aluminum Diethylphosphinate in Flame Retarded Thermoplastic Polyurethane. Polym. Test. 2019, 74, 196–204. [Google Scholar] [CrossRef]
- Tan, W.; Ren, Y.; Guo, Y.; Liu, Y.; Liu, X.; Qu, H. A Novel Multi-Claw Reactive Flame Retardant Derived from DOPO for Endowing Lyocell Fabric with High Effective Flame Retardancy. Cellulose 2022, 29, 6941–6962. [Google Scholar] [CrossRef]

| Samples | FRC-10 | FRC-15 | FRC-20 |
|---|---|---|---|
| FRC (wt%) | 10 | 15 | 20 |
| Weight gain (%) | 11.5 ± 0.2 | 16.0 ± 0.5 | 18.6 ± 0.3 |
| Samples | Weight Gain (%) | After-Flame Time (s) | After-Glow Time (s) | Char Length (cm) |
|---|---|---|---|---|
| Control cotton | - | 41 | 43 | 0 |
| FRC-10 | 11.5 ± 0.2 | 0 | 0 | 5.7 ± 0.2 |
| FRC-15 | 16.0 ± 0.5 | 0 | 0 | 5.1 ± 0.1 |
| FRC-20 | 18.6 ± 0.3 | 0 | 0 | 4.8 ± 0.2 |
| FRC-10 after 50 LCs | 8.0 ± 0.2 | 0 | 0 | 7.0 ± 0.3 |
| FRC-15 after 50 LCs | 11.3 ± 0.4 | 0 | 0 | 6.5 ± 0.1 |
| FRC-20 after 50 LCs | 14.0 ± 0.2 | 0 | 0 | 5.2 ± 0.2 |
| Samples | WG (%) | LOI (%) | P Atomic Percentage (%) | Durability | LOI (%) after 50 LCs | P % after 50 LCs | |
|---|---|---|---|---|---|---|---|
| Author | FRC-20 | 18.6 | 48.4 | 2.67 | AATCC 61-2013 3A | 44.6 | 2.46 |
| (Jia et al., 2017) [36] | APTTP-treated cotton | 22.4 | 43.8 | - | AATCC 61-2006 | 26.9 | - |
| (Ding et al., 2022) [37] | AATMPEG-treated cotton | 17.1 | 45.0 | 3.52 | AATCC 61-2013 2A | 33.0 | 3.14 |
| (Zhao et al., 2022) [18] | DETA-P-treated cotton | 20.1 | 40.7 | 2.84 | AATCC 61-2013 2A | 29.0 | 2.13 |
| (Lu et al., 2022) [38] | ASTP-treated cotton | 33.1 | 45.2 | 2.58 | AATCC 61-2013 2A | 32.1 | 2.30 |
| Samples | PHRR (kW/m2) | TPHRR (s) | FGR ((kW/m2)/s) | THR (MJ/m2) | CO2/CO | TSR (m2/m2) | Residue (%) |
|---|---|---|---|---|---|---|---|
| Control cotton | 297.25 | 33 | 9.01 | 7.13 | 101.62 | 2.94 | 5.0 |
| FRC-20 | 33.11 | 78 | 0.42 | 4.39 | 4.52 | 39.14 | 18.3 |
| Samples | Whiteness | Tensile Strength (N) | Bending Length (mm) | ||
|---|---|---|---|---|---|
| Warp | Weft | Warp | Weft | ||
| Control cotton | 90.1 | 1845 ± 10 | 1613 ± 7 | 53.0 ± 1.8 | 48.0 ± 1.9 |
| FRC-10 | 82.2 | 1441 ± 12 | 1294 ± 21 | 59.8 ± 0.9 | 49.7 ± 3.3 |
| FRC-15 | 80.2 | 1374 ± 9 | 1215 ± 15 | 61.2 ± 2.6 | 51.9 ± 0.7 |
| FRC-20 | 80.0 | 1273 ± 11 | 1176 ± 13 | 62.5 ± 1.2 | 52.7 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, G.; Zhang, F. Phosphamide-Based Washing-Durable Flame Retardant for Cotton Fabrics. Materials 2024, 17, 630. https://doi.org/10.3390/ma17030630
Li J, Zhang G, Zhang F. Phosphamide-Based Washing-Durable Flame Retardant for Cotton Fabrics. Materials. 2024; 17(3):630. https://doi.org/10.3390/ma17030630
Chicago/Turabian StyleLi, Jinhao, Guangxian Zhang, and Fengxiu Zhang. 2024. "Phosphamide-Based Washing-Durable Flame Retardant for Cotton Fabrics" Materials 17, no. 3: 630. https://doi.org/10.3390/ma17030630
APA StyleLi, J., Zhang, G., & Zhang, F. (2024). Phosphamide-Based Washing-Durable Flame Retardant for Cotton Fabrics. Materials, 17(3), 630. https://doi.org/10.3390/ma17030630






